[F1ANNEX VIU.K. METHODS OF ANALYSIS FOR THE DETERMINATION OF CONSTITUENTS OF ANIMAL ORIGIN FOR THE OFFICIAL CONTROL OF FEED
Textual Amendments
1. PURPOSE AND SCOPE U.K.
The determination of constituents of animal origin in feed shall be performed by light microscopy or polymerase chain reaction (PCR) in accordance with the provisions laid down in this Annex.
These two methods make it possible to detect the presence of constituents of animal origin in feed materials and compound feed. However, they do not make it possible to calculate the amount of such constituents in feed materials and compound feed. Both methods have a limit of detection below 0,1 % (w/w).
The PCR method makes it possible to identify the taxonomic group of constituents of animal origin present in feed materials and compound feed.
These methods shall apply for the control of the application of the prohibitions laid down in Article 7(1) and Annex IV to Regulation (EC) No 999/2001 and in Article 11(1) of Regulation (EC) No 1069/2009.
Depending on the type of feed being tested, these methods may be used, within one single operational protocol, either on their own or combined together in accordance with the standard operating procedures (SOP) established by the EU reference laboratory for animal proteins in feedingstuffs (EURL-AP) and published on its website(1).
2. METHODS U.K.
2.1. Light microscopy U.K.
2.1.1. [F2Principle U.K.
The constituents of animal origin which may be present in feed materials and compound feed sent for analysis are identified on the basis of typical and microscopically identifiable characteristics like muscle fibres and other meat particles, cartilage, bones, horn, hair, bristles, blood, milk globules, lactose crystals, feathers, egg shells, fish bones and scales.]
Textual Amendments
2.1.2. Reagents and equipment U.K.
2.1.2.1. Reagents U.K.
2.1.2.1.1. Concentrating agent U.K.
2.1.2.1.1.1. Tetrachloroethylene (specific gravity 1,62) U.K.
2.1.2.1.2. Staining reagent U.K.
2.1.2.1.2.1.Alizarin Red solution (dilute 2,5 ml 1M hydrochloric acid in 100 ml water and add 200 mg Alizarin Red to this solution)U.K.
2.1.2.1.3. Mounting media U.K.
2.1.2.1.3.1. Lye (NaOH 2,5 % w/v or KOH 2,5 % w/v) U.K.
2.1.2.1.3.2. [F2Glycerol (undiluted, viscosity: 1 490 cP) or a mounting medium with equivalent properties for non-permanent slide preparation] U.K.
2.1.2.1.3.3. Norland ® Optical Adhesive 65 (viscosity: 1 200 cP) or a resin with equivalent properties for permanent slide preparation U.K.
2.1.2.1.4. Mounting media with staining properties U.K.
2.1.2.1.4.1.Lugol solution (dissolve 2 g potassium iodide in 100 ml water and add 1 g iodine while frequently shaking)U.K.
2.1.2.1.4.2. Cystine reagent (2 g lead acetate, 10 g NaOH/100 ml water) U.K.
2.1.2.1.4.3.Fehling’s reagent (prepared before use from equals parts (1/1) of two stock solutions A and B. Solution A: dissolve 6,9 g copper (II) sulphate pentahydrate in 100 ml water. Solution B: dissolve 34,6 g potassium sodium tartrate tetrahydrate and 12 g NaOH in 100 ml water)U.K.
2.1.2.1.4.4.Tetramethylbenzidine/Hydrogen peroxide. (dissolve 1 g 3,3’,5,5’ tetramethylbenzidine (TMB) in 100 ml glacial acetic acid and 150 ml water. Before use, mix 4 parts of this TMB solution with 1 part 3 % hydrogen peroxide)U.K.
2.1.2.1.5. Rinsing agents U.K.
2.1.2.1.5.1. Ethanol ≥ 96 % (technical grade) U.K.
2.1.2.1.5.2. Acetone (technical grade) U.K.
2.1.2.1.6. Bleaching reagent U.K.
2.1.2.1.6.1. Commercial sodium hypochlorite solution (9 - 14 % active chlorine) U.K.
2.1.2.2. Equipment U.K.
2.1.2.2.1. Analytical balance with an accuracy of 0,001 g U.K.
2.1.2.2.2. [F2Grinding equipment: knife or rotor mill. If a rotor mill is used, mill sieves ≤ 0,5 mm shall be prohibited] U.K.
2.1.2.2.3. [F2Sieves with square meshes of 0,25 mm and 1 mm width. With the exception of sample pre-sieving, the diameter of the sieves should not exceed 10 cm to avoid loss of materials. Calibration of sieves is not required] U.K.
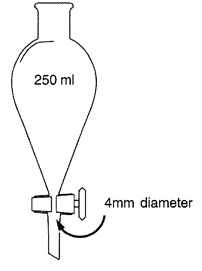
2.1.2.2.4.Conical glass separation funnel with a content of 250 ml with Teflon or ground glass stopcock at the base of the cone. Stopcock opening diameter shall be ≥ 4mm. Alternatively, a conical bottomed settling beaker may be used provided the laboratory has demonstrated that detection levels are equivalent to that obtained using the conical glass separation funnel.U.K.
Separation funnel U.K.
2.1.2.2.5.Stereomicroscope covering at least a 6,5× to 40× final magnification rangeU.K.
2.1.2.2.6.Compound microscope covering at least a 100× to 400× final magnification range with transmitted light bright field. Polarised light and differential interferential contrast can additionally be usedU.K.
2.1.2.2.7. Standard laboratory glassware U.K.
2.1.2.2.8. Equipment for slide preparation: classical microscope slides, hollow slides, coverslips (20 × 20 mm), tweezers, fine spatula U.K.
[F32.1.2.2.9. Laboratory oven U.K.
Textual Amendments
2.1.2.2.10. Centrifuge U.K.
2.1.2.2.11. Filter paper: qualitative cellulose filter (pore size 4-11 μm)] U.K.
2.1.3. Sampling and sample preparation U.K.
2.1.3.1. [F2Sampling U.K.
A representative sample, taken in accordance with the provisions laid down in Annex I to this Regulation shall be used.]
2.1.3.2. Precautions to be taken U.K.
In order to avoid laboratory cross-contamination, all reusable equipment shall be carefully cleaned before use. Separation funnel pieces shall be disassembled before cleaning. Separation funnel pieces and glassware shall be pre-washed manually and then washed in a washing machine. Sieves shall be cleaned by using a brush with stiff synthetic hairs. A final cleaning of sieves with acetone and compressed air is recommended after sieving of fatty material like fishmeal.
2.1.3.3. Preparation of samples other than fat or oil U.K.
2.1.3.3.1. [F2Sample drying : samples with a moisture content > 14 % shall be dried prior to handling according to Annex III to this Regulation.]U.K.
2.1.3.3.2. [F2Sample pre-sieving : in order to collect information on possible environmental contamination of the feed, it is recommended to pre-sieve at 1 mm pelleted feeds and kernels and to subsequently prepare, analyse, and report separately on the two resulting fractions, which must be considered as distinct samples.]U.K.
2.1.3.3.3. Sub-sampling and grinding : at least 50 g of the sample shall be sub-sampled for analysis and subsequently ground.U.K.
2.1.3.3.4. Extraction and preparation of the sediment : a portion of 10 g (accurate to 0,01 g) of the ground sub-sample shall be transferred into the separation funnel or conical bottomed settling beaker and 50 ml of tetrachloroethylene shall be added. The portion transferred into the funnel shall be limited to 3 g in case of fishmeal or other pure animal products, mineral ingredients or premixes which generate more than 10 % of sediment. The mixture shall be vigorously shaken for at least 30 s and at least 50 ml more of tetrachloroethylene shall be added cautiously while washing down the inside surface of the funnel to remove any adhering particles. The resulting mixture shall be left to stand for at least 5 minutes before the sediment is separated off by opening the stopcock.U.K.
If a conical bottomed settling beaker is used then the mixture shall be vigorously stirred for at least 15 s and any particles adhering to the side of the beaker shall be carefully washed down the inside surface with at least 10 ml of clean tetrachloroethylene. The mixture shall be left to stand for 3 minutes and then stirred again for 15 seconds and any particles adhering to the side of the beaker shall be carefully washed down the inside surface with at least 10 ml of clean tetrachloroethylene. The resulting mixture shall be left to stand for at least 5 minutes and then the liquid fraction is removed and discarded by careful decanting, taking care not to lose any of the sediment.
[F2The sediment shall be collected on a filter paper placed into a funnel to allow the separation of the remaining TCE while avoiding fat deposition into the sediment. The sediment shall be dried. It is recommended to subsequently weigh the sediment (accurate to 0,001 g) to control the sedimentation step. Lastly, the sediment shall be sieved at 0,25 mm and the two resulting fractions shall be examined, unless sieving is not deemed necessary.]
2.1.3.3.5. Extraction and preparation of the flotate : after recovery of the sediment with the method described above, two phases should remain in the separation funnel: a liquid one consisting of tetrachloroethylene and a solid one made of floating material. This solid phase is the flotate and shall be recovered by pouring off completely tetrachloroethylene from the funnel by opening the stopcock. By inverting the separation funnel, the flotate shall be transferred into a large Petri dish and air dried in a fumehood. If more than 5 % of the flotate consists of particles > 0,50 mm, it shall be sieved at 0,25 mm and the two resulting fractions shall be examined.U.K.
2.1.3.3.6. Preparation of raw material : a portion of at least 5 g of the ground sub-sample shall be prepared. If more than 5 % of the material consists of particles > 0,50 mm, it shall be sieved at 0,25 mm and the two resulting fractions shall be examined.U.K.
2.1.3.4. Preparation of samples consisting of fat or oil U.K.
The following protocol shall be followed for the preparation of samples consisting of fat or oil:
if the fat is solid, it shall be warmed in a oven until it is liquid.
by using a pipette, 40 ml of fat or oil shall be transferred from the bottom of the sample to a centrifugation tube.
centrifuge during 10 minutes at 4 000 r.p.m.
if the fat is solid after centrifugation, it shall be warmed in an oven until it is liquid.
repeat the centrifugation during 5 minutes at 4 000 r.p.m.
by using a small spoon or a spatula, one half of the decanted impurities shall be transferred to microscopic slides for examination, Glycerol is recommended as mounting medium.
the remaining impurities shall be used for preparing the sediment as described in point 2.1.3.3.
2.1.3.5. Use of staining reagents U.K.
In order to facilitate the correct identification of the constituents of animal origin, the operator may use staining reagents during the sample preparation in accordance with guidelines issued by the EURL-AP and published on its website.
In case Alizarin Red solution is used to colour the sediment, the following protocol shall apply:
the dried sediment shall be transferred into a glass test tube and rinsed twice with approximately 5 ml of ethanol (each time a vortex of 30 s shall be used, the solvent shall be let settle about 1 min 30 s and poured off).
the sediment shall be bleached by adding at least 1 ml sodium hypochlorite solution. The reaction shall be allowed to continue for 10 min. The tube shall be filled with water, the sediment shall be let settle 2-3 min, and the water and the suspended particles shall be poured off gently.
the sediment shall be rinsed twice more with about 10 ml of water (a vortex shall be used for 30 s, let settle, and pour off the water each time).
2 to 10 drops of the Alizarin Red solution shall be added and the mixture shall be vortexed. The reaction shall be let occur for 30 s and the coloured sediment shall be rinsed twice with approximately 5 ml ethanol followed by one rinse with acetone (each time a vortex of 30 s shall be used, the solvent shall be let settle about 1 min and poured off).
the coloured sediment shall be dried.
2.1.4. Microscopic examination U.K.
2.1.4.1. Slide preparation U.K.
[F2Microscopic slides shall be prepared from the sediment and, depending on the operator’s choice, from either the flotate or the raw material.]
A sufficient number of slides shall be prepared in order to ensure that a complete examination protocol as laid down in point 2.1.4.2 can be carried-out.
Microscopic slides shall be mounted with the adequate mounting medium in accordance with the SOP established by the EURL-AP and published on its website. The slides shall be covered with coverslips.
[F22.1.4.2. Observation flowchart for the detection of animal particles in compound feed and feed material U.K.
The prepared microscopic slides shall be observed in accordance with the observation flowcharts laid down in diagrams 1 and 2.
The microscopic observations shall be conducted using the compound microscope on the sediment and, depending on the operator’s choice, either on the flotate or on the raw material. The stereomicroscope may be used in addition to the compound microscope for the coarse fractions. Each slide shall be screened entirely at various magnifications. Precise explanations on how to use the observation flowcharts are detailed by a SOP established by the EURL-AP and published on its website.
The minimum numbers of slides to be observed at each step of the observation flowcharts shall be strictly respected, unless the entire fraction material does not permit to reach the stipulated slide number, for instance when no sediment is obtained. No more than 6 slides per determination shall be used for recording of the number of particles.
When additional slides are prepared on the flotate or the raw material using a more specific mounting medium with staining properties, as laid down in point 2.1.2.1.4, to further characterise structures (e.g. feathers, hairs, muscle or blood particles) which have been detected on slides prepared by other mounting media, as laid down in point 2.1.2.1.3, the number of particles shall be counted based on a number of slides per determination not exceeding 6, including the additional slides with a more specific mounting medium.
In order to facilitate the identification of the particles’ nature and origin, the operator may use support tools like decision support systems, image libraries and reference samples.]
2.1.4.3. [F2Number of determinations U.K.
Determinations shall be performed on different sub-samples of 50 g each.
If following the first determination carried out in accordance with the observation flowchart laid down in diagram 1, no animal particles are detected, no additional determination is necessary and the result of the analysis shall be reported using the terminology laid down in point 2.1.5.1.
If, following the first determination carried out in accordance with the observation flowchart laid down in diagram 1, one or more animal particles of a given nature (i.e. terrestrial vertebrate or fish) are detected, and the nature of the particles found confirms the declared content of the sample, no second determination is necessary. If the number of the animal particles of a given nature detected during this first determination is higher than 5, the result of the analysis shall be reported per animal nature using the terminology laid down in point 2.1.5.3. Otherwise, the result of the analysis shall be reported per animal nature using the terminology laid down in point 2.1.5.2.
In other cases, including when no declaration of content has been provided to the laboratory a second determination shall be carried out from a new sub-sample.
If, following the second determination carried out in accordance with the observation flowchart laid down in diagram 2, the sum of the animal particles of a given nature detected over the two determinations is higher than 10, the result of the analysis shall be reported per animal nature using the terminology laid down in point 2.1.5.3. Otherwise, the result of the analysis shall be reported per animal nature using the terminology laid down in point 2.1.5.2.]
2.1.5. [F2Expression of the results U.K.
When reporting the results, the laboratory shall indicate on which type of material the analysis has been carried-out (sediment, flotate or raw material). The reporting shall clearly indicate how many determinations have been carried-out and if sieving of the fractions prior to slide preparation, in accordance with the last paragraph of point 2.1.3.3.4., was not performed.
The laboratory report shall at least contain information on the presence of constituents derived from terrestrial vertebrates and from fish.
The different situations shall be reported in the following ways.
No animal particle of a given nature detected:
‘As far as was discernible using a light microscope, no particle derived from terrestrial vertebrates was detected in the submitted sample.’
‘As far as was discernible using a light microscope, no particle derived from fish was detected in the submitted sample.’
Between 1 and 5 animal particles of a given nature detected when only one determination has been performed, or between 1 and 10 particles of a given nature detected in case of two determinations (the number of detected particles is below the decision limit established in the standard operating procedures (SOP) of the EU reference laboratory for animal proteins in feedingstuffs (EURL-AP) and published on its website(2)):
When only one determination has been performed:
‘As far as was discernible using a light microscope, no more than 5 particles derived from terrestrial vertebrates were detected in the submitted sample. The particles were identified as … [bone, cartilage, muscle, hair, horn…]. This low level presence is below the decision limit established for this microscopic method.’
‘As far as was discernible using a light microscope, no more than 5 particles derived from fish were detected in the submitted sample. The particles were identified as … [fishbone, fish scale, cartilage, muscle, otolith, gill…]. This low level presence, is below the decision limit established for this microscopic method.’
When two determinations have been performed:
‘As far as was discernible using a light microscope, no more than 10 particles derived from terrestrial vertebrates were detected over the two determinations in the submitted sample. The particles were identified as … [bone, cartilage, muscle, hair, horn…]. This low level presence is below the decision limit established for this microscopic method.’
‘As far as was discernible using a light microscope, no more than 10 particles derived from fish were detected over the two determinations in the submitted sample. The particles were identified as … [fishbone, fish scale, cartilage, muscle, otolith, gill…]. This low level presence is below the decision limit established for this microscopic method.’
Additionally:
In case of sample pre-sieving, the laboratory report shall mention in which fraction (sieved fraction, pelleted fraction or kernels) the animal particles have been detected insofar as the detection of animal particles only in the sieved fraction may be the sign of an environmental contamination.
When only animal particles which cannot be categorised as either terrestrial vertebrates or fish are detected (e.g. muscle fibres), the report shall mention that only such animal particles were detected and that it cannot be excluded that they originate from terrestrial vertebrates
More than 5 animal particles of a given nature detected when only one determination has been performed, or more than 10 particles of a given nature detected in case of two determinations:
When only one determination has been performed:
‘As far as was discernible using a light microscope, more than 5 particles derived from terrestrial vertebrates were detected in the submitted sample. The particles were identified as … [bone, cartilage, muscle, hair, horn…].’
‘As far as was discernible using a light microscope, more than 5 particles derived from fish were detected in the submitted sample. The particles were identified as … [fishbone, fish scale, cartilage, muscle, otolith, gill…].’
When two determinations have been performed:
‘As far as was discernible using a light microscope, more than 10 particles derived from terrestrial vertebrates were detected over the two determinations in the submitted sample. The particles were identified as … [bone, cartilage, muscle, hair, horn…].’
‘As far as was discernible using a light microscope, more than 10 particles derived from fish were detected over the two determinations in the submitted sample. The particles were identified as … [fishbone, fish scale, cartilage, muscle, otolith, gill…].’
Additionally:
In case of sample pre-sieving, the laboratory report shall mention in which fraction (sieved fraction, pelleted fraction or kernels) the animal particles have been detected insofar as the detection of animal particles only in the sieved fraction may be the sign of an environmental contamination.
When only animal particles which cannot be categorised as either terrestrial vertebrates or fish are detected (e.g. muscle fibres), the report shall mention that only such animal particles were detected and that it cannot be excluded that they originate from terrestrial vertebrates.]
2.2. PCR U.K.
2.2.1. Principle U.K.
Deoxyribonucleic acid (DNA) fragments of animal origin which may be present in feed materials and compound feed are detected by a genetic amplification technique through PCR, targeting species-specific DNA sequences.
The PCR method first requires a DNA extraction step. The amplification step shall be applied afterwards to the so-obtained DNA extract, in order to detect the animal species targeted by the assay.
2.2.2. Reagents and equipment U.K.
2.2.2.1. Reagents U.K.
2.2.2.1.1. Reagents for DNA extraction step U.K.
Only reagents approved by the EURL-AP and published on its website shall be used.
2.2.2.1.2. Reagents for genetic amplification step U.K.
2.2.2.1.2.1. Primers and probes U.K.
Only primers and probes with sequences of oligonucleotides validated by the EURL-AP shall be used(3).
2.2.2.1.2.2. Master Mix U.K.
Only Master Mix solutions which do not contain reagents susceptible to lead to false results due to presence of animal DNA shall be used(4).
2.2.2.1.2.3. Decontamination reagents U.K.
2.2.2.1.2.3.1. Hydrochloric acid solution (0,1 N) U.K.
2.2.2.1.2.3.2. Bleach (solution of sodium hypochlorite at 0,15 % of active chlorine) U.K.
2.2.2.1.2.3.3. Non-corrosive reagents for decontaminating costly devices like analytical balances (e.g. DNA Erase TM of MP Biomedicals) U.K.
2.2.2.2. Equipment U.K.
2.2.2.2.1. Analytical balance with an accuracy of 0,001 g U.K.
2.2.2.2.2. Grinding equipment U.K.
2.2.2.2.3. Thermocycler enabling real-time PCR U.K.
2.2.2.2.4. Microcentrifuge for microfuge tubes U.K.
2.2.2.2.5. Set of micropipettes allowing to pipet from 1 μl up to 1 000 μl U.K.
2.2.2.2.6.Standard molecular biology plastic-ware: microfuge tubes, filtered plastic tips for micropipettes, plates suitable for the thermocycler.U.K.
2.2.2.2.7. Freezers to store samples and reagents U.K.
2.2.3. Sampling and sample preparation U.K.
2.2.3.1. Sampling U.K.
A representative sample, taken in accordance with the provisions laid down in Annex I, shall be used.
2.2.3.2. Sample preparation U.K.
The preparation of laboratory samples up to DNA extraction shall comply with the requirements set out in Annex II. At least 50 g of the sample shall be sub-sampled for analysis and subsequently ground.
The sample preparation shall be performed in a room different from the ones dedicated to DNA extraction and to genetic amplification reactions as described by ISO 24276.
Two test portions of at least 100 mg each shall be prepared.
2.2.4. DNA extraction U.K.
The DNA extraction shall be performed on each test portion prepared using the SOP established by the EURL-AP and published on its website.
Two extraction controls shall be prepared for each extraction series as described by ISO 24276.
an extraction blank control,
a positive DNA extraction control.
2.2.5. Genetic amplification U.K.
The genetic amplification shall be performed using the methods validated for each species requiring identification. These methods are laid down in the SOP established by the EURL-AP and published on its website. Each DNA extract shall be analysed at least at two different dilutions in order to evaluate inhibition.
Two amplification controls shall be prepared per species target as described by ISO 24276.
a positive DNA target control shall be used for each plate or series of PCR assays,
an amplification reagent control (also called no template control) shall be used for each plate or series of PCR assays.
2.2.6. Interpretation and expression of results U.K.
When reporting the results, the laboratory shall indicate at least the weight of the test portions used, the extraction technique used, the number of determinations carried-out and the limit of detection of the method.
Results shall not be interpreted and reported if the positive DNA extraction control and the positive DNA target controls do not provide positive results for the target under assay while the amplification reagent control is negative.
In case results from the two test portions are not consistent, at least the genetic amplification step shall be repeated. If the laboratory suspects that the DNA extracts can be the cause of the inconsistency, a new DNA extraction and a subsequent genetic amplification shall be performed before interpreting the results.
The final expression of the results shall be based on the integration and the interpretation of the results of the two test portions in accordance with the SOP established by the EURL-AP and published on its website.
2.2.6.1. Negative result U.K.
A negative result shall be reported as follows:
No DNA from X was detected in the submitted sample (with X being the animal species or group of animal species that is targeted by the assay).
2.2.6.2. Positive result U.K.
A positive result shall be reported as follows:
DNA from X was detected in the submitted sample (with X being the animal species or group of animal species that is targeted by the assay).]
[F1http://eurl.craw.eu/]
[F1The list of these primers and probes for each animal species targeted by the assay is available on the EURL-AP website.]
[F1Examples of Master Mixes that are functional are available on the EURL-AP website.]