ANNEXU.K.
Chapter II of Annex D to Directive 64/432/EEC is replaced by the following:
‘CHAPTER IIU.K. TESTS FOR ENZOOTIC BOVINE LEUKOSIS
Tests for enzootic bovine leukosis shall be carried out by the agar gel immuno-diffusion test (AGID) under the conditions described in Sections A and B or by the enzyme-linked immunosorbent assay (ELISA) under the conditions described in Section C. The agar gel immuno-diffusion test may only be used for the testing of individual samples. If test results are the subject of a duly-substantiated challenge, an additional check shall be carried out by means of the agar gel immuno-diffusion test.
The AGID and ELISA shall be standardised against the E05 serum, which shall be the official EU standard serum, to be supplied by the:
Friedrich-Loeffler-Institut
Federal Research Institute for Animal Health
OIE Reference Laboratory for Enzootic Bovine Leukosis (EBL)
Südufer 10
17493 Greifswald — Insel Riems
Germany.
A. Agar gel immuno-diffusion test for enzootic bovine leukosis
1.The antigen to be used in the test shall contain bovine leukosis virus glycoprotein. The antigen shall be standardised against the E05 serum.
2.The State institutes, national reference laboratories or official institutes designated in accordance with Article 6a for coordinating standards and methods of diagnosis of the tests for enzootic bovine leukosis shall be made responsible for calibrating the standard working antigen of the laboratory against the E05 serum.
3.The standard antigens used in the laboratory shall be submitted at least once a year to the State institutes, national reference laboratories or official institutes designated in accordance with Article 6a, for testing against the E05 serum. Apart from such standardisation, the antigen in use may be calibrated in accordance with the method described in Section B.
4.The reagents of the tests shall consist of:
(a)
antigen: the antigen shall contain specific glycoprotein of enzootic bovine leukosis virus which has been standardised against the E05 serum;
(c)
known positive control serum;
(d)
agar gel:
0,8 % agar,
8,5 % NaCl,
0,05 M Tris-buffer pH 7,2,
15 ml of this agar shall be introduced into a petri dish of 85 mm diameter, resulting in a depth of 2,6 mm of agar.
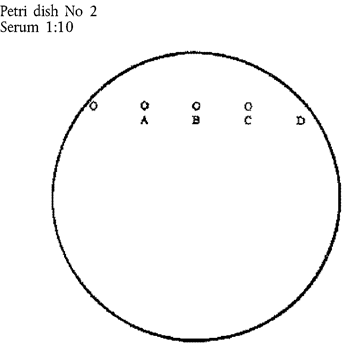
5.A test pattern of seven moisture-free wells shall be cut in the agar to the bottom of the plate; the pattern shall consist of one central well and six wells in a circle around it.
Diameter of central well: 4 mm
Diameter of peripheral wells: 6 mm
Distance between central and peripheral wells: 3 mm
6.The central well shall be filled with the standard antigen. Peripheral wells 1 and 4 described in B.3 are filled with the known positive serum; wells 2, 3, 5 and 6 with the test sera. The wells shall be filled until the meniscus disappears.
7.This results in the following quantities being obtained:
antigen: 32 μl,
control serum: 73 μl,
test serum: 73 μl.
8.Incubation shall be for 72 hours at room temperature (20 to 27 °C) in a closed humid chamber.
9.The test may be read at 24 and 48 hours but a final result shall not be obtained before 72 hours:
(a)
a test serum is positive if it forms a specific precipitation line with the bovine leukosis virus (BLV) antigen and forms a complete line of identity with the control serum;
(b)
a test serum is negative if it does not form a specific precipitation line with the BLV antigen and if it does not bend the line of the control serum;
(c)
the reaction cannot be considered conclusive if it:
(i)
bends the line of the control serum towards the BLV antigen well without forming a visible precipitin line with the antigen; or
(ii)
if it cannot be read either as negative or as positive.
In inconclusive reactions the test may be repeated and concentrated serum utilised.
10.Any other well configuration or pattern may be utilised provided that the E05 serum diluted 1:10 in negative serum can be detected as positive.
B. Method for antigen standardisation
1.Solutions and materials required:
(a)
40 ml of 1,6 % agarose in 0,05 M Tris/HCl buffer, pH 7,2 with 8,5 % NaCl;
(b)
15 ml of a bovine leukosis serum, having antibody only to bovine leukosis virus glycoproteins, diluted 1:10 in 0,05 M Tris/HCl buffer, pH 7,2 with 8,5 % NaCl;
(c)
15 ml of a bovine leukosis serum, having antibody only to bovine leukosis virus glycoproteins, diluted 1:5 in 0,05 M Tris/HCl buffer, pH 7,2 with 8,5 % NaCl;
(d)
four plastic petri dishes with a diameter of 85 mm;
(e)
a punch with a diameter of 4 to 6 mm;
(g)
the antigen which is to be standardised;
2.Procedure:
Dissolve the agarose (1,6 %) in the Tris/HCl buffer by carefully heating to 100 °C. Place in 56 °C water bath for approximately 1 hour. Also, place the bovine leukosis serum dilutions in a 56 °C water bath.
Now mix 15 ml of the 56 °C agarose solution with the 15 ml bovine leukosis serum (1:10), quickly shake and pour 15 ml into each of two petri dishes. Repeat this procedure with the bovine leukosis serum diluted 1:5.
When the agarose has hardened, holes shall be made in it as follows:
3.Addition of antigen:
(a)
petri dishes 1 and 3:
(i)
well A — undiluted reference antigen;
(ii)
well B — 1:2 diluted reference antigen;
(iii)
wells C and E — reference antigen;
(iv)
well D — undiluted test antigen;
(b)
petri dishes 2 and 4:
(i)
well A — undiluted test antigen;
(ii)
well B — 1:2 diluted test antigen;
(iii)
well C — 1:4 diluted test antigen;
(iv)
well D — 1:8 diluted test antigen.
4.Additional instructions:
(a)
the experiment shall be carried out with two serum dilutions (1:5 and 1:10) in order to achieve optimal precipitation;
(b)
if the precipitation diameter is too small with both dilutions, then the serum shall be further diluted;
(c)
if the precipitation diameter in both dilutions is too large and faint, then a lower serum shall be chosen;
(d)
the final concentration of the agarose shall be 0,8 %; that of the sera 5 and 10 % respectively;
(e)
plot the measured diameters in the following coordinate system. The dilution of the antigen to be tested with the same diameter as the reference antigen is the working dilution.

C. Enzyme-linked immunosorbent assay (ELISA) for detecting enzootic bovine leukosis
1.The material and reagents to be used shall be as follows:
(a)
solid-phase microplates, cuvettes or any other solid phase;
(b)
the antigen is fixed to the solid phase with or without the aid of polyclonal or monoclonal catching antibodies. If antigen is coated directly to the solid phase, all test samples giving positive reactions have to be retested against the control antigen. The control antigen should be identical to the antigen except that the BLV antigens are absent. If catching antibodies are coated to the solid phase, the antibodies shall not react to antigens other than BLV antigens;
(c)
the biological fluid to be tested;
(d)
a corresponding positive and negative control;
(f)
a substrate adapted to the enzyme used;
(g)
a stopping solution, if necessary;
(h)
solutions for the dilution of the test samples for preparations of the reagents and for washing;
(i)
a reading system appropriate to the substrate used.
2.Standardisation and sensitivity of test
The sensitivity of the ELISA shall be of such a level that the E05 serum is scored positive when diluted 10 times (serum samples) or 250 times (milk samples) more than the dilution obtained of individual samples when these are included in pools. In assays where samples (serum and milk) are tested individually, the E05 serum diluted 1 to 10 (in negative serum) or 1 to 250 (in negative milk) shall be scored positive when tested in the same assay dilution as used for the individual test samples. The institutes referred to in point 2 of Section A shall be responsible for checking the quality of the ELISA, and in particular for determining, for each production batch, the number of samples to be pooled on the basis of the count obtained for the E05 serum.
3.Conditions for use of the ELISA for enzootic bovine leukosis
(a)
ELISAs may be used on serum and milk samples.
(b)
Where ELISAs are used for certification purposes in accordance with Article 6(2)(c) or for the establishment and maintenance of a herd status in accordance with Annex D(I), pooling of samples of serum or milk shall be carried out in such a way that the samples taken for examination can be undoubtedly related to the individual animals included in the pool. Any confirmatory test shall be carried out on samples taken from individual animals.
(c)
Where ELISAs are used on a sample of bulk milk this sample shall be taken from the milk collected from a herd with at least 30 % of dairy cows in milk. Any confirmatory test shall be carried out on samples of serum or milk taken from individual animals.’