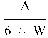ANNEX IIMETHOD FOR DETERMINING URIC ACID
Scope and Field of Application
1. This method is for the determination of uric acid and its salts in dried poultry waste and in feeding stuffs containing dried poultry waste.
Principle
2. Uric acid is extracted with neutral ethanotic formaldehyde solution, precipitated as silver magnesium urate, redissolved in sodium thiosulphate solution and determined spectrophotometrically.
Reagents
3.—3.1 Sodium hydroxide solution: dissolve 50 g sodium hydroxide in 50 ml water, mix well and store in a suitable plastic container.
3.2 Formaldehyde solution: the strength of the commercially available solution should be checked as follows: mix 3 ml formaldehyde solution with 50 ml IN sodium hydroxide solution and 25 ml hydrogen peroxide solution (20 volumes). Heat on a steam bath until effervescence stops. Cool, and titrate with IN hydrochloric acid using phenolphthalein indicator. Carry out a blank titration using 3 ml water in place of the formaldehyde.
3.3 Neutral ethanolic formaldehyde solution: mix an appropriate volume of formaldehyde solution (3.2) containing 17.5 g of formaldehyde with 250 ml water and 500 ml ethanol. Adjust the pH of the solution to 7.0 with 0.1N sodium bhydroxide solution. Dilute to 1,000 ml with water, mix and gain adjust the pH to 7.0 if necessary.
3.4 Scucinate buffer solution: dissolve by heating, 29.5 g of succinic acid in 750 ml water and 20 ml sodium hydroxide solution (3.1). Cool, add an appropriate volume of formaldehyde solution (3.2) containing 17.5 g of formaldehyde, mix well and adjust the pH to 6.0 with sodium hydorxide solution (3.1). Dilute to 1,000 ml with water, mix and gain adjust the pH to 6.0 if necessary.
3.5 sodium thiosulphate solution: 25 g sodium thiosulphate (Na2S2O3.5H2O) per 1,000 ml.
3.6 Silver lactate solution: dissolve, by heating, 3 g silver lactate in 50 ml water and 1 ml lactic acid. Dilute to 100 ml with water, filter, and store in dark glassware. Do not expose to strong light.
3.7 Ammoniacal magnesium solution: dissolve 8.75 g magnesium sulphate (MgSO4.7H2O) and 17.5 g ammonium chloride in 50 ml water. Add 30 ml ammonia solution (d = 0.88 g/ml) mix well and dilute to 100 ml with water.
3.8 Benedict and Hitchcock reagent: mix 35 ml silver lactate solution (3.6) with 15 ml ammoniacal magnesium solution (3.7). Add 50 ml ammonia solution (d = 0.88 g /ml). Mix well, Prepare immediately before use.
3.9 Standard uric acid solution: weigh to the nearest 0.1 mg, 250 mg of uric acid and transfer to a 150 ml round-bottomed flask fitted with a reflux condenser. Add 100 ml ethanolic formaldehyde solution (3.3) and boil under reflux on a steam bath for 30 minutes, shaking frequently. Cool, transfer the solution to a 250 ml graduated flask, wash the round-bottomed flask with ethanolic formaldehyde solution (3.3) and combine the washings with the uric acid solution. Dilute to the mark with ethanolic formaldehyde solution (3.3) and mix. 1 ml contains 1 mg of uric acid.
3.10 Light petroleum, boiling range 40–60°C.
Apparatus
4.—4.1 Spectrophotometer, with 10 mm silica cells.
4.2 Percolation tubes, glass. Upper part: approximately 240 mm long, 18 mm internal diameter, lower part: approximately 120 mm long, 8 mm internal diameter.
Procedure
Extraction of Uric Acid
5.1.1 From dried poultry waste:
5.1.1Weigh to the nearest 0.001 g, about 0.4 g dried poultry waste and place in a 150 ml round-bottomed flask. Add 60 ml ethanolic formaldehyde solution (3.3), fit a reflux condenser onto the flask and heat on a steam bath for 1 hour. Cool and filter by suction through a sintered glass crucible (porosity 4) into a 100 ml graduated flask. Wash out the round-bottomed flask with 3 × 10 ml portions of ethanolic formaldehyde solution (3.3) passing each portion through the crucible into the graduated flask. Dilute to 100 ml with ethanolic formaldehyde solution and mix.
5.1.2 From feeding stuffs:
5.1.2Weigh to the nearest 0.001 g, between 4 g and 5 g of prepared sample. Transfer to a glass percolation tube (4.2) fitted with a small paper cup to retain the feed. Remove the fat from the feed by extraction with light petroleum (3.10). Transfer quantitatively the defatted sample to a 150 ml round-bottomed flask and remove the residual solvent with a slow current of air. Continue as in 5.1.1, second sentence “… Add 60 ml ethanolic formaldehyde solution (3.3) …”.
Determination
5.2 Transfer by pipette 20 ml of the sample extract prepared as in 5.1.1 or 5.1.2 to a 50 ml centrifuge tube. Add 10 ml of Benedict and Hitchcock reagent 93.8), mix well and allow to stand in the dark for 1 hour. Centrifuge at 2,000 rpm for 15 minutes, pour off the supernatant liquid and allow to drain for 10 minutes. Carefully wipe off any remaining liquid without disturbing the precipitate, and add 20 ml sodium thiosulphate solution (3.5) to each tube. Dissolve the precipitate by stirring with a thin glass rod. Transfer by pipette 5 ml of this solution into a 200 mg graduated flask containing 40 ml succinate buffer solution (3.4). Dilute to 200 ml with water and mix well. Measure the absorbance of the solution at 294 mm in 10 mm silica cells against a solution prepared by mixing 5 ml sodium thiosulphate solution (3.5) with 40 ml succinate buffer solution (3.4) and diluting to 200 ml with water. Determine the quantity of uric acid present by reference to the calibration curve (5.3).
Calibration Curve
5.3 Into a series of 50 ml centrifuge tubes, transfer by pipette 2, 4, 6, 8, 10 and 12 ml standard uric acid solution (3.9) (corresponding to 2, 4, 6, 8, 10 and 12 mg of uric acid) and make up to 20 ml with ethanolic formaldehyde solution (3.3). Add to each tube 10 ml Benedict and Hitchcock reagent (3.8), mix well and stand in the dark for 1 hour. Continue as in 5.2 from “… Centrifuge at 2,000 rpm. …”, Measure the absorbances of the solutions and plot the calibration curve using absorbances as the ordinates and the corresponding quantities of uric acid, in mg (as shown above) as the abseissae.
Expression of the Results
6. The uric acid nitrogen content per cent of the sample is given by the formula
where:
A
=
mg uric acid (in the aliquot volume of the sample extract) as determined by photometric measurement; and
W
=
weight of sample in grams.