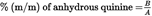- Latest available (Revised)
- Original (As adopted by EU)
Fourth Commission Directive of 11 October 1985 on the approximation of the laws of the Member States relating to methods of analysis necessary for checking the composition of cosmetic products (85/490/EEC)
You are here:
- Directives originating from the EU
- 1985 No. 490
- ANNEX
- IDENTIFICATION AND DETERMINATION OF...
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: IDENTIFICATION AND DETERMINATION OF QUININE
Status:
EU Directives are published on this site to aid cross referencing from UK legislation. Since IP completion day (31 December 2020 11.00 p.m.) no amendments have been applied to this version.
IDENTIFICATION AND DETERMINATION OF QUININE U.K.
A.IDENTIFICATIONU.K.
1.SCOPE AND FIELD OF APPLICATIONU.K.
This method is intended to detect the presence of quinine in shampoo and hair lotions.
2.PRINCIPLEU.K.
Identification is done by thin layer chromatography on silica gel. Detection of quinine is by the blue fluorescence of quinine in acidic conditions at 360 nm.
For further confirmation, the fluorescence can be eliminated by bromine vapours, and ammonia vapours will cause a yellowish fluorescence to appear.
3.REAGENTSU.K.
All reagents should be of analytical purity.
3.1.Silica gel plates, without fluorescence indicators, 0,25 mm thick, 200 mm × 200 mmU.K.
3.2.Developing solvent: toluene /diethyl ether /dichloromethane /diethylamine /20/20/20/8 (v/v/v/v).U.K.
3.3.Methanol.U.K.
3.4.Sulphuric acid (96 %;  ).U.K.
).U.K.
3.5.Diethyl ether.U.K.
3.6.Developing agent: carefully add 5 ml of sulphuric acid (3.4) to 95 ml of diethyl ether (3.5) in a cooled container.U.K.
3.7.Bromine.U.K.
3.8.Ammonium hydroxide solution (28 %;  ).U.K.
).U.K.
3.9.Quinine, anhydrous.U.K.
3.10.Standard solution: weigh accurately about 100,0 mg of anhydrous quinine (3.9) into a standard flask and dissolve in 100 ml of methanol (3.3).U.K.
4.APPARATUSU.K.
4.1.Normal equipment for thin layer chromatography.U.K.
4.2.Ultrasonic bath.U.K.
4.3.Millipore filter, FH 0,5 ƒm or equivalent with suitable filtration equipment.U.K.
5.PROCEDUREU.K.
5.1. Preparation of the sample U.K.
Weigh accurately a quantity of the sample which may contain approximately 100 mg of quinine into a 100 ml standard flask, dissolve and make up to the mark with methanol (3.3).
Stopper the flask and leave for one hour at room temperature in an ultrasonic vibrator (4.2). Filter (4.3) and use the filtrate for the chromatography.
5.2. Thin layer chromatography U.K.
Deposit 1,0 μl of standard solution (3.10) and 1,0 μl of sample solution (5.1) on the silica gel plate (3.1). Develop the chromatogram over a distance of 150 mm using solvent 3.2. in a tank previously saturated with solvent (3.2).
5.3. Development U.K.
5.3.1.Dry the plate at room temperature.U.K.
5.3.21.Spray with reagent 3.6.U.K.
5.3.3.Leave the plate to dry for one hour at room temperature.U.K.
5.3.4.Observe under the light from a UV lamp adjusted to a wavelength of 360 nm. Quinine appears as a fluorescent intense blue spot.U.K.
By way of example the table below gives the values of the RF of the main alkaloids related to quinine when developed with solvent 3.2.
| Alkaloid | RF |
|---|---|
| Quinine | 0,20 |
| Quinidine | 0,29 |
| Cinchonine | 0,33 |
| Cinchonidine | 0,27 |
| Hydroquinidine | 0,17 |
5.3.5.For further confirmation that quinine is present, the plate is exposed for approximately one hour to bromine vapour (3.7). The fluorescence disappears. When the same plate is exposed to ammonia vapour (3.8), the spots reappear with a brown colour, and when the plate is again examined under UV light at 360 nm a yellowish fluorescence can be observed.U.K.
Detection limit: 0,1 μg of quinine.
B.DETERMINATIONU.K.
1.SCOPE AND FIELD OF APPLICATIONU.K.
This method describes the determination of quinine. It may be used to determine the maximum permitted concentration of 0,5 % (m/m) in shampoos and 0,2 % in hair lotions.
2.DEFINITIONU.K.
The quinine content determined by this method is expressed as a percentage by mass (% m/m) of the product.
3.PRINCIPLEU.K.
After appropriate treatment of the product to be analyzed the determination is done by high-performance liquid chromatography (HPLC).
4.REAGENTSU.K.
All reagents should be of analytical purity and suitable for HPLC.
4.1.Acetonitrile.U.K.
4.2.Potassium dihydrogenorthophosphate (KH2PO4).U.K.
4.3.Orthophosphoric acid (85 %;  ).U.K.
).U.K.
4.4.Tetramethylaminium bromide.U.K.
4.5.Quinine, anhydrous.U.K.
4.6.Methanol.U.K.
4.7.Orthophosphoric acid solution (0,1 M): weigh 11,53 g of orthophosphoric acid (4.3) and dissolve in 1 000 nl of water in a graduated flask.U.K.
4.8.Potassium dihyrogenorthophosphate solution (0,1 M): weigh 13,6 g of potassium dihydrogenorthophosphate (4.2) and dissolve in 1 000 ml of water in a graduated flask.U.K.
4.9.Tetramethylammonium bromide solution: dissolve 15,40 g of tetramethylammonium bromide (4.4) in 1 000 ml of water in a graduated flask.U.K.
4.10Eluant: orthophosphoric acid (4.7) /potassium dihydrogenorthophosphate (4.8) /tetramethylammonium bromide (4.9)/water/acetonitrile (4.1) 10/50/100/340/90 (v/v/v/v/v).U.K.
The composition of this mobile phase may be changed in order to achieve a resolution factor R ≥ 1,5.
where
=
retention times, in minutes, of the peaks,
=
peak widths at half height, in millimetres,
=
the chart speed, in millimetres per minute.
4.11.Silica treated with octadecylsilane, 10 μm.U.K.
4.12.Standard solutions: weigh accurately approximately 5,0, 10,0, 15,0 and 20,0 mg respectively of quinine anhydrous (4.5) into a set of 100 ml standard flasks. Make up to the mark with methanol (4.6) and shake the contents of the flasks until the quinine dissolves. Filter each sample through a 0,5 μm filter.U.K.
5.APPARATUSU.K.
5.1.Usual laboratory equipment.U.K.
5.2.Ultrasonic bath.U.K.
5.3.High-performance liquid chromatography equipment with a variable wavelength detector.U.K.
5.4.Column: length: 250 mm; internal diameter: 4,6 mm; filling: silica (4.11).U.K.
5.5.Millipore filter FH 0,5 μm, or equivalent, with suitable filtration apparatus.U.K.
6.PROCEDUREU.K.
6.1. Sample preparation U.K.
Weigh accurately into a 100 ml standard flask a quantity of the product sufficient to contain 10,0 mg of anhydrous quinine, add 20 ml of methanol (4.6) and place the flask in an ultrasonic bath (5.2) for 20 minutes. Make up to the mark with methanol (4.6). Mix the solution and then filter an aliquot (5.5).
6.2. Chromatography U.K.
Flowrate: 1,0 ml/min.
Detector wavelength (5.3): 332 nm.
Injection volume: 10 μl of filtered solution (6.1).
Measurement: peak area.
6.3. Calibration curve U.K.
Inject at least three times 10,0 μl of each reference solution (4.12), measure the area of the peaks, and calculate the average area at each concentration.
Produce the calibration curve and verify that it is rectilinear.
7.CALCULATIONU.K.
7.1.From the calibration curve (6.3) determine the quantity in μg of anhydrous quinine present in the volume injected (6.2).U.K.
7.2.The concentration of anhydrous quinine in the sample, as a percentage by mass (% m/m), is obtained by the following formula:U.K.
where
is the quantity, in micrograms, of anhydrous quinine determined in the 10 microlitres of the filtered solution (6.1).
is the mass of the sample in grams (6.1).
8.REPEATABILITY(1) U.K.
For an anhydrous quinine content of 0,5 % (m/m), the difference between the results of two determinations performed in parallel on the same sample must not exceed 0,02 %.
For an anhydrous quinine content of 0,2 % (m/m), the difference between the results of two determinations performed in parallel on the same sample must not exceed 0,01 %.
ISO 5725.
Options/Help
Print Options
PrintThe Whole Directive
PrintThe Whole Annex
PrintThis Division only
You have chosen to open the Whole Directive
The Whole Directive you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources