Commission Regulation (EC) No 504/2008
of 6 June 2008
implementing Council Directives 90/426/EEC and 90/427/EEC as regards methods for the identification of equidae
(Text with EEA relevance) (repealed)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Directive 90/426/EEC of 26 June 1990 on animal health conditions governing the movement and import from third countries of equidae(1), and in particular Article 4(4) thereof,
Having regard to Council Directive 90/427/EEC of 26 June 1990 on the zootechnical and genealogical conditions governing intra-Community trade in equidae(2), and in particular Article 4(2)(c) and (d), the second indent of Article 6(2) and the first subparagraph of Article 8(1) thereof,
Having regard to Council Directive 94/28/EC of 23 June 1994 laying down the principles relating to the zootechnical and genealogical conditions applicable to imports from third countries of animals, their semen, ova and embryos, and amending Directive 77/504/EEC on pure-bred breeding animals of the bovine species(3), and in particular Article 3(4) thereof,
Whereas:
(1) Commission Decision 93/623/EEC of 20 October 1993 establishing the identification document (passport) accompanying registered equidae(4) introduces a method to identify registered equidae during their movements for animal health control purposes.
(2) Commission Decision 2000/68/EC of 22 December 1999 amending Commission Decision 93/623/EEC and establishing the identification of equidae for breeding and production(5), lays down rules on the identification document to accompany equidae during movement.
(3) Decisions 93/623/EEC and 2000/68/EC have been implemented differently by the Member States. In addition, the identification of equidae in those Decisions is linked to movement, while in Community legislation concerning other livestock species, animals are identified, inter alia for disease control purposes, regardless of their movement status. In addition, that two-tier system of equidae for breeding and production on the one side and registered equidae on the other side may lead to the issuing of more than one identification document for a single animal which can only be counteracted by applying to the animal an indelible, but not necessarily visible, mark on the occasion of the primary identification of the animal.
(4) The outline diagram included in the identification document set out in Decision 93/623/EEC is not fully compatible with similar information required by international organisations handling equidae for competitions and races and by the World Organisation for Animal Health (OIE). This Regulation should therefore establish an outlinediagram which is appropriate to the needs of the Community and in line with those internationally accepted requirements.
(5) Imports of equidae continue to be subject to the conditions laid down in Directive 90/426/EEC, and in particular in Commission Decision 93/196/EEC of 5 February 1993 on animal health conditions and veterinary certification for imports of equidae for slaughter(6), and Commission Decision 93/197/EEC of 5 February 1993 on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production(7).
(6) When the customs procedures laid down in Council Regulation (EC) No 2913/92 of 12 October 1992 establishing the Community Customs Code(8) are applied, it is necessary to refer in addition to Council Regulation (EEC) No 706/73 of 12 March 1973 concerning the Community arrangements applicable to the Channel Islands and the Isle of Man for trade in agricultural products(9). Regulation (EEC) No 706/73 stipulates that as from 1 September 1973, the Community rules are applicable in the matter of veterinary legislation, but excludes Community zootechnical legislation. The present Regulation should apply without prejudice to that Regulation.
(7) Regulation (EC) No 1760/2000 of the European Parliament and of the Council of 17 July 2000 establishing a system for the identification and registration of bovine animals and regarding the labelling of beef and beef products(10) provides a definition of a keeper of animals. By contrast, Article 4(2) of Directive 90/426/EEC refers to the owner or breeder of the animal. Council Directive 92/35/EEC of 29 April 1992 laying down control rules and measures to combat African horse sickness(11) provides for a combined definition of owner and keeper. As under Community and national legislation, the owner of an equine animal is not necessarily the person responsible for the animal, it is appropriate to clarify that primarily the keeper of the equine animal, who may be the owner, should be responsible for the identification of equine animals in accordance with the present Regulation.
(8) In the interests of consistency of Community legislation, the methods for the identification of equidae provided for in this Regulation should apply without prejudice to Commission Decision 96/78/EC of 10 January 1996 laying down the criteria for entry and registration of equidae in stud books for breeding purposes(12).
(9) Those methods should be in line with the principles established by breeding organisations approved in accordance with Commission Decision 92/353/EEC of 11 June 1992 laying down the criteria for the approval or recognition of organisations and associations which maintain or establish stud books for registered equidae(13). In accordance with that Decision, it is for the organisation or association which maintains the stud book of the origin of the breed to establish principles on a system for identifying equidae and on the division of the stud book into classes and on the lineages entered in the stud book.
(10) In addition, the certificate of origin, referred to in Article 4(2)(d) of Directive 90/427/EEC, to be incorporated in the identification document should mention all necessary information to ensure that equidae which are moved between different stud books are entered in the class of the stud book the criteria of which they meet.
(11) In accordance with the third indent of Article 1 of Commission Decision 96/510/EC of 18 July 1996 laying down the pedigree and zootechnical certificates for the importation of breeding animals, their semen, ova and embryos(14) the pedigree and zootechnical certificate for registered equidae must be conform to the identification document as laid down in Decision 93/623/EEC. It is therefore necessary to clarify that any reference to Decision 93/623/EEC, but also to Decision 2000/68/EC, should be construed as reference to the present Regulation.
(12) As all equidae born in or imported into the Community in accordance with this Regulation should be identified by a single identification document, special provisions are necessary when the animals’ status as equidae for breeding and production is changed into registered equidae as defined in Article 2(c) of Directive 90/426/EEC.
(13) Member States should be able to establish specific regimes for the identification of equidae roaming under wild or semi-wild conditions in defined areas or territories, including nature reserves, for the sake of consistency with the second paragraph of Article 2 of Directive 92/35/EEC.
(14) Electronic identifiers (transponders) for equidae are already in wide practical use at international level. That technology should be used to ensure a close link between the equine animal and the means of identification. Equidae should be marked with a transponder, although provision should be made for alternative methods used for the verification of the identity of the animal provided that those alternative methods provide equivalent guarantees to prevent multiple issuing of identification documents.
(15) While equidae must always be accompanied by their identification document in accordance with current Community legislation, provision should be made to derogate from that requirement when it is impossible or even impractical with the view to the retention of the identification document throughout the lifetime of the equine animal, or where such document was not issued taking into account the slaughter of the animal before it reaches the required maximum age for identification.
(16) Those derogations should be applied without prejudice to Article 14 of Council Directive 2003/85/EC of 29 September 2003 on Community measures for the control of foot-and-mouth disease(15), which allows derogations from certain disease control measures for identified equidae on holdings where an outbreak of that disease has been confirmed.
(17) Member States should also be permitted to allow a simplified identification document to be used for equidae being moved within their territory. Plastic cards with embedded computer chips (smart cards) have been introduced as data storage devices in various areas. It should be possible to issue such smart cards as an option in addition to the identification document and to use them under certain conditions in place of the identification document accompanying equidae during movements within a Member State.
(18) In accordance with Article 8 of Commission Regulation (EC) No 2076/2005 of 5 December 2005 laying down transitional arrangements for the implementation of Regulations (EC) No 853/2004, (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council(16) food chain information requirements for equidae are to be implemented by the end of 2009.
(19) Provisions are necessary in case the original identification document issued in accordance with this Regulation for lifetime was lost. Those provisions should as much as possible exclude the unlawful possession of more than one identification document in order to describe correctly the animal's status as intended for slaughter for human consumption. Where sufficient and verifiable information is available, a duplicate document should be issued which is marked as such, and generally excludes the animal from the food chain; in other cases a replacement document should be issued, equally marked as such, that in addition will downgrade a previously registered equine animal to an equine for breeding and production.
(20) In accordance with Articles 4 and 5 of Directive 90/426/EEC, the identification document is an instrument to immobilise equidae in case of an outbreak of a disease on the holding where they are kept or bred. It is therefore necessary to provide for the suspension of the validity of that document for movement purposes in the event of an outbreak of certain diseases by an appropriate entry in the identification document.
(21) On the death of the equine animal other than by slaughter at a slaughterhouse, the identification document should be returned to the issuing body by the authority supervising the processing of the dead animal in accordance with Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption(17), and it should be ensured that the transponder, or any alternative methods, including marks, used to verify the identity of the equine animal, cannot be recycled.
(22) To prevent transponders from entering the food chain, meat from animals from which it has not been possible to remove the transponder at the time of slaughter should be declared unfit for human consumption in accordance with Chapter V of Section II of Annex I to Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption(18).
(23) The standardisation of the place of implantation of transponders and the recording of that place in the identification documents should make it easier to locate implanted transponders.
(24) In accordance with Article 2 of Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety(19), live animals prepared for placing on the market for human consumption are defined as food. That Regulation provides for far-reaching responsibilities of food business operators throughout all stages of the production of food, including traceability of food-producing animals.
(25) Equidae for breeding and production, as well as registered equidae, may become equidae for slaughter as defined in Article 2(d) of Directive 90/426/EEC at a certain stage of their lifetime. Meat of solipeds, synonymous for equidae, is defined in Annex I to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin(20).
(26) In accordance with paragraph 7 of Section III of Annex II to Regulation (EC) No 853/2004, the slaughterhouse operator is to receive, check and act upon food chain information providing details on the origin, history and management of animals intended for food production. The competent authority may allow food chain information on domestic solipeds to be sent to the slaughterhouse at the same time as the animals, rather than being sent in advance. The identification document accompanying equidae for slaughter should therefore form part of that food chain information.
(27) In accordance with paragraph 1 of Chapter III of Section II of Annex I to Regulation (EC) No 854/2004 the official veterinarian is to verify compliance with the food business operator's duty to ensure that animals accepted for slaughter for human consumption are properly identified.
(28) In accordance with paragraph 8 of Section III of Annex II to Regulation (EC) No 853/2004, the food business operators are to check passports accompanying domestic solipeds to ensure that the animal is intended for slaughter for human consumption and if they accept the animal for slaughter they are to give the passport to the official veterinarian.
(29) Without prejudice to Council Regulation (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin(21) and Council Directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of ß-agonists(22), the administration of veterinary medicinal products to equidae is subject to Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products(23).
(30) Article 10(2) and (3) of Directive 2001/82/EC provides for specific derogations for equidae from Article 11 of that Directive, relating to the treatment of food producing animals with medicinal products that have an established maximum residue limit for species other than the target species or are authorised for a different condition, provided that those equidae are identified in accordance with Community legislation and specifically marked in their identification document as not intended for slaughter for human consumption or as intended for slaughter for human consumption following a withdrawal period of at least six months after they have been treated with substances listed in Commission Regulation (EC) No 1950/2006 of 13 December 2006 establishing, in accordance with Directive 2001/82/EC of the European Parliament and of the Council on the Community code relating to veterinary medicinal products, a list of substances essential for the treatment of equidae(24).
(31) In order to maintain control over the issuing of identification documents, a minimum set of relevant data relating to the issuing of such documents should be recorded in a database. The databases in different Member States should cooperate in accordance with Council Directive 89/608/EEC of 21 November 1989 on mutual assistance between the administrative authorities of the Member States and cooperation between the latter and the Commission to ensure the correct application of legislation on veterinary and zootechnical matters(25) to facilitate the exchange of data.
(32) The Universal Equine Life Number (UELN) system has been agreed worldwide between the major horse-breeding and competition organisations. It has been developed on the initiative of the World Breeding Federation for Sport Horses (WBFSH), the International Stud Book Committee (ISBC), the World Arabian Horse Organization (WAHO), the European Conference of Arabian Horse Organisations (ECAHO), the Conférence Internationale de l’Anglo-Arabe (CIAA), the Fédération Equestre Internationale (FEI) and the Union Européenne du Trot (UET) and information on this system can be consulted on the UELN website(26).
(33) The UELN system is suitable for the registration of both registered equidae and equidae for breeding and production and allows computerised networks to be brought in gradually to ensure that the animals’ identity can continue to be verified in accordance with Article 6 of Directive 90/427/EEC in the case of registered equidae.
(34) When codes are assigned to databases, those codes and the format of the recorded identification numbers of individual animals should in no way conflict with the established UELN system. Therefore, the list of assigned UELN codes should be consulted before any new code is assigned to a database.
(35) Article 7(3) of Directive 90/426/EEC requires the official veterinarian to record the identification number or identification document number of the slaughtered equidae, and to forward to the competent authority at the place of dispatch, at the latter’s request, an attestation to the effect that the equine animal has been slaughtered. In accordance with Article 4(4)(i) of that Directive, after registered equidae are slaughtered, their identification document are to be returned to the body that issued them. These requirements should also apply to identification documents issued for equidae for breeding and production. Recording a UELN-compatible life number and using it to identify the authorities or bodies which issued the identification document should facilitate compliance with those requirements. Where possible, Member States should use the liaison bodies they have designated in accordance with Article 35 of Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules(27).
(36) Veterinary supervision necessary to provide the animal health guarantees in accordance with Articles 4 and 5 of Directive 90/426/EEC can only be ensured if the holding as defined in Article 2(a) of that Directive is known to the competent authority. Similar requirements result from the application of food law in relation to equidae as food-producing animals. However, due to the frequency of movements of equidae, in comparison with other livestock, it should not be attempted to establish a real-time habitual traceability of equidae. Identification of equidae should therefore be a first step of a system for the identification and registration of equidae to be completed in the framework of the New Community Animal Health Policy.
(37) With a view to the uniform application of Community legislation on the identification of equidae in the Member States and to ensure that it is clear and transparent, Decisions 93/623/EEC and 2000/68/EC should be repealed and replaced by this Regulation.
(38) Transitional measures should be provided for in order to allow the Member States to adapt to the rules laid down in this Regulation.
(39) The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health and the Standing Committee on Zootechnics,
HAS ADOPTED THIS REGULATION:
CHAPTER IU.K.SUBJECT MATTER, SCOPE AND DEFINITIONS
Article 1U.K.Subject matter and scope
1.This Regulation lays down rules on the identification of equidae:
(a)born in the Community; or
(b)released for free circulation in the Community in accordance with the customs procedure defined in Article 4(16)(a) of Regulation (EEC) No 2913/92.
2.This Regulation shall be without prejudice to:
(a)Regulation (EEC) No 706/73 and Decision 96/78/EC; and
(b)measures taken by Member States to register holdings keeping equidae.
Article 2U.K.Definitions
1.For the purposes of this Regulation, the definitions in Article 2(a) and (c) to (f), (h) and (i) of Directive 90/426/EEC and Article 2(c) of Directive 90/427/EEC shall apply.
2.The following definitions shall also apply:
(a)‘keeper’ means any natural or legal person having ownership of, or in the possession of, or charged with the keeping of, an equine animal, whether or not for financial reward, and whether or not on a permanent or on a temporary basis, including during transportation, at markets, or during competitions, races or cultural events;
(b)‘transponder’ means a read-only passive radio frequency identification device:
complying with standard ISO 11784 and applying HDX or FDX-B technology; and
capable of being read by a reading device compatible with standard ISO 11785, at a minimum distance of 12 cm;
(c)‘equidae’ or ‘equine animals’ means wild or domesticated soliped mammals of all species within the genus Equus of the family Equidae, and their crosses;
(d)‘unique life number’ means a unique 15-digit alphanumeric code compiling information on the individual equine animal and the database and country where such information is first recorded in accordance with the coding system of the Universal Equine Life Number (UELN) and comprising:
a six-digit UELN-compatible identification code for the database referred to in Article 21(1); followed by
a nine-digit individual identification number assigned to the equine animal.
(e)‘smart card’ means a plastic device with an embedded computer chip capable of storing data and transmitting them electronically to compatible computer systems.
CHAPTER IIU.K.IDENTIFICATION DOCUMENT
Article 3U.K.General principles and obligation to identify equidae
1.Equidae referred to in Article 1(1) shall not be kept unless they are identified in accordance with this Regulation.
2.Where the keeper has no ownership of the equine animal he shall act within the framework of this Regulation on behalf of and in agreement with the natural or legal person having the ownership of the equine animal (the owner).
3.For the purpose of this Regulation, the system for the identification of equidae shall be comprised of the following elements:
(a)a single lifetime identification document;
(b)a method to ensure an unequivocal link between the identification document and the equine animal;
(c)a database recording under a unique identification number the identification details relating to the animal for which an identification document was issued to a person recorded in that database.
Article 4U.K.Issuing bodies for identification documents for equidae
1.Member States shall ensure that the identification document referred to in Article 5(1) for registered equidae is issued by the following bodies (issuing bodies):
(a)the organisation or association officially approved or recognised by the Member State, or by the official agency of the Member State concerned, both as referred to in the first indent of Article 2(c) of Directive 90/427/EEC, which manages the stud book for that breed of animal, as referred to in Article 2(c) of Directive 90/426/EEC; or
(b)a branch with its headquarters in a Member State of an international association or organisation which manages horses for competition or racing, as referred to in Article 2(c) of Directive 90/426/EEC.
2.The identification documents issued by the authorities in a third country issuing pedigree certificates in accordance with the third indent of Article 1 of Decision 96/510/EC shall be deemed valid in accordance with this Regulation for registered equidae referred to in Article 1(1)(b).
3.The issuing body for the identification document referred to in Article 5(1) for equidae for breeding and production shall be designated by the competent authority.
4.The issuing bodies referred to in paragraphs 1, 2 and 3 shall act in accordance with this Regulation, in particular with the provisions in Articles 5, 8 to 12, 14, 16, 17, 21 and 23.
5.Member States shall draw up and keep up to date the list of issuing bodies and make this information available to the other Member States and the public on a website.
The information on the issuing bodies shall include at least the contact details necessary to comply with the requirements of Article 19.
In order to assist the Member States in making those up to date lists available, the Commission shall provide a website to which each Member State shall provide a link to its national website.
6.The lists of issuing bodies in third countries referred to in paragraph 2 shall be prepared and updated in accordance with the following conditions:
(a)the competent authority of the third country in which the issuing body is situated guarantees that:
the issuing body complies with paragraph 2;
in the case of an issuing body approved in accordance with Directive 94/28/EEC, it must comply with the information requirement referred to in Article 21(3) of this Regulation;
lists of issuing bodies are drawn up, kept up to date and communicated to the Commission;
(b)the Commission shall:
provide the Member States with regular notifications concerning new or updated lists that it has received from the competent authorities of the third countries concerned in accordance with point (a)(iii);
arrange for up-to-date versions of those lists to be made available to the public;
where necessary, include the matter related to the list of issuing bodies in third countries, without undue delay, on the agenda of the Standing Committee on Zootechnics for decision in accordance with the procedure referred to in Article 11(2) of Council Directive 88/661/EEC(28).
Article 5U.K.Identification of equidae born in the Community
1.Equidae born in the Community shall be identified by means of a single identification document in accordance with the model identification document for equidae set out in Annex I (identification document or passport). It shall be issued for the lifetime of the equine animal.
The identification document shall be in a printed indivisible format and contain entries for the insertion of the information required under the following Sections thereof:
(a)in the case of registered equidae, Sections I to X;
(b)in the case of equidae for breeding and production, at least Sections I, III, IV and VI to IX.
2.The issuing body shall ensure that no identification document is issued for an equine animal unless at least Section I thereof is duly completed.
3.Without prejudice to Article 1(1) of Decision 96/78/EC, and notwithstanding the provisions of paragraph 1(a) and paragraph 2 of this Article, registered equidae shall be identified in the identification document according to the rules of the issuing bodies referred to in Article 4(1) or (2) of this Regulation.
4.For registered equidae, the issuing body, as referred to in Article 4(1)(a) and (2) of this Regulation, shall complete in Section II of the identification document the information in the certificate of origin, as referred to in Article 4(2)(d) of Directive 90/427/EEC.
In accordance with the principles of the approved or recognised breeding organisation keeping the stud book of the origin of the breed of the registered equine animal concerned, the certificate of origin must contain full pedigree information, the section of the stud book referred to in Article 2 or 3 of Decision 96/78/EC and, where established, the class of the main section in which the equine animal is entered.
5.For the purpose of obtaining an identification document, an application shall be submitted by the keeper, or, where specifically required by law in the Member State where the animal is born, by the owner, within the time limits provided for in paragraph 6 of this Article and Article 7(1) for an identification document referred to in paragraph 1 of this Article, to the issuing body referred to in Article 4(1), (2) or (3), and all information necessary to comply with this Regulation shall be supplied.
6.Without prejudice to Article 13(1), equidae born in the Community shall be identified in accordance with this Regulation before 31 December of the year of birth of the equine animal or within six months following the date of birth, whatever date occurs later.
By way of derogation from the first subparagraph, Member States may decide to limit that maximum permitted period for identifying the equine animal to six months.
Member States making use of the derogation provided for in the second subparagraph shall inform the Commission and the other Member States.
7.The order of Sections and their numbering must remain unaltered in the identification document, except in the case of Section I that may be placed centrefold in the identification document.
8.The identification document shall not be duplicated or replaced, except as provided for in Articles 16 and 17.
Article 6U.K.Derogation from the completion of Section I of the identification document
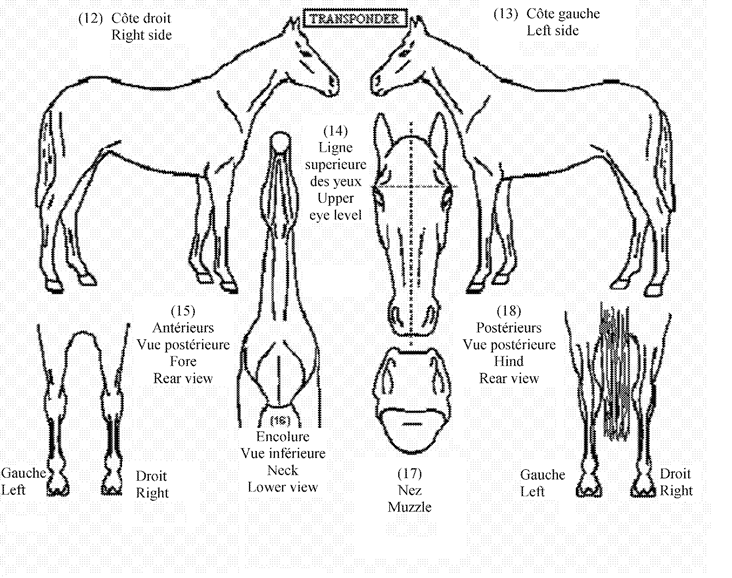
By way of derogation from Article 5(2), where a transponder is implanted in accordance with Article 11, or an individual, indelible and visible alternative mark is applied in accordance with Article 12, the information in points 3(b) to (h) of Part A of Section I and in points 12 to 18 in the outline diagram in Part B of Section I of the identification document need not be completed, or a photograph or print displaying details sufficient to identify the equine animal may be used instead of completing that outline diagram.
The derogation provided for in the first paragraph shall be without prejudice to the rules on identifying equidae laid down by the issuing bodies referred to in Article 4(1), (2) and (3).
Article 7U.K.Derogations concerning the identification of certain equidaeliving under wild or semi-wild conditions
1.By way of derogation from Article 5(1), (3) and (5), the competent authority may decide that equidae constituting defined populations living under wild or semi-wild conditions in certain areas, including nature reserves, to be defined by that authority, shall be identified in accordance with Article 5 only when they are removed from such areas or brought into domestic use.
2.Member States intending to make use of the derogation provided for in paragraph 1 shall notify the Commission of the population and the areas concerned:
(a)within six months of the date of entry into force of this Regulation; or
(b)before making use of that derogation.
Article 8U.K.Identification of imported equidae
1.The keeper or, where specifically required by law in the Member State where the animal is imported, the owner, shall apply for an identification document, or for the registration of the existing identification document in the database of the appropriate issuing body in accordance with Article 21, within 30 days of the date of completion of the customs procedure, as defined in Article 4(16)(a) of Regulation (EC) No 2913/92, where:
(a)equidae are imported into the Community; or
(b)the temporary admission defined in Article 2(i) of Directive 90/426/EEC is converted into permanent entry in accordance with Article 19(iii) of that Directive.
2.Where an equine animal, as referred to in paragraph 1 of this Article, is accompanied by papers that do not comply with Article 5(1) or lack certain information required in accordance with this Regulation, the issuing body shall on request of the keeper or, where specifically required by law in the Member State where the animal is imported, the owner:
(a)complete those papers so that they meet the requirements of Article 5; and
(b)record the identification details of that equine animal and the complementary information in the database in accordance with Article 21.
3.Where the papers accompanying the equidae as referred to in paragraph 1 of this Article cannot be amended to meet the requirements of Article 5(1) and (2), they shall not be considered valid for identification purposes in accordance with this Regulation.
Where the papers referred to in the first subparagraph are surrendered to or invalidated by the issuing body, that fact shall be recorded in the database referred to in Article 21 and the equidae shall be identified in accordance with Article 5.
CHAPTER IIIU.K.CHECKS REQUIRED PRIOR TO ISSUE OF IDENTIFICATION DOCUMENTS AND TRANSPONDERS
Article 9U.K.Verification of single identification documents issued for equidae
Before issuing an identification document, the issuing body, or the person acting on its behalf, shall take all appropriate measures to:
verify that no such identification document has already been issued for the equine animal concerned;
prevent the fraudulent issuing of multiple identification documents for an individual equine animal.
Those measures shall at least involve consulting the appropriate papers and electronic records available, checking the animal for any signs or marks indicative of any previous identification and applying the measures provided for in Article 10.
Article 10U.K.Measures to detect previous active marking of equidae
1.The measures referred to in Article 9 shall include, at least, measures to detect:
(a)any transponder previously implanted, using a reading device complying with ISO standard 11785 and capable of reading HDX and FDX-B transponders at least when the reader is in direct contact with the body surface on the spot where under normal circumstances a transponder is implanted;
(b)any clinical signs indicating that a transponder previously implanted has been surgically removed;
(c)any other alternative mark on the animal applied in accordance with Article 12(3)(b).
2.Where the measures provided for in paragraph 1 indicate the existence of a previously implanted transponder, or any other alternative mark applied in accordance with Article 12(3)(b), the issuing body shall take the following measures:
(a)in the case of equidae born in a Member State, it shall issue a duplicate or replacement identification document in accordance with Articles 16 or 17;
(b)in the case of imported equidae, it shall act in accordance with Article 8(2).
3.Where the measures provided for in paragraph 1(b) indicate the existence of a transponder previously implanted, or the measures provided for in paragraph 1(c) indicate the existence of any other alternative mark, the issuing body shall enter this information in an appropriate way in Part A and in the outline diagram in Part B of Section I of the identification document.
4.Where the undocumented removal of a transponder or alternative mark referred to in paragraph 3 of this Article is confirmed in an equine animal born in the Community, the issuing body, as referred to in Article 4(1) or (3), shall issue a replacement identification document in accordance with Article 17.
Article 11U.K.Electronic methods of identity verification
1.The issuing body shall ensure that at the time it is first identified, the equine animal is actively marked by the implantation of a transponder.
Member States shall lay down the minimum qualification required for the intervention referred to in the first subparagraph or designate the person or profession entrusted with such operations.
2.The transponder shall be implanted parenterally under aseptic conditions between poll and withers in the middle of the neck in the area of the nuchal ligament.
However, the competent authority may authorise the implantation of the transponder at a different place on the neck of the equine animal, provided that such alternative implantation does not compromise the welfare of the animal and does not increase the risk of migration of the transponder compared to the method referred to in the first subparagraph.
3.When the transponder is implanted in accordance with paragraphs 1 and 2, the issuing body shall enter the following information in the identification document:
(a)in point 5 of Part A of Section I, at least the last 15 digits of the code transmitted by the transponder and displayed by the reader following implantation, together with, where appropriate, a self-adhesive sticker with a bar code or a print of that bar code encoding at least those last 15 digits of the code transmitted by the transponder;
(b)in point 11 of Part A of Section I, the signature and stamp of the person referred to in paragraph 1 who carried out the identification and implanted the transponder;
(c)in points 12 or 13 of the outline diagram in Part B of Section I, depending on the side where the transponder was implanted, the place where the transponder has been implanted into the equine animal.
4.By way of derogation from paragraph 3(a) of this Article, where the measures provided for in Article 26(2) are implemented for an equine animal marked with a previously implanted transponder which does not comply with the standards defined in Article 2(2)(b), the name of the manufacturer or the reading system shall be inserted in point 5 of Part A of Section I in the identification document.
5.Where Member States lay down rules to ensure, in accordance with the standards referred to in Article 2(2)(b), the uniqueness of the numbers displayed by the transponders implanted by issuing bodies referred to in Article 4(1)(a) that are approved in accordance with Decision 92/353/EEC by the competent authorities of that Member State, those rules shall be applied without compromising the system of identification laid down by the issuing body in another Member State or third country that carried out the identification in accordance with this Regulation on request of the keeper or, where specifically required by law in the Member State where the animal is born, of the owner.
Article 12U.K.Alternative methods for identity verification
1.By way of derogation from Article 11(1), Member States may authorise the identification of equidae by suitable alternative methods, including marks, that provide equivalent scientific guarantees that, alone or in combination, ensure that the identity of the equine animal can be verified and that effectively prevent the double issuing of identification documents (alternative method).
The issuing body shall ensure that no identification document is issued for an equine animal, unless the alternative method referred to in the first subparagraph is entered in point 6 or 7 of Part A of Section I of the identification document and recorded in the database in accordance with Article 21(1)(f).
2.Where an alternative method is used, the keeper shall provide the means of accessing that identification information or shall, if applicable, bear the costs of verifying the identity of the animal.
3.Member States shall ensure that:
(a)alternative methods as the sole means of the identity verification of equidae are not used in the majority of equidae identified in accordance with this Regulation;
(b)visible marks applied to equidae for breeding and production cannot be confused with those reserved on their territory for registered equidae.
4.Member States intending to make use of the derogation provided for in paragraph 1 shall make this information available to the Commission, other Member States and the public on a website.
In order to assist the Member States in making that information available, the Commission shall provide a website to which each Member State shall provide a link to its national website.
CHAPTER IVU.K.MOVEMENT AND TRANSPORT OF EQUIDAE
Article 13U.K.Movement and transport of registered equidae and equidae for breeding and production
1.The identification document shall accompany registered equidae and equidae for breeding and production at all times.
2.By way of derogation from paragraph 1, the identification document need not accompany equidae referred to in that paragraph on the occasions when they are:
(a)stabled or on pasture, and the identification document can be produced without delay by the keeper;
(b)moved temporarily on foot either:
in the vicinity of the holding within a Member State so that the identification document can be produced within a period of three hours; or
during transhumance of equidae to and from summer grazing grounds and the identification documents can be produced at the holding of departure;
(c)unweaned and accompany their dam or foster mare;
(d)participating in a training or test of an equestrian competition or event which requires them to leave the competition or event venue;
(e)moved or transported in an emergency situation relating to the equine animals themselves or, without prejudice to the second subparagraph of Article 14(1) of Directive 2003/85/EC, to the holding on which they are kept.
Article 14U.K.Derogation for certain movements and transport without or with simplified identification documents
1.By way of derogation from Article 13(1), the competent authority may authorise the movement or transport within the same Member State of equidae referred to in that paragraph not accompanied by their identification document, provided they are accompanied by a smart card issued by the body that issued their identification document and containing the information set out in Annex II.
2.Member States, making use of the derogation provided for in paragraph 1 of this Article, may grant derogations to each other covering movements or transport of the equidae referred to in Article 13(1) within their own territories.
They shall notify the Commission of their intention to grant such derogations.
3.The issuing body shall issue a temporary document comprising at least a reference to the unique life number and, where available, the transponder code, allowing the equine animal to be moved or transported within the same Member State for a period not exceeding 45 days, during which the identification document is surrendered to the issuing body or the competent authority for the purpose of updating identification details.
4.Where, during the period referred to in paragraph 3, an equine animal is transported to another Member State or through another Member State to a third country, it shall, irrespective of its registration status, be accompanied, in addition to the temporary document, by a health certificate in accordance with Annex C to Directive 90/426/EEC. If the animal is not marked with a transponder or if the animal is not identified by an alternative method in accordance with Article 12 of this Regulation, that health certificate must be completed with a description in accordance with Section I of the identification document.
Article 15U.K.Movements and transport of equidae for slaughter
1.The identification document issued in accordance with Articles 5(1) or 8 shall accompany equidae for slaughter while they are being moved or transported to the slaughterhouse.
2.By way of derogation from paragraph 1, the competent authority may authorise an equine animal for slaughter which has not been identified in accordance with Article 5, to be transported directly from the holding of birth to the slaughterhouse within the same Member State provided that:
(a)the equine animal is less than 12 months old and has visible dental stars of the temporary lateral incisors;
(b)there is an uninterrupted traceability from the holding of birth to the slaughterhouse;
(c)during transport to the slaughterhouse the equine animal is individually identifiable in accordance with Articles 11 or 12;
(d)the consignment is accompanied by the food chain information in accordance with Section III of Annex II to Regulation (EC) No 853/2004 that shall include a reference to the individual identification referred to in point (c) of this paragraph.
3.Article 19(1)(b), (c) and (d) shall not apply in the case of the movement or transport of equidae for slaughter in accordance with paragraph 2 of this Article.
CHAPTER VU.K.DUPLICATION, REPLACEMENT AND SUSPENSION OF IDENTIFICATION DOCUMENTS
Article 16U.K.Duplicate identification documents
1.Where the original identification document is lost, but the equine animal’s identity can be established, notably through the code transmitted by the transponder or the alternative method, and an ownership declaration is available, the issuing body, as referred to in Article 4(1), shall issue a duplicate identification document with a reference to the unique life number and shall clearly mark the document as such (duplicate identification document).
In such cases, the equine animal shall be classified in Part II of Section IX of the duplicate identification document as not intended for slaughter for human consumption.
Details of the duplicate identification document issued and the equine animal’s classification in Section IX thereof shall be entered by reference to the unique life number in the database, as referred to in Article 21.
2.By way of derogation from the second subparagraph of paragraph 1, the competent authority may decide to suspend the equine animal’s status as intended for slaughter for human consumption for a period of six months where the keeper can satisfactorily demonstrate within 30 days of the declared date of loss of the identification document that the equine animal’s status as intended for slaughter for human consumption has not been compromised by any medicinal treatment.
To that effect, the competent authority shall enter the date of commencement of the six-month suspension period in the first column of Part III of Section IX of the duplicate identification document, and complete the third column thereof.
3.Where the lost original identification document was issued by an issuing body referred to in Article 4(2) in a third country, the duplicate identification document shall be issued by that original issuing body and routed to the keeper or, where specifically required by law in the Member State where the equine animal is located, to the owner, via the issuing body or competent authority in that Member State.
In such cases, the equine animal shall be classified in Part II of Section IX of the duplicate identification document as not intended for slaughter for human consumption and the entry in the database as referred to in Article 21(1)(l) adapted accordingly.
However, the duplicate identification document may be issued by an issuing body referred to in Article 4(1)(a) which registers equidae of that breed or by an issuing body referred to in Article 4(1)(b) which registers equidae for that purpose in the Member State where the equine animal is located, where the original issuing body in the third country has so agreed.
4.Where the lost original identification document has been issued by an issuing body which is no longer in existence, the duplicate identification document shall be issued by an issuing body in the Member State where the equine animal is located in accordance with paragraph 1.
Article 17U.K.Replacement identification document
Where the original identification document is lost and the identity of the equine animal cannot be established, the issuing body as referred to in Article 4(3) in the Member State where the equine animal is located shall issue a replacement identification document (replacement identification document) which shall be clearly marked as such and meet the requirements of Article 5(1)(b).
In such cases, the equine animal shall be classified in Part II of Section IX of the replacement identification document as not intended for slaughter for human consumption.
Details of the replacement identification document issued and the equine animal’s registration status and classification in Section IX thereof shall be adapted accordingly in the database as referred to in Article 21 by reference to the unique life number.
Article 18U.K.Suspension of identification documents for movement purposes
The official veterinarian shall suspend the validity for movement purposes of the identification document by making an appropriate entry in Section VIII thereof where an equine animal is kept on or comes from a holding which is:
subject to a prohibition order as referred to in Article 4(5) of Directive 90/426/EEC; or
situated in a Member State or part thereof that is not free of African horse sickness.
CHAPTER VIU.K.DEATH OF EQUIDAE AND EQUIDAE INTENDED FOR SLAUGHTER FOR HUMAN CONSUMPTION AND MEDICATION RECORD
Article 19U.K.Death of equidae
1.On the slaughter or death of the equine animal, the following measures shall be taken:
(a)the transponder shall be protected from subsequent fraudulent use, notably by its recovery, destruction or disposal in situ;
(b)the identification document shall be rendered invalid at least by stamping it ‘invalid’ on the first page;
(c)an attestation shall be communicated to the issuing body, either directly or through the contact point referred to in Article 23(4), with reference to the equine animal’s unique life number to the effect that the equine animal has been slaughtered, was killed or died, including the date of death of the animal; and
(d)the invalidated identification document shall be destroyed.
2.The measures provided for in paragraph 1 shall be carried out by or under the supervision of:
(a)the official veterinarian:
in case of slaughter or killing for disease control purposes, in accordance with Article 4(4)(i) of Directive 90/426/EEC; or
following slaughter, in accordance with Article 7(3) of Directive 90/426/EEC; or
(b)the competent authority defined in Article 2(1)(i) of Regulation (EC) No 1774/2002, in the case of disposal or processing of the carcass in accordance with Articles 4 or 5 of that Regulation.
3.Where, as required in paragraph 1(a), the transponder cannot be recovered from an equine animal slaughtered for human consumption, the official veterinarian shall declare the meat or the part of the meat containing the transponder unfit for human consumption in accordance with Chapter V(1)(n) of Section II of Annex I to Regulation (EC) No 854/2004.
4.By way of derogation from paragraph 1(d), and without prejudice to the rules printed in the identification document by the issuing body, Member States may implement procedures to return the invalidated document to the issuing body.
5.In all cases of death or loss of the equine animal not referred to in this Article, the keeper shall return the identification document to the appropriate issuing body referred to in Article 4(1), (2) or (3) within 30 days of the death or loss of the animal.
Article 20U.K.Equidae intended for slaughter for human consumption and medication record
1.An equine animal shall be deemed to be intended for slaughter for human consumption, unless it is irreversibly declared as not so intended in Part II of Section IX of the identification document, by the signature of:
(a)the keeper or owner on his/her own discretion, or
(b)the keeper and the veterinarian responsible, acting in accordance with Article 10(2) of Directive 2001/82/EC.
2.Prior to any treatment in accordance with Article 10(2) of Directive 2001/82/EC or to any treatment by use of a medicinal product authorised in accordance with Article 6(3) of that Directive, the veterinarian responsible shall ascertain the equine animal’s status as either intended for slaughter for human consumption, which is the default case, or not intended for slaughter for human consumption as set out in Part II of Section IX of the identification document.
3.Where the treatment referred to in paragraph 2 of this Article is not permitted for an equine animal intended for slaughter for human consumption, the veterinarian responsible shall ensure that in accordance with the derogation provided for in Article 10(2) of Directive 2001/82/EC the equine animal concerned is irreversibly declared as not intended for slaughter for human consumption by:
(a)completing and signing Part II of Section IX of the identification document; and
(b)invalidating Part III of Section IX of the identification document.
4.Where an equine animal is to be treated under the conditions referred to in Article 10(3) of Directive 2001/82/EC, the veterinarian responsible shall enter in Part III of Section IX of the identification document the requisite details of the medicinal product containing substances essential for the treatment of equidae listed in Regulation (EC) No 1950/2006.
The veterinarian responsible shall enter the date of last administration, as prescribed, of that medicinal product and shall, acting in accordance with Article 11(4) of Directive 2001/82/EC, inform the keeper of the date when the withdrawal period established in accordance with Article 10(3) of that Directive will lapse.
CHAPTER VIIU.K.RECORDS AND PENALTIES
Article 21U.K.Database
1.When issuing the identification document, or registering previously issued identification documents, the issuing body shall record at least the following information concerning the equine animal in its database:
(a)the unique life number;
(b)the species;
(c)the sex;
(d)the colour;
(e)the date (day, month and year) of birth;
(f)if applicable, at least the last 15 digits of the code transmitted by the transponder, or the code transmitted by a radio frequency identification devise not complying with the standard defined in Article 2(2)(b) together with information on the required reading system, or the alternative method;
(g)the country of birth;
(h)the date of issue and any amendment of the identification document;
(i)the name and address of the person to whom the identification document is issued;
(j)the status as registered equidae or equidae for breeding and production;
(k)the name of the animal (birth name and where applicable the commercial name);
(l)the known status of the animal as not intended for slaughter for human consumption;
(m)information concerning any duplicate and replacement identification documents in accordance with Articles 16 and 17;
(n)the notified date of death of the animal.
2.The issuing body shall keep the information referred to in paragraph 1 of this Article on record in its database for at least 35 years or until at least two years from the date of death of the equine animal communicated in accordance with Article 19(1)(c).
3.Immediately after recording the information referred to in paragraph 1 of this Article, the issuing body shall communicate the information referred to in points (a) to (f) and (n) of that paragraph to the central database in the Member State where the equine animal was born, if such central database has been made available in accordance with Article 23.
Article 22U.K.Communication of code of databases of issuing bodies
The Member States shall make the names, addresses, including communication details, and six-digit UELN-compatible identification code of the databases of the issuing bodies available to the other Member States and the public on a website.
In order to assist the Member States in making such information available, the Commission shall provide a website to which each Member State shall provide a link to its national website.
Article 23U.K.Central databases and their cooperation and contact points
1.A Member State may decide that the issuing body is to incorporate the information referred to in Article 21 relating to equidae born or identified on its territory in a central database or that the issuing body’s database is to be networked with that central database (the central database).
2.The Member States shall cooperate in the operation of their central databases in accordance with Directive 89/608/EEC.
3.The Member States shall make the name, address and six-digit UELN-compatible identification code of their central databases available to the other Member States and the public on a website.
In order to assist the Member States in making such information available, the Commission shall provide a website to which each Member State shall provide a link to its national website.
4.Member States shall provide a contact point to receive the attestation referred to in Article 19(1)(c) for further distribution to the respective issuing bodies approved on their territory.
That contact point may be a liaison body referred to in Article 35 of Regulation (EC) No 882/2004.
Details about the contact point, which may be incorporated in the central database, shall be made available to other Member States and the public on a website.
In order to assist the Member States in making such information available, the Commission shall provide a website to which each Member State shall provide a link to its national website.
Article 24U.K.Penalties
The Member States shall lay down the rules on penalties applicable to infringements of this Regulation and shall take all measures necessary to ensure that they are implemented. The penalties laid down shall be effective, proportionate and dissuasive.
The Member States shall notify those provisions to the Commission by 30 June 2009 at the latest. Any subsequent amendments affecting them shall be notified to the Commission without delay.
CHAPTER VIIIU.K.TRANSITIONAL AND FINAL PROVISIONS
Article 25U.K.Repeal
Decisions 93/623/EEC and 2000/68/EC are repealed with effect from 1 July 2009.
References to the repealed Decisions shall be construed as references to this Regulation.
Article 26U.K.Transitional provisions
1.Equidae which are born by 30 June 2009 at the latest, and identified by that date in accordance with Decisions 93/623/EEC or 2000/68/EC, shall be deemed to be identified in accordance with this Regulation.
The identification documents for those equidae shall be registered in accordance with Article 21(1) of this Regulation by 31 December 2009 at the latest.
2.Equidae which are born by 30 June 2009 at the latest, but not identified by that date in accordance with Decisions 93/623/EEC or 2000/68/EC, shall be identified in accordance with this Regulation by 31 December 2009 at the latest.
Article 27U.K.Entry into force
This Regulation shall enter into force on the 20th day following that of its publication in the Official Journal of the European Union.
It shall apply from 1 July 2009.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 6 June 2008.
For the Commission
Androulla Vassiliou
Member of the Commission
ANNEX IU.K.
IDENTIFICATION DOCUMENT FOR EQUIDAEPASSPORTU.K.
General — InstructionsU.K.
These instructions are drawn up to assist the user and do not impede on the rules laid down in Regulation (EC) No 504/2008.
I.The passport must contain all instructions needed for their use and the details of the issuing body in French, English and one of the official language(s) of the Member State or country where the issuing body has its headquarters.U.K.
II.Information shown on the passportU.K.
A.The passport must contain the following information:U.K.
1.Sections I and II — IdentificationU.K.
The equine animal shall be identified by the competent authority. The identification number shall clearly identify the animal and the body which issued the identification document and shall be UELN compatible.
In point 5 of Section I space must be provided for at least 15 digits of the transponder code.
In case of registered equidae the passport shall contain the pedigree and the studbook class in which the animal is entered in accordance with the rules of the approved breeding organisation issuing the passport.
2.Section III — OwnerU.K.
The name of the owner or his agent/representative must be stated where required by the issuing body.
3.Section IV — Recording of identity checksU.K.
Whenever laws and regulations so require, checks conducted on the identity of the equine animal must be recorded by the competent authority.
4.Sections V and VI — Vaccination recordU.K.
All vaccinations must be recorded in Section V (equine influenza only) and in Section VI (all other vaccinations). The information may take the form of a sticker.
5.Section VII — Laboratory health testsU.K.
The results of all tests carried out to detect transmissible diseases must be recorded.
6.Section VIII — Validity of document for movement purposesU.K.
Invalidation/revalidation of the document in accordance with Article 4(4) of Directive 90/426/EEC and list of compulsorily notifiable diseases.
7.Section IX — Administration of veterinary medicinal productsU.K.
Parts I and II or Part III of this Section must be duly completed in accordance with the instructions set out in this Section.
B.The passport may contain the following information:U.K.
Section X — Basic health requirements
SECTION IU.K.Part A — Identification details
SECTION IU.K.Part B — Outline Diagram
SECTION IIU.K.Certificat d'origineCertificate of Origin
SECTION IIIU.K.[Only to be completed if required by and in accordance with the rules of the organisations referred to in Article 2(c) of Directive 90/426/EEC]
| Note for the issuing body [not to be printed in identification document]: The text in points 1 to 4 of this Section, or parts thereof, only to be printed where in accordance with the rules of the organisations referred to in Article 2(c) of Directive 90/426/EEC. | |||||
| Date d'enregistrement par l'organisation, l'association ou le service officielDate of registration, by the organisation, association, or official agency | Nom du propriétaireName of owner | Adresse du propriétaireAddress of owner | Nationalité du propriétaireNationality of owner | Signature du propriétaireSignature of owner | Cachet de l'organisation, association ou service officiel et signatureOrganization, association or official agency stamp and signature |
|---|---|---|---|---|---|
SECTION IVU.K.
| DateDate | Ville et paysTown and country | Motif du contrôle (concours, certificat sanitaire, etc.)Reason for check (event, health certificate, etc.) | Signature, nom en capitales et qualité de la personne ayant vérifié l'identitéSignature, name (in capital letters) and capacity of official verifying the identification |
|---|---|---|---|
SECTION VU.K.
| DateDate | LieuPlace | PaysCountry | Vaccin/Vaccine | Nom en capitales et signature du vétérinaireName (in capital letters) and signature of veterinarian | ||
|---|---|---|---|---|---|---|
| NomName | Numéro du lotBatch number | Maladie(s)Disease(s) | ||||
SECTION VIU.K.
| DateDate | LieuPlace | PaysCountry | Vaccin/Vaccine | Nom en capitales et signature du vétérinaireName (in capital letters) and signature of veterinarian | ||
|---|---|---|---|---|---|---|
| NomName | Numéro du lotBatch number | Maladie(s)Disease(s) | ||||
SECTION VIIU.K.
| Date de prélèvementSampling date | Maladies transmissibles concernéesTransmissible disease tested for | Nature de l’examenType of test | Résultat de l’examenResult of test | Laboratoire officiel d’analyse du prélèvementOfficial laboratory to which sample is sent | Nom en capitales et signature du vétérinaireName (in capital letters) and signature of veterinarian |
|---|---|---|---|---|---|
SECTION VIIIU.K.
INVALIDATION/REVALIDATION DU DOCUMENT DANS LE CADRE DES MOUVEMENTS Conformément à l’article 4, paragraphe 4, de la directive 90/426/CEE U.K. INVALIDATION/REVALIDATION OF THE DOCUMENT FOR MOVEMENT PURPOSES U.K. in accordance with Article 4(4) of Directive 90/426/EEC U.K.
| DateDate | LieuPlace | Validité du documentValidity of document | MaladieDisease[insert figure as mentioned below] | Nom en capitales et signature du vétérinaire officielName in capitals and signature of official veterinarian | |
|---|---|---|---|---|---|
| Validité suspendueValidity suspended | Validité rétablieValidity re-established | ||||
MALADIES À DÉCLARATION OBLIGATOIRE — COMPULSORILY NOTIFIABLE DISEASES U.K.
1.Peste équine — African horse sicknessU.K.
2.Stomatite vésiculeuse — vesicular stomatitisU.K.
3.Dourine — dourineU.K.
4.Morve — glandersU.K.
5.Encéphalomyélites équines (sous toutes ses formes, y compris la VEE) — equine encephalomyelitis (all types including VEE)U.K.
6.Anémie infectieuse — equine infectious anaemiaU.K.
7.Rage — rabiesU.K.
8.Fièvre charbonneuse — anthraxU.K.
SECTION IXU.K.Administration of veterinary medicinal products
SECTION XU.K.Exigences sanitaires de baseLes exigences ne sont pas valables pour l'introduction dans la CommunautéBasic health requirementsThese requirements are not valid to enter the Community
Je soussigné(29) certifie que l'équidé décrit dans ce passeport satisfait aux conditions suivantes:
I, the undersigned(29), hereby certify that the equine animal described in this passport satisfies the following conditions:
il a été examiné ce jour, ne présente aucun signe clinique de maladie et est apte au transport;
it has been examined this day, presents no clinical sign of disease and is fit for transport;
il n'est pas destiné à l'abattage dans le cadre d'un programme national d'éradication d'une maladie transmissible;
it is not intended for slaughter under a national eradication programme for a transmissible disease;
il ne provient pas d'une exploitation faisant l'objet de mesures de restriction pour des motifs de police sanitaire et n'a pas été en contact avec des équidés d'une telle exploitation;
it does not come from a holding subject to restrictions for animal health reasons and has not been in contact with equidae on such a holding;
à ma connaissance, il n'a pas été en contact avec des équidés atteints d'une maladie transmissible au cours des 15 jours précédant l'embarquement.
to the best of my knowledge, it has not been in contact with equidae affected by a transmissible disease during the 15 days prior to loading.
LA PRÉSENTE CERTIFICATION EST VALABLE 10 JOURS À COMPTER DE LA DATE DE SA SIGNATURE PAR LE VÉTÉRINAIRE OFFICIEL
THIS CERTIFICATION IS VALID FOR 10 DAYS FROM THE DATE OF SIGNATURE BY THE OFFICIAL VETERINARIAN
| DateDate | LieuPlace | Pour des raisons épidémiologiques particulières, un certificat sanitaire séparé accompagne le présent passeportFor particular epidemiological reasons, a separate health certificate accompanies this passport | Nom en capitales et signature du vétérinaire officielName in capital letters and signature of official veterinarian |
|---|---|---|---|
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) | |||
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) | |||
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) | |||
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) | |||
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) | |||
| Oui/non (barrer la mention inutile) Yes/no (delete as appropriate) |
ANNEX IIU.K.Information stored on the smart card
The smart card shall contain at least the following:
Visible information:
issuing body
unique life number
name
sex
colour
the last 15 digits of the code transmitted by the transponder (as appropriate)
photo of the equine animal;
Electronic information accessible by use of standard software:
at least all compulsory information in Part A of Section I of the identification document.
OJ L 224, 18.8.1990, p. 42. Directive as last amended by Directive 2006/104/EC (OJ L 363, 20.12.2006, p. 352).
OJ L 298, 3.12.1993, p. 45. Decision as amended by Decision 2000/68/EC (OJ L 23, 28.1.2000, p. 72).
OJ L 86, 6.4.1993, p. 7. Decision as last amended by Regulation (EC) No 1792/2006 (OJ L 362, 20.12.2006, p. 1).
OJ L 86, 6.4.1993, p. 16. Decision as last amended by Regulation (EC) No 1792/2006.
OJ L 302, 19.10.1992, p. 1. Regulation as last amended by Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
OJ L 68, 15.3.1973, p. 1. Regulation as amended by Regulation (EEC) No 1174/86 (OJ L 107, 24.4.1986, p. 1).
OJ L 204, 11.8.2000, p. 1. Regulation as last amended by Regulation (EC) No 1791/2006.
OJ L 157, 10.6.1992, p. 19. Directive as last amended by Commission Decision 2007/729/EC (OJ L 294, 13.11.2007, p. 26).
OJ L 210, 20.8.1996, p. 53. Decision as amended by Decision 2004/186/EC (OJ L 57, 25.2.2004, p. 27).
OJ L 306, 22.11.2003, p. 1. Directive as last amended by Directive 2006/104/EC.
OJ L 338, 22.12.2005, p. 83. Regulation as last amended by Regulation (EC) No 1246/2007 (OJ L 281, 25.10.2007, p. 21).
OJ L 273, 10.10.2002, p. 1. Regulation as last amended by Commission Regulation (EC) No 1432/2007 (OJ L 320, 6.12.2007, p. 13).
OJ L 139, 30.4.2004, p. 206; corrected version (OJ L 226, 25.6.2004, p. 83). Regulation as last amended by Regulation (EC) No 1791/2006.
OJ L 31, 1.2.2002, p. 1. Regulation as last amended by Commission Regulation (EC) No 575/2006 (OJ L 100, 8.4.2006, p. 3).
OJ L 139, 30.4.2004, p. 55; corrected version (OJ L 226, 25.6.2004, p. 22). Regulation as last amended by Commission Regulation (EC) No 1243/2007 (OJ L 281, 25.10.2007, p. 8).
OJ L 224, 18.8.1990, p. 1. Regulation as last amended by Commission Regulation (EC) No 61/2008 (OJ L 22, 25.1.2008, p. 8).
OJ L 125, 23.5.1996, p. 3. Directive as amended by Directive 2003/74/EC of the European Parliament and of the Council (OJ L 262, 14.10.2003, p. 17).
OJ L 311, 28.11.2001, p. 1. Directive as last amended by Directive 2004/28/EC (OJ L 136, 30.4.2004, p. 58).
http://www.ueln.net
OJ L 165, 30.4.2004, p. 1; corrected version (OJ L 191, 28.5.2004, p. 1) Regulation as last amended by Regulation (EC) No 1791/2006.
Ce document doit être signé dans les 48 heures précédant le déplacement international de l’équidé.
This document must be signed within 48 hours prior to international transport of equine animal.