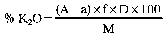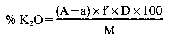11.DETERMINATION OF WATER-SOLUBLE POTASSIUM
SCOPE
1. This method is for the determination of water-soluble potassium.
FIELD OF APPLICATION
2. All the potassium fertilisers listed in Group 3(a) of Section A and Groups 1, 3 and 4 of Section B and Group 2 of Section C of the Table in Schedule 1 of the Fertilisers Regulations 1990().
PRINCIPLE
3. The potassium is extracted with water and after the removal of interfering substances, the potassium is precipitated in a slightly alkaline medium in the form of potassium tetraphenylborate (KTPB).
REAGENTS
4.—4.1 Formaldehyde, 25-30% solution, filter if necessary before use.
4.2 Potassium chloride.
4.3 Sodium hydroxide, 10 N solution. Care should be taken to ensure that the sodium hydroxide is free from potassium.
4.4 Indicator solution: dissolve 0.5 g phenolphtalein in 100 ml 90% ethanol.
4.5 EDTA solution: 4 g of the dihydrated disodium salt of ethylenediaminetetra-acetic acid (EDTA) per 100 ml. Store this reagent in a plastic container.
4.6 STPB solution: dissolve 32.5 g sodium tetraphenylborate in 480 ml of water, add 2 ml sodium hydroxide solution (4.3) and 20 ml of a magnesium chloride solution (100 g of MgCL2.6H2O per litre). Stir for fifteen minutes and filter through a fine, ashless filter. Store this reagent in a plastic container.
4.7 Liquid for washing: dilute 20 ml of the STPB solution (4.6) to 1 litre with water.
4.8 Bromine water: saturated bromine solution in water.
APPARATUS
5.—5.1 Filter crucibles with a porosity of 5 to 20 microns.
5.2 Oven regulated at 120±10°C.
PREPARATION OF THE SAMPLE
6. See Method 1.
In the case of potassium salts the sample must be ground fine enough in order that a representative sample is obtained for analysis. For these products, Method 1, paragraph 6(a) must be used.
PROCEDURE
Extraction
7.—7.1 Weigh to the nearest 0.001g, 10 g of the prepared sample (5 g for potassium salts containing more than 50% of potassium oxide or 20 g for fetilisers containing less than 5% of potassium oxide) and place in a 600 ml beaker with approximately 400 ml of water. Bring to the boil and allow it to boil for 30 minutes. Cool, transfer quantitatively into a 1 litre graduated flask, make up the volume, mix and filter into a dry receiver. Discard the first 50 ml of the filtrate.
Note: If the filtrate is dark in colour, transfer by pipette, an aliquot part containing at the most 100 mg of K2O and place in a 100 ml graduated flask, add bromine water and bring to the boil to eliminate any surplus bromine. After cooling make up the volume, filter and quantitatively determine the potassium in an aliquot part of the filtrate.
Determination
7.2 Transfer by pipette an aliquot part of the filtrate containing 25-50 mg of potassium (see Table on page 53) into a 250 ml beaker; make up to 50 ml with water.
To remove interferences, add 10 ml of the EDTA solution (4.5), several drops of the phenolphthalein solution (4.4) and stir in, drop by drop, sdoium hydroxide solution (4.3) until it turns red, then finally add a few more drops of sodium hydroxide to ensure an excess (usually 1 ml of sodium hydroxide is sufficient to neutralise the sample and ensure an excess).
To eliminate most of the ammonia boil gently for 15 minutes. Add water to make the volume up to 60 ml.
Bring the solution to the boil, remove the beaker from the heat and add 10 ml formaldehyde (4.1). Add several drops of phenolphthalein solution (4.4) and if necessary, more sodium hydroxide solution until a distinct red colour appears. Cover the beaker with a watch glass and place it on a steam bath for fifteen minutes.
Weighing the crucible
7.3 Dry the filter crucible (5.1) to constant weight in the oven at 120°C (5.2) (about 15 minutes).
Precipitation
7.4 Remove the beaker from the steam bath, stir in drop by drop 10 ml of the STPB solution (4.6). This addition should take about 2 minutes; allow to stand for at least 10 minutes before filtering.
Filtering and washing
7.5 Filter under vacuum into the weighed crucible, rinse the beaker with the liquid for washing (4.7), wash the precipitate three times with the liquid for washing (60 ml in all of the liquid for washing) and twice with 5 to 10 ml of water.
Drying and weighing
7.6 Wipe the outside of the crucible with a filter paper and place in the oven (5.2) for one and a half hours at temperature of 120°C. Allow the crucible to cool in a desiccator to ambient temperature and weigh rapidly.
Blank test
7.7 Make a blank test under the same conditions (omitting only the sample) and allow for this in the calculation of the final result.
Control test
7.8 Carry out the determination of an aliquot part of an aqueous solution of potassium chloride, containing at the most 40 mg of K2O.
EXPRESSION OF RESULTS
Method of calculation and formulae
8.—8.1 If the quantities and the dilutions shown in the Table are used, the following formulae apply:
% K2O in the fertiliser = (a − a) × F
or
% K in the fertiliser = (A − a) × F’
where:
A = weight in grams of the precipitate from the sample
a = weight in grams of the precipitate from the blank
F and F’ = factors—see Table.
| % of K2O in the fertiliser | % of K in the fertiliser | Sample for analysis (g) | Aliquot part to be taken as a sample for precipitation (ml) | Conversion factor F % K2O g KTPB | Conversion factor F % K g KTPB |
|---|
| 1 — 5 | 0.8 — 4.2 | 20 | 50 | 13.140 | 10.906 |
| 5 — 10 | 4.2 — 8.3 | 10 | 50 | 26.280 | 21.812 |
| 10 — 20 | 8.3 — 16.6 | 10 | 25 | 52.560 | 43.624 |
| 20 — 50 | 16.6 — 41.5 | 10 | 10 | 131.400 | 109.060 |
| more than 50 | more than 41.5 | 5 | 10 | 262.800 | 218.120 |
With samples and dilutions which differ from those shown in the Table use the following formulae:
or
where:
f = conversion factor, KTPB into K2O = 0.1314
f’ = conversion factor, KTPB into K = 0.109
D = dilution factor
M = weight in grams of sample for analysis