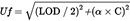- Latest available (Revised)
- Point in Time (13/03/2010)
- Original (As adopted by EU)
Commission Regulation (EC) No 401/2006Show full title
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs (Text with EEA relevance)
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 01/07/20140.53 MB
- Revised 13/03/20100.16 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Division 4.
Version Superseded: 01/07/2014
Alternative versions:
Status:
Point in time view as at 13/03/2010.
Changes to legislation:
There are currently no known outstanding effects for the Commission Regulation (EC) No 401/2006, Division 4..![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
4.METHOD OF ANALYSIS TO BE USED BY THE LABORATORY AND LABORATORY CONTROL REQUIREMENTSU.K.
4.1.DefinitionsU.K.
A number of the most commonly used definitions that the laboratory shall be required to use are the following:
=
Repeatability, the value below which the absolute difference between two single test results obtained under repeatability conditions, namely same sample, same operator, same apparatus, same laboratory, and short interval of time may be expected to lie within a specific probability (typically 95 %) and hence r = 2,8 × sr.
=
Standard deviation, calculated from results generated under repeatability conditions.
=
Relative standard deviation, calculated from results generated under repeatability conditions [(sr / 
=
Reproducibility, the value below which the absolute difference between single test results obtained under reproducibility conditions, namely on identical material obtained by operators in different laboratories, using the standardised test method may be expected to lie within a certain probability (typically 95 %); R = 2,8 × sR.
=
Standard deviation, calculated from results under reproducibility conditions.
=
Relative standard deviation calculated from results generated under reproducibility conditions [(sR / 
4.2.General requirementsU.K.
Methods of analysis used for food control purposes shall comply with the provisions of items 1 and 2 of Annex III to Regulation (EC) No 882/2004.
4.3.Specific requirementsU.K.
4.3.1.Performance criteriaU.K.
Where no specific methods for the determination of mycotoxin levels in foodstuffs are required by Community legislation, laboratories may select any method provided the selected method meets the following criteria:
Performance criteria for aflatoxins
| Note: | |||
| |||
| Criterion | Concentration Range | Recommended Value | Maximum permitted Value |
|---|---|---|---|
| Blanks | All | Negligible | — |
| Recovery — Aflatoxin M1 | 0,01-0,05 μg/kg | 60 to 120 % | |
| > 0,05 μg/kg | 70 to 110 % | ||
| Recovery — Aflatoxins B1, B2, G1, G2 | < 1,0 μg/kg | 50 to 120 % | |
| 1-10 μg/kg | 70 to 110 % | ||
| > 10 μg/kg | 80 to 110 % | ||
| Precision RSDR | All | As derived from Horwitz Equation | 2 × value derived from Horwitz Equation |
| Precision RSDr may be calculated as 0,66 times Precision RSDR at the concentration of interest. | |||
Performance criteria for ochratoxin A
| Level μg/kg | Ochratoxin A | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| < 1 | ≤ 40 | ≤ 60 | 50 to 120 |
| 1-10 | ≤ 20 | ≤ 30 | 70 to 110 |
Performance criteria for patulin
| Level μg/kg | Patulin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| < 20 | ≤ 30 | ≤ 40 | 50 to 120 |
| 20-50 | ≤ 20 | ≤ 30 | 70 to 105 |
| > 50 | ≤ 15 | ≤ 25 | 75 to 105 |
Performance criteria for deoxynivalenol
| Level μg/kg | Deoxynivalenol | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| > 100-≤ 500 | ≤ 20 | ≤ 40 | 60 to 110 |
| > 500 | ≤ 20 | ≤ 40 | 70 to 120 |
Performance criteria for zearalenone
| Level μg/kg | Zearalenone | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| ≤ 50 | ≤ 40 | ≤ 50 | 60 to 120 |
| > 50 | ≤ 25 | ≤ 40 | 70 to 120 |
Performance criteria for Fumonisin B1 and B2
| Level μg/kg | Fumonisin B1 or B2 | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| ≤ 500 | ≤ 30 | ≤ 60 | 60 to 120 |
| > 500 | ≤ 20 | ≤ 30 | 70 to 110 |
Performance criteria for T-2 and HT-2 toxin
| Level μg/kg | T-2 toxin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| 50-250 | ≤ 40 | ≤ 60 | 60 to 130 |
| > 250 | ≤ 30 | ≤ 50 | 60 to 130 |
| Level μg/kg | HT-2 toxin | ||
|---|---|---|---|
| RSDr % | RSDR % | Recovery % | |
| 100-200 | ≤ 40 | ≤ 60 | 60 to 130 |
| > 200 | ≤ 30 | ≤ 50 | 60 to 130 |
Notes to the performance criteria for the mycotoxins
The detection limits of the methods used are not stated as the precision values are given at the concentrations of interest
The precision values are calculated from the Horwitz equation, i.e.:
RSDR = 2(1-0,5logC)
where:
This is a generalised precision equation which has been found to be independent of analyte and matrix but solely dependent on concentration for most routine methods of analysis.
4.3.2.‘Fitness-for-purpose’ approachU.K.
In the case where there are a limited number of fully validated methods of analysis, alternatively, a ‘fitness-for-purpose’ approach, defining a single parameter, a fitness function, to evaluate the acceptability of methods of analysis may be used. A fitness function is an uncertainty function that specifies maximum levels of uncertainty regarded as fit for purpose.
Given the limited number of methods of analysis, fully validated by a collaborative trial, especially for the determination of T-2 and HT-2 toxin, the uncertainty function approach, specifying the maximum acceptable uncertainty, may also be used to assess the suitability (the ‘fitness-for-purpose’) of the method of analysis to be used by the laboratory. The laboratory may use a method which produces results within the maximum standard uncertainty. The maximum standard uncertainty may be calculated using the following formula:
where:
Uf is the maximum standard uncertainty (μg/kg)
LOD is the limit of detection of the method (μg/kg)
α is a constant, numeric factor to be used depending on the value of C. The values to be used are set out in the table hereafter
C is the concentration of interest (μg/kg).
If the analytical method provides results with uncertainty measurements less than the maximum standard uncertainty the method shall be considered being equally suitable to one which meets the performance criteria given in point 4.3.1.
| Table | |
| Numeric values to be used for α as constant in formula set out in this point, depending on the concentration of interest | |
| C (μg/kg) | α |
|---|---|
| ≤ 50 | 0,2 |
| 51-500 | 0,18 |
| 501-1 000 | 0,15 |
| 1 001-10 000 | 0,12 |
| > 10 000 | 0,1 |
4.4.Estimation of measurement uncertainty, recovery calculation and reporting of results(1) U.K.
The analytical result must be reported corrected or uncorrected for recovery. The manner of reporting and the level of recovery must be reported. The analytical result corrected for recovery shall be used for controlling compliance.
The analytical result must be reported as x +/– U whereby x is the analytical result and U is the expanded measurement uncertainty.
U is the expanded measurement uncertainty, using a coverage factor of 2 which gives a level of confidence of approximately 95 %.
For food of animal origin, the taking into account of the measurement uncertainty can also be done by establishing the decision limit (CCα) in accordance with Commission Decision 2002/657/EC(2) (point 3.1.2.5. of the Annex — the case of substances with established permitted limit).
The present interpretation rules of the analytical result in view of acceptance or rejection of the lot apply to the analytical result obtained on the sample for official control. In case of analysis for defence or referee purposes, the national rules apply.
4.5.Laboratory quality standardsU.K.
Laboratory must comply with the provisions of Article 12 of Regulation (EC) No 882/2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules(3).
More details on procedures for the estimation of measurement uncertainty and on procedures for assessing recovery can be found in the report ‘Report on the relationship between analytical results, measurement uncertainty, recovery factors and the provisions of EU food and feed legislation’ — http://europa.eu.int/comm/food/food/chemicalsafety/contaminants/report-sampling_analysis_2004_en.pdf
OJ L 221, 17.8.2002, p. 8. Decision as last amended by Decision 2004/25/EC (OJ L 6, 10.1.2004, p. 38).
See also the transitional arrangements provided for in article 18 of Commission Regulation (EC) No 2076/2005 of 5 December 2005 laying down transitional arrangements for the implementation of Regulation (EC) No 853/2004, 854/2004 and 882/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and 854/2004 (OJ L 338, 22.12.2005, p. 83).
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Whole Annex
PrintThis Division only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources