- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Pwynt Penodol mewn Amser (18/04/2012)
- Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE)
Commission Regulation (EC) No 152/2009Dangos y teitl llawn
Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance)
You are here:
- Dangos Graddfa Ddaearyddol(e.e. Lloegr, Cymru, Yr Alban aca Gogledd Iwerddon)
- Dangos Llinell Amser Newidiadau
Rhagor o Adnoddau
PDF o Fersiynau Diwygiedig
- ddiwygiedig 16/11/20202.10 MB
- ddiwygiedig 24/05/20171.85 MB
- ddiwygiedig 26/04/20171.62 MB
- ddiwygiedig 17/07/20142.16 MB
- ddiwygiedig 01/01/20142.40 MB
- ddiwygiedig 12/02/20132.46 MB
- ddiwygiedig 18/04/20122.15 MB
Pan adawodd y DU yr UE, cyhoeddodd legislation.gov.uk ddeddfwriaeth yr UE a gyhoeddwyd gan yr UE hyd at ddiwrnod cwblhau’r cyfnod gweithredu (31 Rhagfyr 2020 11.00 p.m.). Ar legislation.gov.uk, mae'r eitemau hyn o ddeddfwriaeth yn cael eu diweddaru'n gyson ag unrhyw ddiwygiadau a wnaed gan y DU ers hynny.
Mae'r eitem hon o ddeddfwriaeth yn tarddu o'r UE
Mae legislation.gov.uk yn cyhoeddi fersiwn y DU. Mae EUR-Lex yn cyhoeddi fersiwn yr UE. Mae Archif Gwe Ymadael â’r UE yn rhoi cipolwg ar fersiwn EUR-Lex o ddiwrnod cwblhau’r cyfnod gweithredu (31 Rhagfyr 2020 11.00 p.m.).
Changes over time for: Division B.
Version Superseded: 17/07/2014
Status:
Point in time view as at 18/04/2012.
Changes to legislation:
There are currently no known outstanding effects by UK legislation for Commission Regulation (EC) No 152/2009, Division
B.
.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
[F1B. DETERMINATION OF THE LEVELS OF DIOXINS (PCDD/PCDF) AND PCBs U.K.
CHAPTER I U.K. Methods of sampling and interpretation of analytical results
1. Purpose and scope U.K.
The samples intended for the official control of the levels of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), dioxin-like polychlorinated biphenyls (PCBs) (1) and non-dioxin-like PCBs in feed shall be taken in accordance with the provisions of Annex I. The quantitative requirements in relation to the control of substances or products uniformly distributed throughout the feed as provided for in point 5.A of Annex I shall be applied. Aggregate samples thus obtained shall be considered representative for the lots or sub-lots from which they are taken. Compliance with maximum levels laid down in Directive 2002/32/EC shall be established on the basis of the levels determined in the laboratory samples.
For the purposes of this Part of Annex V, the definitions laid down in Annex I to Commission Decision 2002/657/EC of 14 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results (2) shall apply.
2. Compliance of the lot or sub-lot with the specification U.K.
2.1. As regards non-dioxin-like PCBs U.K.
The lot complies with the specification if the analytical result does not exceed the maximum level of non-dioxin-like PCBs laid down by Directive 2002/32/EC, taking into account the measurement uncertainty.
The lot does not comply with the specification if the upper-bound (3) analytical result confirmed by duplicate analysis (4) exceeds the maximum level laid down by Directive 2002/32/EC, taking into account the measurement uncertainty.
The measurement uncertainty shall be taken into account according to one of the following approaches:
by calculating the expanded uncertainty, using a coverage factor of 2 which gives a level of confidence of approximately 95 %. A lot or sub-lot is non-compliant if the measured value minus U is above the maximum level,
by establishing the decision limit (CCα) in accordance with point 3.1.2.5 of Annex I to Decision 2002/657/EC. A lot or sub-lot is non-compliant if the measured value is equal to or above the CCα.
These interpretation rules shall apply for the analytical result obtained on the sample for official control. In case of analysis for defence or reference purposes, the national rules shall apply.
2.2. As regards PCDD/F and dioxin-like PCBs U.K.
The lot complies with the specifications if the analytical result of a single analysis,
performed by a screening method with a false-compliant rate below 5 %, indicates that the level does not exceed the respective maximum level of PCDD/PCDFs and the sum of PCDD/PCDFs and dioxin-like PCBs laid down by Directive 2002/32/EC,
performed by a confirmatory method, does not exceed the respective maximum level of PCDD/PCDFs and the sum of PCDD/PCDFs and dioxin-like PCBs laid down by Directive 2002/32/EC, taking into account the measurement uncertainty.
For screening assays a cut-off value shall be established for decisions on sample compliance with the respective levels of interest set for either PCDD/PCDFs, or for the sum of PCDD/PCDFs and dioxin-like PCBs.
The lot does not comply with the specification if the upper-bound (5) analytical result obtained with a confirmatory method and confirmed by duplicate analysis exceeds the maximum level laid down by Directive 2002/32/EC, taking into account the measurement uncertainty (6) .
The measurement uncertainty shall be taken into account according to one of the following approaches:
by calculating the expanded uncertainty, using a coverage factor of 2 which gives a level of confidence of approximately 95 %. A lot or sub-lot is non-compliant if the measured value minus U is above the maximum level. In case of a separate determination of PCDD/PCDFs and dioxin-like-PCBs, the sum of the estimated expanded uncertainty of the separate analytical results of PCDD/PCDFs and dioxin-like PCBs shall be used for the sum of PCDD/PCDFs and dioxin-like PCBs,
by establishing the decision limit (CCα) in accordance with point 3.1.2.5 of the Annex I to Decision 2002/657/EC. A lot or sub-lot is non-compliant if the measured value is equal to or above the CCα.
These interpretation rules shall apply for the analytical result obtained on the sample for official control. In case of analysis for defence or reference purposes, the national rules shall apply.
3. Results exceeding action thresholds as laid down in Annex II to Directive 2002/32/EC U.K.
Action thresholds serve as a tool for the selection of samples in those cases where it is necessary to identify a source of contamination and to take measures to reduce or eliminate it. Screening methods shall establish appropriate cut-off values for the selection of these samples. The efforts necessary to identify a source and to reduce or eliminate the contamination shall be deployed only if exceedance of the action thresholds is confirmed by duplicate analysis using a confirmatory method and taking into account the measurement uncertainty (7) .
CHAPTER II U.K. Sample preparation and requirements for methods of analysis used in official control of the levels of dioxins (PCDD/PCDF) and dioxin-like PCBs in feed
1. Field of application U.K.
The requirements set out in this Annex shall be applied where feed is analysed for the official control of the levels of 2,3,7,8-substituted polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dioxin-like PCBs) and for regulatory purposes.
Monitoring for the presence of PCDD/Fs and dioxin-like PCBs in feed may be performed with two different goals:
Selection of those samples with levels of PCDD/Fs and dioxin-like PCBs that exceed the maximum levels, or the action thresholds. This approach may involve a screening method allowing cost-effective high sample-throughput, thus increasing the chance to discover new incidents with high exposure and health risks of consumers. Screening methods may comprise bioanalytical methods and GC/MS methods. Their application should aim at avoiding false-compliant results. The concentration of PCDD/Fs and the sum of PCDD/Fs and dioxin-like PCBs in those samples with significant levels needs to be determined/confirmed by a confirmatory method.
Determination of the levels of PCDD/Fs and dioxin-like PCBs in feed samples in the range of low background levels. This is important in order to follow time trends, exposure assessment of the population and to build a database for possible re-evaluation of action and maximum levels. This goal is achieved by confirmatory methods enabling the PCDD/Fs and dioxin-like PCBs to be identified and quantified unequivocally at the level of interest. These methods can be used for confirmation of results obtained by screening methods and for determination of low background levels in feed monitoring. They are also important for establishing congener patterns in order to identify the source of a possible contamination. At present such methods utilise high-resolution gas chromatography/high resolution mass spectrometry (HRGC/HRMS).
2. Classification of methods by their degree of quantification (8) U.K.
2.1. Qualitative methods give a yes/no response on the presence of analytes of interest, with no quantified indication of the concentration of the putative analyte. Qualitative methods may have the potential for providing semi-quantitative results but are used solely for report of a yes/no decision as indication of levels above or below certain ranges, e.g. limit of detection, limit of quantification or cut-off values. U.K.
For the control of maximum levels and action thresholds for PCDD/PCDFs and dioxin-like PCBs in feed, screening methods may be applied which are based on the comparison of the analytical result with a cut-off value and give a yes/no decision for indication for the possible exceedance of the level of interest.
2.2. Semi-quantitative methods are methods which give an approximate indication of the concentration of the putative analyte, while the numerical result does not meet the requirements for quantitative methods. They may be used to provide information on the range of the analyte concentration in order for the analyst to decide on the calibration range for the confirmatory test subsequently to be performed and for quality control purposes. For example, the following methods shall be considered as semi-quantitative methods: U.K.
methods based on the use of biological principles like cell-based assays, receptor-assays or immunoassays, hereinafter bioanalytical methods, which are able to detect the analytes of interest, include a calibration curve, give a yes/no decision for indication for the possible exceedance of the level of interest and allow to report the result as bioanalytical equivalents (BEQ), being an indication of the TEQ value in the sample;
physicochemical test (e.g. Gas chromatography-Mass spectrometry/Mass spectrometry (GC-MS/MS) or Gas chromatography/Low resolution mass spectrometry (GC/LRMS)) where the measured method precision characteristics do not meet the requirements for quantitative tests.
2.3. Quantitative methods meet the same requirements for accuracy, dynamic range and precision as confirmatory methods. When quantification is required, quantitative methods shall be validated as confirmatory methods. U.K.
3. Background U.K.
For calculation of toxic equivalent (TEQ) concentrations, the concentrations of the individual substances in a given sample shall be multiplied by their respective toxic equivalency factor (TEF), as established by the World Health Organisation and listed in the Appendix to this Annex, and subsequently summed to give the total concentration of dioxin-like compounds expressed as TEQs.
For the purposes of this Part B of Annex V, the accepted specific limit of quantification of an individual congener shall be the concentration of an analyte in the extract of a sample which produces an instrumental response at two different ions to be monitored with an S/N (signal/noise) ratio of 3:1 for the less intensive signal and fulfilment of identification criteria as described, for example, in standard prEN 16215 (Animal feed – Determination of dioxins and dioxin-like PCBs by Gas chromatography/High resolution mass spectrometry (GC/HRMS) and of indicator PCBs by GC/HRMS) and/or in EPA method 1613 revision B.
Bioanalytical screening methods will not give results at the congener level but merely an indication (9) of the TEQ level, expressed in bioanalytical equivalents (BEQ) to acknowledge the fact that not all compounds present in a sample extract that produce a response in the test may obey all requirements of the TEQ-principle.
Screening and confirmatory methods can only be applied for control of a certain matrix if the methods are sensitive enough to detect levels reliably at the level of interest (action threshold or maximum level).
4. Quality assurance requirements U.K.
4.1. Measures shall be taken to avoid cross-contamination at each stage of the sampling and analysis procedure. U.K.
4.2. The samples shall be stored and transported in glass, aluminium, polypropylene or polyethylene containers suitable for storage without any influence on the levels of PCDD/PCDFs and dioxin-like PCBs in the samples. Traces of paper dust shall be removed from the sample container. U.K.
4.3. The sample storage and transportation shall be performed in a way that maintains the integrity of the feed sample. U.K.
4.4. In so far as relevant, each laboratory sample shall be finely grinded and mixed thoroughly using a process that has been demonstrated to achieve complete homogenisation (for example, ground to pass a 1 mm sieve). Samples shall be dried before grinding if the moisture content is too high. U.K.
4.5. Control of reagents, glassware and equipment for possible influence of TEQ- or BEQ-based results shall be carried out. U.K.
4.6. A blank analysis shall be performed by carrying out the entire analytical procedure omitting only the sample. U.K.
4.7. For bioanalytical methods, all glassware and solvents used in analysis shall be tested to be free of compounds that interfere with the detection of target compounds in the working range. Glassware shall be rinsed with solvents or heated at temperatures suitable to remove traces of PCDD/PCDFs, dioxin-like compounds and interfering compounds from its surface. U.K.
4.8. Sample quantity used for the extraction shall be sufficient to fulfil the requirements with respect to a sufficiently low working range including the concentrations of interest. U.K.
4.9. The specific sample preparation procedures used for the products under consideration shall follow internationally accepted guidelines. U.K.
5. Requirements for laboratories U.K.
5.1. In accordance with the provisions of Regulation (EC) No 882/2004, laboratories shall be accredited by a recognised body operating in accordance with ISO Guide 58 to ensure that they are applying analytical quality assurance. Laboratories shall be accredited following the EN ISO/IEC 17025 standard. U.K.
5.2. Laboratory proficiency shall be proven by the continuous successful participation in inter-laboratory studies for the determination of PCDD/PCDFs and dioxin-like PCBs in relevant feed matrices and concentration ranges. U.K.
5.3. Laboratories applying screening methods for the routine control of samples shall establish a close cooperation with laboratories applying the confirmatory method, both for quality control and confirmation of the analytical result of suspected samples. U.K.
6. Basic requirements to be met by analytical procedure for dioxins (PCDD/PCDFs) and dioxin-like PCBs U.K.
6.1. Low working range and limits of quantification U.K.
For PCDD/PCDFs, detectable quantities shall be in the upper femtogram (10 –15 g) range because of extreme toxicity of some of these compounds. For most PCB congeners limit of quantification in the nanogram (10 –9 g) range is already sufficient. For the measurement of the more toxic dioxin-like PCB congeners (in particular non-ortho substituted congeners) the lower end of the working range shall reach the low picogram (10 –12 g) levels. For all other PCB congeners a limit of quantification in the nanogram (10 –9 g) range is sufficient.
6.2. High selectivity (specificity) U.K.
6.2.1. A distinction is required between PCDD/PCDFs and dioxin-like PCBs and a multitude of other, co-extracted and possibly interfering compounds present at concentrations up to several orders of magnitude higher than those of the analytes of interest. For GC/MS methods, a differentiation among various congeners is required, such as between toxic (for example, the seventeen 2,3,7,8-substituted PCDD/PCDFs, and twelve dioxin-like PCBs) and other congeners. U.K.
6.2.2. Bioanalytical methods shall be able to detect the target compounds as the sum of PCDD/PCDFs, and/or dioxin-like PCBs. Sample clean-up shall aim at removing compounds causing false-non-compliant results or compounds that may decrease the response, causing false-compliant results. U.K.
6.3. High accuracy (trueness and precision, bioassay apparent recovery) U.K.
6.3.1. For GC/MS methods, the determination shall provide a valid estimate of the true concentration in a sample. High accuracy is required to avoid the rejection of a sample analysis result on the basis of poor reliability of the determined TEQ level. Accuracy is expressed as trueness (difference between the mean value measured for an analyte in a certified material and its certified value, expressed as percentage of this value) and precision (RSD R relative standard deviation calculated from results generated under reproducibility conditions). U.K.
6.3.2. For bioanalytical methods, the bioassay apparent recovery shall be determined. Bioassay apparent recovery means the BEQ level calculated from the TCDD or PCB 126 calibration curve corrected for the blank and then divided by the GC/HRMS determined TEQ level. It aims at correcting factors like the loss of PCDD/PCDFs and dioxin-like compounds during the extraction and clean-up steps, co-extracted compounds increasing or decreasing the response (agonistic and antagonistic effects), the quality of the curve fit, or differences between the toxic equivalency factor (TEF) and the relative potency (REP) values. The bioassay apparent recovery is calculated from suitable reference samples with representative congener patterns around the level of interest. U.K.
6.4. Validation in the range of level of interest and general quality control measures U.K.
6.4.1. Laboratories shall demonstrate the performance of a method in the range of the level of interest, for example, 0,5 ×, 1 × and 2 × the level of interest with an acceptable coefficient of variation for repeated analysis, during the validation procedure and during routine analysis. U.K.
6.4.2. Regular blank controls and spiking experiments or analysis of control samples (preferably, if available, certified reference material) shall be performed as internal quality control measures. Quality control charts for blank controls, spiking experiments or analysis of control samples shall be recorded and checked to make sure the analytical performance is in accordance with the requirements. U.K.
6.5. Limit of quantification U.K.
6.5.1. For a bioanalytical screening method, the establishment of the limit of quantification (LOQ) is not an indispensable requirement but the method shall prove that it can differentiate between the blank and the cut-off value. When providing a BEQ level, a reporting level shall be established to deal with samples showing a response below this level. The reporting level shall be demonstrated to be different from procedure blank samples at least by a factor of 3, with a response below the working range. It shall therefore be calculated from samples containing the target compounds around the required minimum level, and not from an S/N ratio or an assay blank. U.K.
6.5.2. The LOQ for a confirmatory method shall be about one fifth of the level of interest. U.K.
6.6. Analytical criteria U.K.
For reliable results from confirmatory or screening methods, the following criteria shall be met for the TEQ or BEQ value, respectively, whether determined as total TEQ (as sum of PCDD/PCDFs and dioxin-like PCBs) or separately for PCDD/PCDFs and dioxin-like PCBs:
| a With respect to the maximum levels. | ||
| Screening with bioanalytical or physico-chemical methods | Confirmatory methods | |
|---|---|---|
| False-compliant rate a | < 5 % | |
| Trueness | – 20 % to + 20 % | |
| Repeatability (RSD r ) | < 20 % | |
| Within-laboratory reproducibility (RSD R ) | < 25 % | < 15 % |
6.7. Specific requirements for screening methods U.K.
6.7.1. Both GC/MS and bioanalytical methods may be used for screening. For GC/MS methods the requirements laid down in point 7 shall be met. For cell-based bioanalytical methods specific requirements are laid down in point 8. U.K.
6.7.2. Laboratories applying screening methods for the routine control of samples shall establish a close cooperation with laboratories applying the confirmatory method. U.K.
6.7.3. Performance verification of the screening method is required during routine analysis, by analytical quality control and on-going method validation. There shall be a continuous programme for the control of compliant results. U.K.
6.7.4. Check on possible suppression of the cell response and cytotoxicity: U.K.
20 % of the sample extracts shall be measured in routine screening without and with 2,3,7,8-TCDD added corresponding to the level of interest, to check if the response is possibly suppressed by interfering substances present in the sample extract. The measured concentration of the spiked sample shall be compared to the sum of the concentration of the unspiked extract plus the spiking concentration. If this measured concentration is more than 25 % lower than the calculated (sum) concentration, this is an indication of potential signal suppression and the respective sample shall be submitted to GC/HRMS confirmatory analysis. Results shall be monitored in quality control charts.
6.7.5. Quality control on compliant samples: U.K.
Approximately 2 to 10 % of the compliant samples, depending on sample matrix and laboratory experience, shall be confirmed by GC/HRMS.
6.7.6. Determination of false-compliant rates from quality control data: U.K.
The rate of false-compliant results from screening of samples below and above the maximum level or the action threshold shall be determined. Actual false-compliant rates shall be below 5 %. When a minimum of 20 confirmed results per matrix/matrix group is available from the quality control of compliant samples, conclusions on the false-compliant rate shall be drawn from this database. The results from samples analysed in ring trials or during contamination incidents, covering a concentration range up to for example 2 × maximum level (ML), may also be included in the minimum of 20 results for evaluation of the false-compliant rate. The samples shall cover most frequent congener patterns, representing various sources.
Although screening assays shall preferentially aim at detecting samples exceeding the action threshold, the criterion for determining false-compliant rates is the maximum level, taking into account the measurement uncertainty of the confirmatory method.
6.7.7. Suspected non-compliant samples from screening shall always be verified by a confirmatory method of analysis (GC/HRMS). These samples may also be used to evaluate the rate of false-non-compliant results. For screening methods, the rate of false-non-compliant results shall be the fraction of results confirmed to be compliant from GC/HRMS confirmatory analysis, while in previous screening the sample has been declared to be suspected to be non-compliant. Evaluation of the advantageousness of the screening method shall be based on comparison of false-non-compliant samples with the total number of samples checked. This rate shall be low enough to make the use of a screening tool advantageous. U.K.
6.7.8. At least under validation conditions, bioanalytical methods shall provide a valid indication of the TEQ level, calculated and expressed as BEQ. U.K.
Also for bioanalytical methods carried out under repeatability conditions, the intra-laboratory RSD r would typically be smaller than the reproducibility RSD R .
7. Specific requirements for gc/ms methods to be complied with for screening or confirmatory purposes U.K.
7.1. General requirements U.K.
The difference between upper-bound level and lower-bound level shall not exceed 20 % for feed with a contamination of about 1 ng WHO-TEQ/kg product with 12 % moisture content (based on the sum of PCDD/Fs and dioxin-like PCBs). For lower contamination levels, for example 0,5 ng WHO-TEQ/kg product, the difference between upper-bound and lower-bound level may be in the range of 25 % to 40 %.
7.2. Control of recoveries U.K.
7.2.1. Addition of 13 C-labelled 2,3,7,8-chlorine substituted internal PCDD/PCDF standards and of 13 C-labelled internal dioxin-like PCB standards shall be carried out at the very beginning of the analytical method e.g. prior to extraction in order to validate the analytical procedure. At least one congener for each of the tetra- to octa-chlorinated homologous groups for PCDD/PCDFs and at least one congener for each of the homologous groups for dioxin-like PCBs shall be added (alternatively, at least one congener for each mass spectrometric selected ion recording function used for monitoring PCDD/PCDFs and dioxin-like PCBs). In the case of confirmatory methods, all 17 13 C-labelled 2,3,7,8-substituted internal PCDD/PCDF standards and all 12 13 C-labelled internal dioxin-like PCB standards shall be used. U.K.
7.2.2. Relative response factors shall also be determined for those congeners for which no 13 C-labelled analogue is added by using appropriate calibration solutions. U.K.
7.2.3. For feed of plant origin and feed of animal origin containing less than 10 % fat, the addition of the internal standards shall be mandatory prior to extraction. For feed of animal origin containing more than 10 % fat, the internal standards shall be added either before or after fat extraction. An appropriate validation of the extraction efficiency shall be carried out, depending on the stage at which internal standards are introduced and on whether results are reported on product or fat basis. U.K.
7.2.4. Prior to GC/MS analysis, 1 or 2 recovery (surrogate) standard(s) shall be added. U.K.
7.2.5. Control of recovery is required. For confirmatory methods, the recoveries of the individual internal standards shall be in the range of 60 to 120 %. Lower or higher recoveries for individual congeners, in particular for some hepta- and octa- chlorinated dibenzo-p-dioxins and dibenzofurans, shall be acceptable on the condition that their contribution to the TEQ value does not exceed 10 % of the total TEQ value (based on sum of PCDD/PCDF and dioxin-like PCBs). For GC/MS screening methods, the recoveries shall be in the range of 30 to 140 %. U.K.
7.3. Removal of interfering substances U.K.
Separation of PCDD/PCDFs from interfering chlorinated compounds such as non-dioxin-like PCBs and chlorinated diphenyl ethers shall be carried out by suitable chromatographic techniques (preferably with a florisil, alumina and/or carbon column).
Gas-chromatographic separation of isomers shall be < 25 % peak to peak between 1,2,3,4,7,8-HxCDF and 1,2,3,6,7,8-HxCDF.
7.4. Calibration with standard curve U.K.
The range of the calibration curve shall cover the relevant range of levels of interest.
8. Specific requirements for bioanalytical methods U.K.
Bioanalytical methods are methods based on the use of biological principles like cell-based assays, receptor-assays or immunoassays. This point 8 establishes requirements for bioanalytical methods in general.
A screening method in principle classifies a sample as compliant or suspected to be non-compliant. For this, the calculated BEQ level is compared to the cut-off value (see 8.3). Samples below the cut-off value are declared compliant, samples equal or above the cut-off value are suspected to be non-compliant, requiring analysis by a confirmatory method. In practice, a BEQ level corresponding to 2/3 of the maximum level may serve as the most suitable cut-off value ensuring a false-compliant rate below 5 % and an acceptable rate for false-non-compliant results. With separate maximum levels for PCDD/Fs and for the sum of PCDD/Fs and dioxin-like PCBs, checking compliance of samples without fractionation requires appropriate bioassay cut-off values for PCDD/Fs. For checking of samples exceeding the action thresholds, an appropriate percentage of the respective level of interest would suit as cut-off value.
Furthermore, in the case of certain bioanalytical methods, an indicative level expressed in BEQs may be given for samples in the working range and exceeding the reporting limit (see 8.1.1 and 8.1.6).
8.1. Evaluation of the test response U.K.
8.1.1. General requirements U.K.
When calculating the concentrations from a TCDD calibration curve, values at the lower and higher end of the curve will show a high variation (high coefficient of variation (CV)). The working range is the area where this CV is smaller than 15 %. The lower end of the working range (reporting limit) shall be set at least by a factor of 3 above the procedure blanks. The upper end of the working range is usually represented by the EC 70 value (70 % of maximal effective concentration), but lower if the CV is higher than 15 % in this range. The working range shall be established during validation. Cut-off values (see point 8.3) shall be well within the working range.
Standard solutions and sample extracts shall be tested at least in duplicate. When using duplicates, a standard solution or a control extract tested in 4 to 6 wells divided over the plate shall produce a response or concentration (only possible in the working range) based on a CV < 15 %.
8.1.2. Calibration U.K.
8.1.2.1. Calibration with standard curve U.K.
Levels in samples shall be estimated by comparison of the test response with a calibration curve of TCDD (or PCB 126 or a PCDD/PCDF/dioxin-like PCB standard mixture) to calculate the BEQ level in the extract and subsequently in the sample.
Calibration curves shall contain 8 to 12 concentrations (at least in duplicates), with enough concentrations in the lower part of the curve (working range). Special attention shall be paid to the quality of the curve-fit in the working range. As such, the R 2 value is of little or no value in estimating the goodness of fit in nonlinear regression. A better fit shall be achieved by minimising the difference between calculated and observed levels in the working range of the curve, for example by minimising the sum of squared residuals.
The estimated level in the sample extract shall be subsequently corrected for the BEQ level calculated for a matrix/solvent blank sample (to account for impurities from solvents and chemicals used), and the apparent recovery (calculated from the BEQ level of suitable reference samples with representative congener patterns around the level of interest). To perform a recovery correction, the apparent recovery shall be within the required range (see point 8.1.4). Reference samples used for recovery correction shall comply with the requirements laid down in point 8.2.
8.1.2.2. Calibration with reference samples U.K.
Alternatively, a calibration curve prepared from at least four reference samples (see point 8.2.4: one matrix blank, plus three reference samples at 0,5 ×, 1,0 × and 2,0 × the level of interest) around the level of interest may be used, eliminating the need to correct for blank and recovery. In this case, the test response corresponding to 2/3 of the maximum level (see point 8.3) may be calculated directly from these samples and used as cut-off value. For checking of samples exceeding the action thresholds, an appropriate percentage of these action thresholds would suit as cut-off value.
8.1.3. Separate determination of PCDD/PCDFs and dioxin-like PCBs U.K.
Extracts may be split into fractions containing PCDD/PCDFs and dioxin-like PCBs, allowing a separate indication of PCDD/PCDFs and dioxin-like PCB TEQ levels (in BEQ). A PCB 126 standard calibration curve shall preferentially be used to evaluate results for the fraction containing dioxin-like PCBs.
8.1.4. Bioassay apparent recoveries U.K.
The ‘bioassay apparent recovery’ shall be calculated from suitable reference samples with representative congener patterns around the level of interest and expressed as percentage of the BEQ level in comparison to the TEQ level. Depending on the type of assay and TEFs (10) used, the differences between TEF and REP factors for dioxin-like PCBs can cause low apparent recoveries for dioxin-like PCBs in comparison to PCDD/PCDFs. Therefore, if a separate determination of PCDD/PCDFs and dioxin-like PCBs is performed, bioassay apparent recoveries shall be: for dioxin-like PCBs 25 % to 60 %, for PCDD/PCDFs 50 % to 130 % (ranges apply for the TCDD calibration curve). As the contribution of dioxin-like PCBs to the sum of PCDD/PCDFs and dioxin-like PCBs can vary between different matrices and samples, bioassay apparent recoveries for the sum of PCDD/PCDFs and dioxin-like PCBs reflect these ranges and shall be between 30 % and 130 %. Any implication of substantially revised TEF values for the Union legislation for PCDD/PCDFs and dioxin-like PCBs requires the revision of these ranges.
8.1.5. Control of recoveries for clean-up U.K.
The loss of compounds during the clean-up shall be checked during validation. A blank sample spiked with a mixture of the different congeners shall be submitted to clean-up (at least n = 3) and the recovery and variability checked by GC/HRMS analysis. The recovery shall be within 60 % to 120 % especially for congeners contributing more than 10 % to the TEQ-level in various mixtures.
8.1.6. Reporting limit U.K.
When reporting BEQ levels, a reporting limit shall be determined from relevant matrix samples involving typical congener patterns, but not from the calibration curve of the standards due to low precision in the lower range of the curve. Effects from extraction and clean-up shall be taken into account. The reporting limit shall be set at least by a factor of 3 above the procedure blanks.
8.2. Use of reference samples U.K.
8.2.1. Reference samples shall represent sample matrix, congener patterns and concentration ranges for PCDD/PCDFs and dioxin-like PCBs around the level of interest. U.K.
8.2.2. A matrix blank, and where it is not possible, a procedure blank, and a reference sample at the level of interest shall be included in each test series. These samples shall be extracted and tested at the same time under identical conditions. The reference sample shall show a clearly elevated response in comparison to the blank sample, thus ensuring the suitability of the test. These samples may be used for blank and recovery corrections. U.K.
8.2.3. Reference samples chosen to perform a recovery correction shall be representative for the test samples, meaning that congener patterns may not lead to an underestimation of levels. U.K.
8.2.4. Extra reference samples at e.g. 0,5 × and 2 × the level of interest may be included to demonstrate the proper performance of the test in the range of interest for the control of the level of interest. Combined, these samples may be used for calculating the BEQ levels in test samples (see point 8.1.2.2). U.K.
8.3. Determination of cut-off values U.K.
The relationship between bioanalytical results in BEQ and GC/HRMS results in TEQ shall be established, for example by matrix-matched calibration experiments, involving reference samples spiked at 0, 0,5 ×, 1 × and 2 × maximum level, with 6 repetitions on each level (n = 24). Correction factors (blank and recovery) may be estimated from this relationship but shall be checked in accordance with point 8.2.2.
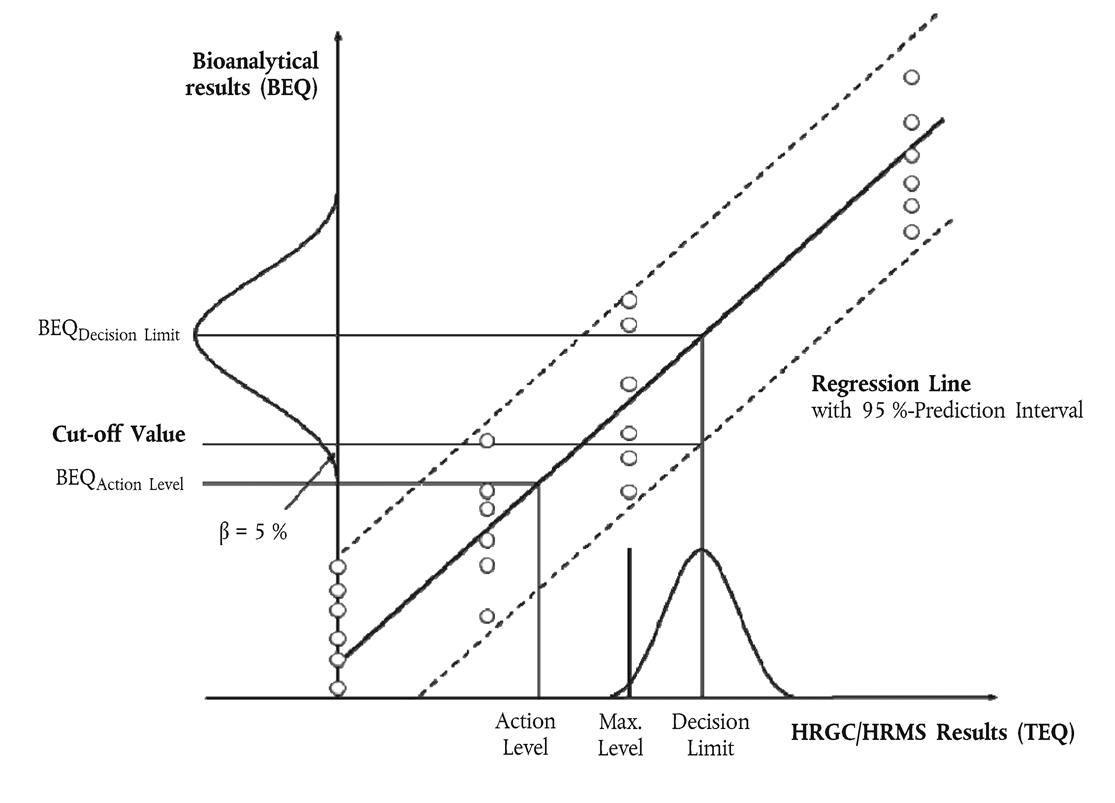
Cut-off values shall be established for decisions over sample compliance with maximum levels or for the control of action thresholds, if relevant, with the respective levels of interest set for either PCDD/PCDFs and dioxin-like PCBs alone, or for the sum of PCDD/PCDFs and dioxin-like PCBs. They are represented by the lower end-point of the distribution of bioanalytical results (corrected for blank and recovery) corresponding to the GC/HRMS decision limit based on a 95 % level of confidence, implying a false-compliant rate < 5 %, and on a RSD R < 25 %. The GC/HRMS decision limit is the maximum level, taking into account the measurement uncertainty.
The cut-off value (in BEQ) may be calculated in accordance with one of the approaches set out in points 8.3.1, 8.3.2 and 8.3.3 (see Figure 1):
Use of the lower band of the 95 % prediction interval at the GC/HRMS decision limit:
with:
BEQ corresponding to the GC/HRMS decision limit, being the maximum level including measurement uncertainty
residual standard deviation
Student factor (α = 5 %, f = degrees of freedom, single-sided)
total number of calibration points (index j)
number of repetitions on each level
GC/HRMS sample concentration (in TEQ) of calibration point i
Calculation from bioanalytical results (corrected for blank and recovery) of multiple analyses of samples (n ≥ 6) contaminated at the GC/HRMS decision limit, as the lower end-point of the data distribution at the corresponding mean BEQ value:
Cut-off value = BEQ DL – 1,64 × SD R
With:
standard deviation of bioassay results at BEQ DL , measured under within-laboratory reproducibility conditions
Calculation as mean value of bioanalytical results (in BEQ, corrected for blank and recovery) from multiple analysis of samples (n ≥ 6) contaminated at 2/3 the level of interest, based on the observation that this level will be around the cut-off value determined under point 8.3.1 or point 8.3.2:
Restrictions to cut-off values:
BEQ-based cut-off values calculated from the RSD R achieved during validation using a limited number of samples with different matrix/congener patterns may be higher than the TEQ-based levels of interest due to a better precision than attainable in routine when an unknown spectrum of possible congener patterns has to be controlled. In such cases, cut-off values shall be calculated from an RSD R = 25 %, or two thirds of the level of interest shall be preferred.
8.4. Performance characteristics U.K.
8.4.1. Tests on the repeatability of bioanalytical methods shall be carried out to obtain information on the standard deviation within and between test series. Repeatability shall be below 20 %, intra-laboratory reproducibility below 25 %. This shall be based on the calculated levels in BEQ after blank and recovery correction. U.K.
8.4.2. As part of the validation process, the test shall be shown to discriminate between a blank sample and a level at the cut-off value, allowing the identification of samples above the corresponding cut-off value (see point 8.1.2). U.K.
8.4.3. Target compounds, possible interferences and maximum tolerable blank levels shall be defined. U.K.
8.4.4. The percent standard deviation in the response or concentration calculated from the response (only possible in working range) of a triplicate determination of a sample extract may not be above 15 %. U.K.
8.4.5. The uncorrected results of the reference sample(s) expressed in BEQ (blank and level of interest) shall be used for evaluation of the performance of the bioanalytical method over a constant time period. U.K.
8.4.6. Quality control charts for procedure blanks and each type of reference sample shall be recorded and checked to make sure the analytical performance is in accordance with the requirements, in particular for the procedure blanks with regard to the requested minimum difference to the lower end of the working range and for the reference samples with regard to within-laboratory reproducibility. Procedure blanks shall be controlled in a manner to avoid false-compliant results when subtracted. U.K.
8.4.7. The results from the GC/HRMS analyses of suspected samples and 2 to 10 % of the compliant samples (minimum of 20 samples per matrix) shall be collected and used to evaluate the performance of the screening method and the relationship between BEQ and TEQ. This database may be used for the re-evaluation of cut-off values applicable to routine samples for the validated matrices. U.K.
8.4.8. Successful method performance may also be demonstrated by participation in ring trials. The results from samples analysed in ring trials, covering a concentration range up to e.g. 2 × maximum level, may be included in the evaluation of the false-compliant rate, if a laboratory is able to demonstrate its successful performance. The samples shall cover most frequent congener patterns, representing various sources. U.K.
8.4.9. During incidents, the cut-off values may be re-evaluated, reflecting the specific matrix and congener patterns of this single incident. U.K.
9. Reporting of the results U.K.
9.1. Confirmatory methods U.K.
9.1.1. In so far as the used analytical procedure makes it possible, the analytical results shall contain the levels of the individual PCDD/PCDF and dioxin-like PCB congeners and be reported as lower-bound, upper-bound and medium-bound in order to include a maximum of information in the reporting of the results and thereby enabling the interpretation of the results according to specific requirements. U.K.
9.1.2. The report shall include the method used for extraction of PCDD/PCDFs, dioxin-like PCBs and lipids. U.K.
9.1.3. The recoveries of the individual internal standards shall be made available in case the recoveries are outside the range referred to in point 7.2.5, in case the maximum level is exceeded and in other cases upon request. U.K.
9.1.4. As the uncertainty of measurement is to be taken into account when deciding about the compliance of a sample, this parameter shall be made available. Thus, analytical results shall be reported as x +/– U whereby x is the analytical result and U is the expanded measurement uncertainty using a coverage factor of 2 which gives a level of confidence of approximately 95 %. In the case of a separate determination of PCDD/PCDFs and dioxin-like-PCBs, the sum of the estimated expanded uncertainty of the separate analytical results of PCDD/PCDFs and dioxin-like PCBs shall be used for the sum of PCDD/Fs and dioxin-like PCBs. U.K.
9.1.5. If the uncertainty of measurement is taken into account by applying CCα (as described in point 2.2), this parameter shall be reported. U.K.
9.1.6. The results shall be expressed in the same units and with at least the same number of significant figures as the maximum levels laid down in Directive 2002/32/EC. U.K.
9.2. Bioanalytical screening methods U.K.
9.2.1. The result of the screening shall be expressed as ‘ compliant ’ or ‘ suspected to be non-compliant ’ ( ‘ suspected ’ ). U.K.
9.2.2. In addition, a result for PCDD/PCDF and/or dioxin-like PCBs expressed in BEQ, and not TEQ, may be given. U.K.
9.2.3. If measurement uncertainty on the calculated BEQ level is given, for example as standard deviation, it shall be based on at least a triplicate analysis of the sample, including extraction, clean up and determination of the test response. U.K.
9.2.4. Samples with a response below the reporting limit shall be expressed as ‘ lower than the reporting limit ’ . U.K.
9.2.5. For each type of sample matrix, the report shall mention the level of interest on which the evaluation is based. U.K.
9.2.6. The report shall mention the type of the test applied, the basic test principle and the kind of calibration. U.K.
9.2.7. The report shall include the method used for extraction of PCDD/PCDFs, dioxin-like PCBs and lipids. U.K.
CHAPTER III U.K. Sample preparation and requirements for methods of analysis used in official control of the levels of non-dioxin-like PCBs (PCB # 28, 52, 101, 138, 153, 180)
1. Applicable detection methods U.K.
Gas chromatography/Electron capture detection (GC/ECD), GC/LRMS, GC/MS-MS, GC/HRMS or equivalent methods.
2. Identification and confirmation of analytes of interest U.K.
2.1. Relative retention time in relation to internal standards or reference standards (acceptable deviation of +/– 0,25 %). U.K.
2.2. Gas chromatographic separation of all six indicator PCBs (PCB 28, PCB 52, PCB 101, PCB 138, PCB 153 and PCB 180) from interfering substances, especially co-eluting PCBs, in particular if levels of samples are in the range of legal limits and non-compliance is to be confirmed. U.K.
Note: Congeners often found to co-elute are for example PCB 28/31, PCB 52/69 and PCB 138/163/164. For GC/MS also possible interferences from fragments of higher chlorinated congeners shall be considered. U.K.
2.3. Requirements for GC/MS techniques U.K.
Monitoring of at least:
two specific ions for HRMS;
two specific ions of m/z > 200 or three specific ions of m/z > 100 for LRMS;
1 precursor and 2 product ions for MS-MS.
Maximum permitted tolerances for abundance ratios for selected mass fragments:
Relative deviation of abundance ratio of selected mass fragments from theoretical abundance or calibration standard for target ion (most abundant ion monitored) and qualifier ion(s):
| a Sufficient number of mass fragments with relative intensity > 10 % available, therefore not recommendable to use qualifier ion(s) with a relative intensity of less than 10 % compared to the target ion. | ||
| Relative intensity of qualifier ion(s) compared to target ion | GC-EI-MS (relative deviation) | GC-CI-MS, GC-MS n (relative deviation) |
|---|---|---|
| > 50 % | ± 10 % | ± 20 % |
| > 20 % to 50 % | ± 15 % | ± 25 % |
| > 10 % to 20 % | ± 20 % | ± 30 % |
| ≤ 10 % | ± 50 % a | ± 50 % a |
2.4. Requirements for GC/ECD techniques U.K.
Results exceeding the tolerance shall be confirmed with two GC columns with stationary phases of different polarity.
3. Demonstration of performance of method U.K.
The performance of the method shall be validated in the range of the level of interest (0,5 to 2 times the level of interest) with an acceptable coefficient of variation for repeated analysis (see requirements for intermediate precision in point 8).
4. Limit of quantification U.K.
The blank values shall not be higher than 30 % of the level of contamination corresponding to the maximum level (11) .
5. Quality control U.K.
Regular blank controls, analysis of spiked samples, quality control samples, participation in inter-laboratory studies on relevant matrices.
6. Control of recoveries U.K.
6.1. Suitable internal standards with physico-chemical properties comparable to analytes of interest shall be used. U.K.
6.2. Addition of internal standards: U.K.
Addition to products (before extraction and clean-up process).
6.3. Requirements for methods using all six isotope-labelled indicator PCB congeners: U.K.
results shall be corrected for recoveries of internal standards;
recoveries of isotope-labelled internal standards shall be between 50 and 120 %;
lower or higher recoveries for individual congeners with a contribution to the sum of the six indicator PCBs below 10 % are acceptable.
6.4. Requirements for methods using not all six isotope-labelled internal standards or other internal standards: U.K.
recovery of internal standard(s) shall be controlled for every sample;
recoveries of internal standard(s) shall be between 60 and 120 %;
results shall be corrected for recoveries of internal standards.
6.5. The recoveries of unlabelled congeners shall be checked by spiked samples or quality control samples with concentrations in the range of the level of interest. Recoveries for these congeners shall be considered acceptable, if they are between 70 and 120 %. U.K.
7. Requirements for laboratories U.K.
In accordance with the provisions of Regulation (EC) No 882/2004, laboratories shall be accredited by a recognised body operating in accordance with ISO Guide 58 to ensure that they are applying analytical quality assurance. Laboratories shall be accredited following the EN ISO/IEC 17025 standard.
8. Performance characteristics: criteria for the sum of the six indicator PCBs at the level of interest U.K.
| Trueness | – 30 to + 30 % |
|---|---|
| Intermediate precision (RSD%) | ≤ 20 % |
| Difference between upper- and lower-bound calculation | ≤ 20 % |
9. Reporting of the results U.K.
9.1. In so far as the used analytical procedure makes it possible, the analytical results shall contain the levels of the individual PCB congeners and be reported as lower-bound, upper-bound and medium-bound in order to include a maximum of information in the reporting of the results and thereby enabling the interpretation of the results according to specific requirements. U.K.
9.2. The report shall include the method used for extraction of PCBs and lipids. U.K.
9.3. The recoveries of the individual internal standards shall be made available in case the recoveries are outside the range referred to in point 6, in case the maximum level is exceeded and in other cases upon request. U.K.
9.4. As the uncertainty of measurement is to be taken into account when deciding about the compliance of a sample, this parameter shall also be made available. Thus, analytical results shall be reported as x +/- U whereby x is the analytical result and U is the expanded measurement uncertainty using a coverage factor of 2 which gives a level of confidence of approximately 95 %. U.K.
9.5. If the uncertainty of measurement is taken into account by applying CCα (as described in point 2.1 of Chapter I), this parameter shall be reported. U.K.
9.6. The results shall be expressed in the same units and with at least the same number of significant figures as the maximum levels laid down in Directive 2002/32/EC.] U.K.
Textual Amendments
[F1Table of TEF (= toxic equivalency factors) for dioxins, furans and dioxin-like PCBs:
WHO-TEFs for human risk assessment based on the conclusions of the World Health Organisation (WHO) – International Programme on Chemical Safety (IPCS) expert meeting which was held in Geneva in June 2005 (Martin van den Berg et al., The 2005 World Health Organisation Re-evaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-like Compounds. Toxicological Sciences 93(2), 223–241 (2006)).
| Abbreviations used: ‘ T ’ = tetra; ‘ Pe ’ = penta; ‘ Hx ’ = hexa; ‘ Hp ’ = hepta; ‘ O ’ = octa; ‘ CDD ’ = chlorodibenzodioxin; ‘ CDF ’ = chlorodibenzofuran; ‘ CB ’ = chlorobiphenyl. | |
| Congener | TEF value |
|---|---|
| Dibenzo-p-dioxins (PCDDs) and Dibenzo-p-furans (PCDFs) | |
| 2,3,7,8-TCDD | 1 |
| 1,2,3,7,8-PeCDD | 1 |
| 1,2,3,4,7,8-HxCDD | 0,1 |
| 1,2,3,6,7,8-HxCDD | 0,1 |
| 1,2,3,7,8,9-HxCDD | 0,1 |
| 1,2,3,4,6,7,8-HpCDD | 0,01 |
| OCDD | 0,0003 |
| 2,3,7,8-TCDF | 0,1 |
| 1,2,3,7,8-PeCDF | 0,03 |
| 2,3,4,7,8-PeCDF | 0,3 |
| 1,2,3,4,7,8-HxCDF | 0,1 |
| 1,2,3,6,7,8-HxCDF | 0,1 |
| 1,2,3,7,8,9-HxCDF | 0,1 |
| 2,3,4,6,7,8-HxCDF | 0,1 |
| 1,2,3,4,6,7,8-HpCDF | 0,01 |
| 1,2,3,4,7,8,9-HpCDF | 0,01 |
| OCDF | 0,0003 |
| ‘ Dioxin-like ’ PCBs Non-ortho PCBs + Mono-ortho PCBs | |
| Non-ortho PCBs | |
| PCB 77 | 0,0001 |
| PCB 81 | 0,0003 |
| PCB 126 | 0,1 |
| PCB 169 | 0,03 |
| Mono-ortho PCBs | |
| PCB 105 | 0,00003 |
| PCB 114 | 0,00003 |
| PCB 118 | 0,00003 |
| PCB 123 | 0,00003 |
| PCB 156 | 0,00003 |
| PCB 157 | 0,00003 |
| PCB 167 | 0,00003 |
| PCB 189 | 0,00003 |
[F1The concept of ‘ upper-bound ’ requires using the limit of quantification for the contribution of each non-quantified congener. The concept of ‘ lower-bound ’ requires using zero for the contribution of each non-quantified congener. The concept of ‘ medium-bound ’ requires using half of the limit of quantification calculating the contribution of each non-quantified congener.]
[F1The duplicate analysis is necessary to exclude the possibility of internal cross-contamination or an accidental mix-up of samples. The first analysis, taking into account the measurement uncertainty is used for verification of compliance. In case the analysis is performed in the frame of a contamination incident, confirmation by duplicate analysis might be omitted in case the samples selected for analysis are through traceability linked to the contamination incident.]
[F1The concept of ‘ upper-bound ’ requires using the limit of quantification for the contribution of each non-quantified congener to the toxic equivalent (TEQ). The concept of ‘ lower-bound ’ requires using zero for the contribution of each non-quantified congener to the TEQ. The concept of ‘ medium-bound ’ requires using half of the limit of quantification calculating the contribution of each non-quantified congener to the TEQ.]
[F1The duplicate analysis is necessary to exclude the possibility of internal cross-contamination or an accidental mix-up of samples. The first analysis, taking into account the measurement uncertainty is used for verification of compliance. In case the analysis is performed in the frame of a contamination incident, confirmation by duplicate analysis might be omitted in case the samples selected for analysis are through traceability linked to the contamination incident.]
[F1Identical explanation and requirements for duplicate analysis for control of action thresholds as in footnote (5) for maximum levels.]
[F1Adapted to PCDD/Fs and dioxin-like compounds from ‘ Guidelines for the validation of screening methods for residues of veterinary medicines ’ , EU Reference Laboratories (EURLs) for residues of veterinary medicines and contaminants in food of animal origin in Fougères, Berlin and Bilthoven, 20/1/2010 , http://ec.europa.eu/food/food/chemicalsafety/residues/lab_analysis_en.htm]
[F1Bioanalytical methods are not specific to those congeners included in the TEF scheme. Other structurally related AhR-active compounds may be present in the sample extract which contribute to the overall response. Therefore, bioanalytical results cannot be an estimate but rather an indication of the TEQ level in the sample.]
[F1Current requirements are based on the TEFs published in: M. Van den Berg et al., Toxicol Sci 93 (2), 223–241 (2006).]
[F1It is highly recommendable to have a lower contribution of the reagent blank level to the level of a contaminant in a sample. It is in the responsibility of the laboratory to control the variation of blank levels, in particular, if the blank levels are subtracted.]
Options/Cymorth
Print Options
PrintThe Whole Regulation
PrintThe Whole Annex
PrintThis Division only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
Y Rhestrau you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE): Mae'r wreiddiol version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Pwynt Penodol mewn Amser: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
Gweler y wybodaeth ychwanegol ochr yn ochr â’r cynnwys
Rhychwant ddaearyddol: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Dangos Llinell Amser Newidiadau: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel adopted version that was used for the EU Official Journal
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Llinell Amser Newidiadau
Mae’r llinell amser yma yn dangos y fersiynau gwahanol a gymerwyd o EUR-Lex yn ogystal ag unrhyw fersiynau dilynol a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig.
Cymerir dyddiadau fersiynau’r UE o ddyddiadau’r dogfennau ar EUR-Lex ac efallai na fyddant yn cyfateb â’r adeg pan ddaeth y newidiadau i rym ar gyfer y ddogfen.
Ar gyfer unrhyw fersiynau a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig, bydd y dyddiad yn cyd-fynd â’r dyddiad cynharaf y daeth y newid (e.e. ychwanegiad, diddymiad neu gyfnewidiad) a weithredwyd i rym. Am ragor o wybodaeth gweler ein canllaw i ddeddfwriaeth ddiwygiedig ar Ddeall Deddfwriaeth.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel adopted fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill