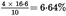ANNEXU.K.METHODS OF ANALYSIS OF THE COMPONENTS OF FEEDING-STUFFS
12.DETERMINATION OF SUGARU.K.
1.Purpose and scopeU.K.
This method makes it possible to determine the amount of reducing sugars and total sugars after inversion, expressed as glucose or where appropriate as sucrose, converting by the factor 0·95. It is applicable to compound feeding-stuffs. Special methods are provided for other feeding-stuffs. Where necessary, lactose should be measured separately and taken into account when calculating the results.
2.PrincipleU.K.
The sugars are extracted in dilute ethanol; the solution is clarified with Carrez solutions I and II. After eliminating the ethanol, the quantities before and after inversion are determined by the Luff-Schoorl method.
3.ReagentsU.K.
3.1.40% ethanol (v/v) d: 0·948 at 20 oC, neutralised to phenolphthalein.U.K.
3.2.Carrez solution I: dissolve in water 21·9 g of zinc acetate Zn (CH3 COO)2 · 2H2O and 3 g of glacial acetic acid. Make up to 100 ml with water.U.K.
3.3.Carrez solution II: dissolve in water 10·6 g of potassium ferrocyanide [X1K 4 Fe (CN) 6 · 3H 2 O]. Make up to 100 ml with water.U.K.
3.4.0·1% solution (w/v) of methyl orange.U.K.
3.5.Hydrochloric acid 4 N.U.K.
3.6.Hydrochloric acid 0·1 N.U.K.
3.7.Sodium hydroxide solution 0·1 N.U.K.
3.8.Luff-Schoorl reagent:U.K.
Stirring carefully, pour the citric acid solution (3.8.2) into the sodium carbonate solution (3.8.3). Add the copper sulphate solution (3.8.1) and make up to 1 litre with water. Leave to settle overnight and filter. Check the normality of the reagent thus obtained (Cu 0·1 N; Na2 CO3 2 N). The solution's pH should be approximately 9·4.
3.8.1.Copper sulphate solution: dissolve 25 g of copper sulphate A.R., Cu SO4 · 5H2O, free from iron, in 100 ml of water.U.K.
3.8.2.Citric acid solution: dissolve 50 g of citric acid A.R., C6H8O7 · H2O in 50 ml of water.U.K.
3.8.3.Sodium carbonate solution: dissolve 143·8 g of anhydrous sodium carbonate A.R. in approximately 300 ml of warm water. Leave to cool.U.K.
3.9.Sodium thiosulphate solution 0·1 N.U.K.
3.10.Starch solution: add a mixture of 5 g of soluble starch in 30 ml of water to 1 litre of boiling water. Boil for three minutes, leave to cool and if necessary add 10 mg of mercuric iodide as a preservative.U.K.
3.11.Sulphuric acid 6 N.U.K.
3.12.30% solution (w/v) of potassium iodide.U.K.
3.13.Granulated pumice stone boiled in hydrochloric acid, washed in water and dried.U.K.
3.14.3-methylbutan-l-ol.U.K.
4.ApparatusU.K.
Mixer (tumbler): approximately 35 to 40 rpm.
5.ProcedureU.K.
5.1.Extraction of sampleU.K.
Weigh 2.5 g of the sample to the nearest mg and place in a 250 ml volumetric flask. Add 200 ml of ethanol (3.1) and mix in the tumbler for one hour. Add 5 ml of Carrez solution I (3.2) and stir for one minute. Add 5 ml of Carrez solution II (3.3) and again stir for one minute. Make up to volume with ethanol (3.1), homogenise and filter. Remove 200 ml of the filtrate and evaporate to approximately half volume in order to eliminate most of the ethanol. Transfer the evaporation residue quantitatively to a 200 ml volumetric flask using warm water, cool, bring up to volume with water, homogenise and filter if necessary. This solution will be used to determine the amount of reducing sugars and, after inversion, of total sugars.
5.2.Determination of reducing sugarsU.K.
Using a pipette, remove not more than 25 ml of the solution containing less than 60 mg of reducing sugars expressed as glucose. If necessary, make up to 25 ml with distilled water and determine the content of reducing sugars by the Luff-Schoorl method. The result is expressed as the percentage content of glucose in the sample.
5.3.Determination of total sugars after inversionU.K.
Using a pipette take 50 ml of the solution and transfer to a 100 ml volumetric flask. Add a few drops of methyl orange solution (3.4) then, carefully and stirring continuously, add hydrochloric acid 4 N (3.5) until the liquid turns a definite red. Add 15 ml of hydrochloric acid 0·1 N (3.6), immerse the flask in a fast boiling water bath and keep there for thirty minutes. Cool rapidly to approximately 20 oC and add 15 ml of sodium hydroxide solution 0·1 N (3.7). Make up to 100 ml with water and homogenise. Remove not more than 25 ml containing less than 60 mg of reducing sugars expressed as glucose. If necessary, make up to 25 ml with distilled water and determine the content of reducing sugars by the Luff-Schoorl method. The result is expressed as the percentage of glucose or, where appropriate, sucrose, by multiplying by the factor 0·95.
5.4.Titration by the Luff-Schoorl methodU.K.
Using a pipette, take 25 ml of Luff-Schoorl reagent (3.8) and transfer to a 300 ml Erlenmeyer flask; add exactly 25 ml of the clarified sugar solution. Add 2 granules of pumice stone (3.13), heat, stirring by hand, over a free flame of medium height and bring the liquid to the boil in approximately two minutes. Place the Erlenmeyer immediately on an asbestos-coated wire gauze with a hole approximately 6 cm in diameter under which a flame has been lit. The flame shall be regulated in such a way that only the base of the Erlenmeyer is heated. Fit a reflux condenser to the Erlenmeyer flask, Boil for exactly ten minutes. Cool immediately in cold water and after approximately five minutes titrate as follows:
Add 10 ml of potassium iodide solution (3.12) and immediately afterwards (carefully, because of the risk of abundant foaming), add 25 ml of sulphuric acid 6 N (3.11). Titrate with sodium thiosulphate solution 0·1 N (3.9) until a dull yellow colour appears, add the starch indicator (3.10) and complete titration.
Carry out the same titration on an accurately measured mixture of 25 ml of Luff-Schoorl reagent (3.8) and 25 ml of water, after adding 10 ml of potassium iodide solution (3.12) and 25 ml of sulphuric acid 6 N (3.11) without boiling.
6.Calculation of resultsU.K.
Using the table establish the amount of glucose in mg which corresponds to the difference between the values of the two titrations, expressed in mg of sodium thiosulphate 0·1 N.
Express the result as a percentage of the sample.
7.Special proceduresU.K.
7.1.In the case of feeding-stuffs which are rich in molasses and other feeding-stuffs which are not particularly homogeneous, weigh out 20 g and place with 500 ml of water in a 1 litre volumetric flask. Mix for one hour in the tumbler. Clarify using Carrez 1 (3.2) and II (3.3) reagents as described under 5.1, this time however using four times the quantities of each reagent. Bring up to volume with 80% ethanol (v/v).U.K.
Homogenise and filter. Eliminate the ethanol as described under 5.1. If there is no dextrinised starch, bring up to volume with distilled water.
7.2.In the case of molasses and straight feeding-stuffs which are rich in sugar and almost starch-free (carobs, dried beetroot cossettes etc.), weigh out 5 g, place in a 250 ml volumetric flask, add 200 ml of distilled water and mix in the tumbler for one hour, or more if necessary. Clarify using Carrez I (3.2) and II (3.3) reagents as described under 5.1. Bring up to volume with cold water, homogenise and filter. In order to determine the amount of total sugars, continue as described under 5.3.U.K.
8.ObservationsU.K.
8.1.In order to prevent foaming it is advisable to add (irrespective of the volume) approximately 1 ml of 3-methylbutan-l-ol (3.14) before boiling with Luff-Schoorl reagent.U.K.
8.2.The difference between the content of total sugars after inversion, expressed as glucose, and the content of reducing sugars, expressed as glucose, multiplied by 0·95, gives the percentage content of sucrose.U.K.
8.3.In order to determine the content of reducing sugars, excluding lactose, two methods may be adopted:U.K.
8.3.1.for an approximate calculation, multiply by 0·675 the lactose content established by a different method of analysis and subtact the result obtained from the content of reducing sugars.U.K.
8.3.2.For an accurate calculation of reducing sugars, excluding lactose, the same sample must be used for the two final determinations. One of the analyses is carried out on part of the solution obtained under 5.1, the other on part of the solution obtained during the determination of lactose by the method laid down for that purpose (after fermenting the other types of sugar and clarifying).U.K.
In both cases the amount of sugar present is determined by the Luff-Schoorl method and calculated in mg of glucose. One of the values is subtracted from the other and the difference is expressed as a percentage of the sample.
Example
The two volumes taken correspond, for each determination, to a sample of 250 mg.
In the first case 17 ml of sodium thiosulphate solution 0·1 N corresponding to 44·2 mg of glucose is consumed; in the second, 11 ml, corresponding to 27·6 mg of glucose.
The difference is 16·6 mg of glucose.
The content of reducing sugars (excluding lactose), calculated as glucose, is therefore:
Table of values for 25 ml of Luff Schoorl reagent
ml of Na2 S2 O3 0·1 N, two minutes'heating, ten minutes' boiling
| Na2 S2 O30·1 N | Glucose, fructose invert sugarsC6 H12 O6 | LactoseC12 H22 O11 | MaltoseC12 H22 O11 | Na2 S2 O30·1 N | |||
|---|---|---|---|---|---|---|---|
| ml | mg | difference | mg | difference | mg | difference | ml |
| 1 | 2·4 | 2·4 | 3·6 | 3·7 | 3·9 | 3·9 | 1 |
| 2 | 4·8 | 2·4 | 7·3 | 3·7 | 7·8 | 3·9 | 2 |
| 3 | 7·2 | 2·5 | 11·0 | 3·7 | 11·7 | 3·9 | 3 |
| 4 | 9·7 | 2·5 | 14·7 | 3·7 | 15·6 | 4·0 | 4 |
| 5 | 12·2 | 2·5 | 18·4 | 3·7 | 19·6 | 3·9 | 5 |
| 6 | 14·7 | 2·5 | 22·1 | 3·7 | 23·5 | 4·0 | 6 |
| 7 | 17·2 | 2·6 | 25·8 | 3·7 | 27·5 | 4·0 | 7 |
| 8 | 19·8 | 2·6 | 29·5 | 3·7 | 31·5 | 4·0 | 8 |
| 9 | 22·4 | 2·6 | 33·2 | 3·8 | 35·5 | 4·0 | 9 |
| 10 | 25·0 | 2·6 | 37·0 | 3·8 | 39·5 | 4·0 | 10 |
| 11 | 27·6 | 2·7 | 40·8 | 3·8 | 43·5 | 4·0 | 11 |
| 12 | 30·3 | 2·7 | 44·6 | 3·8 | 47·5 | 4·1 | 12 |
| 13 | 33·0 | 2·7 | 48·4 | 3·8 | 51·6 | 4·1 | 13 |
| 14 | 35·7 | 2·8 | 52·2 | 3·8 | 55·7 | 4·1 | 14 |
| 15 | 38·5 | 2·8 | 56·0 | 3·9 | 59·8 | 4·1 | 15 |
| 16 | 41·3 | 2·9 | 59·9 | 3·9 | 63·9 | 4·1 | 16 |
| 17 | 44·2 | 2·9 | 63·8 | 3·9 | 68·0 | 4·2 | 17 |
| 18 | 47·1 | 2·9 | 67·7 | 4·0 | 72·2 | 4·3 | 18 |
| 19 | 50·0 | 3·0 | 71·7 | 4·0 | 76·5 | 4·4 | 19 |
| 20 | 53·0 | 3·0 | 75·7 | 4·1 | 80·9 | 4·5 | 20 |
| 21 | 56·0 | 3·1 | 79·8 | 4·1 | 85·4 | 4·6 | 21 |
| 22 | 59·1 | 3·1 | 83·9 | 4·1 | 90·0 | 4·6 | 22 |
| 23 | 62·2 | 88·0 | 94·6 | 23 | |||