Commission Implementing Regulation (EU) 2019/1793
of 22 October 2019
on the temporary increase of official controls and emergency measures governing the entry into the Union of certain goods from certain third countries implementing Regulations (EU) 2017/625 and (EC) No 178/2002 of the European Parliament and of the Council and repealing Commission Regulations (EC) No 669/2009, (EU) No 884/2014, (EU) 2015/175, (EU) 2017/186 and (EU) 2018/1660
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation)(), and in particular Article 34(6)(a), Article 47(2)(b), Article 54(4)(a) and (b) and Article 90(c) thereof,
Having regard to Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety(), and in particular Article 53(1)(b) thereof,
Whereas:
(1) Regulation (EU) 2017/625 integrates into a single legislative framework the rules applicable to official controls on animals and goods entering the Union in order to verify compliance with Union agri-food chain legislation. For that purpose, it repeals and replaces Regulation (EC) No 882/2004 of the European Parliament and of the Council() and other Union acts governing official controls in specific areas.
(2) Pursuant to Regulation (EU) 2017/625, certain categories of animals and goods from certain third countries are always to be presented at border control posts for official controls to be performed prior to their entry into the Union. In addition, Article 47(1)(d) and (e) of Regulation (EU) 2017/625 stipulate that goods subject to measures requiring a temporary increase of official controls or emergency measures respectively, should be subject to official controls at border control posts at their entry into the Union.
(3) In that regard, pursuant to Regulation (EU) 2017/625, certain goods from certain third countries should be subject to a temporary increase of official controls at border control posts in those cases where the Commission has decided, by means of implementing acts, that these controls are necessary due to a known or emerging risk or because there is evidence of widespread serious non-compliance with the Union agri-food chain legislation. To that effect, the Commission should establish the list of such goods, indicating their codes from the Combined Nomenclature (CN) as laid down in Annex I to Commission Regulation (EEC) No 2658/87() (hereinafter, ‘the list’) and update the list as necessary to reflect any developments in that regard.
(4) The list referred to in recital (3) should at this stage consist of an updated list of food and feed of non-animal origin set out in Commission Regulation (EC) No 669/2009(), which lays down rules concerning the increased level of official controls to be carried out at designated points of entry into the Union on imports of certain food and feed of non-animal origin from certain third countries. It is therefore appropriate to set out in Annex I to this Regulation the list of food and feed of non-animal origin from certain third countries to be subject to a temporary increase of official controls at the entry into the Union, in accordance with Article 47(2)(b) of Regulation (EU) 2017/625.
(5) Moreover, the Commission should establish rules on the frequency of identity checks and physical checks for the food and feed of non-animal origin from certain third countries subject to a temporary increase of controls, in accordance with Article 54(4)(a) of Regulation (EU) 2017/625, taking into account in particular the level of risk associated with the hazard under consideration and the frequency of border rejections.
(6) Regulation (EU) 2017/625 and delegated and implementing acts adopted pursuant to Articles 47 to 64 of that Regulation, provide for one single system of official controls which applies to the areas covered by Commission Implementing Regulations (EU) No 884/2014(), (EU) 2015/175(), (EU) 2017/186(), (EU) 2018/1660() and by Regulation (EC) No 669/2009. For this reason, and since the rules covered in these regulations are substantively linked, as they all concern the imposition of additional measures governing the entry into the Union of certain food and feed from certain third countries due to an identified risk and which apply depending on the gravity of the risk, it is appropriate to facilitate the correct and comprehensive application of the relevant rules by establishing in a single act the provisions concerning the temporary increase of official controls on certain food and feed of non-animal origin and the emergency measures currently set out in these Regulations.
(7) The food and feed of non-animal origin subject to the emergency measures set out in Commission Implementing Regulations (EU) No 884/2014, (EU) 2015/175, (EU) 2017/186 and (EU) 2018/1660 still pose a serious risk to public health which cannot be contained satisfactorily by means of measures taken by the Member States. It is therefore appropriate to set out in Annex II to this Regulation a list of food and feed of non-animal origin subject to emergency measures which consists of the updated lists of food and feed of non-animal origin laid down in Commission Implementing Regulations (EU) No 884/2014, (EU) 2015/175, (EU) 2017/186 and (EU) 2018/1660. In addition, the scope of the entries in the aforementioned lists should be amended to include forms of the products other than the ones currently laid down therein, where those other forms present the same risk. It is therefore appropriate to amend all the entries concerning groundnuts to include oilcake and other solid residues, whether or not ground or in the form of pellets, resulting from the extraction of groundnut oil, as well as the entry concerning peppers from India to include roasted peppers (sweet or other than sweet).
(8) With a view to ensure a proper control of the risks to public health, compound food containing any of the food of non-animal origin listed in Annex II to this Regulation due to the risk of contamination by aflatoxins, in a quantity above 20 % of either a single product or as the sum of products listed, and falling within the CN Codes laid down in Annex II should also be included in the list referred to in recital 7.
(9) Moreover, the Commission should establish rules on the frequency of identity checks and physical checks for the food and feed subject to emergency measures pursuant to this Regulation, in accordance with Article 54(4)(b) of Regulation (EU) 2017/625. It is therefore appropriate to establish such rules in this Regulation, taking into account in particular the level of risk associated with the hazard under consideration and the frequency of border rejections.
(10) Measures requiring a temporary increase of official controls and emergency measures set out in this Regulation should apply to food and feed intended for placing on the Union market since those goods represent a risk from a public health perspective.
(11) As regards consignments sent as trade samples, laboratory samples or as display items for exhibitions, which are not intended to be placed on the market, consignments of a non-commercial nature intended for private use or consumption within the custom territory of the Union and consignment intended for scientific purposes, having regard to the low risk that such consignments pose to public health, it would be disproportionate to impose a requirement that these consignments be subject to official controls at border control posts and be accompanied by an official certificate or by the results of the sampling and laboratory analyses in accordance with this Regulation. However, in order to avoid misuse, this Regulation should apply to these consignments in the case where their gross weight exceeds a certain weight limit.
(12) Measures requiring a temporary increase of official controls and emergency measures set out in this Regulation should not apply to food and feed on board means of transport operating internationally which are not unloaded and are intended for consumption by the crew and passengers since the placing on the Union market is very limited.
(13) The maximum levels of mycotoxins, including of aflatoxins in food are established by Commission Regulation (EC) No 1881/2006() and in feed by Directive 2002/32/EC of the European Parliament and of the Council(). The maximum levels for pesticide residues are established by Regulation (EC) No 396/2005 of the European Parliament and of the Council(). The European Union Reference Laboratory for Dioxins and polychlorinated biphenyls (PCBs) in food and feed has carried out a study on the correlation between pentachlorophenol (PCP) and dioxins in contaminated guar gum from India. From this study it can be concluded that guar gum containing a level of PCP below the Maximum Residue Limit (MRL) of 0,01 mg/kg does not contain unacceptable levels of dioxins. Therefore compliance with the MRL on PCP, ensures in this specific case also a high level of human health protection as regards dioxins.
(14) In relation to the rules referred to in recital (13), the provisions on sampling and analyses for the control of mycotoxins, including aflatoxins, in food are established by Commission Regulation (EC) No 401/2006() and in feed by Commission Regulation (EC) No 152/2009(). The provisions on sampling for the official control of pesticide residues are established by Commission Directive 2002/63/EC(). With a view to ensure uniform methods of sampling and laboratory analyses in third countries and Member States, the sampling and the analyses for food and feed required by this Regulation should be carried out in accordance with the aforementioned Union rules on sampling and analyses both in Member States and third countries.
(15) Moreover, in order to ensure uniform sampling procedures and analytical reference methods for the control of Salmonella in food subject to this Regulation in third countries and Member States, this Regulation should lay down such sampling procedures and analytical reference methods.
(16) Model official certificates for the entry into the Union of certain food and feed are laid down in Commission Implementing Regulations (EU) No 884/2014, (EU) 2015/175, (EU) 2017/186 and (EU) 2018/1660. In order to facilitate the performance of official controls at the entry into the Union it is appropriate to establish a single model official certificate for the entry into the Union of food and feed subject to special conditions for the entry into the Union pursuant to this Regulation.
(17) Such official certificates should be issued either on paper or in electronic form. Therefore, it is appropriate to establish common requirements as regards issuance of official certificates in both cases, in addition to the requirements laid down in Chapter VII of Title II of Regulation (EU) 2017/625. In this regard, Article 90(f) of Regulation (EU) 2017/625 makes provisions for the establishment by the Commission of rules for the issuance of electronic certificates and for the use of electronic signatures including in relation to official certificates issued in accordance with this Regulation. In addition, provisions should be made in this Regulation to ensure that the requirements for official certificates not submitted in IMSOC laid down in Commission Implementing Regulation (EU) 2019/628() also apply to official certificates issued in accordance with this Regulation.
(18) Model certificates are included in the electronic system TRACES, set up by Commission Decision 2003/623/EC(), to facilitate and accelerate administrative procedures at Union borders and to enable electronic communication between the competent authorities which helps preventing possible fraudulent or deceptive practices in respect of the official certificates. Since 2003, computer technology has evolved considerably and the TRACES system has been modified to improve the quality, processing and secure exchange of data. In accordance with Article 133(4) of Regulation (EU) 2017/625, the TRACES system is to be integrated into the Information Management System for Official Controls referred to in Article 131 of Regulation (EU) 2017/625 (IMSOC). The model official certificate laid down in this Regulation should therefore be adapted to IMSOC.
(19) Point (c) of Article 90 of Regulation (EU) 2017/625 empowers the Commission to lay down, by means of implementing acts, rules concerning the procedures to be followed for the issuance of replacement certificates. To avoid misuse and abuse, it is important to define the cases where a replacement official certificate may be issued and the requirements that need to be met by such certificate. Such cases have been laid down in Implementing Regulation (EU) 2019/628 in relation to official certificates issued in accordance with that Regulation. With a view to ensure a coherent approach, it is appropriate to provide that, in the case of issuing replacement certificates, official certificates issued in accordance with this Regulation should be replaced in accordance with the procedures for the replacement certificates laid down in Implementing Regulation (EU) 2019/628.
(20) Provisions should be established to regularly review whether modifications of the lists out in Annexes I and II to this Regulation, including of the frequency of identity and physical checks, are necessary. This should take into account new information related to risks and non-compliance, such as the data resulting from notifications received through the Rapid Alert System for Food and Feed (RASFF), data and information concerning consignments and the results of the documentary, identity and physical checks communicated by the Member States to the Commission, reports and information received from third countries, information resulting from the controls carried out by the Commission in third countries and information exchanged between the Commission and Member States and between the Commission and the European Food Safety Authority.
(21) The rules to be established by the Commission in accordance with Articles 34(6)(a), 47(2)(b), and 54(4)(a) of Regulation (EU) 2017/625 are substantively linked since they all concern requirements on official controls at the entry into the Union on certain goods from certain third countries subject to a temporary increase of official controls at their entry into the Union and should therefore apply from the same date. To facilitate the correct and comprehensive application of those rules, it is appropriate to establish them in a single act.
(22) The rules to be established by the Commission in accordance with Articles 54(4)(b) and 90 (c) of Regulation (EU) 2017/625 and with Article 53(1)(b) of Regulation (EC) No 178/2002 are substantively linked since they all concern requirements for the entry into the Union of goods subject to emergency measures pursuant to Article 53(1)(b) of Regulation (EC) No 178/2002 and should therefore apply from the same date. To facilitate the correct and comprehensive application of those rules, it is appropriate to establish them in a single act.
(23) For the purposes of simplification and rationalization, the rules laid down in Commission Regulations (EC) No 669/2009, (EU) No 884/2014, (EU) 2017/186, (EU) 2015/175 and (EU) 2018/1660 are consolidated into this Regulation. These Regulations should therefore be repealed and replaced with this Regulation.
(24) The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed,
HAS ADOPTED THIS REGULATION:
SECTION 1 COMMON PROVISIONS
Article 1Subject matter and scope
1.This Regulation lays down:
(a)the list of food and feed of non-animal origin from certain third countries subject to a temporary increase of official controls at their entry into the Union, established in Annex I, falling within the CN Codes and TARIC classifications laid down in that Annex, in accordance with Article 47(2)(b) of Regulation (EU) 2017/625;
(b)special conditions governing the entry into the Union of the following categories of consignments of food and feed due to the risk of contamination by mycotoxins, including aflatoxins, pesticide residues, pentachlorophenol and dioxins and microbiological contamination, in accordance with Article 53(1)(b) of Regulation (EC) No 178/2002:
(i)
consignments of food and feed of non-animal origin from third countries or parts of those third countries listed in Table 1 to Annex II and falling within the CN Codes and TARIC classifications laid down in that Annex;
(ii)
consignments of compound food containing any of the food listed in Table 1 to Annex II due to the risk of contamination by aflatoxins in a quantity above 20 % of either a single product or as the sum of those products and falling within the CN Codes laid down in Table 2 to that Annex;
(c)rules on the frequency of identity checks and physical checks for the consignments of food and feed referred to in points (a) and (b) of this paragraph;
(d)rules on the methods to be used for sampling and for laboratory analyses for the consignments of food and feed referred to in points (a) and (b) of this paragraph, in accordance with Article 34(6)(a) of Regulation (EU) 2017/625;
(e)rules concerning the model official certificate required to accompany consignments of food and feed referred to in point (b) of this paragraph and the requirements for such official certificate, in accordance with Article 53(1)(b) of Regulation (EC) No 178/2002;
(f)rules for the issuance of replacement official certificates required to accompany consignments of food and feed referred to in point (b) of this paragraph, in accordance with Article 90(c) of Regulation (EU) 2017/625.
2.This Regulation applies to consignments of food and feed referred to in points (a) and (b) of paragraph 1 intended for placing on the Union market.
3.This Regulation does not apply to the following categories of consignments of food and feed unless their gross weight exceeds 30 kg:
(a)consignments of food and feed sent as trade samples, laboratory samples or as display items for exhibitions, which are not intended to be placed on the market;
(b)consignments of food and feed which form part of passengers personal luggage and are intended for personal consumption or use;
(c)non-commercial consignments of food and feed sent to natural persons which are not intended to be placed on the market;
(d)consignments of food and feed intended for scientific purposes.
4.This Regulation does not apply to food and feed referred to in points (a) and (b) of paragraph 1 on board means of transport operating internationally which are not unloaded and are intended for consumption by the crew and passengers.
5.In case of doubt on the intended use of the food and feed referred in points (b) and (c) of paragraph (3), the burden of proof lies with the owner of the personal luggage and with the recipient of the consignment, respectively.
Article 2Definitions
1.For the purposes of this Regulation, the following definitions shall apply:
(a)‘consignment’ means ‘consignment’ as defined in Article 3(37) of Regulation (EU) 2017/625;
(b)‘placing on the market’ means ‘placing on the market’ as defined in point (8) of Article 3 of Regulation (EC) No 178/2002.
2.However, for the purposes of Articles 7, 8, 9, 10 and 11 and of Annex IV, a ‘consignment’ means:
(a)a ‘lot’ as referred to in Annex I to Regulation (EC) No 401/2006 and in Annex I to Regulation (EC) No 152/2009, in relation to food and feed listed in Annex II due to contamination risk by mycotoxins, including aflatoxins;
(b)a ‘lot’ as referred to in the Annex to Directive 2002/63/EC, in relation to food and feed listed in Annex II due to contamination risk by pesticides and pentachlorophenol.
Article 3Sampling and analyses
The sampling and the analyses to be carried out by competent authorities at border control posts or at control points referred to in Article 53(1)(a) of Regulation (EU) 2017/625, as part of physical checks on consignments of food and feed referred to in Article 1(1)(a) and (b), or in third countries for the purposes of the results of analyses which are required to accompany the consignments of food and feed referred to in Article 1(1)(b) as provided for in this Regulation shall be performed in accordance with the following requirements:
(a)
for food listed in Annexes I and II due to possible contamination risk by mycotoxins, including aflatoxins, the sampling and the analyses shall be performed in accordance with Regulation (EC) No 401/2006;
(b)
for feed listed in Annexes I and II due to possible contamination risk by mycotoxins, including aflatoxins, the sampling and the analyses shall be performed in accordance with Regulation (EC) No 152/2009;
(c)
for food and feed listed in Annexes I and II due to possible non-compliance with the maximum allowed levels of pesticides residues the sampling shall be performed in accordance with Directive 2002/63/EC;
(d)
for guar gum listed in Annex II due to possible contamination with pentachlorophenol and dioxins the sampling for the analysis of pentachlorophenol shall be performed in accordance with Directive 2002/63/EC and the sampling and analyses for the control of dioxins in feed shall be performed in accordance with Regulation (EC) No 152/2009;
(e)
for food listed in Annexes I and II due to the risk of presence of Salmonella, the sampling and the analyses for the control of Salmonella shall be performed in accordance with the sampling procedures and the analytical reference methods laid down in Annex III;
(f)
the methods of sampling and analyses referred to in the footnotes to Annexes I and II shall be applied in relation to hazards other than those referred to in points (a), (b), (c), (d) and (e).
Article 4Release for free circulation
The custom authorities shall only allow the release for free circulation of consignments of food and feed listed in Annexes I and II upon presentation of a duly finalised Common Health Entry Document (CHED) as provided for in Article 57(2)(b) of Regulation (EU) 2017/625, which confirms that the consignment is in compliance with the applicable rules referred to in Article 1(2) of that Regulation.
SECTION 2 TEMPORARY INCREASE OF OFFICIAL CONTROLS AT BORDER CONTROL POSTS AND CONTROL POINTS ON CERTAIN FOOD AND FEED FROM CERTAIN THIRD COUNTRIES
Article 5List of food and feed of non-animal origin
1.Consignments of food and feed listed in Annex I shall be subject to a temporary increase of official controls at border control posts at their entry into the Union and at control points.
2.The identification of the food and feed referred to in paragraph 1 for official controls shall be made on the basis of the codes from the Combined Nomenclature and the TARIC sub-division indicated in Annex I.
Article 6Frequency of identity checks and physical checks
1.The competent authorities at border control posts and at control points referred to in Article 53(1)(a) of Regulation (EU) 2017/625 shall carry out identity and physical checks, including sampling and laboratory analyses, on consignments of food and feed listed in Annex I at the frequency set out in that Annex.
2.The frequency of identity and physical checks set out in an entry in Annex I shall be applied as an overall frequency for all products falling under that entry.
SECTION 3 SPECIAL CONDITIONS GOVERNING THE ENTRY INTO THE UNION OF CERTAIN FOOD AND FEED FROM CERTAIN THIRD COUNTRIES
Article 7Entry into the Union
1.Consignments of food and feed listed in Annex II shall only enter into the Union in accordance with the conditions laid down in this section.
2.The identification of the food and feed referred to in paragraph 1 for official controls shall be made on the basis of the codes from the Combined Nomenclature and the TARIC sub-division indicated in Annex II.
3.Consignments referred to in paragraph 1 shall be subject to official controls at border control posts at their entry into the Union and at control points.
Article 8Frequency of identity checks and physical checks
1.The competent authorities at border control post and at control points referred to in Article 53(1)(a) of Regulation (EU) 2017/625 shall carry out identity and physical checks, including sampling and laboratory analyses, on consignments of food and feed listed in Annex II, at the frequency set out in that Annex.
2.The frequency of identity and physical checks set out in an entry in Annex II shall be applied as an overall frequency for all products falling under that entry.
3.Compound food listed in Table 2 to Annex II which contains products falling only under one entry in Table 1 to Annex II shall be subject to the overall frequency of identity and physical checks set out in Table 1 to Annex II for that entry.
4.Compound food listed in Table 2 to Annex II which contains products falling under several entries for the same hazard in Table 1 to Annex II shall be subject to the highest overall frequency of identity and physical checks set out in Table 1 to Annex II for these entries.
Article 9Identification code
1.Each consignment of food and feed listed in Annex II shall be identified with an identification code.
2.Each individual bag or packaging form of the consignment shall be identified with that identification code.
3.By way of derogation from paragraph 2, in case of consignments of food and feed listed in Annex II due to the risk of contamination by mycotoxins and where the packaging is combining several small packages, it is not necessary for the identification code of the consignment to be mentioned individually on all the separate small packages as long as it is mentioned at least on the package combining these small packages.
Article 10Results of sampling and analyses performed by the competent authorities of the third country
1.Each consignment of food and feed listed in Annex II shall be accompanied by the results of sampling and analyses performed on that consignment by the competent authorities of the third country of origin or of the country where the consignment is consigned from if that country is different from the country of origin.
2.On the basis of the results referred to in paragraph 1, the competent authorities shall ascertain:
(a)compliance with Regulation (EC) No 1881/2006 and Directive 2002/32/EC on maximum levels of relevant mycotoxins, for consignments of food and feed listed in Annex II due to contamination risk by mycotoxins;
(b)compliance with Regulation (EC) No 396/2005 on maximum residue levels of pesticides, for consignments of food and feed listed in Annex II due to contamination risk by pesticide residues;
(c)that the product does not contain more than 0,01 mg/kg pentachlorophenol (PCP), for consignments of food and feed listed in Annex II due to contamination risk by pentachlorophenol and dioxins;
(d)the absence of Salmonella in 25 g, for consignments of food listed in Annex II due to risk of microbiological contamination by Salmonella.
3.Each consignment of food and feed listed in Annex II due to contamination risk by pentachlorophenol and dioxins shall be accompanied by an analytical report which shall comply with the requirements set out in Annex II.
The analytical report shall include the results of the analyses referred to in paragraph 1.
4.The results of sampling and analyses referred to in paragraph 1 shall bear the identification code of the consignment to which they relate referred to in Article 9(1).
5.The analyses referred to in paragraph 1 shall be performed by laboratories accredited in accordance with the standard ISO/IEC 17025 on ‘General requirements for the competence of testing and calibration laboratories’.
Article 11Official certificate
1.Each consignment of food and feed listed in Annex II shall be accompanied by an official certificate in accordance with the model set out in Annex IV (‘official certificate’).
2.The official certificate shall comply with the following requirements:
(a)it shall be issued by the competent authority of the third country of origin or of the third country where the consignment is consigned from if that country is different from the country of origin;
(b)it shall bare the identification code of the consignment to which it relates referred to in Article 9(1);
(c)it shall be issued before the consignment to which it relates leaves the control of the competent authority of the third country issuing the certificate;
(d)it shall be valid for not more than four months from the date of issue, but in any case no longer than six months from the date of the results of the laboratory analyses referred to in paragraph 1 of Article 10.
3.An official certificate which is not submitted in the Information Management System for Official Controls referred to in Article 131 of Regulation (EU) 2017/625 (IMSOC) by the competent authority of the third country issuing the certificate shall also meet the requirements for model official certificates not submitted in IMSOC laid down in Article 3 of Implementing Regulation (EU) 2019/628.
4.Competent authorities may issue a replacement official certificate only in accordance with the rules laid down in Article 5 of Implementing Regulation (EU) 2019/628.
5.The official certificate referred to in paragraph 1 shall be completed on the basis of the notes set out in Annex IV.
SECTION 4 FINAL PROVISIONS
Article 12Updates to Annexes
The Commission shall review the lists set out in Annexes I and II on a regular basis not exceeding a period of six months, in order to take into account new information related to risks and non-compliance.
Article 13Repeal
1.Regulations (EC) No 669/2009, (EU) No 884/2014, (EU) 2017/186, (EU) 2015/175 and (EU) 2018/1660 are repealed with effect from 14 December 2019.
2.References to Regulations (EC) No 669/2009, (EU) No 884/2014, (EU) 2017/186, (EU) 2015/175 and (EU) 2018/1660 shall be construed as references to this Regulation.
3.References to ‘the designated point of entry within the meaning of point (b) of Article 3 of Regulation (EC) No 669/2009’ or to ‘the designated point of entry’ in acts other than those referred to in paragraph 1 shall be construed as references to a ‘border control post’ within the meaning of Article 3(38) of Regulation (EU) 2017/625.
4.References to ‘the common entry document (CED) referred to in point (a) of Article 3 of Regulation (EC) No 669/2009’, to ‘the common entry document (CED) referred to in Annex II to Regulation (EC) No 669/2009’ or to ‘the common entry document (CED)’ in acts other than those referred to in paragraph 1 shall be construed as references to the ‘Common Health Entry Document (CHED)’ referred to in Article 56 of Regulation (EU) 2017/625.
5.References to the definition laid down in Article 3(c) of Regulation (EC) No 669/2009 in acts other than those referred to in paragraph 1 shall be construed as references to the definition of ‘consignment’ laid down in Article 3(37) of Regulation (EU) 2017/625.
Article 14Transitional period
1.The reporting obligations set out in Article 15 of Regulation (EC) No 669/2009, Article 13 of Regulation (EU) No 884/2014, Article 12 of Regulation (EU) 2018/1660, Article 12 of Regulation (EU) 2015/175 and Article 12 of Regulation (EU) 2017/186 shall continue to apply until 31 January 2020.
Such reporting obligations shall cover the period until 31 December 2019.
2.The reporting obligations referred to in paragraph 1 shall be deemed to be satisfied where Member States have registered in TRACES the common entry documents issued by their respective competent authorities in accordance with Regulation (EC) No 669/2009, Regulation (EU) No 884/2014, Regulation (EU) 2015/175, Regulation (EU) 2017/186 and Regulation (EU) 2018/1660 during the reporting period set out in the provisions referred to in paragraph 1.
3.Consignments of food and feed listed in Annex II accompanied by the relevant certificates issued before 14 February 2020 in accordance with the provisions of Regulation (EU) No 884/2014, Regulation (EU) 2018/1660, Regulation (EU) 2015/175 and Regulation (EU) 2017/186 respectively in force on 13 December 2019 shall be authorised for the entry into the Union until 13 June 2020.
Article 15Entry into force and date of application
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 14 December 2019.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 22 October 2019.
For the Commission
The President
Jean-Claude Juncker
ANNEX IFood and feed of non-animal origin from certain third countries subject to a temporary increase of official controls at border control posts and control points
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Food and feed(intended use) | CN code | TARIC sub-division | Country of origin | Hazard | Frequency of physical and identity checks (%) |
|---|
| | | | Bolivia (BO) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | 10 | Brazil (BR) | Salmonella | 20 |
| | ex 0813 40 95;
ex 0810 90 75
| 10
10
| China (CN) | Pesticide residues | 20 |
| | | 11 | China (CN) | Salmonella | 20 |
| | | | China (CN) | Pesticide residues | 20 |
| | | | Dominican Republic (DO) | Pesticide residues | 20 |
| | | | Dominican Republic (DO) | Pesticide residues | 50 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| | | |
Yardlong beans
(Vigna unguiculata spp. sesquipedalis, vigna unguiculata spp. unguiculata)
(Food — fresh, chilled or frozen)
| ex 0708 20 00;
ex 0710 22 00
| 10
10
| | | |
| | | | Egypt (EG) | Pesticide residues | 20 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| | | |
| | | | Ethiopia (ET) | Salmonella | 50 |
| | | | Georgia (GE) | Aflatoxins | 50 |
| | | | | | |
| | | 40 | | | |
| | ex 2008 19 19;
ex 2008 19 95;
ex 2008 19 99
| 30
20
30
| | | |
| | | | Ghana (GH) | Sudan dyes | 50 |
| ex 1511 90 19;
1511 90 99
| 90 | | | |
| | ex 0709 99 90;
ex 0710 80 95
| 20
30
| India (IN) | Pesticide residues | 10 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| India (IN) | Pesticide residues | 20 |
| | | | Kenya (KE) | Pesticide residues | 5 |
| | | 20 | Cambodia (KH) | Pesticide residues | 50 |
Yardlong beans
(Vigna unguiculata spp. sesquipedalis, vigna unguiculata spp. unguiculata)
(Food — fresh, chilled or frozen vegetables)
| ex 0708 20 00;
ex 0710 22 00
| 10
10
| Cambodia (KH) | Pesticide residues | 50 |
| | | 11; 19 | Lebanon (LB) | Rhodamine B | 50 |
| | | 93 | Lebanon (LB) | Rhodamine B | 50 |
Peppers (sweet or other than sweet) (Capsicum spp.)
(Food — dried, roasted, crushed or ground)
| | | Sri Lanka (LK) | Aflatoxins | 50 |
ex 0904 21 90;
ex 0904 22 00;
ex 2008 99 99
| 20
11; 19
79
| | | |
| | | | Madagascar (MG) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | 20 | Malaysia (MY) | Pesticide residues | 20 |
| | | | Nigeria (NG) | Salmonella | 50 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| Pakistan (PK) | Pesticide residues | 20 |
Raspberries
(Food — frozen)
| ex 0811 20 11;
ex 0811 20 19;
0811 20 31
| 10
10
| Serbia (RS) | Norovirus | 10 |
| | | | Sudan (SD) | Salmonella | 50 |
| | ex 1207 70 00;
ex 1208 90 00;
ex 2008 99 99
| 10
10
50
| Sierra Leone (SL) | Aflatoxins | 50 |
| | | | Senegal (SN) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | 11; 19 | Syria (SY) | Rhodamine B | 50 |
| | | 93 | Syria (SY) | Rhodamine B | 50 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| Thailand (TH) | Pesticide residues | 10 |
| | | | Turkey (TR) | Sulphites | 10 |
| | | | | | |
| | | | Turkey (TR) | Ochratoxin A | 5 |
Lemons (Citrus limon, Citrus limonum)
(Food – fresh, chilled or dried)
| | | Turkey (TR) | Pesticide residues | 10 |
| | | 30 | Turkey (TR) | Pesticide residues | 10 |
| | | | Turkey (TR) | Pesticide residues | 10 |
Unprocessed whole, ground, milled, cracked, chopped apricot kernels intended to be placed on the market for the final consumer
(Food)
| | 20 | Turkey (TR) | Cyanide | 50 |
| | ex 0709 60 99
ex 0710 80 59
| 20
20
| Uganda (UG) | Pesticide residues | 20 |
| | | | Uganda (UG) | Salmonella | 50 |
| | | | United States (US) | Aflatoxins | 10 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | United States (US) | Aflatoxins | 10 |
| | | | | | |
Pistachios, roasted
(Food)
| ex 2008 19 13;
ex 2008 19 93
| 20
20
| | | |
| | | | Uzbekistan (UZ) | Sulphites | 50 |
| | | | | | |
| | | 72 | Vietnam (VN) | Pesticide residues | 50 |
| | | 20 | | | |
| | | 30 | | | |
| | | 40 | | | |
| | ex 0709 99 90
ex 0710 80 95
| 20
30
| Vietnam (VN) | Pesticide residues | 50 |
| | ex 0709 60 99;
ex 0710 80 59
| 20
20
| Vietnam (VN) | Pesticide residues | 50 |
ANNEX IIFood and feed from certain third countries subject to special conditions for the entry into the Union due to contamination risk by mycotoxins, including aflatoxins, pesticide residues, pentachlorophenol and dioxins and microbiological contamination
| 1. Food and feed of non-animal origin referred to in Article 1(1)(b)(i) |
| Food and feed (intended use) | CN code | TARIC sub-division | Country of origin | Hazard | Frequency of physical and identity checks (%) |
|---|
| | | | Argentina (AR) | Aflatoxins | 5 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Azerbaijan (AZ) | Aflatoxins | 20 |
| | | | | | |
| | ex 0813 50 39;
ex 0813 50 91;
ex 0813 50 99
| 70
70
70
| | | |
| | ex 2007 10 10;
ex 2007 10 99;
ex 2007 99 39;
ex 2007 99 50;
ex 2007 99 97
| 70
40
05; 06
33
23
| | | |
| | ex 2008 19 12;
ex 2008 97 14;
ex 2008 97 16;
ex 2008 97 18;
ex 2008 97 32;
ex 2008 97 34;
ex 2008 97 36;
ex 2008 97 38;
ex 2008 97 51;
ex 2008 97 59;
ex 2008 97 72;
ex 2008 97 74;
ex 2008 97 76;
ex 2008 97 78;
ex 2008 97 92;
ex 2008 97 93;
ex 2008 97 94;
ex 2008 97 96;
ex 2008 97 97;
ex 2008 97 98;
ex 2008 19 12;
ex 2008 19 19;
ex 2008 19 92;
ex 2008 19 95;
ex 2008 19 99
| 15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
30
30
30
20
30
| | | |
| | | 40 | | | |
| | | | | | |
| | ex 2008 19 12;
ex 2008 19 19;
ex 2008 19 92;
ex 2008 19 95;
ex 2008 19 99
| 30
30
30
20
30
| | | |
| | | 20 | | | |
| | | | Brazil (BR) | Aflatoxins | 50 |
| | ex 0813 50 31;
ex 0813 50 39;
ex 0813 50 91;
ex 0813 50 99
| 20
20
20
20
| | | |
| | | | Brazil (BR) | Aflatoxins | 10 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | China (CN) | Aflatoxins | 20 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Egypt (EG) | Aflatoxins | 20 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Ethiopia (ET) | Aflatoxins | 50 |
Ginger, saffron, turmeric (curcuma), thyme, bay leaves, curry and other spices
(Food — dried spices)
| | | | | |
| | | | Ghana (GH) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Gambia (GM) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Indonesia (ID) | Aflatoxins | 20 |
| | | 10 | India (IN) | Salmonella | 10 |
Peppers (sweet or other than sweet) (Capsicum spp.)
(Food — dried, roasted, crushed or ground)
| | | India (IN) | Aflatoxins | 20 |
ex 0904 22 00;
ex 0904 21 90;
ex 2008 99 99
| 11; 19
20
79
| | | |
| | | | India (IN) | Aflatoxins | 20 |
Curry leaves (Bergera/Murraya koenigii)
(Food – fresh, chilled, frozen or dried)
| | 10 | India (IN) | Pesticide residues | 20 |
| | | | India (IN) | Aflatoxins | 10 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | 10 | India (IN) | Pentachlorophenol and dioxins | 5 |
| | | | India (IN) | Salmonella | 20 |
| | | | Iran (IR) | Aflatoxins | 50 |
| | | | | | |
| | ex 0813 50 39;
ex 0813 50 91;
ex 0813 50 99
| 60
60
60
| | | |
| | ex 2007 10 10;
ex 2007 10 99;
ex 2007 99 39;
ex 2007 99 50
ex 2007 99 97
| 60
30
03; 04
32
22
| | | |
| | ex 2008 19 13;
ex 2008 19 93;
ex 2008 97 12;
ex 2008 97 14;
ex 2008 97 16;
ex 2008 97 18;
ex 2008 97 32;
ex 2008 97 34;
ex 2008 97 36;
ex 2008 97 38;
ex 2008 97 51;
ex 2008 97 59;
ex 2008 97 72;
ex 2008 97 74;
ex 2008 97 76;
ex 2008 97 78;
ex 2008 97 92;
ex 2008 97 93;
ex 2008 97 94;
ex 2008 97 96;
ex 2008 97 97;
ex 2008 97 98;
| 20
20
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
| | | |
| | | 50 | | | |
| | ex 1207 70 00;
ex 1208 90 00;
ex 2008 99 99
| 10
10
50
| Nigeria (NG) | Aflatoxins | 50 |
| | | | Sudan (SD) | Aflatoxins | 50 |
| | | | | | |
| | | | | | |
| | 2008 11 91;
2008 11 96;
2008 11 98
| | | | |
| | | | | | |
| | | | Turkey (TR) | Aflatoxins | 20 |
| | | 50 | | | |
| | ex 2007 10 10;
ex 2007 10 99;
ex 2007 99 39;
ex 2007 99 50;
ex 2007 99 97
| 50
20
01; 02
31
21
| | | |
| | ex 2008 97 12;
ex 2008 97 14;
ex 2008 97 16;
ex 2008 97 18;
ex 2008 97 32;
ex 2008 97 34;
ex 2008 97 36;
ex 2008 97 38;
ex 2008 97 51;
ex 2008 97 59;
ex 2008 97 72;
ex 2008 97 74;
ex 2008 97 76;
ex 2008 97 78;
ex 2008 97 92;
ex 2008 97 93;
ex 2008 97 94;
ex 2008 97 96;
ex 2008 97 97;
ex 2008 97 98;
ex 2008 99 28
ex 2008 99 34;
ex 2008 99 37;
ex 2008 99 40;
ex 2008 99 49;
ex 2008 99 67;
ex 2008 99 99
| 11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
11
10
10
10
10
60
95
60
| | | |
| | | 60 | | | |
| | | | Turkey (TR) | Aflatoxins | 5 |
| | | | | | |
| | ex 0813 50 39;
ex 0813 50 91;
ex 0813 50 99
| 70
70
70
| | | |
| | ex 2007 10 10;
ex 2007 10 99;
ex 2007 99 39;
ex 2007 99 50
ex 2007 99 97
| 70
40
05; 06
33
23
| | | |
| | ex 2008 97 12;
ex 2008 97 14;
ex 2008 97 16;
ex 2008 97 18;
ex 2008 97 32;
ex 2008 97 34;
ex 2008 97 36;
ex 2008 97 38;
ex 2008 97 51;
ex 2008 97 59;
ex 2008 97 72;
ex 2008 97 74;
ex 2008 97 76;
ex 2008 97 78;
ex 2008 97 92;
ex 2008 97 93;
ex 2008 97 94;
ex 2008 97 96;
ex 2008 97 97;
ex 2008 97 98;
ex 2008 19 12;
ex 2008 19 19;
ex 2008 19 92;
ex 2008 19 95;
ex 2008 19 99
| 15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
15
30
30
30
20
30
| | | |
| | | 40 | | | |
| | | | | | |
| | ex 2008 19 12;
ex 2008 19 19;
ex 2008 19 92;
ex 2008 19 95;
ex 2008 19 99
| 30
30
30
20
30
| | | |
| | | 20 | | | |
| | | | Turkey (TR) | Aflatoxins | 50 |
| | | | | | |
| | ex 0813 50 39;
ex 0813 50 91;
ex 0813 50 99
| 60
60
60
| | | |
| | ex 2007 10 10;
ex 2007 10 99
| 60
30
| | | |
| | ex 2007 99 39;
ex 2007 99 50;
ex 2007 99 97;
ex 2008 19 13;
ex 2008 19 93;
ex 2008 97 12;
ex 2008 97 14;
ex 2008 97 16;
ex 2008 97 18;
ex 2008 97 32;
ex 2008 97 34;
ex 2008 97 36;
ex 2008 97 38;
ex 2008 97 51;
ex 2008 97 59;
ex 2008 97 72;
ex 2008 97 74;
ex 2008 97 76;
ex 2008 97 78;
ex 2008 97 92;
ex 2008 97 93;
ex 2008 97 94;
ex 2008 97 96;
ex 2008 97 97;
ex 2008 97 98;
| 03; 04
32
22
20
20
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
19
| | | |
| | | 50 | | | |
| | | 11, 19 | Turkey (TR) | Pesticide residues | 20 |
| | | 10 | Vietnam (VN) | Pesticide residues | 10 |
| 2. Compound food referred to in Article 1(1)(b)(ii) |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Compound food containing any of the individual products listed in table 1 of this Annex due to risk of contamination by aflatoxins in a quantity above 20 % of either a single product or as the sum of products listed |
| CN Code | Description |
|---|
| ex 1704 90 | Sugar confectionery (including white chocolate), not containing cocoa, other than chewing gum, whether or not sugar-coated |
| ex 1806 | Chocolate and other food preparations containing cocoa |
| ex 1905 | Bread, pastry, cakes, biscuits and other bakers' wares, whether or not containing cocoa; communion wafers, empty cachets of a kind suitable for pharmaceutical use, sealing wafers, rice paper and similar products |
ANNEX III
(1) Sampling procedures and analytical reference methods referred to in Article 3(e)
1. Sampling procedures and analytical reference methods for the control of presence of Salmonella in food
(a)
In the case where Annexes I or II to this Regulation provide for the application of the sampling procedures and analytical reference methods laid down in point 1(a) of Annex III to this Regulation, the following rules shall apply:
| |
| Analytical reference method | Weight of consignment | Number of sample units (n) | Sampling procedures | Analytical result required for each sample unit of the same consignment |
|---|
| EN ISO 6579-1 | Less than 20 tonnes | 5 | n sample units are collected of a minimum of 100 g each. If batches are identified in the CHED, the sample units shall be collected from the different batches randomly chosen from the consignment. If batches cannot be identified, the sample units are collected randomly from the consignment. Pooling of sample units is not allowed. Each sample unit shall be tested separately. | No detection of Salmonella in 25 g |
| Greater than or equal to 20 tonnes | 10 |
(b)
In the case where Annexes I or II to this Regulation provide for the application of the sampling procedures and analytical reference methods laid down in point 1(b) of Annex III to this Regulation, the following rules shall apply:
| |
| Analytical reference method | Weight of consignment | Number of sample units (n) | Sampling procedures | Analytical result required for each sample unit of the same consignment |
|---|
| EN ISO 6579-1 | Any weight | 5 | n sample units are collected of a minimum of 100 g each. If batches are identified in the CHED, the sample units shall be collected from the different batches randomly chosen from the consignment. If batches cannot be identified, the sample units are collected randomly from the consignment. Pooling of sample units is not allowed. Each sample unit shall be tested separately. | No detection of Salmonella in 25 g |
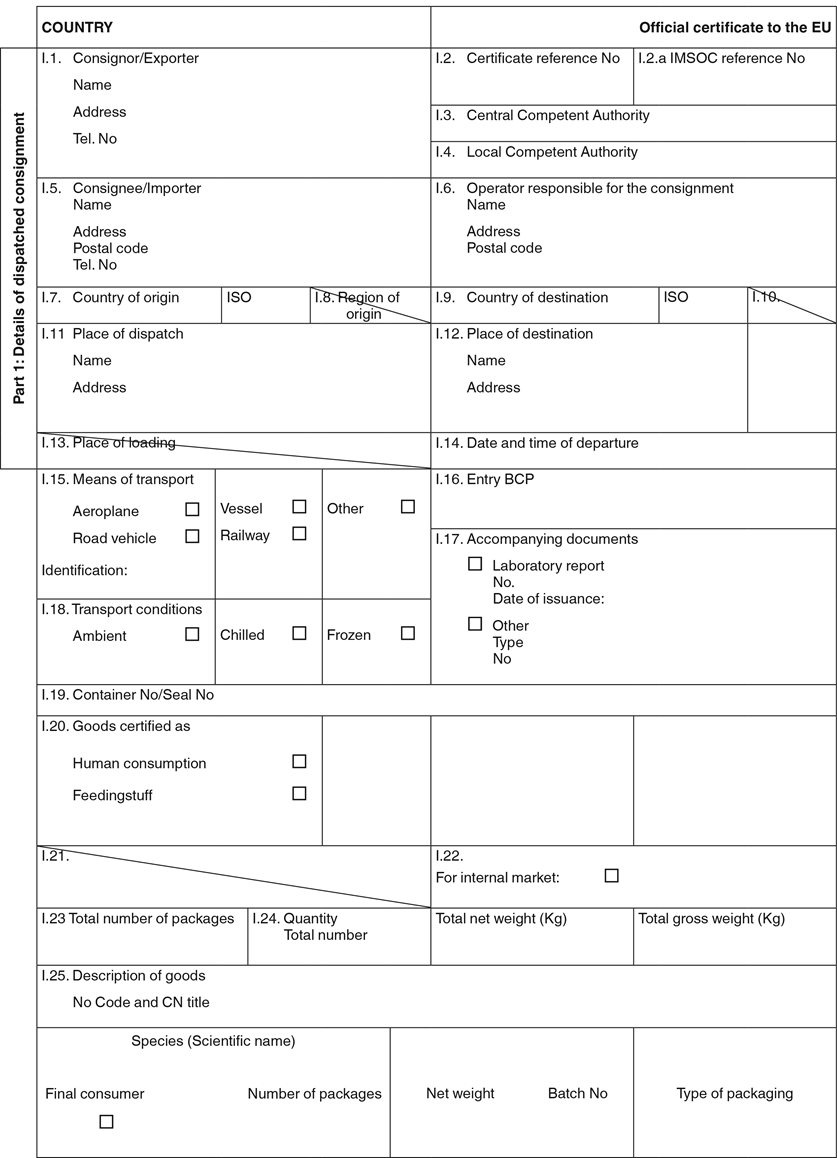
ANNEX IV
MODEL OFFICIAL CERTIFICATE REFERRED TO IN ARTICLE 11 OF COMMISSION IMPLEMENTING REGULATION (EU) 2019/1793 FOR THE ENTRY INTO THE UNION OF CERTAIN FOOD OR FEED
NOTES ON THE COMPLETION OF THE MODEL OFFICIAL CERTIFICATE REFERRED TO IN ARTICLE 11 OF IMPLEMENTING REGULATION (EU) 2019/1793 FOR THE ENTRY INTO THE UNION OF CERTAIN FOOD OR FEED
General
To positively select any option, please tick or mark the relevant box with a cross (X).
Whenever mentioned, ‘ISO’ means the international standard two-letter code for a country, in accordance with the international standard ISO 3166 alpha-2().
Only one of the options may be selected in boxes I.15, I.18, I.20.
Unless otherwise indicated, the boxes are compulsory.
If the consignee, the entry border control post (BCP) or the transport details (that is to say, the means and date) change after the certificate has been issued, the operator responsible for the consignment must advise the competent authority of the Member State of entry. Such a change shall not result in a request for a replacement certificate.
In case the certificate is submitted in IMSOC, the following applies:
the entries or boxes specified in Part I constitute the data dictionaries for the electronic version of the official certificate;
the sequences of boxes in part I of the model official certificate and the size and shape of those boxes are indicative;
where a stamp is required, its electronic equivalent is an electronic seal. Such seal shall comply with the rules for the issuance of electronic certificates referred to in Article 90(f) of Regulation (EU) 2017/625.
Part I: Details of the dispatched consignment
| |
| |
| |
| Country: | The name of the third country issuing the certificate. |
| Box I.1. | Consignor/Exporter: the name and address (street, city and region, province or state, as appropriate) of the natural or legal person dispatching the consignment that must be located in the third country. |
| Box I.2. | Certificate reference No: the unique mandatory code assigned by the competent authority of the third country in accordance with its own classification. This box is compulsory for all certificates not submitted in IMSOC. |
| Box I.2.a | IMSOC reference No: the unique reference code automatically assigned by IMSOC, if the certificate is registered in IMSOC. This box must not be completed if the certificate is not submitted in IMSOC. |
| Box I.3. | Central competent authority: name of the central authority in the third country issuing the certificate. |
| Box I.4. | Local competent authority: if applicable, the name of the local authority in the third country issuing the certificate. |
| Box I.5. | Consignee/Importer: name and address of the natural or legal person to whom the consignment is intended in the Member State. |
| Box I.6. | Operator responsible for the consignment: the name and address of the person in the European Union in charge of the consignment when presented to the BCP and who makes the necessary declarations to the competent authorities either as the importer or on behalf of the importer. This box is optional. |
| Box I.7. | Country of origin: the name and ISO code of the country where the goods are originating from, grown, harvested or produced. |
| Box I.8. | Not applicable. |
| Box I.9. | Country of destination: the name and ISO code of the European Union country of destination of the products. |
| Box I.10. | Not applicable. |
| Box I.11. | Place of dispatch: the name and address of the holdings or establishments from which the products come from.
Any unit of a company in the food or feed sector. Only the establishment shipping the products is to be named. In the case of trade involving more than one third country (triangular movement), the place of dispatch is the last third-country establishment of the export chain from which the final consignment is transported to the European Union.
|
| Box I.12. | Place of destination: this information is optional.
For the placing on the market: the place where the products are sent for final unloading. Give the name, address and approval number of the holdings or establishments of the place of destination, if applicable.
|
| Box I.13. | Place of loading: not applicable. |
| Box I.14. | Date and time of departure: the date when the means of transport departs (aeroplane, vessel, railway or road vehicle). |
| Box I.15. | Means of transport: means of transport leaving the country of dispatch.
Mode of transport: aeroplane, vessel, railway, road vehicle or other. ‘Other’ means modes of transport not covered by Council Regulation (EC) No 1/2005.
Identification of the means of transport: for aeroplanes the flight number, for vessels the ship name(s), for railways the train identity and wagon number, for road transports the registration number plate with trailer number plate if applicable.
In the case of a ferry, state the identification of the road vehicle, the registration number plate with trailer number plate if applicable, and the name of the scheduled ferry.
|
| Box I.16. | Entry BCP: state the name of the BCP and its identification code assigned by IMSOC. |
| Box I.17. | Accompanying documents:
Laboratory report: indicate the reference number and the date of issuance of the report/results of laboratory analyses referred to in Article 10 of Implementing Regulation (EU) 2019/1793 (this Regulation).
Other: the type and reference number of document must be stated when a consignment is accompanied by the other documents such a commercial document (for example, the airway bill number, the bill of lading number or the commercial number of the train or road vehicle).
|
| Box I.18. | Transport conditions: category of required temperature during the transport of products (ambient, chilled, frozen). Only one category may be selected. |
| Box I.19. | Container No/Seal No: if applicable, the corresponding numbers.
The container number must be provided if the goods are transported in closed containers.
Only the official seal number must be stated. An official seal applies if a seal is affixed to the container, truck or rail wagon under the supervision of the competent authority issuing the certificate.
|
| Box I.20. | Goods certified as: state the intended use for products as specified in the relevant European Union official certificate.
Human consumption: concerns only products intended for human consumption.
Feedingstuff: concerns only products intended for animal feed.
|
| Box I.21. | Not applicable |
| Box I.22. | For internal market: for all consignments destined to be placed on the market in the European Union. |
| Box I.23. | Total number of packages: the number of packages. In the case of bulk consignments, this box is optional. |
| Box I.24. | Quantity:
Total net weight: this is defined as the mass of the goods themselves without immediate containers or any packaging.
Total gross weight: overall weight in kilograms. This is defined as the aggregate mass of the products and of the immediate containers and all their packaging, but excluding transport containers and other transport equipment.
|
| Box I.25. | Description of goods: State the relevant Harmonised System code (HS code) and the title defined by the World Customs Organisation as referred to in Council Regulation (EEC) No 2658/87. This customs description shall be supplemented, if necessary, by additional information required to classify the products.
Indicate the species, types of products, the number of packages, type of packaging, batch number, net weight, and final consumer (i.e. products are packed for final consumer).
Species: the scientific name or as defined in accordance with European Union legislation.
Type of packaging: identify the type of packaging according to the definition given in Recommendation No 21 of UN/CEFACT (United Nations Centre for Trade Facilitation and Electronic Business).
|
Part II: Certification
This part must be completed by a certifying officer authorised by the competent authority of the third country to sign the official certificate, as provided for in Article 88(2) of Regulation (EU) 2017/625.
| Box II. | Health information: please complete this part in accordance with the specific European Union health requirements relating to the nature of the products and as defined in the equivalence agreements with certain third countries or in other European Union legislation, such as that for certification.
Select among points II.2.1, II.2.2, II.2.3 and II.2.4, the point corresponding to the category of product and the hazard for which the certification is given.
In case the official certificates is not submitted in IMSOC, the statements which are not relevant must be crossed out, initialled and stamped by the certifying officer, or completely removed from the certificate.
In case the certificate is submitted in IMSOC, the statements which are not relevant must be crossed out or completely removed from the certificate.
|
| Box II.a. | Certificate reference No: same reference code as in box I.2. |
| Box II.b. | IMSOC reference No: same reference code as in box I.2.a. Mandatory only for official certificates issued in IMSOC. |
| Certifying officer: | Official of the competent authority of the third country authorised to sign official certificates by such authorities: Indicate the name in capital letters, qualification and title, where applicable, identification number and original stamp of the competent authority and date of signature. |