- Latest available (Revised)
- Point in Time (01/10/2006)
- Original (As enacted)
Medicines Act 1968
You are here:
- UK Public General Acts
- 1968 c. 67
- Schedules only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Changes over time for: Medicines Act 1968 (Schedules only)
Version Superseded: 19/01/2010
Alternative versions:
Status:
Point in time view as at 01/10/2006.
Changes to legislation:
Medicines Act 1968 is up to date with all changes known to be in force on or before 15 November 2024. There are changes that may be brought into force at a future date. Changes that have been made appear in the content and are referenced with annotations.![]()
Changes to Legislation
Changes and effects yet to be applied by the editorial team are only applicable when viewing the latest version or prospective version of legislation. They are therefore not accessible when viewing legislation as at a specific point in time. To view the ‘Changes to Legislation’ information for this provision return to the latest version view using the options provided in the ‘What Version’ box above.
SCHEDULES
Section 5.
F1SCHEDULE 1U.K. Provisions Relating to Medicines Commission and Committees
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
Section 5
[F2SCHEDULE 1AU.K.PROVISIONS RELATING TO COMMISSION AND COMMITTEES
Textual Amendments
F2Sch. 1A inserted (31.5.2005 for specified purposes, 30.10.2005 in so far as not already in force) by Medicines (Advisory Bodies) Regulations 2005 (S.I. 2005/1094), reg. 1(1)(2)7(2)
InterpretationU.K.
1.In this Act, “Advisory Body” means the Commission or a committee established under section 4 of this Act.
Co-opted membersU.K.
2.(1)Subject to the approval of the Secretary of State, at any meeting of an Advisory Body, that Advisory Body may co-opt additional members.
(2)A co-opted member shall hold office only in relation to the meeting for which he is co-opted.
Expert Advisory GroupsU.K.
3.(1)Subject to paragraph 4 of this Schedule, an Advisory Body, or any two or more Advisory Bodies acting jointly, may, subject to the approval of the Secretary of State, appoint sub-committees, to be known as Expert Advisory Groups.
(2)The Secretary of State may direct an Advisory Body to appoint an Expert Advisory Group to advise on such matters as may be specified in the direction.
(3)An Expert Advisory Group may include or consist of persons who are not members of the Advisory Body or Bodies which appointed that Expert Advisory Group.
(4)Subject to paragraph 4(2) of this Schedule, the Advisory Body or Bodies which appointed the Expert Advisory Group shall appoint one of the members of the Expert Advisory Group as chairman.
(5)At any meeting of an Expert Advisory Group, the chairman of that Group may, after consulting the chairman or chairmen of the Advisory Body or Bodies which appointed that Group, co-opt additional members of that Group.
(6)Members co-opted in accordance with sub-paragraph (5) of this paragraph shall hold office only in relation to the meeting for which they are co-opted.
Appointment by the Commission of Expert Advisory GroupsU.K.
4.(1)The Commission shall establish—
(a)an Expert Advisory Group to be called “the Biologicals Expert Advisory Group”, to advise on the safety, quality and efficacy of medicinal products of biological or bio-technological origin, including vaccines;
(b)an Expert Advisory Group to be called “the Chemistry, Pharmacy and Standards Expert Advisory Group”, to advise on the quality, and quality in relation to safety and efficacy, of medicinal products which are the subject of an application for a product licence under this Act, a marketing authorization under the Marketing Authorisation Regulations[F3, a certificate of registration under the Homoeopathic Regulations, a traditional herbal registration under the Herbal Regulations], or a request for authorisation pursuant to regulation 17 of the Clinical Trials Regulations;
(c)an Expert Advisory Group to be called “the Pharmacovigilance Expert Advisory Group”, to advise on pharmacovigilance and other issues relating to the safety of medicinal products; and
(d)such other Expert Advisory Groups as it considers appropriate.
(2)The chairmen of the Expert Advisory Groups referred to in paragraphs (a) to (c) of sub-paragraph (1) above shall be appointed by the Ministers.
Textual Amendments
F3Words in Sch. 1A para. 4(1)(b) inserted (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(a), Sch. 1 para. 4(a)
Delegation of functions by Advisory BodiesU.K.
5.(1)Subject to sub-paragraph (2) of this paragraph, an Advisory Body may delegate to an Expert Advisory Group such of its functions as it thinks fit.
(2)Subject to sub-paragraph (3) of this paragraph, an Advisory Body may not delegate any function which consists of advising the licensing authority in cases where the licensing authority is required to consult that Advisory Body pursuant to the provisions of—
(a)Part 2 of this Act;
(b)the Clinical Trials Regulations;
[F4(bb)the Herbal Regulations;]
(c)the Homoeopathic Regulations; or
(d)the Marketing Authorisation Regulations.
(3)An Advisory Body may arrange for an Expert Advisory Group to provide advice or assistance in relation to the performance of any function referred to in sub-paragraph (2) of this paragraph.
Textual Amendments
Terms of office of membersU.K.
6.The Ministers may make provision by regulations with respect to one or more of the following matters—
(a)the terms on which members of Advisory Bodies shall hold and vacate office, including the terms on which any person appointed as chairman of such a Body shall hold and vacate office as chairman; and
(b)the terms on which members of Expert Advisory Groups shall hold and vacate office, including the terms on which any person appointed as chairman of such a Group shall hold and vacate office as chairman.
Staff, premises and facilitiesU.K.
7.The Ministers shall provide each Advisory Body and any committee appointed under section 60 of this Act with such staff and such accommodation, services and other facilities as appear to the Ministers to be necessary or expedient for the proper performance of their functions.
Validity of proceedingsU.K.
8.The validity of any proceedings of an Advisory Body, Expert Advisory Group or any committee appointed under section 60 of this Act shall not be affected by—
(a)a vacancy among the members of that Advisory Body, Expert Advisory Group, or committee, or
(b)a defect in the appointment of any member of that Advisory Body, Expert Advisory Group or committee.
ProceedingsU.K.
9.(1)An Advisory Body may, subject to approval by the Secretary of State, make such provision as it thinks fit to regulate its own proceedings.
(2)The Secretary of State may make such provision as he thinks fit to regulate the proceedings of Expert Advisory Groups.
(3)A committee established under section 60 of this Act shall have the power to regulate their procedure.
Remuneration and expenses of membersU.K.
10.The Ministers may pay to the members of each Advisory Body and Expert Advisory Group and of any committee appointed under section 60 of this Act such remuneration (if any) and such allowances as may be determined by the Ministers with the consent of the Treasury.
Expenses of Advisory Bodies and Expert Advisory GroupsU.K.
11.The Ministers shall defray any expenses incurred with their approval by each Advisory Body and Expert Advisory Group or by any committee appointed under section 60 of this Act.
StatusU.K.
12.No Advisory Body, no Expert Advisory Group and no committee appointed under section 60 of this Act shall be taken to be the servant or agent of the Crown or to enjoy any status or immunity of the Crown.]
Section 29.
[F5 SCHEDULE 2 U.K.SUSPENSION, REVOCATION OR VARIATION OF LICENCE
Textual Amendments
F5Sch. 2 substituted (30.10.2005) by Medicines (Advisory Bodies) Regulations 2005 (S.I. 2005/1094), reg. 1(1), Sch. 1 para. 15
Modifications etc. (not altering text)
C1Sch. 2 applied (with modifications) (3.4.1992) by S.I. 1992/605, reg. 2(1)(2),Sch.
Sch. 2 extended (with modifications) (14.2.1994) by S.I. 1994/105, reg. 19, Sch.4
C2Sch. 2 amendment to earlier affecting provision SI 1992/605 Sch. (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(b), Sch. 4 para. 6(b)
C3Sch. 2 amendment to earlier affecting provision SI 1994/105 Sch. 4 (30.10.2005) by Medicines (Homoeopathic Medicinal Products for Human Use) Amendment Regulations 2005 (S.I. 2005/2753), regs. 1(1), 19(2)
Procedure on consultation with appropriate committeeU.K.
1U.K.Subject to paragraph 8 below, where the licensing authority propose, in the exercise of their powers under section 28 of this Act—
(a)to suspend, revoke or vary a product licence on the grounds specified in paragraph (a) or paragraph (c) of subsection (3) of that section, in a case where it appears to the licensing authority that the matters or characteristics in question are such as to affect the safety, efficacy or quality of medicinal products to which the licence relates, or
(b)to suspend, revoke or vary a product licence on any of the grounds specified in paragraph (g) or paragraph (h) of that subsection,
the licensing authority shall not suspend, revoke or vary the licence except after consultation with the appropriate committee.
2(1)Where the appropriate committee are consulted under the preceding paragraph and are of the provisional opinion that, on such grounds as are mentioned in that paragraph, they may have to advise the licensing authority that the product licence ought to be revoked, varied or suspended, the appropriate committee shall notify the holder of the licence accordingly.U.K.
(2)A person who has been so notified may, within the time allowed, give notice of his wish to make written or oral representations to the appropriate committee.
(3)The appropriate committee shall give the holder of the licence an opportunity to make such representations in accordance with sub-paragraphs (4) to (7) of this paragraph.
(4)Subject to sub-paragraph (5) of this paragraph, the holder of the licence shall provide the appropriate committee with—
(a)his written representations or a written summary of the oral representations he intends to make; and
(b)any documents on which he wishes to rely in support of those representations,
before the end of the period of six months beginning with the date of the notice referred to in sub-paragraph (2) of this paragraph, or within such shorter period as the appropriate committee may specify in the notification under sub-paragraph (1) of this paragraph.
(5)If the holder of the licence so requests, the appropriate committee may extend the time limit referred to in sub-paragraph (4) of this paragraph, up to a maximum period of twelve months beginning with the date of the notice referred to in sub-paragraph (2) of this paragraph.
(6)The holder of the licence may not submit any additional written representations or documents once the time limit referred to in sub-paragraphs (4) and (5) of this paragraph has expired, except with the permission of the appropriate committee.
(7)If the holder gave notice of his wish to make oral representations, the appropriate committee shall, after receiving a written summary and any other documents in accordance with sub-paragraph (4) of this paragraph, arrange for the holder to make such representations at a hearing before the committee.
(8)The appropriate committee shall—
(a)take into account such representations as are made in accordance with this paragraph; and
(b)report their findings and advice to the licensing authority, together with the reasons for their advice.
3(1)After receiving the report of the appropriate committee the licensing authority shall—U.K.
(a)decide whether to continue with the proposal to revoke, vary or suspend the product licence; and
(b)take the report into account when making their decision.
(2)The licensing authority shall then notify the holder of the licence of—
(a)the decision made pursuant to sub-paragraph (1) of this paragraph; and
(b)the advice given to them by the appropriate committee and the reasons for that advice.
4U.K.If—
(a)the appropriate committee was consulted under paragraph 1 of this Schedule;
(b)the committee did not give a provisional opinion under paragraph 2(1) of this Schedule; and
(c)the licensing authority propose—
(i)to determine the matter in a way which differs from the advice of the committee, or
(ii)to suspend, revoke or vary the licence on grounds not relating to safety, quality or efficacy,
the authority shall notify the holder of the licence accordingly.
(2)A notification given under sub-paragraph (1) of this paragraph shall state—
(a)the advice of the committee and the reasons stated by the committee for that advice; and
(b)the proposals of the licensing authority and the reasons for them.
5(1)Subject to sub-paragraph (4) of this paragraph, a person to whom a notification has been given under paragraph 3(2) of this Schedule may, within the time allowed, notify the licensing authority that he wishes to appear before and be heard by a person appointed by the licensing authority with respect to the decision.U.K.
(2)A person to whom a notification has been given under paragraph 4(1) of this Schedule may, within the time allowed—
(a)notify the licensing authority that he wishes to appear before and be heard by a person appointed for the purpose by the licensing authority, or
(b)make representations in writing to the licensing authority with respect to the proposal referred to in the notification.
(3)If the applicant makes written representations in accordance with sub-paragraph (2)(b) of this paragraph, the licensing authority shall take those representations into account before determining the matter.
(4)Sub-paragraph (1) of this paragraph shall not apply where—
(a)the person has not made any representations in accordance with paragraph 2(4) to (7) of this Schedule; and
(b)the decision of the licensing authority was in accordance with the advice of the appropriate committee.
Modifications etc. (not altering text)
C4Sch. 2 para. 5(4) applied (with modifications) (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(b), Sch. 5 para. 6(4)
C5Sch. 2 para. 5(4)(a) applied (with modifications) (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(b), Sch. 5 para. 2(5)
C6Sch. 2 para. 5(4)(a) applied (with modifications) (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(b), Sch. 5 para. 11(4)
Procedure in other casesU.K.
6.(1)This paragraph applies where the licensing authority propose, in the exercise of the powers conferred by section 28 of this Act—U.K.
(a)to suspend, revoke or vary a licence under Part 2 of this Act, other than a product licence; or
(b)to suspend, revoke or vary a product licence where the holder of the licence has been given neither—
(i)notice of any provisional opinion or any advice of the appropriate committee which led to that proposal under paragraphs 2 and 3 of this Schedule; nor
(ii)notice of that proposal under paragraph 4 of this Schedule,
and the provisions of paragraph 8 of this Schedule do not apply.
(2)The licensing authority shall notify the holder of the licence of—
(a)their proposals;
(b)the reasons for them; and
(c)the date (not being earlier than twenty-eight days from the date of the notification) on which it is proposed that the suspension, revocation or variation should take effect.
(3)The holder of the licence may, before the date specified in the notification—
(a)notify the licensing authority of his wish to appear before and be heard by a person appointed by the licensing authority with respect to the decision; or
(b)make representations in writing to the licensing authority with respect to the proposal referred to in the notification.
(4)If the applicant makes written representations in accordance with sub-paragraph (3)(b) of this paragraph, the licensing authority shall take those representations into account before determining the matter.
Hearing before person appointedU.K.
7(1)If the holder of the licence gives notice under paragraph 5 or 6 of this Schedule of his wish to appear before and be heard by a person appointed by the licensing authority, the authority shall—U.K.
(a)make that appointment; and
(b)arrange for the applicant to have an opportunity of appearing before that person.
(2)The person appointed—
(a)shall not be, or at any time have been, a member of—
(i)the Commission on Human Medicines or any of its Expert Advisory Groups,
(ii)the Medicines Commission formerly established under section 2 of this Act or any of its committees, or
(iii)a committee established under section 4 of this Act, or any sub-committee of such a committee; and
(b)shall not be an officer or servant of any Minister of the Crown.
(3)Subject to sub-paragraph (4) of this paragraph, the holder of the licence shall provide the person appointed with—
(a)a written summary of the oral representations he intends to make; and
(b)any documents on which he wishes to rely in support of those representations,
before the end of the period of three months beginning with the date of the notice referred to in sub-paragraph (1) of this paragraph.
(4)If the holder of the licence so requests, the person appointed may, after consulting the licensing authority, extend the time limit referred to in sub-paragraph (3) of this paragraph, up to a maximum period of six months beginning with the date of the notice referred to in sub-paragraph (1) of this paragraph.
(5)If the holder of the licence fails to comply with the time limit in sub-paragraph (3) of this paragraph, or, where he has been granted an extended time limit under sub-paragraph (4) of this paragraph, that time limit—
(a)he may not appear before or be heard by the person appointed, and
(b)the licensing authority shall decide whether to grant or refuse the licence, or to grant it otherwise than in accordance with the application, and notify the applicant accordingly.
(6)The holder of the licence may not submit any additional written representations or documents once the time limit has expired, except with the permission of the person appointed.
(7)At the hearing before the person appointed, both the holder of the licence and the licensing authority may make representations.
(8)If the holder of the licence so requests the hearing shall be in public.
(9)After the hearing—
(a)the person appointed shall provide a report to the licensing authority; and
(b)the licensing authority shall take this report into account and decide whether to revoke, vary or suspend the licence.
(10)The licensing authority shall then—
(a)notify the holder of the licence of their decision;
(b)if the holder so requests, provide the holder with a copy of the report of the person appointed.
Modifications etc. (not altering text)
C7Sch. 2 para. 7 excluded (30.10.2005) by Medicines (Advisory Bodies) (No.2) Regulations 2005 (S.I. 2005/2754), reg. 1(2)(b), Sch. 5 para. 19(3)(a)(ii)
Procedure in cases of urgencyU.K.
8U.K.Notwithstanding anything in paragraphs 1 to 7 of this Schedule, where it appears to the licensing authority that in the interests of safety it is necessary to suspend a licence under Part 2 of this Act with immediate effect, the licensing authority may do so, for a period not exceeding three months.
9U.K.If the licence is a product licence, the licensing authority shall report the suspension forthwith to the appropriate committee.
10.U.K.If, after the suspension has taken effect—
(a)it appears to the licensing authority; or
(b)in the case of a product licence, they are advised by the appropriate committee,
that it is necessary to consider whether the licence ought to be further suspended, or ought to be revoked or varied, the licensing authority (subject to paragraph 11 of this Schedule) shall proceed in accordance with such of the provisions of paragraphs 1 to 7 of this Schedule as are applicable in the circumstances.
11.(1)This paragraph applies where, in the circumstances specified in paragraph 10 of this Schedule, the licensing authority proceed as mentioned in that paragraph and any proceedings under paragraphs 1 to 7 of this Schedule relating to a further suspension of the licence have not been finally disposed of before the end of the period—U.K.
(a)for which the licence was suspended under paragraph 8 of this Schedule; or
(b)for which it has been further suspended under this paragraph.
(2)If it appears to the licensing authority to be necessary in the interests of safety to do so, the authority may further suspend the licence for a period which (in the case of each such further suspension) shall not exceed three months.
(3)The provisions of section 27(7) of this Act shall, with the necessary modifications, have effect for the purpose of determining the date on which any proceedings are taken to be finally disposed of.
InterpretationU.K.
12U.K.In this Schedule, the “the time allowed” means the period of twenty-eight days from the date of the relevant notification, or such longer period as the licensing authority may allow in any particular case.]
SCHEDULE 3 U.K. SAMPLING
Modifications etc. (not altering text)
C8Sch. 3 modified (3.4.1992) by S.I. 1992/605, regs. 2(3), 3
Sch. 3 applied (3.10.1994) by S.I. 1994/2328, reg. 11(c)
Sch. 3 applied (with modifications) (1.1.1995) by S.I. 1994/3144, reg.10, Sch. 4
Sch. 3 applied (31.3.1997) by S.I. 1997/322, reg. 34, Sch.5
Sch. 3 applied (1.2.2000) by S.I. 2000/7, reg. 5
C9Sch. 3 applied (with modifications) (1.5.2004) by Medicines for Human Use (Clinical Trials) Regulations 2004 (S.I. 2004/1031), regs. 1, 47, Schs. 9
C10Sch. 3 applied (with modifications) (30.10.2005) by Medicines (Traditional Herbal Medicinal Products for Human Use) Regulations 2005 (S.I. 2005/2750), regs. 1(a), 11, Schs. 4 (with Sch. 6)
C11Sch. 3 amendment to earlier affecting provision SI 2004/1031 reg. 47 Sch. 9 (29.8.2006) by Medicines for Human Use (Clinical Trials) Amendment Regulations 2006 (S.I. 2006/1928), regs. 1(1), 32
IntroductoryU.K.
1(1)The provisions of this Schedule shall have effect where a person authorised in that behalf by an enforcement authority (in this Schedule referred to as a “sampling officer") obtains a sample of any substance or article—U.K.
(a)for the purpose of ascertaining whether there is or has been, in connection with that substance or article, any contravention of any provisions of this Act or of any regulations or order made thereunder which, by or under any provisions of sections 108 to 110 of this Act, that authority (in this Schedule referred to as “the relevant enforcement authority”) is required or empowered to enforce, or
(b)otherwise for any purpose connected with the performance by that authority of their functions under this Act or under any such regulations or order,
and the sampling officer obtains the sample by purchase or in the exercise of any power conferred by section 112 of this Act.
(2)In this Schedule “public analyst”, [F6except in relation to Northern Ireland, has the meaning assigned to it by section 27 of the Food Safety Act 1990], and in relation to Northern Ireland has the meaning assigned to it by [F7Article 27(1) of the Food Safety (Northern Ireland) Order 1991].
Textual Amendments
F6Words in Sch. 3 para. 1(2) substituted (E.W.S.) by Food Safety Act 1990 (c. 16, SIF 53:1, 2), ss. 54, 59(1), Sch. 3 para. 12
F7Words in Sch. 3 para. 1(2) substituted (N.I.) (21.5.1991) by S.I. 1991/762, art. 51(1), Sch. 2 para.10; S.R. 1991/175, art. 2(1).
Division of sampleU.K.
2U.K.The sampling officer shall forthwith divide the sample into three parts, each part to be marked and sealed or fastened up in such manner as its nature will permit.
3U.K.If the sample was purchased by the sampling officer, otherwise than from an automatic machine, he shall supply one part of the sample to the seller.
4U.K.If the sampling officer obtained the sample from an automatic machine, then—
(a)if a person’s name, and an address in the United Kingdom, are stated on the machine as being the name and address of the owner of the machine, the sampling officer shall supply one part of the sample to that person;
(b)in any other case, the sampling officer shall supply one part of the sample to the occupier of the premises on which the machine stands or to which it is affixed.
5U.K.If the sample is of goods consigned from outside the United Kingdom and was taken by the sampling officer before delivery to the consignee, the sampling officer shall supply one part of the sample to the consignee.
6U.K.If, in a case not falling within any of paragraphs 3 to 5 of this Schedule, the sample was obtained by the sampling officer at the request or with the consent of a purchaser, the sampling officer shall supply one part of the sample to the seller.
7U.K.If, in a case not falling within any of paragraphs 3 to 6 of this Schedule, the sample was taken in transit, the sampling officer shall supply one part of the sample to the consignor.
8U.K.In any case not falling within any of paragraphs 3 to 7 of this Schedule the sampling officer shall supply one part of the sample to the person appearing to him to be the owner of the substance or article from which the sample was taken.
9U.K.In every case falling within any of paragraphs 3 to 8 of this Schedule the sampling officer shall inform the person to whom the part of the sample in question is supplied that the sample has been obtained for the purpose of analysis or other appropriate examination.
10U.K.Of the remaining parts of the sample into which the sample is divided in accordance with paragraph 2 of this Schedule, the sampling officer, unless he decides not to submit the sample for analysis or other appropriate examination, shall—
(a)retain one part for future comparison, and
(b)submit the other part for analysis or examination in accordance with the following provisions of this Schedule.
11U.K.Where a sample consists of substances or articles enclosed in unopened containers, and it appears to the sampling officer that to open the containers and divide the contents into parts—
(a)is not reasonably practicable, or
(b)might affect the composition or impede the proper analysis or other examination of the contents,
the sampling officer may divide the sample into parts by dividing the containers into three lots without opening them.
12U.K.Section 127 of this Act shall have effect in relation to supplying any part of a sample in pursuance of the preceding paragraphs as it has effect in relation to the service of a document.
13U.K.If after reasonable inquiry the sampling officer is unable to ascertain the name of a person to whom, or the address at which, a part of a sample ought to be supplied in pursuance of the preceding paragraphs, he may retain that part of the sample instead of supplying it.
Notice to person named on containerU.K.
14(1)Where it appears to the sampling officer that a substance or article of which he has obtained a sample was manufactured or assembled by a person whose name and address in the United Kingdom are stated on its container, and who is not a person to whom a part of the sample is required to be supplied under the preceding provisions of this Schedule, the sampling officer, unless he decides not to submit the sample for analysis or other appropriate examination, shall serve notice on that person—U.K.
(a)stating that the sample has been obtained by the sampling officer, and
(b)specifying the person from whom the sampling officer purchased it, or, if he obtained it otherwise than by purchase, the place from which he obtained it.
(2)The notice required to be served under the preceding sub-paragraph shall be served before the end of the period of three days beginning with the day on which the sample was obtained.
Analysis or other examination of sampleU.K.
15U.K.If the sampling officer decides to submit the sample for analysis or other appropriate examination, he shall—
(a)submit it for analysis to the public analyst for the area in which the sample was obtained, or, if for the time being there is no public analyst for that area, then to the public analyst for some other area, or
(b)submit it for other appropriate examination to the person having the management or control of any laboratory available for the purpose in accordance with any arrangements made in that behalf by the relevant enforcement authority.
16U.K.Where the relevant enforcement authority is a Minister or the Pharmaceutical Society, and the sampling officer decides to have the sample analysed, he may (instead of submitting it to a public analyst) submit it for analysis to the person having the management or control of any laboratory available for the purpose in accordance with any arrangements made in that behalf by the relevant enforcement authority.
17U.K.Any such arrangements as are mentioned in paragraph 15(b) or paragraph 16 of this Schedule,—
F8(a). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
(b)if F9... they are made by an enforcement authority in England and Wales other than [F10the Secretary of State], shall be arrangements approved by [F10the Secretary of State];
(c)if they are made by an enforcement authority in Scotland other than the Secretary of State, shall be arrangements approved by the Secretary of State;
and any such arrangements as are mentioned in paragraph 15(b) of this Schedule, if made by a [F11district council] in Northern Ireland, shall be arrangements approved by the Minister of Health and Social Services for Northern Ireland.
Textual Amendments
F8Sch. 3 para. 17(a) omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 68(a) (with regs. 2(4), 3)
F9Words in Sch. 3 para. 17(b) omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 68(b) (with regs. 2(4), 3)
F10Words substituted by virtue of S.I. 1968/1699, arts. 2, 5(4)(a)
F11Words substituted by S.R. &O. (N.I.) 1973 No. 211, Sch.
Modifications etc. (not altering text)
C12Functions of Secretary of State in matters only affecting Wales exercisable by Secretary of State for Wales: S.I. 1969/388, art. 2(1)
18(1)Subject to the following sub-paragraph, the person to whom the sample is submitted under paragraph 15 or paragraph 16 of this Schedule shall analyse or examine the sample (as the case may be), or cause the sample to be analysed or examined by some other person under his direction, as soon as practicable.U.K.
(2)If the person to whom the sample is so submitted is a public analyst, and that analyst determines that for any reason an effective analysis of the sample cannot be performed by him or under his direction, he shall send it to the public analyst for some other area, and that other public analyst shall as soon as practicable analyse the sample or cause it to be analysed by some other person under his direction.
19(1)A public analyst who has analysed a sample submitted to him under the preceding provisions of this Schedule, or who has caused such a sample to be analysed by some other person under his direction, shall issue and send to the sampling officer a certificate specifying the result of the analysis.U.K.
(2)A person having the management or control of a laboratory in which a sample submitted to him under the preceding provisions of this Schedule has been analysed or examined, or a person appointed by him for the purpose, shall issue and send to the sampling officer a certificate specifying the result of the analysis or examination.
(3)Any certificate issued under this paragraph shall be in a form prescribed by the Ministers and shall be signed by the person who issues the certificate.
20(1)Any person to whom, in accordance with paragraphs 2 to 8 of this Schedule, a part of the sample is required to be supplied shall, on payment of the prescribed fee to the relevant enforcement authority, be entitled to be supplied with a copy of any certificate as to the result of an analysis or examination which is sent to the sampling officer under paragraph 19 of this Schedule.U.K.
(2)Any regulations prescribing a fee for the purposes of this paragraph shall be made by the Ministers.
Provisions as to evidenceU.K.
21U.K.In any proceedings for an offence under this Act a document produced by one of the parties to the proceedings and purporting to be a certificate issued under paragraph 19 of this Schedule shall be sufficient evidence of the facts stated in the document, unless the other party requires that the person who issued the certificate shall be called as a witness; and, in any proceedings in Scotland, if that person is called as a witness, his evidence shall be sufficient evidence of those facts.
22U.K.In any proceedings for an offence under this Act a document produced by one of the parties to the proceedings, which has been supplied to him by the other party as being a copy of such a certificate, shall be sufficient evidence of the facts stated in the document.
23(1)If in any such proceedings before a magistrates’ court a defendant intends to produce such a certificate, or to require that the person by whom such a certificate was issued shall be called as a witness, a notice of his intention, and (where he intends to produce such a certificate) a copy of the certificate, shall be given to the other party at least three clear days before the day on which the summons is returnable.U.K.
(2)If the preceding sub-paragraph is not complied with, the court may, if it thinks fit, adjourn the hearing on such terms as it thinks proper.
(3)In Scotland, if in any such proceedings in the sheriff court the accused intends to produce such a certificate, or to require that the person by whom such a certificate was issued shall be called as a witness, notice of his intention, and (where he intends to produce such a certificate) a copy of the certificate, shall be given to the procurator fiscal at least three clear days before the day on which the case proceeds to trial.
(4)If sub-paragraph (3) of this paragraph is not complied with, the sheriff may, if he thinks fit, adjourn the diet on such terms as he deems proper.
Analysis under direction of courtU.K.
24(1)In any proceedings for an offence under this Act, where the proceedings relate to a substance or article of which a sample has been obtained as mentioned in paragraph 1 of this Schedule, the part of the sample retained in pursuance of paragraph 10(a) of this Schedule shall be produced as evidence; and the court—U.K.
(a)at the request of either party to the proceedings shall, and
(b)in the absence of any such request may if it thinks fit,
cause that part of the sample to be sent for analysis to the Government Chemist (or, in Northern Ireland, the Government Chemist for Northern Ireland) or to be sent for other appropriate examination to the person having the management or control of a laboratory specified by the court.
(2)If, in a case where an appeal is brought, no action has been taken under the preceding sub-paragraph, the provisions of that sub-paragraph shall have effect in relation to the court by which the appeal is heard.
(3)A person to whom a part of a sample is sent under this paragraph for analysis or other examination shall analyse or examine it, or cause it to be analysed or examined on his behalf, and shall transmit to the court a certificate specifying the result of the analysis or examination.
(4)Any such certificate shall be signed by that person, or signed on his behalf by the person who made the analysis or examination or a person under whose direction it was made.
(5)Any such certificate shall be evidence (and, in Scotland, shall be sufficient evidence) of the facts stated in the certificate unless any party to the proceedings requires that the person by whom it was signed shall be called as a witness; and, in any proceedings in Scotland, if that person is called as a witness, his evidence shall be sufficient evidence of those facts.
25U.K.The costs of any analysis or examination under paragraph 24 of this Schedule shall be paid by the prosecutor or the defendant (or, in Scotland, the accused) as the court may order.
Proof by written statementU.K.
26U.K.In relation to England and Wales section 9 of the M1Criminal Justice Act 1967, and in relation to Northern Ireland any corresponding enactment which may be passed by the Parliament of Northern Ireland, shall not have effect with respect to any document produced as mentioned in paragraph 21 or paragraph 22 of this Schedule or with respect to any certificate transmitted to a court under paragraph 24 of this Schedule.
Marginal Citations
Power to modify sampling provisionsU.K.
27U.K.The Ministers may by order provide that, in relation to substances or articles of any such description as may be specified in the order, the preceding provisions of this Schedule shall have effect subject to such exceptions and modifications as may be specified in the order.
Payment for sample taken under compulsory powersU.K.
28(1)Where a sampling officer takes a sample in the exercise of any power conferred by section 112 of this Act he shall, if payment is demanded, pay the value of the sample to the person to whom a part of the sample is required under paragraph 5, paragraph 7 or paragraph 8 of this Schedule (as the case may be) to be supplied.U.K.
(2)In default of agreement between the sampling officer and the person mentioned in the preceding sub-paragraph, the value of the sample shall be determined by the arbitration of a single arbitrator appointed by the sampling officer and the other person in question or, if they are unable to agree on the appointment of an arbitrator, shall be determined by the county court for the district (or, in Northern Ireland, the division) in which the sample was taken.
(3)In the application of this paragraph to Scotland, for references to an arbitrator there shall be substituted references to an arbiter and for the reference to the county court there shall be substituted a reference to the sheriff.
Application of s. 64 to samplesU.K.
29U.K.Where a medicinal product is taken as a sample by a sampling officer in the exercise of any power conferred by section 112 of this Act, the provisions of subsections (1) to (4) of section 64 of this Act shall have effect as if the taking of the product as a sample were a sale of it to the sampling officer by the person from whom it is taken; and, if the product was prepared in pursuance of a prescription given by a practitioner, those provisions shall so have effect as if, in subsection (1) of that section, for the words “demanded by the purchaser", there were substituted the words “specified in the prescription"
Section 134.
SCHEDULE 4U.K. Provisions relating to Northern Ireland
Modifications etc. (not altering text)
C13Sch. 4 applied (31.3.1997) by S.I. 1997/322, reg. 34, Sch.5
C14Paras. 6, 8, 11 extended by Medicines Act 1971 (c. 69), s. 1(3)(c)
1(1)The Minister of Health and Social Services for Northern Ireland may by order make provision for the application of this Act in relation to druggists subject to such exceptions and modifications as may be specified in the order.U.K.
(2)In this paragraph “druggist” means a person registered in the register of druggists for Northern Ireland made out and maintained under [F12Articles 6 and 9 of the M2Pharmacy (Northern Ireland) Order 1976].
Textual Amendments
F12Words substituted by S.I. 1976/1213 (N.I. 22), Sch. 5 para. 8
Marginal Citations
F132U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F13Sch. 4 paras. 2-5 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(a) (with regs. 2(4), 3)
F133U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F13Sch. 4 paras. 2-5 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(a) (with regs. 2(4), 3)
F134U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F13Sch. 4 paras. 2-5 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(a) (with regs. 2(4), 3)
F135U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F13Sch. 4 paras. 2-5 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(a) (with regs. 2(4), 3)
6U.K.[F14The appropriate Northern Ireland Minister] may in relation to Northern Ireland exercise any power of making an order or regulations which is conferred on the [F15Ministers] by any provision of this Act (except a provision contained in section 15(3)F16..., . . . F17 or section 57(3) of this Act) where in his F18... opinion there are special circumstances which render it expedient to do so.
Textual Amendments
F14Words in Sch. 4 para. 6 substituted (1.10.2006) by The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(b)(i) (with regs. 2(4), 3)
F15Word in Sch. 4 para. 6 substituted (1.10.2006) by The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(b)(ii) (with regs. 2(4), 3)
F16Words in Sch. 4 para. 6 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(b)(iii) (with regs. 2(4), 3)
F17Words repealed by Animal Health and Welfare Act 1984 (c. 40, SIF 2:8), s. 16, Sch. 1 para. 3(11), Sch. 2
F18Words in Sch. 4 para. 6 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(b)(iv) (with regs. 2(4), 3)
F197U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F19Sch. 4 para. 7 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(c) (with regs. 2(4), 3)
8U.K.Every order or regulation under this Act made by the Minister of Health and Social Services for Northern IrelandF20... by virtue of the power conferred by paragraph 1F21... or paragraph 6 of this Schedule, and every regulation made solely by the Minister of Health and Social Services for Northern Ireland under section 120 of this Act, shall be subject to negative resolution within the meaning of section 41(6) of the M3Interpretation Act (Northern Ireland) 1954 as if it were a statutory instrument within the meaning of that Act.
Textual Amendments
F20Words in Sch. 4 para. 8 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(d)(i) (with regs. 2(4), 3)
F21Words in Sch. 4 para. 8 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(d)(ii) (with regs. 2(4), 3)
Marginal Citations
9U.K.In this Schedule [F22“the appropriate Northern Ireland Minister”] —
(a)F23... means the Minister of Health and Social Services for Northern Ireland;
F24(b). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F24(c). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F25...
Textual Amendments
F22Words in Sch. 4 para. 9 substituted (1.10.2006) by The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(e)(i) (with regs. 2(4), 3)
F23Words in Sch. 4 para. 9(a) omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(e)(ii) (with regs. 2(4), 3)
F24Sch. 4 para. 9(b)(c) omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(e)(iii) (with regs. 2(4), 3)
F25Words in Sch. 4 para. 9 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(e)(iv) (with regs. 2(4), 3)
10U.K.In this Act any reference to the Minister of Health and Social Services for Northern Ireland F26..., and any reference which is to be construed as including a reference to [F27that Minister], shall include a reference to the Ministry of Health and Social Services for Northern Ireland F28....
Textual Amendments
F26Words in Sch. 4 para. 10 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(f)(i) (with regs. 2(4), 3)
F27Words in Sch. 4 para. 10 substituted (1.10.2006) by The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(f)(ii) (with regs. 2(4), 3)
F28Words in Sch. 4 para. 10 omitted (1.10.2006) by virtue of The Veterinary Medicines Regulations 2006 (S.I. 2006/2407), reg. 1, Sch. 8 para. 69(f)(iii) (with regs. 2(4), 3)
11U.K.[F29The Statutory Rules (Northern Ireland) Order 1979, except article 5(2)(a) of that Order] (which requires the responsible officer of each rule-making authority making any statutory rules to send copies of them, and certain information, to the Ministry of Finance for Northern Ireland for registration [F29under that Order]), shall not apply to any orders or regulations made under this Act by statutory instrument.
Textual Amendments
F29Words substituted by S.I. 1979/1573, Sch. 4 para. 10
Section 135(1)
SCHEDULE 5U.K. Amendments of Enactments of Parliament of United Kingdom.
The Venereal Disease Act 1917 (c. 21). U.K.
F301. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F30Sch. 5 para. 1 repealed (22.7.2004) by Statute Law (Repeals) Act 2004 (c. 14), Sch. 1 Pt. 17 Group 7
Modifications etc. (not altering text)
C15The text of Sch. 5 paras. 1, 10, 13, 16, 7, Schs. 6, 8 is in the form in which it was originally enacted: it was not reproduced in Statutes in Force and does not reflect any amendments or repeals which may have been made prior to 1.2.1991.
2, 9.U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F31
Textual Amendments
F31Sch. 5 paras. 2–9 repealed by Poisons Act 1972 (c. 66), Sch. 2
The Cancer Act 1939 (c. 13.)U.K.
10In section 4, in subsection (4)(a)(v), for the words “authorised sellers of poisons" there shall be substituted the words “persons lawfully conducting a retail pharmacy business in accordance with section 69 of the Medicines Act 1968".
Modifications etc. (not altering text)
C16The text of Sch. 5 paras. 1, 10, 13, 16, 7, Schs. 6, 8 is in the form in which it was originally enacted: it was not reproduced in Statutes in Force and does not reflect any amendments or repeals which may have been made prior to 1.2.1991.
11U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F32
Textual Amendments
F32Sch. 5 para. 11 repealed by National Health Service Act 1977 (c. 49), Sch. 16
12U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F33
Textual Amendments
F33Sch. 5 para. 12 repealed by National Health Service (Scotland) Act 1978 (c. 29), Sch. 17
F3413U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F34Sch. 5 para. 13 repealed (5.11.1993) by 1993 c. 50, s. 1(1), Sch. 1Pt. XII
14, 15.U.K.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F35
Textual Amendments
F35Sch. 5 paras. 14, 15 repealed by Misuse of Drugs Act 1971 (c. 38), Sch. 6
The Trade Descriptions Act 1968 (c. 29). U.K.
16In section 2, in subsection 5, after the word “section" there shall be inserted “(a)", and at the end of the subsection there shall be inserted the following paragraph:—
“(b) where by virtue of any provision made under Part V of the Medicines Act 1968 (or made under any provisions of the said Part V as applied by an order made under section 104 or section 105 of that Act) anything which, in accordance with this Act, constitutes the application of a trade description to goods is subject to any requirements or restrictions imposed by that provision, any particular description specified in that provision, when applied to goods in circumstances to which those requirements or restrictions are applicable, shall be deemed not to be a trade description.”
Modifications etc. (not altering text)
C17The text of Sch. 5 paras. 1, 10, 13, 16, 7, Schs. 6, 8 is in the form in which it was originally enacted: it was not reproduced in Statutes in Force and does not reflect any amendments or repeals which may have been made prior to 1.2.1991.
[F3617U.K.In section 22, in subsection (2), after the words “the Food and Drugs Act (Northern Ireland) 1958 M4" there shall be inserted the words “or the Medicines Act 1968" ; in paragraph (b) the word “and", where it occurs at the end of that pargraph, shall be omitted ; and at the end of paragraph (c) there shall be inserted the words “and
(d)in relation to the said Act of 1968, so much of Schedule 3 to that Act as is applicable to the circumstances in which the sample was procured,”
at the end of the subsection there shall be inserted the words “or paragraph 27 of Schedule 3 to the said Act of 1968".]
Textual Amendments
F36Sch. 5 para. 17 repealed (E.W.S.) by Food Safety Act 1990 (c. 16, SIF 53:1, 2), ss. 54, 59(4), Sch. 5
Marginal Citations
Section 135(2)
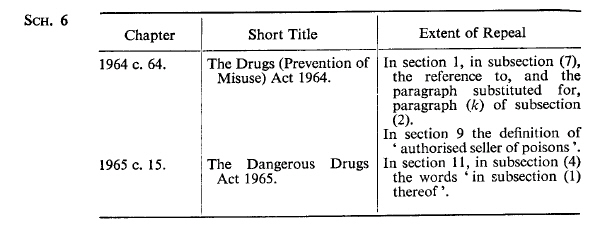
SCHEDULE 6U.K. Enactments of Parliament of United Kingdom Repealed.
Modifications etc. (not altering text)
C18The text of Sch. 6 is in the form in which it was originally enacted: it was not reproduced in Statutes in Force and does not reflect any amendments or repeals which may have been made prior to 1.2.1991
F37F37SCHEDULE 7U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F37Sch. 7 repealed by S.I. 1976/1213 (N.I. 22), Sch. 6 and 1976/1214 (N.I. 23), Sch. 3
Section 135(4)
SCHEDULE 8U.K. Enactments of Parliament of Northern Ireland Repealed.
Modifications etc. (not altering text)
C19The text of Sch. 8 is in the form in which it was originally enacted: it was not reproduced in Statutes in Force and does not reflect any amendments or repeals which may have been made prior to 1.2.1991
Options/Help
Print Options
PrintThe Whole Act
PrintThe Schedules only
You have chosen to open the Whole Act
The Whole Act you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different points in time where a change occurred. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. The first date in the timeline will usually be the earliest date when the provision came into force. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). This date is our basedate. No versions before this date are available. For further information see the Editorial Practice Guide and Glossary under Help.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources