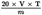METHOD 3
DETERMINATION OF TITRATABLE ACIDITY
1.SCOPE AND FIELD OF APPLICATION
The method determines the titratable acidity of:
acid caseins.
2.DEFINITION
The titratable acidity of acid caseins: the volume in millilitres, of a 0,1 mol/1 standard sodium hydroxide solution required to neutralize an aqueous extract of 1 gram of the product.
3.PRINCIPLE
An aqueous extract of the sample at 60 o C is obtained and filtered. The filtrate is titrated against standard sodium hydroxide using phenolphtalein indicator.
4.REAGENTS
Any water used in the method procedure or in the preparation of reagents shall be freed from carbon dioxide by boiling for 10 minutes before use.
4.1. Sodium hydroxide solution: 0,1 Mol/1.
4.2. Phenolphtalein indicator solution, 10 g/1 in ethanol (95 % V/V) neutralized to the indicator.
5.APPARATUS
5.1. Analytical balance
5.2. Conical flask, 500 ml capacity, with ground neck and fitted with a ground glass stopper.
5.3. One-mark pipette, 100 ml capacity.
5.4. Pipette, suitable for measuring 0,5 ml of indicator solution (4.2).
5.5. Conical flask, 250 ml capacity.
5.6. Measuring cylinder, 250 ml capacity.
5.7. Burette, graduated in 0,1 ml.
5.8. Water bath, capable of being controlled at a temperature of 60 oC ± 2 oC.
5.9. Appropriate filter
6.PROCEDURE
6.1. Preparation of the test sample
As described in Section 1.2 of the General Provisions.
6.2. Test portion
Weigh about 10 grams of the test sample (6.1) to the nearest 10 mg and transfer it to the conical flask (5.2).
6.3. Determination
Using the 250 ml measuring cylinder (5.6), add 200 ml of freshly boiled and cooled water, previously heated to 60 o C. Stopper the flask, mix by swirling and place in the water bath at 60 o C (5.8) for 30 minutes. Shake the flask at intervals of about 10 minutes.
Filter, and cool the filtrate to about 20 oC. The filtrate must be clear.
Transfer 100 ml of the cooled filtrate into the conical flask (5.5), using the pipette (5.3). Add 0,5 ml of the phenolphtalein indicator solution (4.2), using the pipette (5.4). Titrate with the standard volumetric sodium hydroxide solution (4.1), until the appearance of a faint pink colour, persisting for at least 30 seconds. Determine and record the volume used to the nearest 0,01 ml.
7.EXPRESSION OF RESULTS
7.1. Formula and method of calculation
The titratable acidity of the acid casein is given by:
where:
is the volume, in millilitres, of the standard volumetric sodium hydroxide solution (4.1) used;
is the strength of the standard volumetric sodium hydroxide solution (4.1) in mol/1;
is the mass, in grams, of the test portion.
Calculate the titratable acidity to two decimal places.
7.2. Repeatability
The difference in results between two determinations carried out simultaneously or in rapid succession on the same sample, by the same analyst, under the same conditions, shall not exceed 0,02 ml of 0,1 mol/1 sodium hydroxide per 1 gram of product.
This repeatability interval should be achieved in 95 % of the times that the method is correctly carried out.