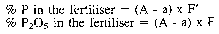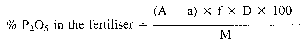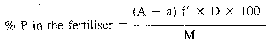SCHEDULE 2METHODS OF ANALYSIS
10.DETERMINATION OF EXTRACTED PHOSPHORUS
“1. SCOPE
This method is for the determination of phosphorus in the extracts from fertilisers.
“2. FIELD OF APPLICATION
The method is applicable to all extracts of fertilisers(1), for the determination of the different forms of phosphorus.
“3. PRINCIPLE
After hydrolysis, phosphorus is precipitated in an acid solution in the form of quinoline phosphomolybdate. The precipitate is collected, washed, dried at 250°C and weighed.
In the above conditions, compounds likely to be found in the solution (mineral and organic acids, ammonium ions, soluble silicates, etc . . .) will not interfere provided that a reagent based on sodium molybdate or ammonium molybdate is used in the precipitation.
“4. REAGENTS
Concentrated nitric acid (d = 1.40 g/ml).
Molybdate reagent:
Preparation of the reagent based on sodium molybdate:
4.2.1 Solution A: dissolve 70 g sodium molybdate dihydrate in 100 ml water.
Solution B: dissolve 60 g citric acid monohydrate in 100 ml water and add 85 ml concentrated nitric acid (4.1).
Solution C: stir solution A into solution B to obtain solution C.
Solution D: to 50 ml water add 35 ml concentrated nitric acid (4.1), add 5 ml freshly distilled quinoline. Add this solution to solution C, mix thoroughly and leave standing overnight in the dark. Make up to 500 ml with water, mix again and filter through a sintered glass funnel (5.3).
Preparation of the reagent based on ammonium molybdate:
4.2.2 Solution A: dissolve 100 g ammonium molybdate in 300 ml water, heating gently and stirring from time to time.
Solution B: dissolve 120 g citric acid monohydrate in 200 ml water and add 170 ml of concentrated nitric acid (4.1).
Solution C: add 10 ml freshly distilled quinoline to 70 ml of concentrated nitric acid (4.1).
Solution D: slowly pour, stirring well, solution A into solution B. After thoroughly mixing, add solution C to this mixture and make up to 1 litre with water. Leave standing for two days in a dark place and filter through a sintered glass funnel (5.3).
The reagents 4.2.1 and 4.2.2 can be used in the same way; both must be kept in the dark in stoppered polyethylene bottles.
“5. APPARATUS
Filter crucible with porosity of 5 to 20 microns.
Drying oven regulated at 250°C f 10°C.
Sintered glass funnel with porosity of 5 to 20 microns.
“6. PROCEDURE
Treatment of the solution
6.1 With a pipette take an aliquot part of fertiliser extract (see the Table) containing about 0.01 g of P2O5 and put it in a 500 ml Erlenmeyer flask. Add 15 ml concentrated nitric acid(2) (4.1) and dilute with water to about 100 ml.
6.2 Hydrolysis
Bring the contents of the Erlenmeyer flask to the boil slowly and keep at this temperature until hydrolysis is completed (this usually takes 1 hour). Care must be taken to avoid losses by splashing and excessive evaporation which would reduce the initial volume by more than half, by fitting a reflux condenser. After hydrolysis make up to the initial volume with distilled water.
Weighing the crucible
6.3 Dry the filter crucible (5.1) for at least 15 minutes in the drying oven (5.2). Cool the crucible in a desiccator and weigh.
Precipitation
6.4 Heat the acid solution in the Erlenmeyer flask until it begins to boil and then precipitate the quinoline phosphomolybdate by adding 40 ml of the precipitating reagent (4.2.1 or 4.2.2)(3) drop by drop, stirring continuously.
Place the Erlenmeyer flask in a steam bath, leave it there for 15 minutes, shaking it from time to time. The solution can be filtered immediately or after it has cooled down.
Filtering and washing
6.5 Filter the solution under vaccum by decantation. Wash the precipitate in the Erlenmeyer flask with 30 ml water. Decant and filter the solution. Repeat this process five times. Quantitatively transfer the rest of the precipitate into the crucible washing in with water. Wash four times with 20 ml water, allowing the liquid to drain from the crucible before each addition.
Drying and weighing
6.6 Wipe the outside of the crucible with a filter paper. Place the crucible in the drying oven (5.5) and keep it there until its weight remains constant at a temperature of 250°C (usually 1.5 minutes): leave it to cool in a desiccator to ambient temperature and weigh rapidly.
Blank test
6.7 For each series of determination, make a blank test under the same conditions (omitting only the sample) and allow for this in the calculation of the final result.
Control test
6.8 Carry out the determination using an aliquot part of a potassium dihydrogen phosphate solution containing 0.01 g of P2O5.
“7. EXPRESSION OF THE RESULTS
If the samples for analysis and dilutions shown in the Table are used the following formulae apply:
where:
A = weight in g of the quinoline phosphomolybdate
a = weight in g of the quinoline phosphomolybdate obtained in the blank test
F and F' = factors given in the last two columns of the Table.
With samples for analysis and dilutions which differ from those of the Table the following formulae apply:—
where:
f = conversion factor, quinoline phosphomolybdate into P20s = 0.03074
f' = conversion factor, quinoline phosphomolybdate into P = 0.013984
D = dilution factor
M = weighing of the sample analysed
| % P2O5 in the fertiliser | % P in the fertiliser | Sample for analysis g | Dilution to ml | Sample ml | Dilution to ml | Sample to be precipitated ml | Quinoline phosphomolybdate conversion factor (F) in percentage P2O5 | Quinoline phosphomolybdate conversion factor (F) in percentage P |
|---|---|---|---|---|---|---|---|---|
| 1-5 | 0.44 -2.2 | 1 2.5 5 | 500 500 500 | — — — | — — — | 100 50 25 | 16.037 12.830 12.830 | 6.992 5.594 5.594 |
| 5-10 | 2.2 -4.4 | 1 2.5 3 5 | 500 500 500 500 | — — — — | — — — — | 50 25 25 10 | 32.074 25.660 21.383 32.074 | 13.984 11.188 9.323 13.984 |
| 10-25 | 4.4 -11.0 | 1 2.5 3 5 | 500 500 500 500 | — — — 50 | — — — 500 | 25 10 10 50 | 64.148 64.148 53.457 64.148 | 27.968 27.968 23.307 27.968 |
| +25 | +11 | 1 2.5 3 5 | 500 500 500 500 | — 50 50 50 | — 500 500 500 | 10 50 50 25 | 160.370 128.296 106.913 128.296 | 69.921 55.937 46.614 55.937 |
Phosphorus soluble in mineral acids, water soluble phosphorus, phosphorus soluble in solutions of ammonium citrate, phosphorus soluble in 2% citric acid and phosphorus soluble in 2% formic acid.
21 ml when the solution to he precipitated contains more than 15 ml of citrate solution (neutral citrate, Petermann or Joulie alkaline citrate).
To precipitate phosphate solutions containing more than 15 ml citrate solution (neutral, Petermann or Joulie) which have been acidified with 21 ml concentrated nitric acid (see footnote to paragraph 6.1) use 80 ml of the precipitating reagent.