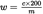- Latest available (Revised)
- Point in Time (31/01/2020)
- Original (As adopted by EU)
Commission Regulation (EC) No 152/2009Show full title
Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance)
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 16/11/20202.10 MB
- Revised 24/05/20171.85 MB
- Revised 26/04/20171.62 MB
- Revised 17/07/20142.16 MB
- Revised 01/01/20142.40 MB
- Revised 12/02/20132.46 MB
- Revised 18/04/20122.15 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Division E.
Alternative versions:
Status:
Point in time view as at 31/01/2020.
Changes to legislation:
There are currently no known outstanding effects by UK legislation for Commission Regulation (EC) No 152/2009, Division E..![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
E.DETERMINATION OF ROBENIDINEU.K.
1,3-bis [(4-chlorobenzylidene)amino]guanidine — hydrochloride
1.Purpose and scopeU.K.
This method makes it possible to determine the levels of robenidine in feed. The limit of quantification is 5 mg/kg.
2.PrincipleU.K.
The sample is extracted with acidified methanol. The extract is dried and an aliquot portion subjected to a clean-up on an aluminium oxide column. Robenidine is eluted from the column with methanol, concentrated, and made up to a suitable volume with mobile phase. The content of robenidine is determined by reversed-phase high-performance liquid chromatography (HPLC) using an UV detector.
3.ReagentsU.K.
3.1.Methanol.U.K.
3.2.Acidified methanol.U.K.
Transfer 4,0 ml hydrochloric acid (ρ20 = 1,18 g/ml) into a 500 ml graduated flask, make up to the mark with methanol (3.1) and mix. This solution shall be freshly prepared before use.
3.3.Acetonitrile, equivalent to HPLC grade.U.K.
3.4.Molecular sieve.U.K.
Type 3A, 8 to 12 mesh beads (1,6-2,5 mm beads, crystalline alumino-silicate, diameter of pores 0,3 mm).
3.5.Aluminium oxide acidic activity grade I for column chromatography.U.K.
Transfer 100 g aluminium oxide into a suitable container and add 2,0 ml of water. Stopper and shake for approximately 20 minutes. Store in a well stoppered container.
3.6.Potassium dihydrogen phosphate solution, c = 0,025 mol/l.U.K.
Dissolve 3,4 g of potassium dihydrogen phosphate in water (HPLC grade) in a 1 000 ml graduated flask, make up to the mark and mix.
3.7.Di-sodium hydrogen phosphate solution, c = 0,025 mol/l.U.K.
Dissolve 3,55 g of anhydrous (or 4,45 g of dihydrate or 8,95 g of dodecahydrate) di-sodium hydrogen phosphate in water (equivalent to HPLC grade) in a 1 litre graduated flask, make up to the mark and mix.
3.8.HPLC mobile phase.U.K.
Mix together the following reagents:
650 ml acetonitrile (3.3),
250 ml water (equivalent to HPLC-grade),
50 ml potassium di-hydrogen phosphate solution (3.6),
50 ml di-sodium hydrogen phosphate solution (3.7).
Filter through a 0,22 μm filter (4.6) and degas the solution, (e.g. by ultrasonification for 10 minutes).
3.9.Standard substance.U.K.
Pure robenidine: 1,3-bis [(4-chlorobenzylidene)amino]guanidine — hydrochloride.
3.9.1. Robenidine stock standard solution: 300 μg/ml U.K.
Weigh to the nearest 0,1 mg, 30 mg of robenidine standard substance (3.9). Dissolve in acidified methanol (3.2) in a 100 ml graduated flask, make up to the mark with the same solvent and mix. Wrap the flask with aluminium foil and store in a dark place.
3.9.2. Robenidine intermediate standard solution: 12 μg/ml U.K.
Transfer 10,0 ml of the stock standard solution (3.9.1) into a 250 ml graduated flask, make up to the mark with the mobile phase (3.8) and mix. Wrap the flask with aluminium foil and store in a dark place.
3.9.3. Calibration solutions U.K.
Into a series of 50 ml calibrated flasks, transfer 5,0, 10,0, 15,0, 20,0 and 25,0 ml of the intermediate standard solution (3.9.2). Make up to the mark with mobile phase (3.8) and mix. These solutions correspond to 1,2, 2,4, 3,6, 4,8 and 6,0 μg/ml of robenidine respectively. These solutions must be freshly prepared before use.
3.10.Water equivalent to HPLC grade.U.K.
4.ApparatusU.K.
4.1.Glass column.U.K.
Constructed of amber glass fitted with a stopcock and a reservoir of approximately 150 ml capacity, internal diameter 10 to 15 mm, length 250 mm.
4.2.Mechanical shaker or magnetic stirrer.U.K.
4.3.Rotary film evaporator.U.K.
4.4.HPLC equipment with variable wavelength ultraviolet detector or diode array detector operating in the range of 250 to 400 nm.U.K.
4.4.1.Liquid chromatographic column: 300 mm x 4 mm, C18 10 μm packing or equivalent.U.K.
4.5.Glass fibre filter paper (Whatman GF/A or equivalent).U.K.
4.6.Membrane filters, 0,22 μm.U.K.
4.7.Membrane filters, 0,45 μm.U.K.
5.ProcedureU.K.
Robenidine is light-sensitive. Amber glassware shall be used in all operations.
5.1.GeneralU.K.
5.1.1.A blank feed shall be analysed to check that neither robenidine nor interfering substances are present.U.K.
5.1.2.A recovery test shall be carried out by analysing the blank feed (5.1.1) which has been fortified by addition of a quantity of robenidine, similar to that present in the sample. To fortify at a level of 60 mg/kg, transfer 3,0 ml of the stock standard solution (3.9.1) to a 250 ml conical flask. Evaporate the solution to ca. 0,5 ml in a stream of nitrogen. Add 15 g of the blank feed, mix and wait for 10 minutes before proceeding with the extraction step (5.2).U.K.
For the purpose of this method, the blank feed shall be similar in type to that of the sample and on analysis robenidine shall not be detected.
5.2.ExtractionU.K.
Weigh to the nearest 0,01 g, approximately 15 g of the prepared sample. Transfer to a 250 ml conical flask and add 100,0 ml of acidified methanol (3.2), stopper and shake for one hour on the shaker (4.2). Filter the solution through a glass fibre filter paper (4.5) and collect the whole filtrate in a 150 ml conical flask. Add 7,5 g molecular sieve (3.4), stopper and shake for five minutes. Filter immediately through a glass-fibre filter paper. Retain this solution for the purification step (5.3).
5.3.PurificationU.K.
5.3.1. Preparation of the aluminium-oxide column U.K.
Insert a small glass-wool plug into the lower end of a glass column (4.1) and tamp it down using a glass rod. Weigh out 11,0 g of the prepared aluminium oxide (3.5) and transfer to the column. Care shall be taken to minimise the exposure to the atmosphere during this stage. Gently tap the loaded column at its lower end to settle the aluminium oxide.
5.3.2. Sample purification U.K.
Transfer onto the column by pipette 5,0 ml of the sample extract prepared in (5.2) Rest the pipette tip close to the column wall and allow the solution to be absorbed onto the aluminium oxide. Elute the robenidine from the column using 100 ml methanol (3.1), at a flow rate of 2 to 3 ml/minute and collect the eluate in a 250 ml round bottomed flask. Evaporate the methanol solution to dryness under reduced pressure at 40 oC by means of a rotary film evaporator (4.3). Re-dissolve the residue in 3 to 4 ml of mobile phase (3.8) and transfer quantitatively to a 10 ml graduated flask. Rinse the flask with several 1 to 2 ml portions of mobile phase and transfer these rinsings to the graduated flask. Make up to the mark with the same solvent and mix. An aliquot is filtered through a 0,45 μm membrane filter (4.7). Reserve this solution for HPLC determination (5.4).
5.4.HPLC determinationU.K.
5.4.1. Parameters U.K.
The following conditions are offered for guidance, other conditions may be used provided they yield equivalent results:
Liquid chromatographic column (4.4.1),
HPLC mobile phase (3.8),
Flow rate: 1,5 to 2 ml/minute,
Detector wavelength: 317 nm,
Injection volume: 20 to 50 μl.
Check the stability of the chromatographic system, injecting the calibration solution (3.9.3) containing 3,6 μg/ml several times, until constant peak heights and retention times are achieved.
5.4.2. Calibration graph U.K.
Inject each calibration solution (3.9.3) several times and measure the peak heights (areas) for each concentration. Plot a calibration curve using the mean peak heights or areas of the calibration solutions as the ordinates and corresponding concentrations in μg per ml as abscissae.
5.4.3. Sample solution U.K.
Inject the sample extract (5.3.2) several times, using the same volume as taken for the calibration solutions and determine the mean peak height (area) of the robenidine peaks.
6.Calculation of resultsU.K.
From the mean height (area) of the robenidine peaks of the sample solution determine the concentration of the sample solution in μg/ml by reference to the calibration graph (5.4.2).
The content of robenidine w (mg/kg) in the sample is given by the following formula:
in which:
=
robenidine concentration of the sample solution in μg/ml,
=
weight of the test portion in grams.
7.Validation of the resultsU.K.
7.1.IdentityU.K.
The identity of the analyte can be confirmed by co-chromatography, or by using a diode-array detector by which the spectra of the sample extract and the calibration solution (3.9.3) containing 6 μg/ml are compared.
7.1.1. Co-chromatography U.K.
A sample extract is fortified by addition of an appropriate amount of calibration solution (3.9.3). The amount of added robenidine must be similar to the estimated amount of robenidine found in the sample extract.
Only the height of the robenidine peak shall be enhanced after taking into account both the amount added and the dilution of the extract. The peak width, at half of its maximum height, must be within approximately 10 % of the original width.
7.1.2. Diode-array detection U.K.
The results are evaluated according to the following criteria:
the wavelength of maximum absorption of the sample and of the standard spectra, recorded at the peak apex on the chromatogram, must be the same within a margin determined by the resolving power of the detection system. For diode-array detection, this is typically within approximately 2 nm;
between 250 and 400 nm, the sample and standard spectra recorded at the peak apex on the chromatogram, must not be different for those parts of the spectrum within the range 10 % to 100 % of relative absorbance. This criterion is met when the same maxima are present and at no observed point the deviation between the two spectra exceeds 15 % of the absorbance of the standard analyte;
between 250 and 400 nm, the spectra of the upslope, apex and downslope of the peak produced by the sample extract must not be different from each other for those parts of the spectrum within the range 10 % to 100 % of relative absorbance. This criterion is met when the same maxima are present and when at all observed points the deviation between the spectra does not exceed 15 % of the absorbance of the spectrum of the apex.
If one of these criteria is not met the presence of the analyte has not been confirmed.
7.2.RepeatabilityU.K.
The difference between the results of two parallel determinations carried out on the same sample must not exceed 10 % of the higher result for robenidine content higher than 15 mg/kg.
7.3.RecoveryU.K.
For a fortified blank sample the recovery shall be at least 85 %.
8.Results of a collaborative studyU.K.
An EC collaborative study was arranged in which four samples of poultry and rabbit feed, in meal or pelleted form were analysed by 12 laboratories. Duplicate analyses were performed on each sample. The results are given in the table below:
| Poultry | Rabbit | |||
|---|---|---|---|---|
| Meal | Pellet | Meal | Pellet | |
| Mean [mg/kg] | 27,0 | 27,99 | 43,6 | 40,1 |
| sr [mg/kg] | 1,46 | 1,26 | 1,44 | 1,66 |
| CVr [%] | 5,4 | 4,5 | 3,3 | 4,1 |
| SR [mg/kg] | 4,36 | 3,36 | 4,61 | 3,91 |
| CVR [%] | 16,1 | 12,0 | 10,6 | 9,7 |
| Recovery [%] | 90,0 | 93,3 | 87,2 | 80,2 |
=
standard deviation of repeatability,
=
coefficient of variation of repeatability, %
=
standard deviation of reproducibility,
=
coefficient of variation of reproducibility. %
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Whole Annex
PrintThis Division only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources