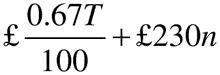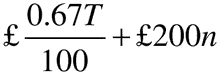SCHEDULE 7U.K.Fees
PART 2U.K.Fees relating to marketing authorisations
Annual fees for marketing authorisationsU.K.
26.—(1) Within 30 days of receiving a written demand from the Secretary of State, a holder of a marketing authorisation must provide the Secretary of State with a statement of turnover for the previous calendar year.
(2) The annual fee, rounded to the next £1, is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(3) In the case of an authorisation holder with a turnover relating to all marketing authorisations held of less than £230,000, the annual fee, rounded to the next £1 is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(4) In this paragraph—
“turnover” means the sales value at manufacturers’ prices of all authorised veterinary medicinal products sold or supplied in the United Kingdom;
“manufacturers’ prices” means the prices charged (excluding value added tax) for authorised products by manufacturers to wholesalers, except to the extent that—
the products are supplied by manufacturers direct to retailers, in which case it means the prices charged for the products by the manufacturers to the retailers reduced by such sum as, in the opinion of the Secretary of State, represents the difference between the prices paid by the retailers and those which could be expected to be charged by the manufacturers to wholesalers according to the practice prevailing during the period in question with regard to such products;
a marketing authorisation holder sells or supplies products that the marketing authorisation holder has neither manufactured nor obtained from the manufacturer, in which case it means the prices paid by the marketing authorisation holder for those products.