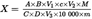Please note that the date you requested in the address for this web page is not an actual date upon which a change occurred to this item of legislation. You are being shown the legislation from , which is the first date before then upon which a change was made.
ANNEX IIIU.K.METHODS OF ANALYSIS TO CONTROL THE COMPOSITION OF FEED MATERIALS AND COMPOUND FEED
G.DETERMINATION OF TRYPTOPHANU.K.
1.Purpose and scopeU.K.
The method makes the determination possible of the total and free tryptophan in feed. It does not distinguish between D- and L- forms.
2.PrincipleU.K.
For the determination of the total tryptophan, the sample is hydrolysed under alkaline conditions with saturated barium hydroxide solution and heated to 110 oC for 20 hours. After hydrolysis internal standard is added.
For the determination of free tryptophan, the sample is extracted under mild acidic conditions in the presence of internal standard.
The tryptophan and the internal standard in the hydrolysate or in the extract are determined by HPLC with fluorescence detection.
3.ReagentsU.K.
3.1.Double distilled water or water of equivalent quality must be used (conductivity < 10 μS/cm).U.K.
3.2.Standard substance: tryptophan (purity/content ≥ 99 %) dried under vacuum over phosphorous pentoxide.U.K.
3.3.Internal standard substance: α-methyl-tryptophan (purity/content ≥ 99 %), dried under vacuum over phosphorous pentoxide.U.K.
3.4.Barium hydroxide octa-hydrate (care shall be taken not to expose the Ba(OH)2 .8 H2O excessively to air in order to avoid formation of BaCO3, which could disturb the determination) (see observation 9.3).U.K.
3.5.Sodium hydroxide.U.K.
3.6.Ortho-phosphoric acid, w (w/w) = 85 %.U.K.
3.7.Hydrochloric acid, ρ201,19 g/ml.U.K.
3.8.Methanol, equivalent to HPLC grade.U.K.
3.9.Light petroleum, boiling range 40-60 oC.U.K.
3.10.Sodium hydroxide solution, c = 1 mol/l:U.K.
Dissolve 40,0 g NaOH (3.5) in water and make up to 1 litre with water (3.1).
3.11.Hydrochloric acid, c = 6 mol/l:U.K.
Take 492 ml HCl (3.7) and make up to 1 litre with water.
3.12.Hydrochloric acid, c = 1 mol/l:U.K.
Take 82 ml HCl (3.7) and make up to 1 litre with water.
3.13.Hydrochloric acid, c = 0,1 mol/l:U.K.
Take 8,2 ml HCl (3.7) and make up to 1 litre with water.
3.14.Ortho-phosphoric acid, c = 0,5 mol/l:U.K.
Take 34 ml ortho-phosphoric acid (3.6) and make up to 1 litre with water (3.1).
3.15.Concentrated solution of tryptophan (3.2), c = 2,5 μmol/ml:U.K.
In a 500 ml volumetric flask dissolve 0,2553 g tryptophan (3.2) in hydrochloric acid (3.13) and make up to the mark with hydrochloric acid (3.13). Store at - 18 oC for a maximum of 4 weeks.
3.16.Concentrated internal standard solution, c = 2,5 μmol/ml:U.K.
In a 500 ml volumetric flask dissolve 0,2728 g α-methyl-tryptophan (3.3) in hydrochloric acid (3.13) and make up to the mark with hydrochloric acid (3.13). Store at - 18 oC for a maximum of 4 weeks.
3.17.Calibration standard solution of tryptophan and internal standard:U.K.
Take 2,0 ml concentrated solution of tryptophan (3.15), and 2,0 ml of concentrated internal standard (α-methyl-tryptophan) solution (3.16). Dilute with water (3.1) and methanol (3.8) to approximately the same volume and to approximately the same concentration of methanol (10 %-30 %) as the finished hydrolysate.
This solution must be prepared freshly before use.
Protect from direct sunlight during preparation.
3.18.Acetic acidU.K.
3.19.1,1,1-trichloro-2-methyl-2-propanol.U.K.
3.20.Ethanolamine w (w/w) > 98 %.U.K.
3.21.Solution of 1 g 1,1,1-trichloro-2-methyl-2-propanol (3.19) in 100 ml methanol (3.8).U.K.
3.22.Mobile phase for HPLC: 3,0 g acetic acid (3.18) + 900 ml water (3.1) +50,0 ml solution (3.21) of 1,1,1-trichloro-2-methyl-2-propanol (3.19) in methanol (3.8) (1g/100ml). Adjust pH to 5,0 using ethanolamine (3.20). Make up to 1 000 ml with water (3.1).U.K.
4.ApparatusU.K.
4.1.HPLC equipment with a spectrofluorometric detector.U.K.
4.2.Liquid chromatographic column, 125 mm x 4 mm, C18, 3 μm packing, or equivalent.U.K.
4.3.pH-meter.U.K.
4.4.Polypropylene flask, capacity 125 ml, with wide neck and screw cap.U.K.
4.5.Membrane filter, 0,45 μm.U.K.
4.6.Autoclave, 110 (± 2) oC, 1,4 (±0,1) bar.U.K.
4.7.Mechanical shaker or magnetic stirrer.U.K.
4.8.Vortex mixer.U.K.
5.ProcedureU.K.
5.1.Preparation of samplesU.K.
The sample is ground to pass through a 0,5 mm sieve. Samples high in moisture must be either air-dried at a temperature not exceeding 50 oC or freeze dried prior to grinding. Samples with high fat content shall be extracted with light petroleum (3.9) prior to grinding.
5.2.Determination of free tryptophan (extract)U.K.
Weigh to the nearest 1 mg an appropriate amount (1-5 g) of the prepared sample (5.1), into a conical flask. Add 100,0 ml hydrochloric acid, (3.13) and 5,0 ml concentrated internal standard solution (3.16). Shake or mix for 60 min. using a mechanical shaker or a magnetic stirrer (4.7). Allow the sediment to settle and pipette 10,0 ml of the supernatant solution into a beaker. Add 5 ml ortho-phosphoric acid (3.14). Adjust the pH to 3 using sodium hydroxide (3.10). Add sufficient methanol (3.8) to give a concentration of between 10 % and 30 % of methanol in the final volume. Transfer to a volumetric flask of appropriate volume and dilute with water to a volume necessary for the chromatography (approx. the same volume as the calibration standard solution (3.17)).
Filter a few ml of the solution through a 0,45 μm membrane filter (4.5) before injection on the HPLC column. Proceed to the chromatography step according to paragraph 5.4.
Protect standard solution and extracts against direct sunlight. If it is not possible to analyse the extracts the same day, the extracts may be stored at 5 oC for a maximum of 3 days.
5.3.Determination of total tryptophan (hydrolysate)U.K.
Weigh to the nearest 0,2 mg from 0,1 to 1 g of the prepared sample (5.1) into the polypropylene flask (4.4). The weighed sample portion shall have a nitrogen content of about 10 mg. Add 8,4 g barium hydroxide octa-hydrate (3.4) and 10 ml water. Mix on a vortex mixer (4.8) or magnetic stirrer (4.7). Leave the teflon coated magnet in the mixture. Wash down the walls of the vessel with 4 ml water. Put on the screw cap and close the flask loosely. Transfer to an autoclave (4.6) with boiling water and steam for 30-60 minutes. Close the autoclave and autoclave at 110 (± 2) oC for 20 hours.
Before opening the autoclave reduce the temperature to just under 100 oC. In order to avoid crystallisation of Ba(OH)2 · 8 H2O, add to the warm mixture 30 ml water which is at room temperature. Shake or stir gently. Add 2,0 ml concentrated internal standard (α-methyl-tryptophan) solution (3.16). Cool the vessels on water/ice bath for 15 minutes.
Then, add 5 ml ortho-phosphoric acid (3.14). Keep the vessel in the cooling bath and neutralise with HCl (3.11) whilst stirring and adjust the pH to 3,0 using HCl (3.12). Add sufficient methanol to give a concentration of between 10 % and 30 % of methanol in the final volume. Transfer to a volumetric flask of appropriate volume and dilute with water to the defined volume necessary for the chromatography (for example 100 ml). The addition of methanol shall not cause precipitation.
Filter a few ml of the solution through a 0,45 μm membrane filter (4.5) before injection on the HPLC column. Proceed to the chromatography step according to paragraph 5.4.
Protect standard solution and hydrolysates against direct sunlight. If it is not possible to analyse the hydrolysates the same day, they may be stored at 5 oC for a maximum of 3 days.
5.4.HPLC determinationU.K.
The following conditions for isocratic elution are offered for guidance; other conditions may be used, provided they yield equivalent results (see also observations 9.1 and 9.2):
| Liquid chromatographic column (4.2): | 125 mm x 4 mm, C18, 3 μm packing or equivalent |
| Column temperature: | Room temperature |
| Mobile phase (3.22): | 3,0 g acetic acid (3.18) + 900 ml water (3.1) +50,0 ml solution (3.21) of 1,1,1-trichloro-2- methyl-2-propanol (3.19) in methanol (3.8) (1 g/100 ml). Adjust pH to 5,0 using ethanolamine (3.20). Make up to 1 000 ml with water (3.1) |
| Flow rate: | 1 ml/min. |
| Total run time: | approx. 34 min. |
| Detection wavelength: | excitation: 280 nm, emission: 356 nm. |
| Injection volume | 20 μl |
6.Calculation of resultsU.K.
The amount of tryptophane (X), in g per 100g sample, is calculated as follows:
=
peak area of internal standard, calibration standard solution (3.17)
=
peak area of tryptophan, extract (5.2) or hydrolysate (5.3)
=
volume in ml (2 ml) of concentrated tryptophan solution (3.15) added to the calibration solution (3.17)
=
concentration in μmol/ml (= 2,5) of concentrated tryptophan solution (3.15) added to calibration solution (3.17)
=
volume in ml of concentrated internal standard solution (3.16) added at the extraction (5.2) (= 5,0 ml) or to the hydrolysate (5.3) (= 2,0 ml)
=
peak area of internal standard, extract (5.2) or hydrolysate (5.3)
=
peak area of tryptophan, calibration standard solution (3.17)
=
volume in ml (= 2,0 ml) of concentrated internal standard solution (3.16) added to calibration standard solution (3.17)
=
sample weight in g (corrected to original weight if dried and/or defatted)
=
molar weight of tryptophan (= 204,23 g/mol)
7.RepeatabilityU.K.
The difference between the results of two parallel determinations carried out on the same sample must not exceed 10 % relative to the highest result.
8.Results of a collaborative studyU.K.
An EC collaborative study (4th intercomparison) was arranged in which three samples were analysed by up to 12 laboratories to certify the method for hydrolysis. Replicate (5) analyses were performed on each sample. The results are given in the following table:
| Sample 1Pig feed | Sample 2Pig feed supplemented with L-tryptophan | Sample 3Feed concentrate for pigs | |
|---|---|---|---|
| L | 12 | 12 | 12 |
| n | 50 | 55 | 50 |
| Mean [g/kg] | 2,42 | 3,4 | 4,22 |
| sr [g/kg] | 0,05 | 0,05 | 0,08 |
| r [g/kg] | 0,14 | 0,14 | 0,22 |
| CVr [%] | 1,9 | 1,6 | 1,9 |
| SR [g/kg] | 0,15 | 0,2 | 0,09 |
| R [g/kg] | 0,42 | 0,56 | 0,25 |
| CVR [%] | 6,3 | 6,0 | 2,2 |
=
number of laboratories submitting results
=
number of single results retained eliminating outliers (identified by Cochran, Dixon outlier test)
=
standard deviation of repeatability
=
standard deviation of reproducibility
=
repeatability
=
reproducibility
=
coefficient of variation of repeatability, %
=
coefficient of variation of reproducibility, %
Another EC collaborative study (3rd intercomparison) was arranged in which two samples were analysed by up to 13 laboratories to certify the method for extraction of free tryptophan. Replicate (5) analyses were performed on each sample. The results are given in the following table:
| Sample 4Wheat and soya mixture | Sample 5Wheat and soya mixture (= sample 4) with added tryptophan (0,457g/kg1) | |
|---|---|---|
| L | 12 | 12 |
| n | 55 | 60 |
| Mean [g/kg] | 0,391 | 0,931 |
| sr [g/kg] | 0,005 | 0,012 |
| r [g/kg] | 0,014 | 0,034 |
| CVr [%] | 1,34 | 1,34 |
| SR [g/kg] | 0,018 | 0,048 |
| R [g/kg] | 0,05 | 0,134 |
| CVR [%] | 4,71 | 5,11 |
=
number of laboratories submitting results
=
number of single results retained after eliminating outliers (identified by Cochran, Dixon outlier test)
=
standard deviation of repeatability
=
standard deviation of reproducibility
=
repeatability
=
reproducibility
=
coefficient of variation of repeatability, %
=
coefficient of variation of reproducibility, %
Another EC intercomparison study was arranged in which four samples were analysed by up to 7 laboratories with the aim of a tryptophan certification for hydrolysis. The results are given below. Replicate (5) analyses were performed on each sample.
| Sample 1Mixed pig feed(CRM 117) | Sample 2Low fat fish meal(CRM 118) | Sample 3Soybean meal(CRM 119) | Sample 4Skimmed milk powder(CRM 120) | |
|---|---|---|---|---|
| L | 7 | 7 | 7 | 7 |
| n | 25 | 30 | 30 | 30 |
| Mean [g/kg] | 2,064 | 8,801 | 6,882 | 5,236 |
| sr [g/kg] | 0,021 | 0,101 | 0,089 | 0,04 |
| r [g/kg] | 0,059 | 0,283 | 0,249 | 0,112 |
| CVr [%] | 1,04 | 1,15 | 1,3 | 0,76 |
| SR [g/kg] | 0,031 | 0,413 | 0,283 | 0,221 |
| R [g/kg] | 0,087 | 1,156 | 0,792 | 0,619 |
| CVR [%] | 1,48 | 4,69 | 4,11 | 4,22 |
=
number of laboratories submitting results
=
number of single results retained after eliminating outliers (identified by Cochran, Dixon outlier test)
=
standard deviation of repeatability
=
standard deviation of reproducibility
=
repeatability
=
reproducibility
=
coefficient of variation of repeatability, %
=
coefficient of variation of reproducibility, %
9.ObservationsU.K.
9.1.Following special chromatographic conditions may give better separation between tryptophan and α-methyl-tryptophan.U.K.
Isocratic elution followed by gradient column cleaning:
| Liquid chromatographic column: | 125 mm x 4 mm, C18, 5 μm packing or equivalent | ||
| Column temperature: | 32 oC | ||
| Mobile phase: | A: 0,01 mol/l KH2PO4/méthanol, 95+5 (V+V). B: methanol | ||
| Gradient program: | 0 min. | 100 % A | 0 % B |
| 15 min. | 100 % A | 0 % B | |
| 17 min. | 60 % A | 40 % B | |
| 19 min. | 60 % A | 40 % B | |
| 21 min. | 100 % A | 0 % B | |
| 33 min. | 100 % A | 0 % B | |
| Flow rate: | 1,2 ml/min. | ||
| Total run time: | approx. 33 min. | ||
9.2.The chromatography will vary according to the type of HPLC and column packing material used. The chosen system must be capable of giving baseline separation between the tryptophan and the internal standard. Moreover it is important that degradation products are well separated from the tryptophan and the internal standard. Hydrolysates without internal standard shall be run in order to check the base line under the internal standard for impurities. It is important that the run time is sufficiently long for the elution of all the degradation products, otherwise late eluting peaks may interfere with subsequent chromatographic runs.U.K.
In the range of operation, the chromatographic system shall give linear response. The linear response shall be measured with a constant (the normal) concentration of the internal standard and varying concentrations of tryptophan. It is of importance that the size of both the tryptophan and internal standard peaks are within the linear range of the HPLC/fluorescence system. If either the tryptophan and/or the internal standard peak(s) is (are) too small or too high the analysis shall be repeated with another sample size and/or a changed final volume.
9.3. Barium hydroxide U.K.
With age barium hydroxide becomes more difficult to dissolve. This results in an unclear solution for the HPLC determination, which may produce low results for tryptophan.