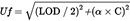- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Pwynt Penodol mewn Amser (13/03/2010)
- Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE)
Commission Regulation (EC) No 401/2006Dangos y teitl llawn
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs (Text with EEA relevance)
You are here:
- Dangos Graddfa Ddaearyddol(e.e. Lloegr, Cymru, Yr Alban aca Gogledd Iwerddon)
- Dangos Llinell Amser Newidiadau
Rhagor o Adnoddau
PDF o Fersiynau Diwygiedig
- ddiwygiedig 01/07/20140.53 MB
- ddiwygiedig 13/03/20100.16 MB
Pan adawodd y DU yr UE, cyhoeddodd legislation.gov.uk ddeddfwriaeth yr UE a gyhoeddwyd gan yr UE hyd at ddiwrnod cwblhau’r cyfnod gweithredu (31 Rhagfyr 2020 11.00 p.m.). Ar legislation.gov.uk, mae'r eitemau hyn o ddeddfwriaeth yn cael eu diweddaru'n gyson ag unrhyw ddiwygiadau a wnaed gan y DU ers hynny.
Mae'r eitem hon o ddeddfwriaeth yn tarddu o'r UE
Mae legislation.gov.uk yn cyhoeddi fersiwn y DU. Mae EUR-Lex yn cyhoeddi fersiwn yr UE. Mae Archif Gwe Ymadael â’r UE yn rhoi cipolwg ar fersiwn EUR-Lex o ddiwrnod cwblhau’r cyfnod gweithredu (31 Rhagfyr 2020 11.00 p.m.).
Changes over time for: ANNEX II
Version Superseded: 01/07/2014
Alternative versions:
Status:
Point in time view as at 13/03/2010.
Changes to legislation:
There are currently no known outstanding effects by UK legislation for Commission Regulation (EC) No 401/2006, ANNEX II.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
ANNEX IIU.K.CRITERIA FOR SAMPLE PREPARATION AND FOR METHODS OF ANALYSIS USED FOR THE OFFICIAL CONTROL OF THE LEVELS OF MYCOTOXINS IN FOODSTUFFS
1.INTRODUCTIONU.K.
1.1.PrecautionsU.K.
As the distribution of mycotoxins is generally non-homogeneous, samples shall be prepared, and especially homogenised, with extreme care.
The complete sample as received by the laboratory shall be homogenized, in case the homogenisation is performed by the laboratory.
For the analysis of aflatoxins, daylight should be excluded as much as possible during the procedure, since aflatoxin gradually breaks down under the influence of ultra-violet light.
1.2.Calculation of proportion of shell/kernel of whole nutsU.K.
The limits fixed for aflatoxins in Regulation (EC) No 466/2001 apply to the edible part. The level of aflatoxins in the edible part can be determined by:
samples of nuts ‘in shell’ can be shelled and the level of aflatoxins is determined in the edible part.
the nuts ‘in shell’ can be taken through the sample preparation procedure. The method of sampling and analysis shall estimate the weight of nut kernel in the aggregate sample. The weight of nut kernel in the aggregate sample shall be estimated after establishing a suitable factor for the proportion of nut shell to nut kernel in whole nuts. This proportion is used to ascertain the amount of kernel in the bulk sample taken through the sample preparation and method of analysis.
Approximately 100 whole nuts shall be taken at random separately from the lot or shall be put aside from each aggregate sample. The ratio may, for each laboratory sample, be obtained by weighing the whole nuts, shelling and re-weighing the shell and kernel portions.
However, the proportion of shell to kernel may be established by the laboratory from a number of samples and so can be assumed for future analytical work. But if a particular laboratory sample is found to be in contravention of any limit, the proportion shall be determined for that sample using the approximately 100 nuts that have been set aside.
2.TREATMENT OF THE SAMPLE AS RECEIVED IN THE LABORATORYU.K.
Each laboratory sample shall be finely grinded and mixed thoroughly using a process that has been demonstrated to achieve complete homogenisation.
In case the maximum level applies to the dry matter, the dry matter content of the product shall be determined on a part of the homogenised sample, using a method that has been demonstrated to determine accurately the dry matter content.
3.REPLICATE SAMPLESU.K.
The replicate samples for enforcement, trade (defence) and reference (referee) purposes shall be taken from the homogenised material unless such procedure conflicts with Member States’ rules as regards the rights of the food business operator.
4.METHOD OF ANALYSIS TO BE USED BY THE LABORATORY AND LABORATORY CONTROL REQUIREMENTSU.K.
4.1.DefinitionsU.K.
A number of the most commonly used definitions that the laboratory shall be required to use are the following:
=
Repeatability, the value below which the absolute difference between two single test results obtained under repeatability conditions, namely same sample, same operator, same apparatus, same laboratory, and short interval of time may be expected to lie within a specific probability (typically 95 %) and hence r = 2,8 × sr.
=
Standard deviation, calculated from results generated under repeatability conditions.
=
Relative standard deviation, calculated from results generated under repeatability conditions [(sr / 
=
Reproducibility, the value below which the absolute difference between single test results obtained under reproducibility conditions, namely on identical material obtained by operators in different laboratories, using the standardised test method may be expected to lie within a certain probability (typically 95 %); R = 2,8 × sR.
=
Standard deviation, calculated from results under reproducibility conditions.
=
Relative standard deviation calculated from results generated under reproducibility conditions [(sR / 
4.2.General requirementsU.K.
Methods of analysis used for food control purposes shall comply with the provisions of items 1 and 2 of Annex III to Regulation (EC) No 882/2004.
4.3.Specific requirementsU.K.
4.3.1.Performance criteriaU.K.
Where no specific methods for the determination of mycotoxin levels in foodstuffs are required by Community legislation, laboratories may select any method provided the selected method meets the following criteria:
Performance criteria for aflatoxins
| Note: | |||
| |||
| Criterion | Concentration Range | Recommended Value | Maximum permitted Value |
|---|---|---|---|
| Blanks | All | Negligible | — |
| Recovery — Aflatoxin M1 | 0,01-0,05 μg/kg | 60 to 120 % | |
| > 0,05 μg/kg | 70 to 110 % | ||
| Recovery — Aflatoxins B1, B2, G1, G2 | < 1,0 μg/kg | 50 to 120 % | |
| 1-10 μg/kg | 70 to 110 % | ||
| > 10 μg/kg | 80 to 110 % | ||
| Precision RSDR | All | As derived from Horwitz Equation | 2 × value derived from Horwitz Equation |
| Precision RSDr may be calculated as 0,66 times Precision RSDR at the concentration of interest. | |||
Performance criteria for ochratoxin A
| Level μg/kg | Ochratoxin A | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| < 1 | ≤ 40 | ≤ 60 | 50 to 120 |
| 1-10 | ≤ 20 | ≤ 30 | 70 to 110 |
Performance criteria for patulin
| Level μg/kg | Patulin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| < 20 | ≤ 30 | ≤ 40 | 50 to 120 |
| 20-50 | ≤ 20 | ≤ 30 | 70 to 105 |
| > 50 | ≤ 15 | ≤ 25 | 75 to 105 |
Performance criteria for deoxynivalenol
| Level μg/kg | Deoxynivalenol | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| > 100-≤ 500 | ≤ 20 | ≤ 40 | 60 to 110 |
| > 500 | ≤ 20 | ≤ 40 | 70 to 120 |
Performance criteria for zearalenone
| Level μg/kg | Zearalenone | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| ≤ 50 | ≤ 40 | ≤ 50 | 60 to 120 |
| > 50 | ≤ 25 | ≤ 40 | 70 to 120 |
Performance criteria for Fumonisin B1 and B2
| Level μg/kg | Fumonisin B1 or B2 | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| ≤ 500 | ≤ 30 | ≤ 60 | 60 to 120 |
| > 500 | ≤ 20 | ≤ 30 | 70 to 110 |
Performance criteria for T-2 and HT-2 toxin
| Level μg/kg | T-2 toxin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| 50-250 | ≤ 40 | ≤ 60 | 60 to 130 |
| > 250 | ≤ 30 | ≤ 50 | 60 to 130 |
| Level μg/kg | HT-2 toxin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| 100-200 | ≤ 40 | ≤ 60 | 60 to 130 |
| > 200 | ≤ 30 | ≤ 50 | 60 to 130 |
Notes to the performance criteria for the mycotoxins
The detection limits of the methods used are not stated as the precision values are given at the concentrations of interest
The precision values are calculated from the Horwitz equation, i.e.:
RSDR = 2(1-0,5logC)
where:
This is a generalised precision equation which has been found to be independent of analyte and matrix but solely dependent on concentration for most routine methods of analysis.
4.3.2.‘Fitness-for-purpose’ approachU.K.
In the case where there are a limited number of fully validated methods of analysis, alternatively, a ‘fitness-for-purpose’ approach, defining a single parameter, a fitness function, to evaluate the acceptability of methods of analysis may be used. A fitness function is an uncertainty function that specifies maximum levels of uncertainty regarded as fit for purpose.
Given the limited number of methods of analysis, fully validated by a collaborative trial, especially for the determination of T-2 and HT-2 toxin, the uncertainty function approach, specifying the maximum acceptable uncertainty, may also be used to assess the suitability (the ‘fitness-for-purpose’) of the method of analysis to be used by the laboratory. The laboratory may use a method which produces results within the maximum standard uncertainty. The maximum standard uncertainty may be calculated using the following formula:
where:
Uf is the maximum standard uncertainty (μg/kg)
LOD is the limit of detection of the method (μg/kg)
α is a constant, numeric factor to be used depending on the value of C. The values to be used are set out in the table hereafter
C is the concentration of interest (μg/kg).
If the analytical method provides results with uncertainty measurements less than the maximum standard uncertainty the method shall be considered being equally suitable to one which meets the performance criteria given in point 4.3.1.
| Table | |
| Numeric values to be used for α as constant in formula set out in this point, depending on the concentration of interest | |
| C (μg/kg) | α |
|---|---|
| ≤ 50 | 0,2 |
| 51-500 | 0,18 |
| 501-1 000 | 0,15 |
| 1 001-10 000 | 0,12 |
| > 10 000 | 0,1 |
4.4.Estimation of measurement uncertainty, recovery calculation and reporting of results(1) U.K.
The analytical result must be reported corrected or uncorrected for recovery. The manner of reporting and the level of recovery must be reported. The analytical result corrected for recovery shall be used for controlling compliance.
The analytical result must be reported as x +/– U whereby x is the analytical result and U is the expanded measurement uncertainty.
U is the expanded measurement uncertainty, using a coverage factor of 2 which gives a level of confidence of approximately 95 %.
For food of animal origin, the taking into account of the measurement uncertainty can also be done by establishing the decision limit (CCα) in accordance with Commission Decision 2002/657/EC(2) (point 3.1.2.5. of the Annex — the case of substances with established permitted limit).
The present interpretation rules of the analytical result in view of acceptance or rejection of the lot apply to the analytical result obtained on the sample for official control. In case of analysis for defence or referee purposes, the national rules apply.
4.5.Laboratory quality standardsU.K.
Laboratory must comply with the provisions of Article 12 of Regulation (EC) No 882/2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules(3).
More details on procedures for the estimation of measurement uncertainty and on procedures for assessing recovery can be found in the report ‘Report on the relationship between analytical results, measurement uncertainty, recovery factors and the provisions of EU food and feed legislation’ — http://europa.eu.int/comm/food/food/chemicalsafety/contaminants/report-sampling_analysis_2004_en.pdf
OJ L 221, 17.8.2002, p. 8. Decision as last amended by Decision 2004/25/EC (OJ L 6, 10.1.2004, p. 38).
See also the transitional arrangements provided for in article 18 of Commission Regulation (EC) No 2076/2005 of 5 December 2005 laying down transitional arrangements for the implementation of Regulation (EC) No 853/2004, 854/2004 and 882/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and 854/2004 (OJ L 338, 22.12.2005, p. 83).
Options/Cymorth
Print Options
PrintThe Whole Regulation
PrintThis Annex only
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE): Mae'r wreiddiol version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Pwynt Penodol mewn Amser: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
Gweler y wybodaeth ychwanegol ochr yn ochr â’r cynnwys
Rhychwant ddaearyddol: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Dangos Llinell Amser Newidiadau: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel adopted version that was used for the EU Official Journal
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Llinell Amser Newidiadau
Mae’r llinell amser yma yn dangos y fersiynau gwahanol a gymerwyd o EUR-Lex yn ogystal ag unrhyw fersiynau dilynol a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig.
Cymerir dyddiadau fersiynau’r UE o ddyddiadau’r dogfennau ar EUR-Lex ac efallai na fyddant yn cyfateb â’r adeg pan ddaeth y newidiadau i rym ar gyfer y ddogfen.
Ar gyfer unrhyw fersiynau a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig, bydd y dyddiad yn cyd-fynd â’r dyddiad cynharaf y daeth y newid (e.e. ychwanegiad, diddymiad neu gyfnewidiad) a weithredwyd i rym. Am ragor o wybodaeth gweler ein canllaw i ddeddfwriaeth ddiwygiedig ar Ddeall Deddfwriaeth.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel adopted fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill