- Latest available (Revised)
- Original (As adopted by EU)
Commission Regulation (EU) No 142/2011Show full title
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (Text with EEA relevance)
You are here:
More Resources
Revised version PDFs
- Revised 08/12/20209.59 MB
- Revised 30/06/20209.58 MB
- Revised 23/06/20209.54 MB
- Revised 08/03/20209.54 MB
- Revised 14/12/20199.44 MB
- Revised 31/07/20199.44 MB
- Revised 16/07/20199.44 MB
- Revised 20/03/20199.75 MB
- Revised 02/08/201710.18 MB
- Revised 01/07/201710.16 MB
- Revised 29/05/20179.92 MB
- Revised 22/02/20179.91 MB
- Revised 23/02/20159.85 MB
- Revised 15/07/20149.35 MB
- Revised 19/03/20149.30 MB
- Revised 01/12/20138.38 MB
- Revised 01/07/20139.54 MB
- Revised 15/03/20139.53 MB
- Revised 14/12/20129.27 MB
- Revised 04/12/20129.27 MB
- Revised 19/08/20118.01 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Status:
This is the original version as it was originally adopted in the EU.
This legislation may since have been updated - see the latest available (revised) version
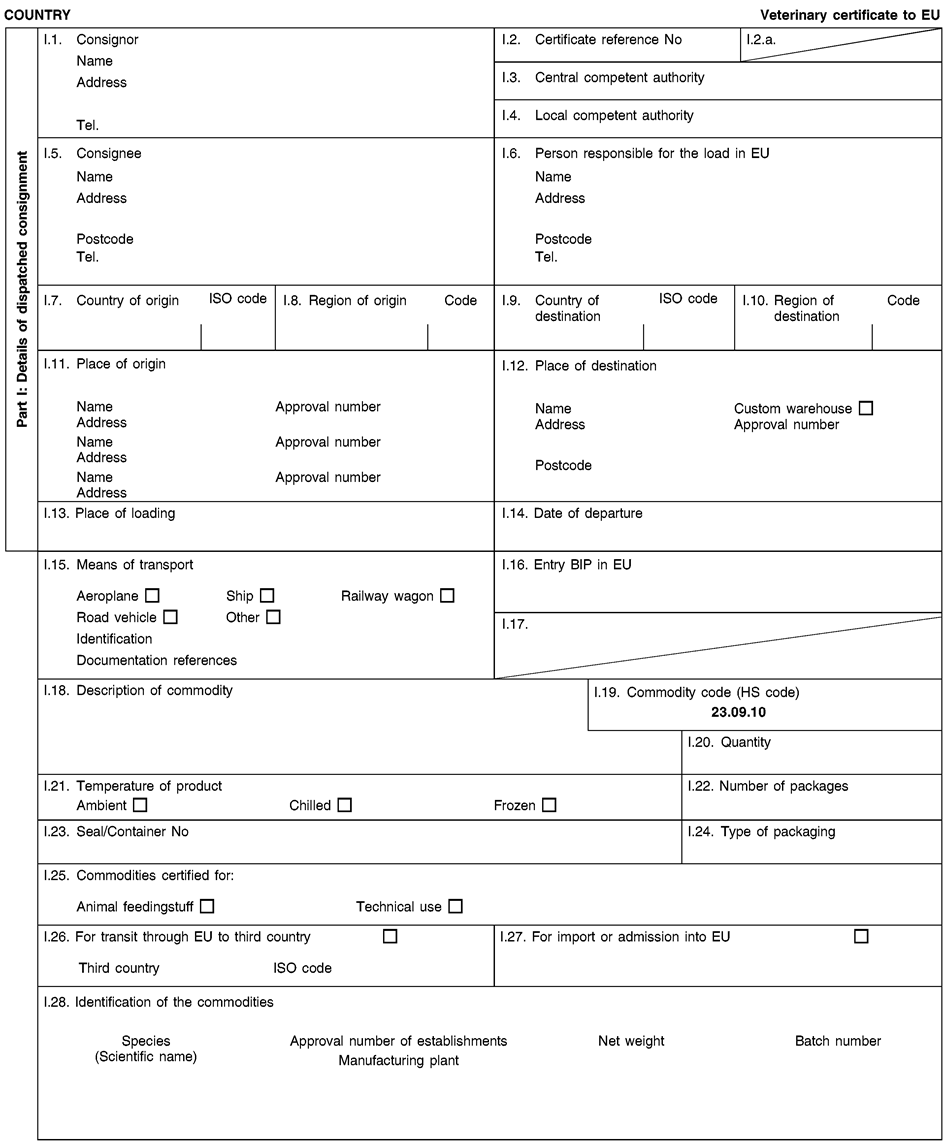
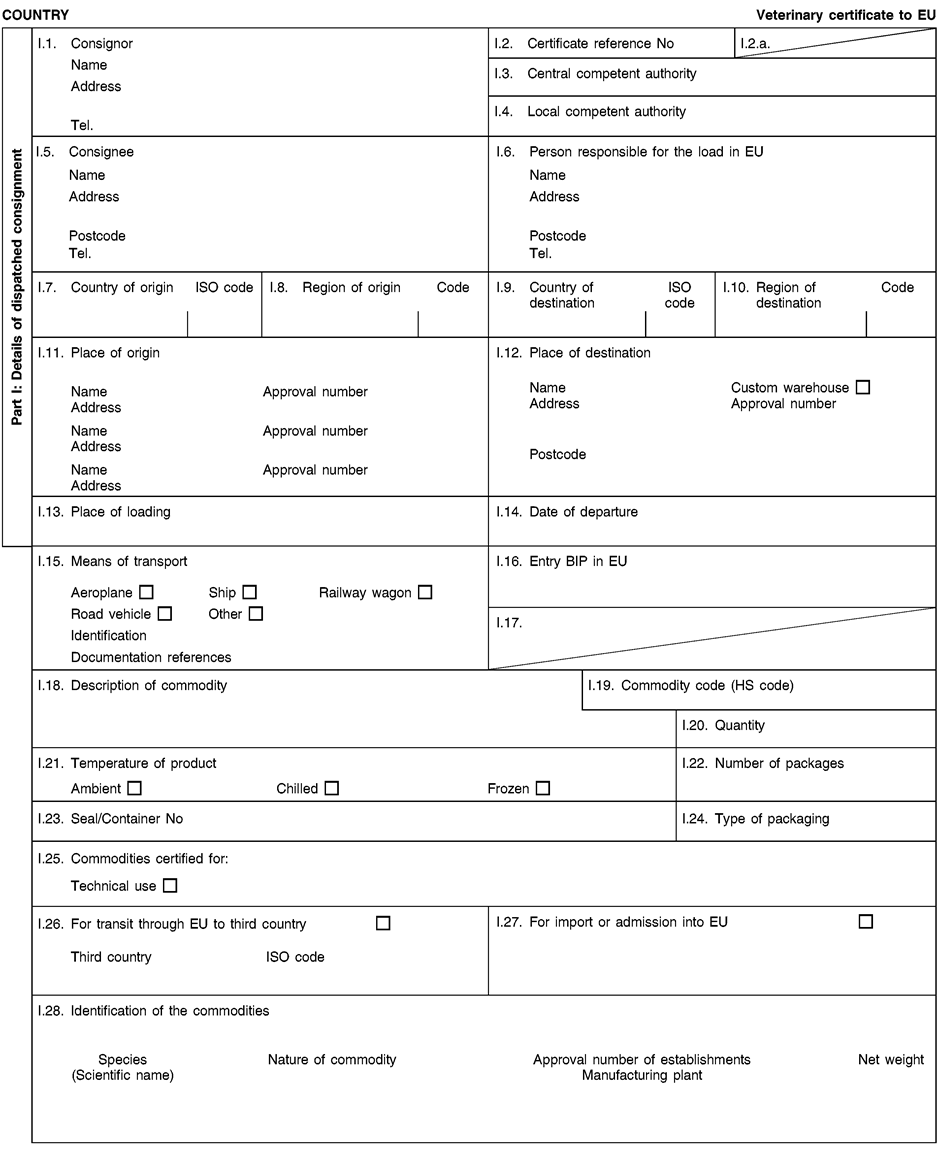
ANNEX XV MODEL HEALTH CERTIFICATES
The model health certificates in this Annex shall apply to the importation from third countries and to the transit through the European Union of the animal by-products and the derived products referred to in the respective model health certificates.
Notes
(a)Veterinary certificates shall be produced by the exporting third country, based on the models set out in this Annex, according to the layout of the model that corresponds to the animal by-products or derived products concerned. They shall contain, in the numbered order that appears in the model, the attestations that are required for any third country and, as the case may be, those supplementary guarantees that are required for the exporting third country or part thereof.
(b)Where the model certificate states that certain statements shall be kept as appropriate, statements which are not relevant may be crossed out and initialled and stamped by the certifying officer, or completely deleted from the certificate.
(c)The original of each certificate shall consist of a single sheet of paper, both sides, or, where more text is required; it shall be in such a form that all sheets of paper needed are part of an integrated whole and indivisible.
(d)It shall be drawn up in at least one of the official languages of the EU Member State in which the inspection at the border post shall be carried out and of the EU Member State of destination. However, these Member States may allow other languages, accompanied, if necessary, by an official translation.
(e)If for reasons of identification of the items of the consignment, additional sheets of paper are attached to the certificate, these sheets of paper shall also be considered as forming part of the original of the certificate by the application of the signature and stamp of the certifying official veterinarian, in each of the sheets of paper.
(f)When the certificate, including additional schedules referred to in e), comprises more than one page, each page shall be numbered – (page number) of (total number of pages) – at the bottom of the page and shall bear the code number of the certificate that has been designated by the competent authority at the top of the page.
(g)The original of the certificate must be completed and signed by an official veterinarian. In doing so, the competent authorities of the exporting country shall ensure that the principles of certification equivalent to those laid down in Directive 96/93/EC are followed.
(h)The colour of the signature shall be different to that of the printing. The same rule applies to stamps other than those embossed or watermark.
(i)The original of the certificate must accompany the consignment at the EU border inspection post.
(j)If health certificates are used for consignments in transit, box No I.5 (‘Consignee’) of the relevant health certificate shall be completed with the name and address of the border inspection post through which the consignment is intended to leave the European Union.
CHAPTER 1 Health certificate
For processed animal protein not intended for human consumption, including mixtures and products other than petfood containing such protein, for dispatch to or for transit through (2) the European Union
CHAPTER 2(A) Health certificate
For milk, milk-based products and milk-derived products not intended for human consumption for dispatch to or transit through (2) the European Union
CHAPTER 2(B) Health certificate
For colostrum and colostrum products from bovine animals not intended for human consumption for dispatch to or transit through (2) the European Union
CHAPTER 3(A) Health certificate
For canned petfood intended for dispatch to or for transit through (2) the European Union
CHAPTER 3(B) Health certificate
For processed petfood other than canned petfood, intended for dispatch to or for transit through (2) the European Union
CHAPTER 3(C) Health certificate
For dogchews intended for dispatch to or for transit through (2) the European Union
CHAPTER 3(D) Health certificate
For raw petfood for direct sale or animal by-products to be fed to fur animals, intended for dispatch to or for transit through (2) the European Union
CHAPTER 3(E) Health certificate
For flavouring innards for use in the manufacture of petfood, intended for dispatch to or for transit through (2) the European Union
CHAPTER 3(F) Health certificate
For animal by-products (3) for the manufacture of petfood, intended for dispatch to or for transit through (2) the European Union
CHAPTER 4(A) Health certificate
For the import of blood and blood products from equidae to be used outside the feed chain, for dispatch to or for transit through (2) the European Union
CHAPTER 4(B) Health certificate
For blood products not intended for human consumption that could be used as feed material, intended for dispatch to or for transit through (2) the European Union
CHAPTER 4(C) Health certificate
For untreated blood products, excluding of equidae, for the manufacture of derived products for purposes outside the feed chain for farmed animals, intended for dispatch to or for transit through (2) the European Union
CHAPTER 4(D) Health certificate
For treated blood products, excluding of equidae, for the manufacture of derived products for purposes outside the feed chain for farmed animals, intended for dispatch to or for transit through (2) the European Union
CHAPTER 5(A) Health certificate
For fresh or chilled hides and skins of ungulates, intended for dispatch to or for transit through (2) the European Union
CHAPTER 5(B) Health certificate
For treated hides and skins of ungulates, intended for dispatch to or for transit through (2) the European Union
CHAPTER 5(C) Official declaration
For treated hides and skins of ruminants and of equidae that are intended for dispatch to or for transit through (1) the European Union and have been kept separate for 21 days or will undergo transport for 21 uninterrupted days before importation
CHAPTER 6(A) Health certificate
For treated game trophies and other preparations of birds and ungulates, being solely bones, horns, hooves, claws, antlers, teeth, hides or skins, for dispatch to or for transit through (2) the European Union
CHAPTER 6(B) Health certificate
For game trophies or other preparations of birds and ungulates consisting of entire parts not having been treated, intended for dispatch to or for transit through (2) the European Union
CHAPTER 7(A) Health certificate
For pig bristles from third countries or regions thereof that are free from African swine fever, intended for dispatch to or for transit through (2) the European Union
CHAPTER 7(B) Health certificate
For pig bristles from third countries or regions thereof that are not free from African swine fever, intended for dispatch to or for transit through (2) the European Union
CHAPTER 8 Health certificate
For animal by-products to be used for purposes outside the feed chain or for trade samples (2), intended for dispatch to or for transit through (2) the European Union
CHAPTER 9 Health certificate
For fish oil not intended for human consumption to be used as feed material or for purposes outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 10(A) Health certificate
For rendered fats not intended for human consumption to be used as feed material, intended for dispatch to or for transit through (2) the European Union
CHAPTER 10(B) Health certificate
For rendered fats not intended for human consumption to be used for certain purposes outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 11 Health certificate
For gelatine and collagen not intended for human consumption to be used as feed material or for purposes outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 12 Health certificate
For hydrolysed protein, dicalcium phosphate and tricalcium phosphate not intended for human consumption to be used as feed material or for uses outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 13 Health certificate
For apiculture by-products intended exclusively for use in apiculture, intended for dispatch to or for transit through (2) the European Union
CHAPTER 14(A) Health certificate
For fat derivatives not intended for human consumption to be used outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 14(B) Health certificate
For fat derivatives not intended for human consumption to be used as feed or outside the feed chain, intended for dispatch to or for transit through (2) the European Union
CHAPTER 15 Health certificate
For egg products not intended for human consumption that could be used as feed material, intended for dispatch to or for transit through (2) the European Union
CHAPTER 16 Model declaration
CHAPTER 17 Health certificate
For processed manure, derived products from processed manure and guano from bats intended for dispatch to or for transit through (2) the European Union
CHAPTER 18 Health certificate
For horns and horn products, excluding horn meal, and hooves and hoof products, excluding hoof meal, intended for the production of organic fertilisers or soil improvers intended for dispatch to or for transit through (2) the European Union
CHAPTER 19 Health certificate
For gelatine not intended for human consumption to be used by the photographic industry, intended for dispatch to the European Union
CHAPTER 20 Model declaration
Declaration for the import from third countries and for the transit through the European Union of intermediate products to be used for the manufacture of medicinal products, veterinary medicinal products, medical devices, in vitro diagnostics and laboratory reagents
Options/Help
Print Options
PrintThe Whole Regulation
PrintThis Annex only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources