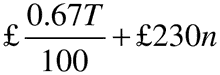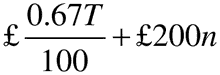- Latest available (Revised)
- Point in Time (14/04/2014)
- Original (As made)
The Veterinary Medicines Regulations 2013
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Changes over time for: PART 2
Version Superseded: 31/12/2020
Alternative versions:
- 01/10/2013- Amendment
- 14/04/2014- Amendment
- 14/04/2014
Point in time - 31/12/2020- Amendment
- 17/05/2024- Amendment
Status:
Point in time view as at 14/04/2014.
Changes to legislation:
There are currently no known outstanding effects for The Veterinary Medicines Regulations 2013, PART 2.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
PART 2U.K.Fees relating to marketing authorisations
Specified pharmaceutical applicationsU.K.
7. The following table sets out the fees relating to a pharmaceutical veterinary medicinal product for—
(a)a national application for a marketing authorisation that is—
(i)a full application under Part 1 of Schedule 1;
(ii)a bibliographic application; or
(iii)an application based on pharmacological equivalence;
(b)an application for a marketing authorisation using the decentralised procedure where the United Kingdom is a concerned member State;
(c)an application for the mutual recognition of a product authorised in another member State.
| Application | Full national application under Part 1 of Schedule 1 (£) | Bibliographic national application (£) | Pharmacologically equivalent national application | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) | ||
|---|---|---|---|---|---|---|
| Reference product authorised in UK (£) | Reference product not authorised in UK (£) | |||||
| Base Fee: | 13,530 | 12,115 | 7,195 | 9,220 | 6,515 | |
| Additional fee if any of the target species is a food-producing animal: | 3,905 | 3,585 | 2,155 | 2,760 | 1,415 | |
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom— | ||||||
| food-producing animal: | 7,465 | 6,595 | 5,885 | 7,495 | 2,630 | |
| non-food-producing animal: | 6,525 | 5,855 | 5,590 | 7,155 | 2,295 | |
| Additional fee for each additional pack type: | 740 | 740 | 605 | 775 | 330 | |
| Additional fee for each additional active ingredient (food-producing animal): | 6,465 | 6,125 | 4,040 | 5,165 | 2,085 | |
| Additional fee for each additional active ingredient (non-food-producing animal): | 4,310 | 4,105 | 3,235 | 4,135 | 1,475 | |
| Additional fee if there is more than one target species, for each additional species (food-producing animal): | 3,970 | 3,565 | 2,425 | 3,100 | 1,280 Applies for a maximum of 2 additional species | |
| Additional fee if there is more than one target species, for each additional species (non- food-producing animal): | 2,495 | 2,090 | 1,550 | 1,980 | 805 Applies for a maximum of 2 additional species | |
| Additional fee for each additional recommended route of administration (food-producing animal): | 2,695 | 2,490 | 1,620 | 2,070 | 940 | |
| Additional fee for each additional recommended route of administration (non- food-producing animal): | 1,215 | 1,010 | 740 | 945 | 405 | |
| Simultaneous applications: fee for each additional product in the application: | 2,895 | 2,895 | 2,895 | 3,705 | 1,685 | |
Decentralised pharmaceutical application where the United Kingdom is the reference member StateU.K.
8. The fee for a decentralised application for a pharmaceutical product where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 7, with the addition of the fees in the following table.
Fees for decentralised pharmaceutical application where the United Kingdom is the reference member State
| Application | Additional fee for a pharmacologically equivalent product (£) | Additional fee otherwise (£) | |
|---|---|---|---|
| Food-producing animal: one member State: | 5,230 | 3,705 | |
| Non-food-producing animal: one member State: | 3,985 | 3,220 | |
| Each additional member State: | 530 | 530 | |
| Simultaneous application: fee for each additional product in the application: | |||
| one member State: | 6,670 | 6,670 | |
| each additional member State: | 120 | 120 | |
Application for a marketing authorisation for an immunological or biosimilar productU.K.
9.—(1) The fee for a national application for a marketing authorisation relating to an immunological or biosimilar product, a decentralised application where the United Kingdom is the concerned member State or the mutual recognition of a product authorised in another member State is in accordance with the following table.
(2) In this paragraph a biosimilar application means an application made in accordance with Article 13(4) of Directive 2001/82/EC and a biosimilar product means a product which is the subject of such an application.
Fees for specified immunological and biosimilar applications
| Application | National application for a marketing authorisation(£) | Decentralised application where the UK is a concerned member State or recognition of a product authorised in another member State (£) | |
|---|---|---|---|
| 1. Immunological or biosimilar product other than in paragraph 2 below: Base fee: | 11,775 | 5,785 | |
| The following fees are in addition to the base fee— | |||
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom, and for each new combination of active ingredients: | 7,405 | 2,490 | |
| Additional fee for each adjuvant or preservative not previously included in a veterinary medicinal product authorised in the United Kingdom and for each new combination of adjuvants or preservatives: | 1,345 | 675 | |
| More than one antigenic component – fee for each additional component: | 1,350 | 405 | |
| More than one species – fee for each additional species: | 5,380 | 1,615 Applies for a maximum of 2 additional species | |
| More than one route of administration – fee for each additional route of administration: | 5,380 | 1,615 | |
| Simultaneous application - fee for each additional product in the application: | 2,895 | 1,685 | |
| 2. Immunological or product that is identical to a product already authorised in the United Kingdom but with a lesser number of antigens and that only contains antigens contained in that product: | 10,430 | 5,380 | |
Decentralised immunological application where the United Kingdom is the reference member StateU.K.
10. The fee for a decentralised application for a marketing authorisation for an immunological product where the United Kingdom is the reference member State is the same as for a national application set out in the previous table, with the addition of the fees in the following table—
Fees for decentralised immunological application where the United Kingdom is the reference member State
| Application | Additional fee (£) | |
|---|---|---|
| One member State: | 3,470 | |
| Each additional member State: | 530 | |
| Simultaneous applications: fee for each additional product in the application: | ||
| one member State: | 6,670 | |
| each additional member State: | 120 | |
Applications for a marketing authorisation using data already assessedU.K.
11. The fees for applications for marketing authorisations using identical data submitted simultaneously or on the basis of information provided under Article 13(c) of Directive 2001/82/EC are in accordance with the following table.
Fees for a marketing authorisation using data already assessed
| Application | Fee (£)per authorisation | |
|---|---|---|
| Decentralised application where the United Kingdom is the reference member State— | ||
| one member State: | 4,165 | |
| each additional member State: | 530 | |
| Any other application: | 945 | |
Application for an exceptional marketing authorisation (pharmaceutical)U.K.
12. The fee for an application for an exceptional marketing authorisation for a pharmaceutical product is in accordance with the following table.
Fees for an exceptional marketing authorisation for a pharmaceutical product
| Application | Provisional (£) | Limited (£) | |
|---|---|---|---|
| Base Fee: | 12,015 | 6,765 | |
| The following fees are in addition to the base fee— | |||
| Additional fee if any of the target species is a food-producing animal: | 3,905 | 1,952 | |
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom— | |||
| food-producing animal: | 5,850 | 3,732 | |
| non-food-producing animal: | 4,910 | 3,262 | |
| Additional fee for each additional pack type: | 710 | 370 | |
| Additional fee for each additional active ingredient (food-producing animal): | 5,955 | 3,232 | |
| Additional fee for each additional active ingredient (non-food-producing animal): | 3,800 | 2,155 | |
| Additional fee if there is more than one target species, for each additional species (food-producing animal): | 2,965 | 1,985 | |
| Additional fee if there is more than one target species, for each additional species (non-food-producing animal): | 1,485 | 1,247 | |
| Additional fee for each additional recommended route of administration (food-producing animal): | 2,185 | 1,347 | |
| Additional fee for each additional recommended route of administration (non-food-producing animal): | 710 | 608 | |
| Simultaneous applications— fee for each additional product in the application: | 2,895 | 1,447 | |
Fees for an application for an exceptional marketing authorisation (immunological)U.K.
13. The fee for an application for an exceptional marketing authorisation for an immunological product is in accordance with the following table.
Fees for an exceptional marketing authorisation for an immunological product
| Application | Provisional (£) | Limited (£) |
|---|---|---|
| Base fee: | 10,810 | 5,887 |
| The following fees are in addition to the base fee— | ||
| Additional fee for each active ingredient not previously included in a veterinary medicinal product authorised in the United Kingdom, and for each new combination of active ingredients: | 5,650 | 3,702 |
| Additional fee for each adjuvant or preservative not previously included in a veterinary medicinal product authorised in the United Kingdom and for each new combination of adjuvants or preservatives: | 1,350 | 672 |
| More than one antigenic component – fee for each additional component: | 1,190 | 675 |
| More than one species – fee for each additional species: | 4,060 | 2,690 |
| More than one route of administration – fee for each additional route of administration: | 4,060 | 2,690 |
| Simultaneous application - fee for each additional product in the application: | 2,895 | 1,447 |
Fee for the conversion from an exceptional to a full marketing authorisationU.K.
14. The fee for the conversion of an exceptional marketing authorisation to a full marketing authorisation is £3,000.
Application for a marketing authorisation relating to a parallel importU.K.
15. The fee for a marketing authorisation for a parallel import is in accordance with the following table.
Parallel imports
| Application | Fee (£) | |
|---|---|---|
| Application where the imported product has been authorised in accordance with the mutual recognition procedure or decentralised procedure, and the United Kingdom is included in these procedures— | ||
| import from one or more member States: | 1,755 | |
| Application to add an additional member State after the marketing authorisation has been granted – fee for each member State: | 455 | |
| Application where the imported product has not been authorised in accordance with the mutual recognition procedure or the decentralised procedure but where the imported product originates from the same manufacturing site as the product authorised in the United Kingdom to which the imported product is considered to be essentially similar: | 2,130 | |
| Any other application – fee for each member State from which the product is imported: | 4,710 | |
Application to change the distribution category of a product authorised through the centralised procedureU.K.
16. The fee to change the distribution category of a product authorised through the centralised procedure is £3,135.
Application for a variation to a marketing authorisation dealt with under national or mutual recognition variation procedures.U.K.
17.—(1) This paragraph applies in relation to an application for a variation to one or more marketing authorisations except where paragraph 18, 19 or 21 applies.
(2) The fees for the variations to which this paragraph applies are set out in the following table.
(3) Where applications are made at the same time seeking an identical change to the terms of more than one marketing authorisation, and those applications are based on identical data, fees are payable as for a grouped variation.
(4) References in this paragraph to a grouped variation being “led” by a particular type of variation indicate that the principal variation in that group is a variation of that type.
| Type of variation | National | UK is the reference member State | UK is a concerned member State | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Single variations; one change for each product | |||||||||
| Extension: | |||||||||
| Change of strength or potency or the addition of a new strength or potency: | 6,670 | - | 1,998 | ||||||
| Change of pharmaceutical form or the addition of a new pharmaceutical form: | 8,415 | - | 2,301 | ||||||
| Change of route of administration, or the addition of a new one, of— | - | ||||||||
| (i) an immunological product, or a pharmaceutical product for a [F1non-food-producing] animal: | 5,390 | - | 1,737 | ||||||
| (ii) a pharmaceutical product for a food-producing animal: | 7,135 | - | 2,058 | ||||||
| Change or addition of a food producing target species: | 9,620 | - | 2,547 | ||||||
| Change of active substance, including: | 8,415 | - | 2,301 | ||||||
| use of a different salt, ester, complex or derivative of the same therapeutic moiety: | |||||||||
| use of a different biologically active substance with a slightly different molecular structure: | |||||||||
| modification of the vector used to produce the antigen or the source material, including a new master cell bank from a different source: | |||||||||
| use of a new ligand or coupling mechanism for a radiopharmaceutical: | |||||||||
| change of the extraction solvent or change of the ratio of herbal drug to herbal drug preparation: | |||||||||
| Change of bioavailability: | 8,415 | - | 2,301 | ||||||
| Change of pharmacokinetics: | 8,415 | - | 2,301 | ||||||
| Simultaneous application: fee for each additional product in the application: | 2,895 | - | 1,011 | ||||||
| Type II: | 2,895 | 6,030 | 1,872 | ||||||
| Type IB: | 885 | 1,325 | 531 | ||||||
| Type IA: | 455 | 685 | 273 | ||||||
| Grouped variations | |||||||||
| Extension-led: | |||||||||
| The fee for an application for an extension-led grouped variation is the fee for that extension as specified above plus — | |||||||||
| (a) if there is one variation in addition to the extension, the fee for that variation as specified above; or | |||||||||
| (b) if there is more than one variation in addition to the extension, the fee that would be payable for a grouped variation of that type as specified below. | |||||||||
| Type II led: | |||||||||
| For the first nine changes: | 6,280 | 12,060 | 3,768 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
| Type IB led: | |||||||||
| For the first nine changes: | 1,770 | 2,650 | 1,062 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
| Type IA led: | |||||||||
| For the first nine changes: | 885 | 1,325 | 531 | ||||||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 2,700 | ||||||
Textual Amendments
F1Words in Sch. 7 para. 17 substituted (14.4.2014) by The Veterinary Medicines (Amendment) Regulations 2014 (S.I. 2014/599), regs. 1, 4(2)
Application for a variation to a marketing authorisation dealt with under worksharing proceduresU.K.
18.—(1) This paragraph applies in relation to an application for a variation to a marketing authorisation dealt with in accordance with worksharing procedures as set out in Article 20 of Commission Regulation (EC) No 1234/.
(2) The fee for a worksharing application, involving marketing authorisations obtained by a national procedure in the United Kingdom only, is the fee specified in the following table in the column headed “UK Only”.
(3) The fee for a worksharing application, involving marketing authorisations obtained through a national procedure in the United Kingdom and any other member State, is specified in the following table by reference to the United Kingdom’s role in the procedure, as “UK Reference Authority”, “UK Co-Reference Authority” or “Other”.
(4) The fee for a worksharing application, involving at least one marketing authorisation obtained through the mutual recognition or decentralised procedure, is specified in the following table by reference to the United Kingdom’s role in the procedure, as “UK Reference Authority”, “UK Co-Reference Authority” or “UK Concerned member State”.
(5) The fee for any kind of variation where the Agency co-ordinates worksharing is £455 for each marketing authorisation.
| Type of application | UK Only | Where the application involves nationally authorised products in more than one member State | Application involves mutually recognised products | ||||||
|---|---|---|---|---|---|---|---|---|---|
UK Only | UK Reference Authority | UK Co-Reference Authority | Other | UK Reference Authority | UK Co-Reference Authority | UK Concerned member State | |||
Worksharing applications The following fees apply for each change to each product: | |||||||||
| Type II | |||||||||
| For the first nine changes: | 6,240 | 12,060 | 7,485 | 12,060 | 13,265 | 6,745 | 3,372 | ||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 2,700 | ||
| Type IB | |||||||||
| For the first nine changes: | 1,770 | 2,650 | 2,120 | 2,650 | 2,915 | 1,905 | 954 | ||
| For each subsequent group of up to ten changes: | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 4,500 | 2,700 | ||
Application for an extension dealt with under the decentralised procedure where the United Kingdom is the reference member StateU.K.
19. The fee for a decentralised application for an extension where the United Kingdom is the reference member State is the same as for a national application as set out in the table in paragraph 17, with the addition of the supplementary fees in the following table (save that, where the application is for the addition of more than one species, only one supplementary fee applies).
Decentralised application for an extension where the United Kingdom is the reference member State
| Application | Supplementary fee (£) | |
|---|---|---|
| Pharmaceutical product for a food-producing animal – one member State: | 3,705 | |
| Pharmaceutical product for a non-food-producing animal – one member State: | 3,220 | |
| Immunological product – one member State: | 3,460 | |
| Each additional member State: | 530 | |
| Simultaneous application: fee for each additional product in the application: | ||
| one member State: | 6,670 | |
| each additional member State: | 120 | |
Provision of information relating to the recognition of a United Kingdom marketing authorisation or an extensionU.K.
20.—(1) Where an application is made for the Secretary of State to provide information to other member States to enable them to recognise a marketing authorisation already granted by the United Kingdom the following fees are payable.
(2) Those fees also apply where a marketing authorisation has been granted in more than one member State, the holder applies for an extension for that marketing authorisation and the United Kingdom acts as reference member State.
(3) Where a valid application to provide information to another member State is received within six months of the original grant of the marketing authorisation, or where the Secretary of State has already provided the information to a member State, and a further valid application is made to provide the information to an additional member State within six months of the date the last information was provided, the fees are—
| Type of application | Fee for a pharmacologically equivalent product(a) | Fee (other products) (£) | ||
|---|---|---|---|---|
(a) This fee is payable if the application for the marketing authorisation was on the basis that the product was pharmacologically equivalent to another veterinary medicinal product. | ||||
| Pharmaceutical product for a food-producing animal – one member State: | 3,940 | 2,440 | ||
| Pharmaceutical product for a non-food-producing animal ‑ one member State: | 2,645 | 1,895 | ||
| Immunological product – one member State: | 2,130 | 2,130 | ||
| Each additional member State: | 535 | 535 | ||
(4) Where the information to be provided relates to a product granted a marketing authorisation using identical data submitted simultaneously or on the basis of information provided under Article 13(c) of Directive 2001/82/EC the fees are—
| Application | Fee (£) | |
|---|---|---|
| Provision of information to— | ||
| one member State: | 4,165 | |
| each additional member State: | 530 | |
(5) In any other case the fees are—
| Type of application | Fee for a pharmacologically equivalent product (£)(a) | Fee (other products) (£) | |
|---|---|---|---|
(a) This fee is payable if the application for the marketing authorisation was on the basis that the product was pharmacologically equivalent to another veterinary medicinal product. | |||
| Pharmaceutical product for a food-producing animal – one member State: | 12,015 | 10,515 | |
| Pharmaceutical product for a non-food-producing animal – one member State: | 8,115 | 7,365 | |
| Immunological product – one member State: | 8,940 | 8,940 | |
| Each additional member State: | 535 | 535 | |
(6) In the case of simultaneous applications, the above fees are payable for each additional product in the application for one member State, with a fee of £115 for each additional product for each additional member State.
Exception for a variation relating to animal testingU.K.
21. If the only purpose of a variation is to remove animal testing or to reduce the numbers of animals used in testing, no fee is payable for the variation in the case of a national authorisation, and the United Kingdom element of the fee for the variation is not payable for an authorisation obtained through the mutual recognition procedure or the decentralised procedure.
Application for the renewal of a national marketing authorisationU.K.
22.—(1) The fee for an application for the renewal of a marketing authorisation is £1,360.
(2) The fee for the first reassessment of an exceptional marketing authorisation is £305, and the fee for each subsequent reassessment is £1,360.
Application for the renewal of a marketing authorisation obtained through mutual recognition or the decentralised procedureU.K.
23. The fee for an application for the renewal of a marketing authorisation obtained through mutual recognition or the decentralised procedure is —
(a)£1,835 if the United Kingdom is the reference member State; and
(b)£1,225 if the United Kingdom is a concerned member State.
Registration of a homeopathic remedyU.K.
24. The fee for an application for the registration of a homeopathic remedy is in accordance with the following table.
Fee for the registration of a homeopathic remedy
| Type of application | Fees(£) | ||
|---|---|---|---|
| If all stocks and the formulation have already been assessed by the Secretary of State— | |||
| not more than five stocks: | 160 | ||
| more than five stocks: | 375 | ||
| If either all the stocks have already been assessed by the Secretary of State but there is a new formulation, or if the formulation has already been assessed by the Secretary of State but one or more of the stocks have not been already assessed— | |||
| not more than five stocks: | 455 | ||
| more than five stocks: | 665 | ||
| If the formulation and at least one of the stocks has not already been assessed by the Secretary of State— | |||
| not more than five stocks: | 760 | ||
| more than five stocks: | 985 | ||
| If the product is already authorised for human use in the United Kingdom, or for human or veterinary use in the United Kingdom or in another member State— | |||
| not more than five stocks: | 160 | ||
| more than five stocks: | 375 | ||
Renewal of a homeopathic remedyU.K.
25. The fee for the renewal of a homeopathic remedy is £320.
Annual fees for marketing authorisationsU.K.
26.—(1) Within 30 days of receiving a written demand from the Secretary of State, a holder of a marketing authorisation must provide the Secretary of State with a statement of turnover for the previous calendar year.
(2) The annual fee, rounded to the next £1, is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(3) In the case of an authorisation holder with a turnover relating to all marketing authorisations held of less than £230,000, the annual fee, rounded to the next £1 is—
where—
T is the annual turnover in the previous calendar year;
and n is the number of active marketing authorisations held at any time during the previous calendar year.
(4) In this paragraph—
“turnover” means the sales value at manufacturers’ prices of all authorised veterinary medicinal products sold or supplied in the United Kingdom;
“manufacturers’ prices” means the prices charged (excluding value added tax) for authorised products by manufacturers to wholesalers, except to the extent that—
the products are supplied by manufacturers direct to retailers, in which case it means the prices charged for the products by the manufacturers to the retailers reduced by such sum as, in the opinion of the Secretary of State, represents the difference between the prices paid by the retailers and those which could be expected to be charged by the manufacturers to wholesalers according to the practice prevailing during the period in question with regard to such products;
a marketing authorisation holder sells or supplies products that the marketing authorisation holder has neither manufactured nor obtained from the manufacturer, in which case it means the prices paid by the marketing authorisation holder for those products.
Auditor’s certificateU.K.
27.—(1) The Secretary of State may at any time require an audit certificate in support of a statement of turnover.
(2) If the holder of the marketing authorisation does not provide an audit certificate before the date stipulated in the demand, an additional fee is payable for that year of £11,300 plus an additional £2,245 in respect of each marketing authorisation held.
(3) If the Secretary of State is not satisfied that the audit certificate provides sufficient assurance that the figures fairly present the financial records of the company, the Secretary of State may require the marketing authorisation holder to produce a further certificate and specify what further assurances are needed; and if these are not provided by the required date, the additional fee specified in sub-paragraph (2) is payable.
(4) Nothing in this paragraph limits the powers of an inspector to examine financial records.
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Whole Schedule
PrintThis Part only
You have chosen to open The Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open The Whole Instrument as a PDF
The Whole Instrument you have selected contains over 200 provisions and might take some time to download.
Would you like to continue?
You have chosen to open the Whole Instrument
The Whole Instrument you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Explanatory Memorandum
Explanatory Memorandum sets out a brief statement of the purpose of a Statutory Instrument and provides information about its policy objective and policy implications. They aim to make the Statutory Instrument accessible to readers who are not legally qualified and accompany any Statutory Instrument or Draft Statutory Instrument laid before Parliament from June 2004 onwards.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Impact Assessments
Impact Assessments generally accompany all UK Government interventions of a regulatory nature that affect the private sector, civil society organisations and public services. They apply regardless of whether the regulation originates from a domestic or international source and can accompany primary (Acts etc) and secondary legislation (SIs). An Impact Assessment allows those with an interest in the policy area to understand:
- Why the government is proposing to intervene;
- The main options the government is considering, and which one is preferred;
- How and to what extent new policies may impact on them; and,
- The estimated costs and benefits of proposed measures.
Timeline of Changes
This timeline shows the different points in time where a change occurred. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. The first date in the timeline will usually be the earliest date when the provision came into force. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). This date is our basedate. No versions before this date are available. For further information see the Editorial Practice Guide and Glossary under Help.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources