- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Gwreiddiol (a wnaed Fel)
The Tobacco Products and Nicotine Inhaling Products (Amendment etc.) (EU Exit) Regulations 2019
You are here:
- Offerynnau Statudol y Deyrnas Unedig
- 2019 No. 41
- Whole Instrument without Schedules
Rhagor o Adnoddau
Status:
Dyma’r fersiwn wreiddiol (fel y’i gwnaed yn wreiddiol).
PART 1Introduction
Citation and commencement
1. These Regulations may be cited as the Tobacco Products and Nicotine Inhaling Products (Amendment etc.) (EU Exit) Regulations 2019 and come into force on exit day.
PART 2Amendment of primary legislation
Amendment of the Tobacco Advertising and Promotion Act 2002
2.—(1) The Tobacco Advertising and Promotion Act 2002(1) is amended as follows.
(2) Omit section 2(4) (prohibition of tobacco advertising in the EEA)(2).
(3) In section 3A(1) (advertising: information society services)(3)—
(a)in paragraph (a), omit “or”;
(b)omit paragraph (b).
(4) In section 4(1) (advertising: exclusions)(4)—
(a)in paragraph (c)—
(i)for “in a country which is not an EEA State”, substitute “outside the United Kingdom”;
(ii)for “one or more of the EEA States (or any part of them)”, substitute “the United Kingdom (or any part of the United Kingdom)”;
(b)in paragraph (d)—
(i)for “an EEA State”, substitute “the United Kingdom”;
(ii)for “one or more EEA States (or any part of them)”, substitute “the United Kingdom (or any part of the United Kingdom)”.
(5) In section 5 (advertising: defences)(5)—
(a)in subsection (1), after “section 3A(1)(a)”, omit “or (b)”;
(b)omit subsection (3A);
(c)in subsection (5)(c), for “an EEA State”, substitute “the United Kingdom”;
(d)omit subsection (5A).
(6) Omit section 7D(3) (displays on a website)(6).
(7) Omit section 8(1A) (displays: Scotland)(7).
(8) Omit section 9(1A) (prohibition of free distributions)(8).
(9) Omit section 11(5) (brandsharing)(9).
(10) In section 21(1) (interpretation), omit the definition of “EEA State”(10).
PART 3Amendment of subordinate legislation
Amendment of the Tobacco Advertising and Promotion (Brandsharing) Regulations 2004
3.—(1) The Tobacco Advertising and Promotion (Brandsharing) Regulations 2004(11) are amended as follows.
(2) In regulation 4, in both paragraph (3)(b) and (7)(b)—
(a)omit “or has subsequently become”;
(b)after “European Economic Area”, insert “or which became part of the European Economic Area after that date but before exit day”.
Amendment of the Standardised Packaging of Tobacco Products Regulations 2015
4.—(1) The Standardised Packaging of Tobacco Products Regulations 2015(12) are amended as follows.
(2) In regulation 2—
(a)in paragraph (1), for the definition of “cross-border distance sale”, substitute—
““cross-border distance sale”, in relation to a tobacco product, means a distance sale to a consumer where, at the time the consumer orders the product from a retailer, the consumer is located in the United Kingdom and the retailer is established in another country;”;
(b)in paragraph (7), omit “where the consumer is located in the United Kingdom”.
Amendment of the Nicotine Inhaling Products (Age of Sale and Proxy Purchasing) Regulations 2015
5.—(1) The Nicotine Inhaling Products (Age of Sale and Proxy Purchasing) Regulations 2015(13) are amended as follows.
(2) In regulation 5(2)—
(a)in sub-paragraph (a), omit the words “or Article 11” to the end;
(b)omit sub-paragraph (b).
Amendment of the Tobacco and Related Products Regulations 2016
6.—(1) Subject to regulation 9, the Tobacco and Related Products Regulations 2016(14) are amended as follows.
(2) In regulation 2(1), for the definition of “retailer”, substitute—
““retailer” means a person who sells, or offers or agrees to sell, a tobacco product or related product to a consumer;”.
(3) In regulation 3—
(a)in paragraph (3), omit “to a consumer located in the United Kingdom”;
(b)for paragraph (4), substitute—
“(4) In these Regulations, “cross-border distance sale” means a distance sale to a consumer where, at the time the consumer orders a product from a retailer, the consumer is located in the United Kingdom and the retailer is established in another country.”.
(4) In regulation 5, for paragraph (3)(a), substitute—
“(a)one of the text warnings with the corresponding colour photograph, as listed in the picture library in Schedule A1; and”.
(5) After regulation 5, insert—
Revision of text warnings, photographs and technical specifications
5A. Regulations may—
(a)amend the text warnings or photographs listed in the picture library in Schedule A1, taking into account scientific and market developments;
(b)modify for the purposes of these Regulations the layout, design and proportions specified in the Combined Health Warnings Decision referred to in regulation 5, taking into account different packet shapes.”.
(6) In regulation 6—
(a)omit paragraph (1)(a);
(b)in paragraph (1)(b)—
(i)for “14”, substitute “13”;
(ii)omit “in a specified set”;
(c)in paragraph (2)—
(i)for “obligations”, substitute “obligation”;
(ii)for “are”, substitute “is”;
(d)in paragraph (3), omit sub-paragraphs (a) to (c).
(7) In regulation 7, after paragraph (4), insert—
“(5) Regulations may amend the wording of the information message in paragraph (2)(b), taking into account scientific and market developments.”.
(8) In regulation 8, after paragraph (9), insert—
“(10) Regulations may amend paragraphs (5) to (8) as regards the precise position of the general warning and the information message on hand rolling tobacco marketed in pouches, taking into account the different shapes of pouches.”.
(9) In regulation 9, after paragraph (11), insert—
“(12) Regulations may amend paragraph (1) by omitting reference to one or more of the products listed in that paragraph if there is a substantial change of circumstances for the product concerned.”.
(10) In regulation 10, after paragraph (4), insert—
“(5) Regulations may amend the wording of the health warning in paragraph (2), taking into account scientific developments.”.
(11) In regulation 13, after paragraph (2), insert—
“(3) Regulations may decrease the maximum emission levels specified in paragraph (1), where this is necessary, based on internationally agreed standards.”.
(12) In regulation 14, after paragraph (4), insert—
“(5) Regulations may modify the methods of measurement of tar, nicotine and carbon monoxide emissions from cigarettes, where this is necessary, based on scientific and technical developments or internationally agreed standards.
(6) Any regulations made under paragraph (5) must integrate standards agreed by the parties to the WHO Framework Convention on Tobacco Control(15) or by the World Health Organization for measurement methods.”.
(13) In regulation 15, after paragraph (2), insert—
“(3) Regulations may—
(a)specify whether a tobacco product has a characterising flavour;
(b)set maximum content levels for additives or a combination of additives that result in a characterising flavour.”.
(14) In regulation 16, after paragraph (3), insert—
“(4) Regulations may—
(a)specify whether a tobacco product contains additives in quantities that increase the toxic or addictive effect, or the CMR properties of that tobacco product at the stage of consumption to a significant or measureable degree;
(b)where an additive or a certain quantity thereof has been shown to increase the toxic or addictive effect of a tobacco product, set maximum content levels for that additive.”.
(15) After regulation 16, insert—
Regulations: procedures for determining characterising flavour
16A.—(1) Regulations may establish procedures for determining whether a tobacco product—
(a)has a characterising flavour; or
(b)contains additives in quantities that increase the toxic or addictive effect, or the CMR properties, of that tobacco product at the stage of consumption to a significant or measureable degree.
(2) Regulations made under paragraph (1) may—
(a)provide for any determination to be made by—
(i)the Secretary of State; or
(ii)a person authorised by the Secretary of State for that purpose;
(b)establish, and provide for the operating procedures of, an independent advisory panel;
(c)be varied from time to time, including to take account of scientific and market developments in relation to tobacco products;
(d)make different provision for different cases or descriptions of case, different circumstances, different purposes or different areas;
(e)be revoked.
(3) Before making regulations under this regulation the Secretary of State must consult such persons (or representatives of such persons) as appear to the Secretary of State to be likely to be substantially affected by them.”.
(16) In regulation 20A(16)—
(a)in paragraph (1)(b), omit “and to the European Commission”;
(b)in paragraph (2)(c), omit “the European Commission or”;
(c)in paragraph (4), omit “or the European Commission”;
(d)in paragraph (5)—
(i)in sub-paragraph (a), after “enterprises”, insert “but with the modifications in paragraph (7)”;
(ii)in sub-paragraph (b), omit “or the European Commission”;
(e)after paragraph (5), insert—
“(6) Regulations may amend the list of additives in Schedule 2, which list must contain additives—
(a)for which initial indications, research, or regulation in jurisdictions outside the United Kingdom exist suggesting that they have one of the properties set out in regulation 20B(1)(a) to (d); and
(b)which are amongst the most commonly used additives by weight or number according to the reporting of ingredients pursuant to regulation 18.
(7) For the purposes of this regulation, the Annex to Commission Recommendation 2003/361/EC concerning the definition of micro, small and medium-sized enterprises(17) is to be read as if—
(a)in Article 2—
(i)in paragraph 1, for “EUR 50 million, and/or an annual balance sheet total not exceeding EUR 43 million” there were substituted “£44,000,000, and/or an annual balance sheet total not exceeding £38,000,000”;
(ii)in paragraph 2, for “EUR 10 million” there were substituted “£8,800,000”;
(iii)in paragraph 3, for “EUR 2 million” there were substituted “£1,750,000”;
(b)in Article 3—
(i)in paragraph 2(a), for “EUR 1 250 000” there were substituted “£1,100,000”;
(ii)in paragraph 2(d), for “EUR 10 million” there were substituted “£8,800,000”;
(iii)in paragraph 5, for “by national or Community rules” there were substituted “under the law of the United Kingdom (or any part of it)”;
(c)in Article 5, in paragraph (b), for “national law” there were substituted “the law of the United Kingdom (or any part of it)”.”.
(17) In regulation 25(18)—
(a)for paragraph (2), substitute—
“(2) Information must be submitted—
(a)in electronic form; and
(b)having regard to such technical requirements and procedures as may be specified in guidance issued by the Secretary of State.”;
(b)omit paragraph (3).
(18) In regulation 26(19)—
(a)in paragraph (a), after “Part;” insert “and”;
(b)omit paragraph (b).
(19) In regulation 31(3)(a), for “a member State” (in both places), substitute “the United Kingdom”.
(20) For regulation 33(2), substitute—
“(2) Information must be submitted—
(a)in electronic form; and
(b)having regard to such technical requirements and procedures as may be specified in guidance issued by the Secretary of State.”.
(21) Omit regulation 34(b).
(22) In regulation 36, after paragraph (10), insert—
“(11) Regulations may amend the technical standards in paragraph (10) for the refill mechanism required by paragraph (8).”.
(23) In regulation 37—
(a)in paragraph (2)—
(i)in sub-paragraph (e), after “toxicity;”, insert “and”;
(ii)omit sub-paragraph (g);
(b)after paragraph (9), insert—
“(10) Regulations may amend the wording of the health warning in paragraph (4) taking into account scientific developments, provided that any such amended warning is factual.”.
(24) In regulation 39—
(a)in paragraph (4), omit “and the competent authority of any other member State in which the product has been supplied or is intended to be supplied,”;
(b)in paragraph (5), omit “or the competent authority of any other member State”.
(25) Omit regulation 40(4).
(26) In regulation 41—
(a)in paragraph (2)—
(i)omit the definition of “the Union market”;
(ii)in the definition of “third country”, for “a state which is not a member State”, substitute “a country other than the United Kingdom”;
(b)omit paragraphs (3) to (5).
(27) In regulation 42(3)(b), for “Union”, substitute “United Kingdom”.
(28) In regulation 43—
(a)omit paragraphs (2) to (4);
(b)in paragraph (5)—
(i)for “Paragraphs (1) and (2) do”, substitute “Paragraph (1) does”;
(ii)in sub-paragraph (b), for “Union”, substitute “United Kingdom”.
(29) Omit regulation 44.
(30) Omit regulation 47.
(31) In regulation 48—
(a)in paragraph (d), after “notifications);”, insert “or”;
(b)in paragraph (e), for “, 43 or 44 (advertising and sponsorship); or”, substitute “or 43 (advertising).”;
(c)omit paragraph (f).
(32) In regulation 50(2), for “, 43 or 44 (advertising and sponsorship)”, substitute “or 43 (advertising)”.
(33) After regulation 53, insert—
“PART 9AFEES
Fees for determining characterising flavour, toxicity, addictiveness or CMR properties
53A.—(1) Regulations may make provision for the charging of fees in connection with the exercise of functions relating to the determination of characterising flavour, toxicity, addictiveness or CMR properties of tobacco products for the purposes of these Regulations.
(2) Regulations made under paragraph (1) may make such provision in relation to fees as may be made by the appropriate authority under paragraph 1(3)(a) or (b) of Schedule 4 to the European Union (Withdrawal) Act 2018 in relation to those functions.”.
(34) Before regulation 54, insert—
Regulations
54ZA.—(1) Any power to make regulations under these Regulations—
(a)is exercisable by the Secretary of State (and in the case of regulations made under regulation 53A, with the consent of the Treasury) by statutory instrument; and
(b)includes power to make—
(i)different provision for different cases or descriptions of case, different circumstances, different purposes or different areas;
(ii)consequential, supplementary, incidental, transitional or transitory provision or savings.
(2) A statutory instrument containing regulations made under—
(a)regulation 53A may not be made unless a draft of the instrument has been laid before, and approved by a resolution of, each House of Parliament;
(b)any other provision of these Regulations is subject to annulment in pursuance of a resolution of either House of Parliament.
Regulations: duty to consult
54ZB. Where the Secretary of State proposes to make regulations under these Regulations which will apply in Wales, Scotland or Northern Ireland, the Secretary of State must consult—
(a)the Welsh Ministers, in respect of any proposed application in Wales;
(b)the Scottish Ministers, in respect of any proposed application in Scotland; and
(c)such Northern Ireland department as the Secretary of State considers appropriate, in respect of any proposed application in Northern Ireland,
before making such regulations.”.
(35) Before Schedule 1, insert—
Regulation 5(3)(a)
“SCHEDULE A1Picture Library (of combined health warnings)
| Number | Text Warning | Corresponding Photograph(NOTE) |
|---|---|---|
(NOTE) Corresponding photographs in numbers 1 to 10 and 13 © Commonwealth of Australia. Corresponding photographs in numbers 11 and 12 © Professor Laurence J Walsh, The University of Queensland.”. | ||
| 1 | Smoking clogs your arteries |  |
| 2 | Don’t let children breathe your smoke |  |
| 3 | Smoking causes blindness |  |
| 4 | Smoking causes lung cancer |  |
| 5 | Smoking doubles your risk of stroke |  |
| 6 | Tobacco smoke is toxic |  |
| 7 | Smoking harms unborn babies |  |
| 8 | Smoking causes peripheral vascular disease |  |
| 9 | Smoking causes emphysema | 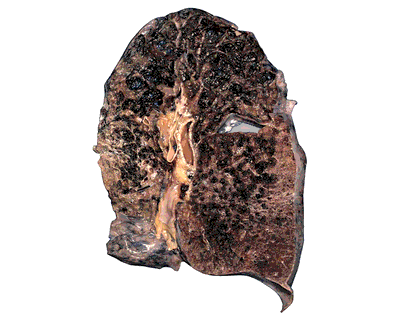 |
| 10 | Quitting will improve your health |  |
| 11 | Smoking damages your gums and teeth |  |
| 12 | Smoking damages your gums and teeth |  |
| 13 | Smoking causes throat cancer |  |
PART 4Amendment and revocation of retained direct EU legislation
Amendment of Commission Implementing Decision 2015/1842
7.—(1) Subject to regulation 9, Commission Implementing Decision (EU) 2015/1842 of 9 October 2015 on the technical specifications for the layout, design and shape of the combined health warnings for tobacco products for smoking is amended as follows.
(2) In Article 3(5)(g)—
(a)for “Annex I to Directive 2014/40/EU”, substitute “Schedule A1 to the Tobacco and Related Products Regulations 2016”; and
(b)omit “, but excluding the numbering”.
(3) Omit Article 5.
Revocation of Commission Implementing Regulation and Decisions
8. The following Regulation and Decisions are revoked—
(a)Commission Implementing Regulation (EU) 2016/779 of 18 May 2016 laying down uniform rules as regards the procedures for determining whether a tobacco product has a characterising flavour;
(b)Commission Implementing Decision (EU) 2015/2183 of 24 November 2015 establishing a common format for the notification of electronic cigarettes and refill containers;
(c)Commission Implementing Decision (EU) 2015/2186 of 25 November 2015 establishing a format for the submission and making available of information on tobacco products;
(d)Commission Implementing Decision (EU) 2016/786 of 18 May 2016 laying down the procedure for the establishment and operation of an independent advisory panel assisting Member States and the Commission in determining whether tobacco products have a characterising flavour.
PART 5Transitional arrangements
Transitional arrangements: supply of existing products
9. Regulation 6(4) and (6) and regulation 7(2) do not apply to a tobacco product where—
(a)the product was produced before exit day; and
(b)the supply of that product takes place at any time before the end of the period of one year beginning with the day after that on which exit day falls.
Signed by authority of the Secretary of State for Health and Social Care.
Steve Brine
Parliamentary Under-Secretary of State,
Department of Health and Social Care
9th January 2019
We consent
Jeremy Quin
Rebecca Harris
Two of the Lords Commissioners of Her Majesty’s Treasury
10th January 2019
Options/Cymorth
Print Options
PrintThe Whole Instrument
PrintThe Instrument without Schedules
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i Deddfwyd neu y’i Gwnaed): Mae'r wreiddiol fersiwn y ddeddfwriaeth fel ag yr oedd pan gafodd ei deddfu neu eu gwneud. Ni wnaed unrhyw newidiadau i’r testun.
Memorandwm Esboniadol
Mae Memoranda Esboniadol yn nodi datganiad byr o ddiben Offeryn Statudol ac yn rhoi gwybodaeth am ei amcan polisi a goblygiadau polisi. Maent yn ceisio gwneud yr Offeryn Statudol yn hygyrch i ddarllenwyr nad oes ganddynt gymhwyster cyfreithiol, ac maent yn cyd-fynd ag unrhyw Offeryn Statudol neu Offeryn Statudol Drafft a gyflwynwyd ger bron y Senedd o Fehefin 2004 ymlaen.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel deddfwyd fersiwn a ddefnyddiwyd am y copi print
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel gwnaed fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
