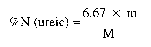Remarks
2. The titration may also be carried out using an indicator (4.26), although the change of colour is more difficult to observe.
7.6.2 Blank test
See 7.2.3.
7.6.3 Expression of result
where:
a = ml of standard solution of sodium or potassium hydroxide (0.1 M) (4.17) used for the blank, carried out in exactly the same conditions as the analysis.
A = ml of standard solution of sodium or potassium hydroxide (0.1 M) (4.17) used for the analysis.
M = mass of the sample, in grams, present in the aliquot part taken for analysis.
where:
a = ml of standard solution of sodium or potassium hydroxide (0.1 M) (4.17) used for the blank, carried out in exactly the same conditions as the analysis.
A = ml of standard solution of sodium or potassium hydroxide (0.1 M) (4.17) used for the analysis.
M = mass of the sample, in grams, present in the aliquot part taken for analysis.
7.6.4 Gravimetric method using xanthydrol
Transfer by pipette into a 100 ml beaker an aliquot portion of the filtrate (7.1) containing not more than 20 mg urea. Add 40 ml acetic acid (4.11). Stir with a glass rod for one minute. Allow any precipitate to settle for five minutes. Filter, wash with a few ml acetic acid (4.11). Add 10 ml xanthydrol solution (4.23) to the filtrate drop by drop, stirring continuously with a glass rod. Allow to stand until the precipitate appears, then stir again for one or two minutes. Allow to stand for one and a half hours. Filter, using a slight reduction in pressure, through a sintered glass crucible (5.6) which has been previously dried and weighed. Wash three times with 5 ml ethanol (4.28), without trying to remove all the acetic acid. Place in an oven at a temperature of 130°C for one hour (do not exceed 145°C). Allow to cool in a desiccator and weigh.
7.6.5 Expression of result
where:
m = mass of the precipitate in grams.
M = mass of the sample, in grams, present in the aliquot part taken for analysis.
where:
m = mass of the precipitate in grams.
M = mass of the sample, in grams, present in the aliquot part taken for analysis.
Correct for the blank.
Although biuret will also be precipitated by xanthydrol, this should not give rise to a significant error in the determination since its level is generally low in absolute value in compound fertilisers.
Note:
Although biuret will also be precipitated by xanthydrol, this should not give rise to a significant error in the determination since its level is generally low in absolute value in compound fertilisers.
7.6.6 Difference method
Ureic N can also be calculated as indicated in the following table:
| Case | Nitric N | Ammoniacal N | Ureic N |
|---|---|---|---|
| 1 | Absent | Present | (7.2.4)-(7.5.3) |
| 2 | Present | Present | (7.3.3)-(7.5.3) |