- Latest available (Revised)
- Point in Time (28/08/2006)
- Original (As adopted by EU)
Commission Decision of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (2006/696/EC) (repealed)
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 01/01/20090.46 MB
- Revised 15/02/20081.67 MB
- Revised 27/10/20071.58 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: ANNEX I
Version Superseded: 15/02/2008
Status:
Point in time view as at 28/08/2006.
Changes to legislation:
There are currently no known outstanding effects for the Commission Decision of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (2006/696/EC) (repealed), ANNEX I.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
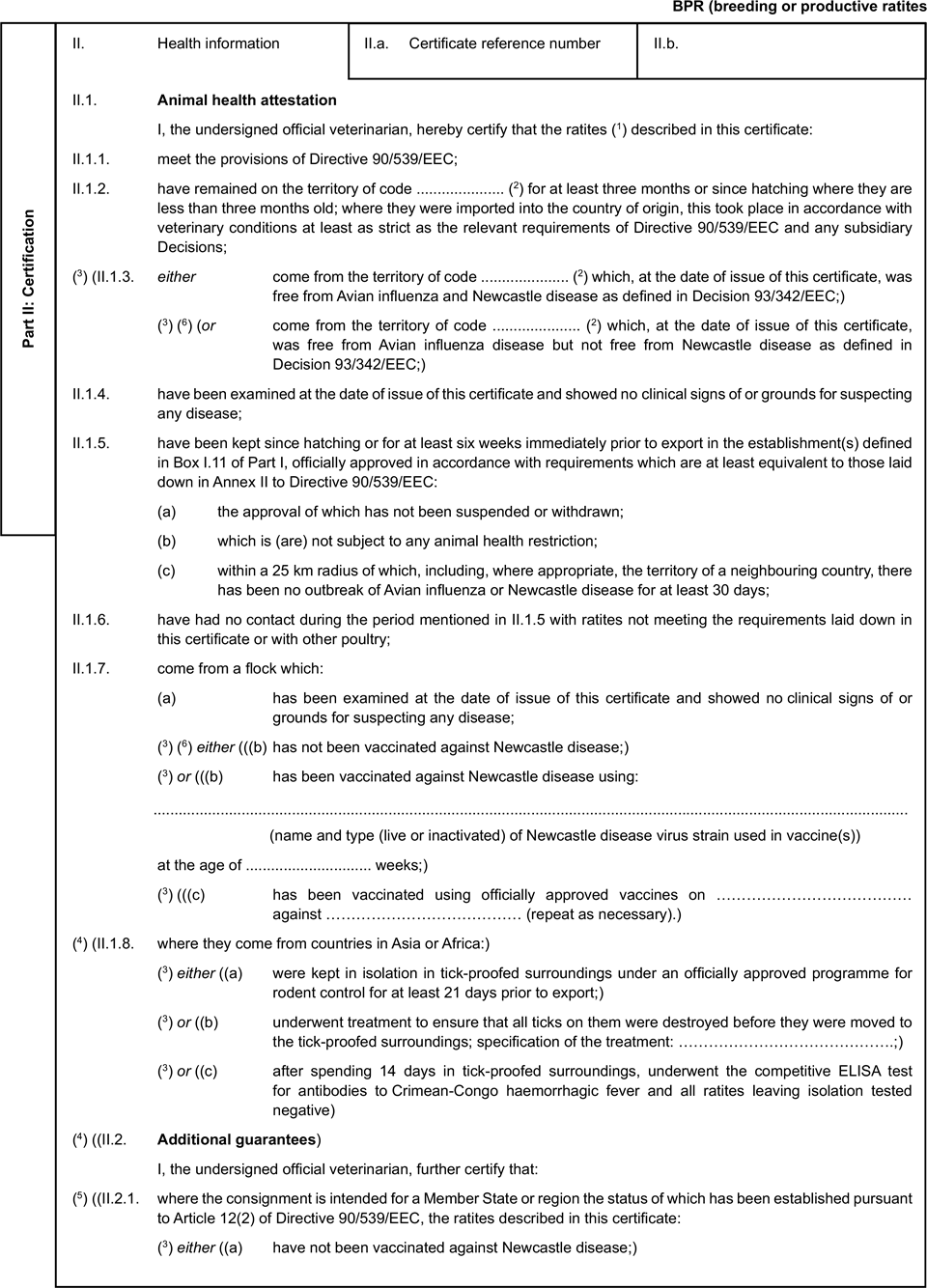
ANNEX IU.K.POULTRY, RATITES INCLUDING HATCHING EGGS OF THESE SPECIES AND SPECIFIED PATHOGEN-FREE EGGS
PART 1U.K.
List of third countries or parts thereofa
| a Without prejudice to specific certification requirements provided for in Community agreements with third countries. | |||||
| b Only applicable until this Acceding State becomes a Member State of the European Union. | |||||
| c Certificates in accordance with the agreement between the European Community and the Swiss Confederation on trade in agricultural products (OJ L 114, 30.4.2002, p. 132). | |||||
| Country | Code of territory | Description of territory | Veterinary certificate | Specific conditions | |
|---|---|---|---|---|---|
| Model(s) | Additional Guarantees | ||||
| 1 | 2 | 3 | 4 | 5 | 6 |
| AR – Argentina | AR-0 | SPF | |||
| AU – Australia | AU-0 | BPP, DOC, HEP, SPF, SRP | |||
| BPR | I | ||||
| DOR | II | ||||
| HER | III | ||||
| BG – Bulgariab | BG-0 | BPP, DOC, HEP, SPF, SRP | |||
| BR – Brazil | BR-0 | SPF | |||
| BR-1 | States of Mato Grosso, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo | BPP, DOC, HEP, SRP | |||
| BR-2 | States of Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Mato Grosso do Sul | BPR, DOR, HEP, HER, SRA | |||
| BW – Botswana | BW-0 | SPF | |||
| BPR | I | ||||
| DOR | II | ||||
| HER | III | ||||
| CA – Canada | CA-0 | BPR, BPP, DOC, DOR, HEP, HER, SRA, SPF, SRP | |||
| CH – Switzerland | CH-0 | c | |||
| CL – Chile | CL-0 | BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP | |||
| HR – Croatia | HR-0 | BPR, BPP, DOR, DOC, HEP, HER, SPF, SRA, SRP | |||
| GL – Greenland | GL-0 | SPF | |||
| IL – Israel | IL-0 | BPR, BPP, DOC, DOR, HEP, HER, SPF, SRP | |||
| IS – Iceland | IS-0 | SPF | |||
| MG – Madagascar | MG-0 | SPF | |||
| MX – Mexico | MX-0 | SPF | |||
| NA – Namibia | NA-0 | SPF | |||
| BPR | I | ||||
| DOR | II | ||||
| HER | III | ||||
| NZ – New Zealand | NZ-0 | BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP | |||
| PM — St Pierre and Miquelon | PM-0 | SPF | |||
| RO – Romaniab | RO-0 | BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP | |||
| TH – Thailand | TH-0 | SPF | |||
| TN – Tunisia | TN-0 | DOR, BPR, BPP, HER, SPF | |||
| TR – Turkey | TR-0 | SPF | |||
| US – United States | US-0 | BPR, BPP, DOC, DOR, HEP, HER, SPF SRA, SRP | |||
| UY – Uruguay | UY-0 | SPF | |||
| ZA – South Africa | ZA-0 | SPF | |||
| BPR | I | ||||
| DOR | II | ||||
| HER | III | ||||
PART 2U.K.
Model veterinary certificatesU.K.
Models:U.K.
:
Model veterinary certificate for breeding or productive poultry other than ratites
:
Model veterinary certificate for breeding or productive ratites
:
Model veterinary certificate for day-old chicks other than of ratites
:
Model veterinary certificate for day-old chicks of ratites
:
Model veterinary certificate for hatching eggs of poultry other than ratites
:
Model veterinary certificate for hatching eggs of ratites
:
Model veterinary certificate for specified pathogen-free (SPF) eggs
:
Model veterinary certificate for slaughter poultry and poultry for restocking game supplies other than ratites
:
Model veterinary certificate for slaughter ratites
Additional guarantees (AG):U.K.
:
Guarantees for breeding and productive ratites coming from regions free from avian influenza but not free from Newcastle disease, certified in accordance with model BPR
:
Guarantees for day-old chicks of ratites coming from regions free from avian influenza but not free from Newcastle disease, certified in accordance with model DOR
:
Guarantees for hatching eggs of ratites coming from third countries free from avian influenza and free or not free from Newcastle disease certified in accordance with model HER
Notes:U.K.
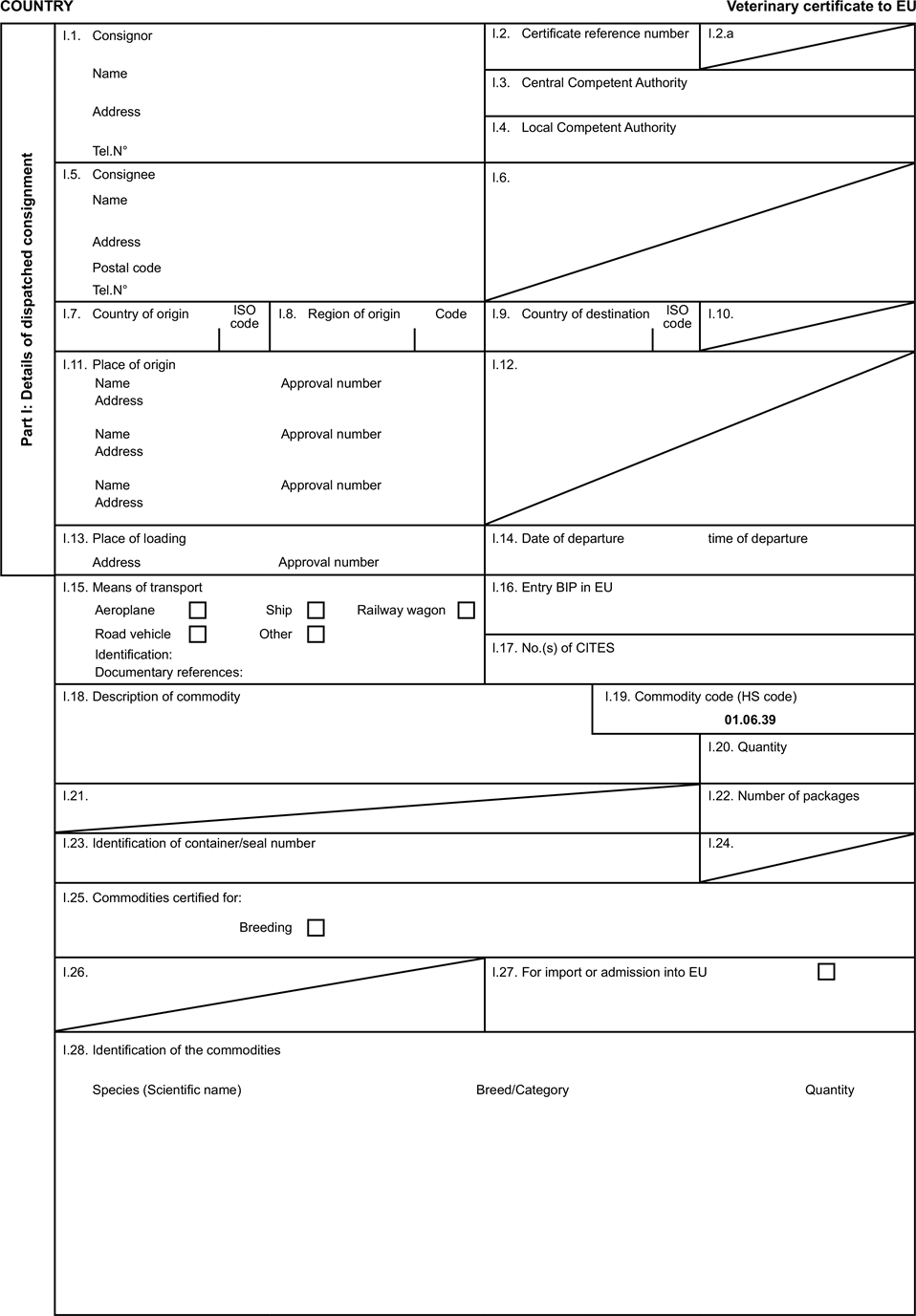
Veterinary certificates based on the models in Part 2 of this Annex or Part 2 of Annex II and following the layout of the model that corresponds to the commodity concerned shall be issued by the exporting third country. They shall contain, in the order appearing in the model, the attestations that are required for any third country and, where applicable, those additional health requirements required for the exporting third country or part thereof.
Where additional guarantees are required by the EU Member State of destination for the commodity concerned, these shall also be entered on the original of the veterinary certificate.
A separate, single certificate must be presented for each consignment of the commodity concerned, exported to the same destination from a territory appearing in columns 2 and 3 of Part 1 of this Annex or in columns 2 and 3 of Part 1 of Annex II and transported in the same railway wagon, lorry, aircraft or ship.
The original of certificates shall consist of a single page printed on both sides or, where more text is required, such that all the pages form a whole and cannot be separated.
The certificate shall be drawn up in at least one official language of the EU Member State where the border inspection takes place and in one official language of the EU Member State of destination. However, those Member States may allow another Community language instead of their own, accompanied, if necessary, by an official translation.
Where additional pages are attached to the certificate for the purposes of identifying the items making up the consignment, such additional pages shall also be considered to form part of the original of the certificate, provided the signature and stamp of the certifying official veterinarian appear on each page.
Where the certificate, including any additional pages as provided for in (e), comprises more than one page, each page shall be numbered ‘–x(page number) of y(total number of pages)–’ on the bottom and shall bear the code number of the certificate allocated by the competent authority on the top.
The original of the certificate must be completed and signed by an official veterinarian not more than 24 hours prior to loading of the consignment for export to the Community. To that end, the competent authorities of the exporting country shall ensure that principles of certification equivalent to those laid down in Directive 96/93/EC are followed.
The colour of the signature shall be different from that of the printing. The same rule shall apply to stamps other than embossed stamps or watermarks.
The original of the certificate must accompany the consignment as far as the EU border inspection post.
The certificate shall be valid for 10 days from the date of issue, unless otherwise stated.
In the case of transport by ship, the term of validity shall be extended by the time taken by the voyage. To that end, the original of a declaration by the ship's master, drawn up in accordance with the addendum to Part 3 of this Annex, shall be attached to the veterinary certificate.
Poultry shall not be transported with other poultry that is either not intended for the European Community or of a lower health status.
During transport to the European Community, poultry shall not be unloaded in the territory of a third country or part of a third country that is not approved for imports of poultry into the Community.
Model veterinary certificate for breeding or productive poultry other than ratites (BPP)U.K.
Model veterinary certificate for breeding or productive ratites (BPR)U.K.
Model veterinary certificate for day-old chicks other than of ratites (DOC)U.K.
Model veterinary certificate for day-old chicks of ratites (DOR)U.K.
Model veterinary certificate for hatching eggs of poultry other than ratites (HEP)U.K.
Model veterinary certificate for hatching eggs of ratites (HER)U.K.
Model veterinary certificate for specified pathogen-free eggs (SPF)U.K.
Model veterinary certificate for slaughter poultry and poultry for restocking game supplies other than ratites (SRP)U.K.
Model veterinary certificate for slaughter ratites (SRA)U.K.
PART 3U.K.
Addendum for transport of poultry by seaU.K.
(To be completed and attached to the veterinary certificate where transport to the European Community border includes transport by ship, even for part of the journey.)U.K.
PART 4U.K.
A.Methods for standardisation of materials and procedures for veterinary tests for imports of poultry and hatching eggsU.K.
Newcastle disease
The sampling and testing methods must comply with the methods described in the Annex to Decision 92/340/EEC on testing of poultry for Newcastle disease prior to movement, in accordance with Article 12 of Directive 90/539/EEC.
Salmonella pullorum
The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
Salmonella gallinarum
The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
Salmonella arizonae
Serological examination: 60 birds to be sampled at the point of lay. Testing must be carried out in accordance with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
Mycoplasma gallisepticum
The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
Mycoplasma meleagridis
The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
B.Sampling and testing procedures for Newcastle disease and avian influenza after importU.K.
During the period referred to in Article 10(1), the official/authorised veterinarian shall take samples from the imported poultry for virological examination, to be tested as follows:
between the 7th and the 15th day of the isolation period, cloacal swabs must be taken from all birds where the consignment contains fewer than 60 birds, and from 60 birds where consignments contain more than 60 birds,
testing of samples for avian influenza and Newcastle disease must be carried out in official laboratories designated by the competent authority, using diagnostic procedures in accordance with Annex III to Council Directive 92/66/EEC(1) and Annex III to Council Directive 92/40/EEC(2),
samples may be pooled, subject to a maximum of five samples from individual birds in each pool,
virus isolates must be sent without delay to the national reference laboratory.
Options/Help
Print Options
PrintThe Whole Decision
PrintThis Annex only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources