2019 No. 696
The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019
Made
Coming into force in accordance with regulation 1
The Secretary of State makes the following Regulations in exercise of the powers conferred by section 8(1) of, and paragraph 21 of Schedule 7 to, the European Union (Withdrawal) Act 2018 M1.
In accordance with paragraph 1(1) of Schedule 7 to the European Union (Withdrawal) Act 2018 a draft of this instrument has been laid before Parliament and approved by a resolution of each House of Parliament.
PART 1Introduction
Citation and CommencementI41
These Regulations may be cited as the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 and come into force on exit day.
ExtentI852F52
Subject to regulation 3, these Regulations extend to England and Wales and Scotland only.
I853F63
The following provisions extend to the United Kingdom—
a
Part 1;
b
regulation 4 and Schedule 1;
c
Part 5 and its related schedules, except—
i
paragraphs 29 to 40 of Schedule 34 (cosmetic product enforcement);
ii
paragraphs 1 and 2 of Schedule 35 (personal protective equipment enforcement);
iii
paragraph 1 of Schedule 36 (gas appliances enforcement);
d
Part 6 and its related schedule.
PART 2Amendment of primary legislation
Amendment of the Hallmarking Act 1973I8544
Schedule 1 contains amendment of the Hallmarking Act 1973 M2.
Amendment of the Weights and Measures Act 1985I8555
Schedule 2 contains amendment of the Weights and Measures Act 1985 M3.
Amendment of the Consumer Protection Act 1987I8566
Schedule 3 contains amendment of the Consumer Protection Act 1987 M4.
PART 3Amendment of subordinate legislation
Amendment of the Measuring Container Bottles (EEC Requirements) Regulations 1977I8577
Schedule 4 contains amendment of the Measuring Container Bottles (EEC Requirements) Regulations 1977 M5.
Amendment of the Measuring Instruments (EEC Requirements) Regulations 1988I8588
Schedule 5 contains amendment of the Measuring Instruments (EEC Requirements) Regulations 1988 M6.
Amendment of the Weights and Measures (Intoxicating Liquor) Order 1988I8599
Schedule 6 contains amendment of the Weights and Measures (Intoxicating Liquor) Order 1988 M7.
Amendment of the Lifting Operations and Lifting Equipment Regulations 1998I86010
Schedule 7 contains amendment of the Lifting Operations and Lifting Equipment Regulations 1998 M8.
Amendment of the Noise Emission in the Environment by Equipment for use Outdoors Regulations 2001I86111
Schedule 8 contains amendment of the Noise Emission in the Environment by Equipment for use Outdoors Regulations 2001 M9.
Amendment of the General Product Safety Regulations 2005I86212
Schedule 9 contains amendment of the General Product Safety Regulations 2005 M10.
Amendment of the Offshore Installations (Safety Case) Regulations 2005I86313
Schedule 10 contains amendment of the Offshore Installations (Safety Case) Regulations 2005 M11.
Amendment of the Weights and Measures (Packaged Goods) Regulations 2006I86414
Schedule 11 contains amendment of the Weights and Measures (Packaged Goods) Regulations 2006 M12.
Amendment of the Supply of Machinery (Safety) Regulations 2008I86515
Schedule 12 contains amendment of the Supply of Machinery (Safety) Regulations 2008 M13.
Amendment of the Aerosol Dispensers Regulations 2009I86616
Schedule 13 contains amendment of the Aerosol Dispensers Regulations 2009 M14.
Amendment of the Accreditation Regulations 2009I86717
Schedule 14 contains amendment of the Accreditation Regulations 2009 M15.
Amendment of the Toys (Safety) Regulations 2011I86818
Schedule 15 contains amendment of the Toys (Safety) Regulations 2011 M16.
Amendment of the Explosives Regulations 2014I86919
Schedule 16 contains amendment of the Explosives Regulations 2014 M17 and associated provision relating to retained direct EU legislation.
Amendment of the Weights and Measures (Revocations) Regulations 2015I87020
Schedule 17 contains amendment of the Weights and Measures (Revocations) Regulations 2015 M18.
Amendment of the Offshore Installations (Offshore Safety Directive) (Safety Case etc.) Regulations 2015I87121
Schedule 18 contains amendment of the Offshore Installations (Offshore Safety Directive) (Safety Case etc.) Regulations 2015 M19.
Amendment of the Pyrotechnic Articles (Safety) Regulations 2015I87222
Schedule 19 contains amendment of the Pyrotechnic Articles (Safety) Regulations 2015 M20.
Amendment of the Electromagnetic Compatibility Regulations 2016I87323
Schedule 20 contains amendment of the Electromagnetic Compatibility Regulations 2016 M21.
Amendment of the Simple Pressure Vessels (Safety) Regulations 2016I87424
Schedule 21 contains amendment of the Simple Pressure Vessels (Safety) Regulations 2016 M22.
Amendment of the Lifts Regulations 2016I87525
Schedule 22 contains amendment of the Lifts Regulations 2016 M23.
Amendment of the Electrical Equipment (Safety) Regulations 2016I87626
Schedule 23 contains amendment of the Electrical Equipment (Safety) Regulations 2016 M24.
Amendment of the Pressure Equipment (Safety) Regulations 2016I87727
Schedule 24 contains amendment of the Pressure Equipment (Safety) Regulations 2016 M25.
Amendment of the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016I87828
Schedule 25 contains amendment of the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016 M26.
Amendment of the Non-automatic Weighing Instruments Regulations 2016I87929
Schedule 26 contains amendment of the Non-automatic Weighing Instruments Regulations 2016 M27.
Amendment of the Measuring Instruments Regulations 2016I88030
Schedule 27 contains amendment of the Measuring Instruments Regulations 2016 M28.
Amendment of the Recreational Craft Regulations 2017 and related amendmentI88131
Schedule 28 contains amendment of the Recreational Craft Regulations 2017 M29 and of Commission Implementing Regulation (EU) 2017/1 of 3 January 2017 on procedures for watercraft identification under Directive 2013/53/EU of the European Parliament and of the Council on recreational and personal watercraft.
Amendment of the Radio Equipment Regulations 2017 and related amendmentsI88232
Schedule 29 contains amendment of the Radio Equipment Regulations 2017 M30 and of Commission Implementing Regulation specifying how to present the information provided for in Article 10(10) of Directive 2014/53/EU of the European Parliament and the Council (EU) 2017/1354.
F3PART 4Amendment of subordinate legislation relating to Northern Ireland
Pt. 4 omitted (31.12.2020 immediately before IP completion day) by virtue of The Product Safety and Metrology etc. (Amendment to Extent and Meaning of Market) (EU Exit) Regulations 2020 (S.I. 2020/676), regs. 1(1), 3
Amendment of the Identification and Traceability of Explosives Regulations (Northern Ireland) 2013F333
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Amendment of the Making Available on the Market and Supervision of Transfers of Explosives Regulations (Northern Ireland) 2016F334
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Amendment of the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations (Northern Ireland) 2017F335
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
PART 5Amendment of retained direct EU legislation
Amendment of Regulation (EC) No 765/2008I88336
Schedule 33 contains amendment of Regulation (EC) No 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93.
Amendment of Regulation (EC) No 1223/2009 and related amendmentsI88437
Schedule 34 contains amendment of Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products, and amendment of the Cosmetic Products Enforcement Regulations 2013 M31, and amendment of related direct EU tertiary legislation.
Amendment of Regulation (EU) 2016/425 and the Personal Protective Equipment (Enforcement) Regulations 2018I88538
Schedule 35 contains amendment of Regulation (EU) 2016/425 of the European Parliament and of the Council of on personal protective equipment and repealing Council Directive 89/686/EEC, and amendment of the Personal Protective Equipment (Enforcement) Regulations 2018 M32.
Amendment of Regulation (EU) 2016/426 and the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018I88639
Schedule 36 contains amendment of Regulation (EU) 2016/426 of the European Parliament and of the Council on appliances burning gaseous fuels and repealing Directive 2009/142/EC, and amendment of the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018 M33.
PART 6Revocations
Revocation of retained direct EU and EEA legislationI88740
Schedule 37 contains revocation of retained direct EU and EEA legislation.
SCHEDULE 1Amendment of the Hallmarking Act 1973
GeneralI8881
The Hallmarking Act 1973 is amended as follows.
I52
In section 2—
a
in subsection (1)(d), after “United Kingdom” insert “
before exit day
”
;
b
for subsection (2A), substitute—
2A
In this section “EEA state” has the meaning which it had under Schedule 1 to the Interpretation Act 1978 before exit day.
I63
In section 22(1), in paragraph (c) of the definition of “sponsor's mark”, after “EEA state” insert “
other than the United Kingdom before exit day
”
.
SCHEDULE 2Amendment of the Weights and Measures Act 1985
GeneralI71
In the Weights and Measures Act 1985 omit section 76 (fees for performance of EU obligations) M34.
SCHEDULE 3Amendment of the Consumer Protection Act 1987
IntroductionI8891
The Consumer Protection Act 1987 is amended in accordance with paragraphs 2 to 6.
Amendment of section 1I82
In section 1(1)—
1
for “shall have effect” substitute “
was enacted
”
;
2
for “is” substitute “
was
”
.
Amendment of section 2I93
In section 2(2)(c) for the words from “a member State” to “member States” substitute “
the United Kingdom
”
.
Amendment of section 4I104
In section 4(1)(a) before “EU” insert “
retained
”
.
Omission of section 8I115
Omit section 8.
Saving for Orders in Council made under section 8I126
An Order in Council made under section 8 continues to have effect despite the repeal of that section by paragraph 5.
SCHEDULE 4Amendment of the Measuring Container Bottles (EEC Requirements) Regulations 1977
IntroductionI8901
The Measuring Container Bottles (EEC Requirements) Regulations 1977 are amended in accordance with paragraphs 2 to 11.
Amendment to regulation 2I132
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
in the definition of “batch”—
i
for “paragraph 1 of Annex II of the Directive” substitute “
Schedule 3
”
;
ii
for “the Directive” in the second place in which it occurs substitute “
these Regulations
”
;
b
for the definition of “importer” substitute—
“importer” means any person who—
a
is established in the UK; and
b
places a measuring container bottle (marked with the UK marking) from a country outside the United Kingdom onto the market;
c
in the definition of “manufacturer” for “EEC sign” substitute “
UK marking
”
;
d
after “production control records” insert—
“UK marking” means the marking in the form set out in Annex 2 of Regulation (EC) No 765/2008M35 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products;
3
After paragraph (1) insert—
1A
Schedules 2 and 3 reproduce provisions of Annexes I and II (respectively) to the Directive with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedules 2 and 3 is a reference to the equivalent provision of Annexes I and II to the Directive as set out in that Schedule.
4
In paragraph (3), for “the schedule” substitute “
Schedule 1
”
.
Amendment to regulation 3I143
In regulation 3 (application), for the words from “only” to the end of the regulation, substitute—
to bottles made of glass, or any other substance having the rigidity and stability that offers the same metrological guarantees as glass, when such bottles—
a
are stoppered or designed to be stoppered and are intended for the storage, transport or delivery of liquids,
b
have a nominal capacity of between 0.05 litre and five litres inclusive, and
c
have metrological characteristics (design characteristics and uniformity of manufacture)
such that they can be used as measuring containers, i.e. when they are filled up to a specified level or to a specified percentage of their brim capacity their contents can be measured with sufficient accuracy.
Amendment to regulation 4I154
In regulation 4 (weights and measures acts) for “EEC sign” substitute “
UK marking
”
.
Amendment to regulation 5I165
For regulation 5 substitute—
5
1
Only measuring container bottles—
a
complying with paragraph 3 of Schedule 2;
b
the actual capacity of which has been checked in accordance with paragraph 4 of Schedule 2; and
c
marked in accordance with paragraph 5.1.1 and 5.1.2 of Schedule 2; shall be marked with the UK marking.
2
The UK marking on a measuring container bottle F7, or where paragraph (2A) applies, on a label affixed to the bottle or on a document accompanying the bottle shall be at least 3mm high and shall be indelible, easily legible and visible.
F82A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to a label affixed to, or a document accompanying, a measuring container bottle.
3
It is the responsibility of the manufacturer to affix the UK marking to certify that a measuring container bottle meets the requirements of these Regulations, including Schedule 2.
Amendment to regulation 6I176
In regulation 6 (manufacturer's identification mark) omit paragraph (3).
Insertion of regulation 7AI187
After regulation 7 insert—
Obligations which are met by complying with obligations in the Directive7A
1
In this regulation any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive.
2
Paragraph (3) applies where, before a measuring container bottle is sold or supplied on the UK market, the manufacturer—
a
has complied with the requirements of the Directive in accordance with Article 2 and Annex 1; and
b
marks the bottle with the EEC sign in accordance with the third subparagraph of paragraph 5 of Annex 1.
3
Where this paragraph applies—
a
the requirements of regulations 5 and of Schedule 2 are to be treated as being satisfied;
b
the definitions of importer and manufacturer in regulation 2(1) apply subject to the modification that the references to “UK marking” are to be read as references to the EEC sign; and
c
regulations 4, 9 and 10 apply subject to the modifications that—
i
any reference to “UK marking” is to be read as a reference to the EEC sign;
ii
any reference to “these Regulations” is to be read as a reference to the Directive.
F9Expiry of regulation 7A7B
1
Subject to paragraphs (2) and (3), regulation 7A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 7A—
a
any measuring container bottle to which paragraph (3) applies may be sold or supplied on the market of Great Britain on or after the expiry of regulation 7A;
b
any obligation to which a person was subject under regulation 7A(2) in respect of a measuring container bottle to which paragraph (3) applies continues to have effect after the expiry of regulation 7A.
3
This paragraph applies to a measuring container bottle which—
a
was sold or supplied on the market of Great Britain prior to the expiry of regulation 7A; and
b
in respect of which the requirements in regulation 7A were met.
Qualifying Northern Ireland Goods7C
1
Where paragraph (2) applies—
a
the requirements of regulation 5 and of Schedule 2 are treated as being satisfied;
b
the definition of “importer” and “manufacturer” in regulation 2(1) apply subject to the modification that the references to “UK marking” are to be read as references to the EEC sign.
2
This paragraph applies where a measuring container bottle—
a
complies with regulation 5, as it applies in Northern Ireland; and
b
is qualifying Northern Ireland goods.
3
In this regulation “qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018.
Amendment to regulation 9I198
In regulation 9 (individual measuring container bottles), for “EEC sign” in each place in which it occurs substitute “
UK marking
”
.
Amendment to regulation 10I209
In regulation 10 (offences)—
a
in paragraphs (1)(a) and (b) and paragraph (7), for “EEC sign” substitute “
UK marking
”
;
b
in paragraph (1)( c), for “in section 2 of Annex I of the Directive” substitute “
at paragraph 1 of Schedule 2
”
.
Amendment to ScheduleI2110
For the heading “Schedule” substitute “
Schedule 1
”
.
Insertion of Schedules 2 and 3I2211
After Schedule 1, insert—
SCHEDULE 2(Annex I to the Directive)
1
Measuring container bottles shall be characterized by the following capacities which are always specified for a temperature of 20C:
1
the nominal capacity Vn is the volume which is marked on the bottle; it is the volume of liquid which the latter is deemed to contain when it is filled in the conditions of use for which it is intended;
1
the brim capacity of a bottle is the volume of liquid it contains when filled to the brim;
1
the actual capacity of a bottle is the volume of liquid it in fact contains when it is filled exactly under the conditions corresponding theoretically to the nominal capacity;
2
There are two methods of filling measuring container bottles:
1
to a constant level,
2
to a constant vacuity.
The distance between the theoretical filling level for the nominal capacity and the brim level and the difference between the brim capacity and the nominal capacity, known as the volume of expansion or vacuity, shall be perceptibly constant for all bottles of the same type, that is, for all bottles made to the same design.
3
The maximum permissible errors (positive or negative) in the capacity of a measuring container bottle, i.e. the greatest differences permitted (positive or negative) at a temperature of 20°C and under the control conditions laid down in Schedule 3, between the actual capacity and the nominal capacity Vn shall be in accordance with the following table:
Normal capacity Vn in millilitres
Maximum permissible errors
as a % of Vn
in millilitres
from 50 to 100
3
from 100 to 200
3
from 200 to 300
6
from 300 to 500
2
from 500 to 1 000
10
from 1 000 to 5 000
1
The maximum permissible error in the brim capacity shall be the same as the maximum permissible error in the corresponding nominal capacity. The systematic exploitation of tolerances shall be prohibited.
4
In practice, the actual capacity of a measuring container bottle shall be checked by determining the quantity of water at 20°C which the bottle actually contains when filled to the level theoretically corresponding to the nominal capacity. It may also be checked indirectly by a method of equivalent accuracy.
5
A measuring container bottle shall bear the following indelible, easily legible and visible indications :
5
on its side, on the bottom rim or on the bottom:
5
an indication of its nominal capacity in litres, centilitres or millilitres in figures at least 6 mm high, if the nominal capacity is greater than 100 cl, 4 mm high if it is from 100 cl down to but not including 20 cl and 3 mm high if it is not more than 20 cl, followed by the symbol for the unit of measurement used or, where appropriate, by the name of the unit in accordance with the Units of Measurement Regulations 1986 M36;
5
the manufacturer's identification mark referred to in regulation 6;
5
the UK marking;
5
On the bottom or on the bottom rim, in such a manner as to avoid confusion with the previous indication, in figures of the same minimum height as those expressing the corresponding nominal capacity, according to the method or methods of filling for which the bottle is intended:
5
an indication of the brim capacity expressed in centilitres and not followed by the symbol cl, and/or
5
an indication of the distance in millimetres from the brim level to the filling level corresponding to the nominal capacity, followed by the symbol mm.
5
Other indications may appear on the bottle provided they do not give rise to confusion with the compulsory indications.
SCHEDULE 3(Annex II to the Directive)
METHOD OF SAMPLING1
A sample of measuring container bottles of the same design and the same manufacture shall be drawn from a batch corresponding, in principle, to an hour's production.
If the result of the check on a batch corresponding to an hour's production is not satisfactory, a second test can be carried out, based either on another sample from a batch corresponding to a longer period of production or, where production has been subject to a check recognized by the Secretary of State, on the results recorded on the manufacturers' check-cards.
The number of measuring container bottles constituting the sample shall be 35 or 40 as determined by an inspector.
MEASURING THE CAPACITY OF THE MEASURING CONTAINER BOTTLES CONSTITUTING THE SAMPLE2
The measuring container bottles shall be weighed empty.
They shall be filled with water at 20C of a known density, up to the filling level appropriate to the method of checking used.
They shall then be weighed in full.
The check shall be carried out by means of a legal measuring instrument, suitable for effecting the necessary operations.
Error in measuring the capacity shall not be greater than one-fifth of the maximum permissible error corresponding to the nominal capacity of the measuring container bottle.
APPLICATION OF THE RESULTS3
3
Use of the standard deviation method
The number of measuring container bottles in the sample is 35.
3
Calculate as follows (see 3.1.4.):
3
the average
3
estimated standard deviation s of the actual capacities Xi of the bottles in the batch.
3
Calculate as follows:
3
The upper limit T s : the sum of the indicated capacity (see Schedule 2 paragraph 5) and of the maximum permissible error corresponding to this capacity.
3
The lower limit Ti : the difference between the indicated capacity (see Schedule 2 paragraph 5) and the maximum permissible error corresponding to this capacity.
3
Acceptance criteria:
The batch shall be declared to comply with the Regulations if the numbers
where k = 1·57
and F = 0·266
3
Calculation of the mean value
Calculate as follows:
— the sum of the 35 actual capacity measurements
— the mean value of the 35 measurements
— the sum of the squares of the 35 measurements
— the square of the sum of the 35 measurements
— the corrected sum:
— the estimated variance:
Hence the estimated standard deviation:
3
Use of the average range method The number of measuring container bottles in the sample is 40.
3
Calculate as follows (see 3.2.4):
3
the average
3
the average range value
3
Calculate as follows:
3
The upper limit T s : the sum of the indicated capacity (see Schedule 2 paragraph 5) and of the maximum permissible error corresponding to this capacity.
3
The lower limit Ti : the difference between the indicated capacity (see Schedule 2 paragraph 5) and the maximum permissible error corresponding to this capacity.
3
Acceptance criterion:
The batch shall be declared to comply with the Regulations if the numbers
where k′ = 0·668,
and F′ = 0·628.
3
Calculation of the mean value
3
to obtain
— the sum of the 40 actual capacity measurements Xi:
— the mean value of these 40 measurements of the 40 actual capacity measurements:
3
To obtain
Divide the sample, in chronological order of selection, into eight sub-samples of five measuring container bottles each. Calculate as follows:
— the range of each of the sub-samples, i.e. the difference between the actual capacity of the largest and the smallest of the five bottles in the sub-sample; eight ranges are thus obtained: R1 ; R2 ; . . . . . . R8
— the sum of the ranges of the eight sub-samples:
The average range
SCHEDULE 5Amendment of the Measuring Instruments (EEC Requirements) Regulations 1988
GeneralI231
1
The Measuring Instruments (EEC Requirements) Regulations 1988, so far as they continue in force by virtue of paragraph 5 of the Schedule to the Weights and Measures (Revocations) Regulations 2015 M37, are amended as follows.
2
In the following, omit “other than the United Kingdom”—
a
regulation 2(1) in the definition of “use for trade”;
b
regulations 10, 12(1) and (2), 13(8), 17(1) and (5), 18, 19(1) and (2), 20(1)(a) and (3) and 21(5);
c
paragraph 1(e) of Schedule 5.
3
In regulation 2 after paragraph (3) insert—
3A
For the purposes of these Regulations but subject to paragraphs (3B) and (3C), any reference to a member State in an Annex to a relevant Directive is to be read as including a reference to the United Kingdom.
3B
Paragraph (3A) does not apply to a reference contained in a reference to the title of a Directive.
3C
For the purposes of these Regulations, the references to the metrological service of a member State in point 5.2.3 of the Annex to the Directive on cold-water meters are to be read, in relation to the conduct of EEC pattern approval by the Secretary of State, as references to the Secretary of State.
4
In regulation 8(3) for “any other member State” substitute “
a member State
”
.
5
In regulation 9(2) after sub-paragraph (b) insert—
ba
a condition requiring such notice to be given to the Secretary of State if such instruments are to be installed in the United Kingdom;
6
After regulation 15 insert—
Conditions in EEC pattern approvals requiring notice of place of installation: effect after EU exit15A
1
This regulation applies where an EEC pattern approval granted before F10IP completion day is subject to a condition requiring notice of the place of installation to be given to the competent authorities of member States in which instruments of the pattern in question are to be installed.
2
For the purposes of these Regulations the condition is to be treated as including a requirement for such notice to be given to the Secretary of State if any such instrument is to be installed in the United Kingdom.
SCHEDULE 6Amendment of the Weights and Measures (Intoxicating Liquor) Order 1988
GeneralI8911
The Weights and Measures (Intoxicating Liquor) Order 1988 is amended as follows.
I242
In article 3A(2), for “European Union” substitute “
United Kingdom
”
.
SCHEDULE 7Amendment of the Lifting Operations and Lifting Equipment Regulations 1998
GeneralI251
1
The Lifting Operations and Lifting Equipment Regulations 1998 are amended as follows.
2
In regulation 2(1) (interpretation)—
a
for “EC declaration of conformity” substitute “
declaration of conformity
”
M38
;
b
for paragraph (b) of that definition, substitute—
- b
the requirements for a declaration of conformity in Article 15 of Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on personal protective equipment and repealing Council Directive 89/686/EEC; or
3
In regulation 9(1)(b)(thorough examination and inspection), for “an EC declaration of conformity” substitute “
a declaration of conformity
”
.
4
In regulation 11(1) (keeping of information), for “an EC declaration of conformity” substitute “
a declaration of conformity
”
.
SCHEDULE 8Amendment of the Noise Emission in the Environment by Equipment for use Outdoors Regulations 2001
IntroductionI8921
The Noise Emission in the Environment by Equipment for use Outdoors Regulations 2001 are amended in accordance with paragraphs 2 to 27.
Amendment to regulation 2I262
Regulation 2 is amended as follows—
a
in paragraph (1)—
i
in sub-paragraph (a) after “equipment for use outdoors” insert “as it has effect immediately before F11IP completion day”
;
ii
omit sub-paragraph (b); and
iii
for sub-paragraph (c) substitute—
c
unless the context otherwise requires, a reference to a numbered regulation or Schedule is a reference to the regulation or Schedule so numbered in these Regulations and a reference to a paragraph in a regulation is a reference to a paragraph in that regulation.
b
in paragraph (2)—
i
before the definition of “CE marking” insert—
“approved body” has the meaning given to it in regulation 13
ii
in the definition of “CE marking”—
aa
for “regulation 11” substitute “
Article 3(c) of the Directive
”
, and
bb
for “Schedule 7” substitute “
Annex IV to the Directive
”
;
iii
omit the definition of “the Commission”;
iv
in the definition of “equipment for use outdoors”, omit from “all the kinds of machinery” to “that is to say”;
F12v
in the definition of “responsible person”—
aa
for “European Union” in the first two places in which it occurs substitute “
United Kingdom
”
;
bb
for “the European Union” in sub-paragraph (c) in the second place in which it occurs substitute “Great Britain”
;
vi
omit the definition of “notified body”; and
vii
after the definition of “sound power level LWA” insert—
“UK marking” means the marking in the form set out in Annex 2 of Regulation (EC) No 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93;
Amendment to regulation 7I283
In regulation 7—
a
in paragraph (2)(c) for “CE” substitute “
UK
”
;
b
in paragraph (2)(d) for “an EC” substitute “
a
”
; and
F13ba
before paragraph (3) insert—
2A
Where paragraph (2B) applies, paragraph (2)(c) is met where the UK marking is affixed to—
a
a label affixed to the equipment; or
b
a document accompanying the equipment.
2B
This paragraph applies to equipment that is placed on the market within a period of 24 months beginning with IP completion day.
c
in paragraph (3), omit “In respect of” to “Kingdom,”.
Amendment to regulation 9I294
In regulation 9, in paragraph (a)—
F15a
in sub-paragraph (i) for “the European Union” substitute “
Great Britain
”
;
F14aa
for sub-paragraph (ii) substitute—
ii
is imported into Great Britain for re-export to Northern Ireland or a country outside of the United Kingdom;
b
for “CE” substitute “
UK
”
.
Amendment to regulation 11I305
In regulation 11—
a
for “CE” substitute “
UK
”
in each place it occurs;
b
for “directives” substitute “
enactments
”
in each place it occurs; F17...
F16ba
at the end of paragraph (2) insert “
or, where regulation 7(2B) applies, to a label affixed to the equipment or to a document accompanying the equipment
”
;
c
in paragraph (4), omit “as published in the Official Journal of the European Union”.
Amendment to regulation 12I316
In regulation 12 omit “and to the Commission”.
Insertion of regulation 12AI327
After regulation 12 insert—
Obligations which are met by complying with obligations in the Directive12A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article of or an Annex to the Directive;
b
“conformity assessment procedure” has the meaning given to it in Article 3(b).
2
Paragraph (3) applies where, before placing equipment on the market or putting it into service in F18Great Britain, a responsible person—
a
ensures that the equipment satisfies the requirements of the Directive concerning noise emission in the environment;
b
ensures that the conformity assessment procedure that applies to the equipment in accordance with Article 14(1) or (2) has been carried out;
c
ensures that the technical documentation referred to in Article 14 and Annexes V to VIII, and any other records and correspondence relating to the relevant conformity assessment procedure set out in those provisions, are prepared in or translated into English;
d
ensures that the equipment bears a CE marking and an indication of the guaranteed sound power level in accordance with Article 11;
e
draws up an EC declaration of conformity, in accordance with Article 8; and
f
ensures that the EC declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulation 7(2) and (3) are to be treated as being satisfied;
b
regulations 7(4), 11, 12, 16(1), 17 and 18 and paragraph 8 of Schedule 13 apply subject to the modifications in paragraph (4); and
c
regulation 10 does not apply.
4
The modifications referred to in paragraph (3)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EC declaration of conformity referred to in Article 8(1);
b
any reference to “technical documentation” is to be read as a reference to the technical documentation referred to in Article 8(3);
c
any reference to “UK marking” is to be read as a reference to the CE marking; and
d
for the purposes of regulation 16(1)—
i
the reference to regulation 7(2)(c) is to be read as a reference to Article 11(2);
ii
the reference to regulation 7(2)(d) is to be read as a reference to Article 8(1); and
iii
the reference to regulation 10 is to be read as a reference to Article 14(1) or (2).
F19Expiry of regulation 12A12B
1
Subject to paragraph (2), regulation 12A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 12A—
a
any equipment which was placed on the market or put into service pursuant to regulation 12A may continue to be made available on the market on or after the expiry of regulation 12A;
b
any obligation to which a person was subject under regulation 12A(2) in respect of equipment placed on the market or put into service pursuant to regulation 12A continues to have effect after the expiry of regulation 12A, in respect of that equipment.
Qualifying Northern Ireland Goods12C
1
Where paragraph (2) applies the requirements in regulation 7(2) and (3) and regulations 10 and 11 are to be treated as being satisfied.
2
This paragraph applies where equipment is—
a
qualifying Northern Ireland goods; and
b
meets the requirements of regulation 16, as it applies in Northern Ireland.
3
In this regulation “qualifying Northern Ireland goods has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018.
Amendment to regulation 13I338
For regulation 13 substitute—
Approved Bodies13
1
For the purposes of these Regulations, an approved body is a body which has been appointed to carry out one or more of the conformity assessment procedures mentioned or referred to in regulation 10(a) and which—
a
has been appointed as an approved body in the United Kingdom pursuant to regulation 14; or
b
2
In this regulation “notified body” means a body which before F20IP completion day has been—
a
appointed as a notified body in the United Kingdom pursuant to regulation 14, as it had effect immediately before F20IP completion day; and
b
notified by the Secretary of State to the European Commission and other member States pursuant to Article 15 of the Directive.
Amendment to regulation 14I349
In regulation 14—
a
for “a notified” substitute “
an approved
”
in each place it occurs;
b
in paragraph (1), after “such persons” insert “
which meet the approved body requirements,
”
;
c
in paragraphs (1), (2)(d), (5)(a) and (5)(b) for “notified” substitute “
approved
”
;
d
in paragraph (2)(e), after “any of” insert “
the approved body requirements or
”
;
e
after paragraph (6) insert—
7
In this regulation “approved body requirements” means the requirements set out in Schedule 12A.
Insertion of regulation 14AI3510
After regulation 14 insert—
Register of approved bodies14A
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
Amendment to regulation 15I3611
In regulation 15—
a
in paragraph (1), for “a notified” in both places in which it occurs substitute “
an approved
”
; and
b
in paragraph (1)(a), for “notified” substitute “
approved
”
.
Amendment to regulation 16I3712
In regulation 16—
a
in paragraph (1)(a), for “CE” substitute “
UK
”
; and
b
in paragraph (1)(b) for “an EC” substitute “
a
”
.
Amendment to regulation 17I3813
In regulation 17—
a
in paragraph (2) for “CE” substitute “
UK
”
;
b
in paragraph (2)(a) and (b) F21omit “established within the European Union”; and
c
in paragraph (3) for “CE” substitute “
UK
”
in each place it occurs.
Transitional provision in relation to EU ExitI2714
After regulation 22, insert—
Transitional provision in relation to EU Exit23
1
In this regulation—
“pre-exit period” means the period beginning with 3rd July 2001 and ending immediately before F22IP completion day;
“product” means equipment to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 8 of the Product Safety and Metrology etc. (Amendments etc.) (EU Exit) Regulations 2019 M39, any obligation to which a person was subject under these Regulations as they had effect immediately before F22IP completion day, continues to have effect as it did immediately before F22IP completion day, in relation to that product.
3
Paragraph (2) does not apply to any obligation to take action outside of the United Kingdom in respect of that product.
4
Where, during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 7(2)(b) as it had effect immediately before F22IP completion day in relation to that product
that action has effect as if it had been done under regulation 7(2)(b) as it has effect on and after F22IP completion day.
Amendment to Schedule 1]I3915
In Schedule 1—
a
for “Annex I” substitute “
Schedule 4
”
in each place it occurs; and
b
for “Annex III” substitute “
Schedule 6
”
in each place it occurs.
Amendment to Schedule 2I4016
In Schedule 2—
a
for “Annex I” substitute “
Schedule 4
”
in each place it occurs; and
b
for “Annex III” substitute “
Schedule 6
”
in each place it occurs.
Amendment to Schedule 3I4117
In the heading to Schedule 3 after “(Extract from Article 12)” insert “
Permissible Sound Power Levels
”
.
Amendment to Schedule 5I4218
In Schedule 5—
a
omit “EC” in each place it occurs;
b
F55omit “established in the European Union” in each place it occurs;
c
for “notified” substitute “
approved
”
;
d
for “this Directive” substitute “
these Regulations
”
in each place it occurs; and
e
for “Community Directives” substitute “
other enactments
”
.
Amendment to Schedule 6I4319
In Schedule 6—
a
under the subheading “Scope”—
i
for “this Annex” substitute “
this Schedule
”
in each place it occurs;
ii
for “this Directive” substitute “
these Regulations
”
in each place it occurs;
iii
for “Article 2(1)” substitute “
regulation 3 (application)
”
in each place it occurs; and
iv
F56omit “in the European Union”;
b
under the subheading “Part A Basic Noise Emission Standard”—
i
in the first line, for “Article 2(1)” substitute “
regulation 3 (application)
”
;
ii
in paragraphs 5 and 6 for “this Directive” substitute “
these Regulations
”
.
Amendment to Schedule 7I4420
In Schedule 7—
a
for “CE” substitute “
UK
”
in each place it occurs;
b
for “taking the following form” the first time it occurs, substitute “
taking the form set out in Annex 2 of Regulation (EC) No 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93
”
the first time it occurs; and
c
omit the first diagram.
Amendment to Schedule 8I4521
In Schedule 8—
a
in paragraph 1—
i
for “Annex” substitute “
Schedule
”
;
F57ia
omit “established within the European Union”;
ii
iii
for “this Directive” substitute “
these Regulations
”
;
iv
for “CE” substitute “
UK
”
;
v
for “in Article 11” substitute “
by regulation 7(2)(c), regulation 11 and Schedule 7
”
;
vi
omit “EC”; and
vii
for “in Article 8” substitute “
by regulation 7(2)(d) and Schedule 5
”
;
b
in paragraph 2—
i
F60omit “established in the European Union” in each place it occurs; and
ii
omit “EC”;
c
in paragraph 3—
i
for “this Directive” substitute “
these Regulations
”
in each place it occurs; and
ii
F61omit “established in the European Union”; and
d
in paragraph 4 for “this Directive” substitute “
these Regulations
”
.
Amendment to Schedule 9I4622
In Schedule 9—
a
in paragraph 1—
i
for “Annex” substitute “
Schedule
”
;
ii
F64omit “established in the European Union” in each place it occurs;
iii
for “this Directive” substitute “
these Regulations
”
;
iv
for “CE” substitute “
UK
”
;
v
for “Article 11” substitute “
regulation 7(2)(c), regulation 11 and Schedule 7
”
; and
vi
for “Article 8” substitute “
regulation 7(2)(d) and Schedule 5
”
;
b
in paragraph 2—
i
F65omit “established in the European Union” in each place it occurs; and
ii
omit “EC”;
c
in paragraph 3—
i
for “this Directive” substitute “
these Regulations
”
in each place it occurs; and
ii
F66omit “established in the European Union”;
d
in paragraph 4 for “this Directive” substitute “
these Regulations
”
;
e
in paragraph 5—
i
for “the notified” substitute “
the approved
”
in each place it occurs;
ii
for “a notified” substitute “
an approved
”
;
iii
F67omit “established in the European Union” in each place it occurs;
F62iiia
omit “established within the European Union”;
iv
for “CE” substitute “
UK
”
;
v
for “an EC” substitute “
a
”
; and
vi
for “Articles 11 and 8” substitute “
regulation 7(2)(c) and (d), regulation 11 and Schedules 5 and 7
”
;
f
in paragraph 6—
i
for “notified” substitute “
approved
”
in each place it occurs;
ii
F68omit “established in the European Union” in each place it occurs;
F63iia
omit “established within the European Union”;
iii
for “this Directive” substitute “
these Regulations
”
in each place it occurs;
iv
for “Article 11” substitute “
regulation 7(2)(c), regulation 11 and Schedule 7
”
in each place it occurs;
v
for “Article 8” substitute “
regulation 7(2)(d) and Schedule 5
”
in each place it occurs;
vi
for “Annex III” substitute “
Schedule 6
”
in each place it occurs; and
vii
for “notifying Member State” substitute “
Secretary of State
”
.
Amendment to Schedule 10I4723
In Schedule 10—
a
in paragraph 1—
i
for “Annex” substitute “
Schedule
”
;
ii
for “this Directive” substitute “
these Regulations
”
;
iii
F69omit “established in the European Union” in each place it occurs;
iv
for “CE” substitute “
UK
”
;
v
for “Article 11” substitute “
regulation 7(2)(c), regulation 11 and Schedule 7
”
vi
omit “EC”; and
vii
for “Article 8” substitute “
regulation 7(2)(d) and Schedule 5
”
;
b
in paragraph 2—
i
F70omit “established in the European Union”;
ii
for “a notified” substitute “
an approved
”
;
iii
for “other notified” substitute “
other approved
”
; and
iv
for “this Directive” substitute “
these Regulations
”
;
c
in paragraph 3—
i
for “notified” substitute “
approved
”
; and
ii
for “this Directive” substitute “
these Regulations
”
in each place it occurs;
d
in paragraph 4—
i
for “this Directive” substitute “
these Regulations
”
;
ii
for “notified” substitute “
approved
”
in each place it occurs; and
iii
for “Annex X” substitute “
Schedule 12
”
;
e
in paragraph 5 F71omit “established in the European Union”.
Amendment to Schedule 11I4824
In Schedule 11—
a
in paragraph 1—
i
for “Annex” substitute “
Schedule
”
;
ii
for “this Directive” substitute “
these Regulations
”
;
iii
F72omit “established in the European Union”;
iv
for “CE” substitute “
UK
”
;
v
for “Article 11” substitute “
regulation 7(2)(c), regulation 11 and Schedule 7
”
;
vi
omit “EC”; and
vii
for “Article 8” substitute “
regulation 7(2)(d) and Schedule 5
”
;
b
in paragraph 3.1—
i
for “a notified” substitute “
an approved
”
;
ii
F73omit “established in the European Union” ;
iii
for “this Directive” substitute “
these Regulations
”
in each place it occurs; and
iv
omit “EC”;
c
in paragraph 3.2 for “Directives” substitute “
enactments
”
;
d
in paragraph 3.3 for “notified” substitute “
approved
”
the first time it occurs;
e
in paragraph 3.4—
F74i
omit “established within the European Union”;
ii
for “notified” substitute “
approved
”
in each place it occurs;
f
in paragraph 4, for “notified” substitute “
approved
”
in each place it occurs;
g
in paragraph 5—
i
for “Annex” substitute “
Schedule
”
; and
ii
for “notified” substitute “
approved
”
;
h
in point 6, for “notified” substitute “
approved
”
in each place it occurs.
Amendment to Schedule 12I4925
In Schedule 12, in the model of conformity certificate—
a
omit “EC” in each place it occurs;
b
for “Issuing Directive Applicable” substitute “
Enactments Applicable
”
; and
c
for “EC Directive Applicable .. ../.. .. ../EC” substitute “
Enactments Applicable …
”
.
Insertion of Schedule 12AI5026
After Schedule 12 insert—
SCHEDULE 12AApproved body requirements
1
1
The body, its director and its staff responsible for carrying out verification operations may be neither the designer, builder, supplier or installer of the equipment nor the authorised representative established in the United Kingdom of any of those parties. They may become involved neither directly nor as authorised representatives in the design, construction, marketing or maintenance of such equipment nor represent the parties engaged in these activities.
2
Sub-paragraph (1) does not preclude the possibility of exchange of technical information between the manufacturer and the body.
2
The body and its staff must carry out the assessments and verifications with the highest degree of professional integrity and technical competence and must be free from all pressures and inducements, particularly financial, which might influence their judgement or the results of their work, especially from persons or groups of persons with an interest in the results of verification.
3
The body must have at its disposal the necessary staff and possess the necessary facilities to enable it to perform properly the technical and administrative tasks connected with inspection and surveillance operations; it must also have access to the equipment required for any special verification.
4
The staff responsible for inspection must have—
a
sound technical and professional training;
b
satisfactory knowledge of the requirements for the assessment of technical documentation;
c
satisfactory knowledge of the requirements for the tests they carry out and adequate practical experience of such tests;
d
the ability to draw up the certificates, records and reports required to authenticate the performance of the tests.
5
The body must be able to demonstrate the impartiality of its inspection staff.
6
The remuneration of the inspection staff must not depend on the number of tests carried out or the results of such tests.
7
The body must have, and must satisfy the Secretary of State that it has, adequate civil liability insurance in respect of its activities.
8
The body must ensure that its staff observe professional secrecy with regard to all information gained in carrying out its tests under these Regulations.
9
Paragraph 8 does not prevent the staff from providing the information to the Secretary of State.
Amendment to Schedule 13I5127
In Schedule 13, in paragraph 8 for “an EC” substitute “
a
”
in each place it occurs.
SCHEDULE 9Amendment of the General Product Safety Regulations 2005
IntroductionI8931
The General Product Safety Regulations 2005 are amended in accordance with paragraphs 2 to 10.
Amendment of regulation 2I522
In regulation 2—
a
omit the definition of “EU law”;
b
omit the definition of “the GPS Directive”;
c
after the definition of “magistrates' court” insert—
“the market” means the F75market of Great Britain;
d
omit the definition of “Member State”;
e
in the definition of “producer”—
i
for “a Member State”, in the first, second and third place it occurs, substitute “
the United Kingdom
”
; and
ii
in paragraph (b)(ii), for the words “importer of the product from a state that is not a Member State into a Member State” substitute “
person established in the United Kingdom that places a product from a country outside the United Kingdom on the market
”
;
f
after the definition of “record” insert—
“relevant enactment” means any retained EU law F76 (as it applies in Great Britain) derived from an EU instrument harmonising the conditions for the marketing of products in the EU but does not include Regulation (EC) No 765/2008 of the European Parliament and the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93;
Amendment of regulation 3I533
In regulation 3—
a
for “rules of EU law” substitute “
any relevant enactment
”
, in both places it occurs;
b
omit “other than the GPS Directive”, in both places it occurs; and
c
in paragraph (2)(a) for “rules” substitute “
provisions of the enactment
”
.
Amendment of regulation 6I544
1
Regulation 6 is amended as follows.
2
In paragraph (1)—
a
for “rules of EU law” substitute “
any relevant enactment
”
; and
b
for “the law” substitute “
any other law
”
.
3
In paragraph (2)—
a
for the words from “giving” to “Directive”, substitute “
(“S”) which meets the conditions in paragraph (2A)
”
;
b
for “that national standard” substitute “
S
”
; and
c
omit the final sentence.
4
After paragraph (2) insert—
2A
The conditions referred to in paragraph (2) are that—
a
the Secretary of State considers S appropriate for the purposes of giving rise to the presumption of conformity; and
b
the Secretary of State has published the reference to S in a manner the Secretary of State considers appropriate.
5
In paragraph (3)—
a
omit sub-paragraph (a);
b
in sub-paragraph (b) omit “other”; and
c
in sub-paragraph (c), for “European Commission” substitute “
Secretary of State
”
.
Amendment of regulation 9I555
In regulation 9—
a
in paragraph (1)(a), omit “and”; and
b
omit paragraph (1)(b).
Amendment of regulation 33I566
1
Regulation 33 is amended as follows.
2
In the heading, omit “and Commission”.
3
At the beginning insert—
A1
The Secretary of State must establish and operate a database containing information relating to market surveillance and product safety.
4
Before paragraph (1) insert—
B1
The database referred to in paragraph (A1) must be designed so as to enable notifications required under paragraph (1), (2) or (4), or under Article 22 of Regulation (EC) 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93, to be made to the Secretary of State through the database.
5
For paragraph (1), substitute—
1
An enforcement authority which has received a notification of a risk under regulation 9(1) shall immediately notify the Secretary of State of the risk through the database referred to in paragraph (A1).
6
In paragraph (2), after “Secretary of State”, in the first place it occurs, insert “
of the action taken through the database referred to in paragraph (A1)
”
;
7
Omit paragraph (3).
8
In paragraph (4)—
a
for “pharmaceutical” substitute “
medicinal
”
;
b
after “Secretary of State”, in the first place it occurs, insert “
of the measure or action taken through the database referred to in paragraph (A1)
”
; and
c
after “withdrawal of any such measure or action” insert “through the database referred to in paragraph (A1).
9
Omit paragraphs (5) to (9).
10
For paragraph (10)(b), substitute—
b
“medicinal product” has the meaning given to it in regulation 2 of the Human Medicines Regulations 2012 M40.
Amendment of regulation 34I577
In regulation 34—
a
in paragraph (1)—
i
for “to (6), (8) or 27(9)”, substitute “
or (4)
”
;
ii
omit “or the Commission”; and
iii
omit “be in writing and shall”; and
b
omit paragraphs (2) and (3).
Omission of regulation 35I588
Omit regulation 35.
Amendment of regulation 36I599
In regulation 36, omit “and competent authorities of other Member States”.
Amendment of regulation 38I6010
In regulation 38, omit paragraph (2).
SCHEDULE 10Amendment of the Offshore Installations (Safety Case) Regulations 2005
IntroductionI611
1
The Offshore Installations (Safety Case) Regulations 2005 are amended as follows.
2
For regulation 2(6)(b) substitute—
b
examination of any design, specification, certificate, marking or other document, or standard relating to those elements or that plant by such persons;
SCHEDULE 11Amendment of the Weights and Measures (Packaged Goods) Regulations 2006
IntroductionI8941
The Weights and Measures (Packaged Goods) Regulations 2006 M41 are amended in accordance with paragraphs 2 to 5.
Amendment to regulation 2I622
In regulation 2 (interpretation) omit the definition of “Member State”.
Amendment to regulation 3I633
In regulation 3 (scope of application)—
a
omit paragraph (5);
b
after paragraph (6) insert—
7
The obligations on the importer set out in regulation 5(1)(b) and regulation 6(1)(c) to ensure the package or outer container is marked with specified contact information do not apply where—
a
b
it is marked with the contact information of the person in that EEA state who packed or imported the package or who arranged for the packer to make up or the importer to import the package.
Amendment to regulation 11I644
Omit regulation 11 (notices to local weights and measures authorities).
Amendment to regulation 15I655
In regulation 15 (offences relating to E-marks) omit paragraph (2).
SCHEDULE 12Amendment of the Supply of Machinery (Safety) Regulations 2008
IntroductionI8951
The Supply of Machinery (Safety) Regulations 2008 are amended in accordance with paragraphs 2 to 34.
Amendment to regulation 2I662
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)(a) at the end insert “
(as it had effect immediately before F79IP completion day)
”
;
3
In paragraph (2)—
a
after the definition of “applicable” insert—
“approved body” has the meaning given to it in regulation 16A;
F80b
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
c
omit the definition of “CE marking”;
d
omit the definition of “Commission”;
e
after the definition of “conformity assessment” insert—
“designated standard” has the meaning given to it in regulation 2A;
f
omit the definition of “harmonised standard”;
g
in the definition of “manufacturer” for “an EEA state” substitute “F81Great Britain”
;
h
omit the definition of—
i
“notified body”;
ii
“notified body criteria”;
iii
“notified body designation”;
iv
“official Community language”;
v
“published harmonised standard”;
i
after the definition of “safety component” insert—
“UK marking” means the marking in the form set out in Annex 2 of Regulation (EC) No 765/2008 setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93;
Insertion of regulation 2AI683
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions; and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to machinery to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 3I694
In regulation 3 (placing on the market and putting into service; supplies outside the EEA and showing at trade fairs not covered)—
F82a
in the heading for “the EEA” substitute “Great Britain”;
b
in each place in which it occurs for “an EEA state” substitute “F83Great Britain”
; and
c
in paragraph (2)(a) in both places in which it occurs for “CE” substitute “
UK
”
.
Amendment to regulation 5I705
In regulation 5 (disapplication where more specific Community safety rules apply)—
a
in the heading omit “Community”;
b
for “EU directives other than the Directive” substitute “
other enactments
F84(as they apply in Great Britain) ”
; and
c
in the second place in which it occurs for “the Directive” substitute “
these Regulations
”
.
Amendment to regulation 7I716
In regulation 7 (supply of machinery: general obligations and prohibition)—
a
in paragraph (2)(e) omit “EC”;
F86b
in paragraph (2)(f)—
i
for “CE” substitute “
UK
”
;
ii
after “machinery” insert “
or where paragraph (2A) applies, or on a label affixed to, or document accompanying, the machinery
”
;
F85ba
after paragraph (2) insert—
2A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the machinery; or
b
a document accompanying the machinery.
c
in paragraph (4)—
i
for “harmonised” substitute “
designated
”
;
ii
omit “the references to which have been published in the Official Journal of the European Union (a “published harmonised standard”)”.
Amendment to regulation 11I727
In regulation 11 (Annex IV machinery manufactured fully in accordance with published harmonised standards and fully covered by such standards)—
a
in the heading and in paragraphs (1)(b)(i) and (1)(b)(ii) for “published harmonised” substitute “
designated
”
;
b
in paragraph (2)(b) for “EC type-” substitute “
Type-
”
.
Amendment to regulation 12I738
In regulation 12 (Annex IV machinery not manufactured fully in accordance with published harmonised standards or not fully covered by such standards)—
a
in the heading and in paragraphs (1)(b)(i) to (iii) for “published harmonised” substitute “
designated
”
;
b
in paragraph (1)(b)(iv) for “harmonised” substitute “
designated
”
;
c
in sub-paragraph (2)(a) for “EC type-” substitute “
Type-
”
.
Insertion of regulation 12AI749
After regulation 12 insert—
Obligations which are met by complying with obligations in the Directive12A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article of or an Annex to the Directive;
b
“CE marking” means the marking referred to in Article 16(1);
c
“harmonised standard” means a harmonised standard within the meaning of Article 2(l), the reference to which has been published in the Official Journal of the European Union.
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing machinery on the market or putting machinery into service, the responsible person—
a
ensures that the machinery satisfies the essential health and safety requirements set out in Annex I;
b
ensures that the technical file referred to in Annex VII, part A is available to the enforcement authorities on request;
c
provides the necessary information, referred to in Article 5(1)(c);
d
carries out the appropriate procedures for assessing conformity in accordance with Article 12;
e
ensures that the technical file, necessary information and records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
f
draws up the EC declaration of conformity in accordance with Annex II, part I, Section A and ensure that it accompanies the machinery;
h
ensures that the EC declaration of conformity is prepared in or translated into English; and
i
affixes the CE marking in accordance with Article 16.
3
Where this paragraph applies—
a
the requirements of regulation 7(2)(a), (b), (c), (e)(i) and (f) are to be treated as being satisfied;
b
regulations 7(2)(e)(ii), 15 and 21 apply subject to the modifications in paragraph (8);
c
Part 8 of Schedule 2 does not apply.
4
Subject to paragraphs (6) and (7) paragraph (5) applies where, before placing partly completed machinery on the market, the responsible person ensures that—
a
the relevant technical documentation referred to in Annex VII part B is prepared in or translated into English;
b
the assembly instructions referred to in Annex VI are prepared in or translated into English; and
c
a declaration of incorporation referred to in Annex II part 1, Section B has been drawn up in or translated into English.
5
Where this paragraph applies the requirements of regulation 8(1) are to be treated as being satisfied.
6
This paragraph applies to machinery listed in Annex IV where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 7(2).
7
Where paragraph (6) applies, paragraph (2)(d) is to be read as requiring the responsible person to have carried out the conformity assessment procedure in Article 12(4).
8
The modifications referred to in paragraph (3)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking.
F87Expiry of regulation 12A12B
1
Subject to paragraph (2), regulation 12A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 12A—
a
any machinery or partly completed machinery which was placed on the market or put into service pursuant to regulation 12A may continue to be made available on the market on or after the expiry of regulation 12A;
b
any obligation to which a person was subject under regulation 12A(2) in respect of machinery or partly completed machinery placed on the market or put into service pursuant to regulation 12A continues to have effect after the expiry of regulation 12A, in respect of that machinery or partly completed machinery.
Qualifying Northern Ireland Goods12C
1
Where paragraph (2) applies the requirements of Part 3, other than those in regulations 12A, 12B and this regulation, are treated as being satisfied.
2
This paragraph applies where—
a
the responsible person has complied with the requirements of Part 3, as that Part applies in Northern Ireland; and
b
the machinery or partly completed machinery is qualifying Northern Ireland goods.
3
In this regulation “qualifying Northern Ireland goods has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018.
Amendment to heading to Part 4I7510
For the heading to Part 4 substitute— “
Part 4
UK Marking
”
.
Amendment to regulation 13I7611
In regulation 13 (CE marked machinery to be taken to comply with Regulations)—
a
in the heading for “CE” substitute “
UK
”
;
b
in paragraph (1)—
i
for “CE” substitute “
UK
”
;
ii
for “an EC” substitute “
a
”
.
F88c
after paragraph (1) insert—
1A
For the purposes of paragraph (1) machinery bears the UK marking if, in accordance with regulation 7(2A), the UK marking is affixed to —
a
a label affixed to the machinery; or
b
a document accompanying the machinery.
Amendment to regulation 14I7712
1
Regulation 14 (machinery covered by more than one Directive) is amended as follows.
2
In the heading for “Directive” substitute “
enactment
”
.
3
In paragraph (1)—
a
for “EU directive” substitute “
enactment
”
;
b
for “the Directive” substitute “
these Regulations
”
;
c
for “the other Directive” substitute “
the other enactment
”
in both places in which it occurs;
d
for “CE” substitute “
UK
”
.
4
In paragraph (2)—
a
in each place in which it occurs for “Directive” substitute “
enactment
”
;
b
in sub-paragraph (c) omit—
i
“as published in the Official Journal of the European Union”;
ii
“EC”;
c
in the text following subparagraph (c) for “CE” substitute “
UK
”
.
Amendment to regulation 15I7813
In regulation 15 (protection of CE marking) in the heading and in each place where it occurs for “CE” substitute “
UK
”
.
Amendment to the heading to Part 5I7914
For the heading to Part 5 substitute— “
Part 5
Approved Bodies
”
.
Omission of regulation 16I8015
Omit regulation 16 (designation and monitoring of UK notified bodies).
Insertion of regulations 16A to 16CI8116
Before regulation 17 insert—
Approved bodies16A
1
An approved body is a person who—
a
on or after F89 IP completion day has been designated to carry out conformity assessment in accordance with regulation 16B, to the extent that the designation remains in effect; or
b
immediately beforeF89 IP completion day was a UK notified body.
2
Paragraph (1) is subject to regulation 17.
Approved body designation16B
1
The Secretary of State may only designate a person to carry out conformity assessment if that person qualifies for approval.
2
A person qualifies for approval if the Secretary of State—
a
is satisfied that the person meets the criteria specified in Annex XI (Part 11 of Schedule 2) (“approved body criteria”); and
b
makes a designation in respect of that person (an “approved body designation”).
3
Where a person meets the assessment criteria laid down in a designated standard (or part of such a standard) the Secretary of State is to presume that the person meets the approved body criteria covered by that standard (or that part of that standard).
4
Where the Secretary of States makes an approved body designation, that designation—
a
must be in writing;
b
must specify the conformity assessment procedures that the person designated may carry out;
c
may relate to all the categories of machinery listed in Annex IV (Part 4 of Schedule 2) or to such of those categories as are specified in the designation;
d
may designate a person for a specified period; and
e
may be made subject to such other conditions as are specified in the designation, including conditions which are to apply upon or following termination of the designation.
5
In making an approved body designation the Secretary of State may have regard (in addition to the approved body criteria) to any other matter which appears to the Secretary of State to be relevant.
Monitoring16C
1
The Secretary of State must, from time to time, carry out an inspection of each approved body with a view to verifying that it—
a
meets the approved body criteria;
b
complies with any condition to which its designation is subject—
i
in accordance with regulation 16B(4)(e);
c
complies with these Regulations.
2
An approved body must comply with any request of the Secretary of State to provide information relevant to determining its compliance with the approved body criteria, these Regulations, or any condition to which its designation is subject.
Amendment to regulation 17I8217
Regulation 17 (duration, variation and termination of designations) is amended as follows—
a
in each place in which it occurs—
i
for “a notified” substitute “
an approved
”
;
ii
for “a UK notified” substitute “
an approved
”
;
iii
for “the UK notified” substitute “
the approved
”
;
iv
for “another notified” substitute “
another approved
”
;
v
for “another UK notified” substitute “
another approved
”
.
b
after paragraph (6) insert—
7
The activities undertaken as an approved body referred to in paragraph (6) include any activities that the body has undertaken as a UK notified body.
Amendment to regulation 18I8318
In regulation 18 (functions of UK notified bodies) in the heading and in each place in which it occurs—
a
for “notified” substitute “
approved
”
;
b
for “UK notified” substitute “
approved
”
c
for “a UK notified” substitute “
an approved
”
;
d
for “the UK notified” substitute “
the approved
”
.
Amendment to regulation 19I8419
In regulation 19 (fees) in each place in which it occurs for “a UK notified” substitute “
an approved
”
.
Insertion of Regulation 19AI8520
After regulation 19 insert—
Register of approved bodies19A
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved: and
iv
any restriction on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
Amendment to regulation 21I8621
In regulation 21 (non-compliance with CE marking requirements)—
a
in the heading and in each place in which it occurs for “CE” substitute “
UK
”
;
F92aa
in paragraph (1) after “affixed to it” insert “
(or, where regulation 7(2A) applies, to a label affixed to it or a document accompanying it)
”
(four times);
b
in paragraph (1)(a) in both places in which it occurs for “the Directive” substitute “
these Regulations
”
;
c
in paragraph (1)(b)(ii) omit “EC”.
Insertion of regulations 30 and 31I6722
After regulation 29 insert—
Transitional provisions in relation to EU Exit30
1
In this regulation—
“pre-exit period” means the period beginning with 29 December 2009 and ending immediately before F93IP completion day;
“product” means machinery to which these Regulations apply.
2
Where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 12 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M42, any obligation to which a person was subject under these Regulations as they had effect immediately before F93IP completion day, continues to have effect as it did immediately before F93IP completion day, in relation to that product.
3
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action to comply with regulations 10 to 12 as they had effect immediately before F93IP completion day in relation to that product
that action has effect as if it had been done under regulations 10 to 12 as they have effect on and after F93IP completion day.
31
1
In this regulation—
“information requirements” means the requirements set out in—
- a
section 1.7.3 of Schedule 2, Part 1 that all machinery must be marked visibly, legibly and indelibly with the business name and full address of the manufacturer and where applicable the manufacturer's authorised representative; and
- b
section 4.3.1 of Schedule 2, Part 1 that each length of lifting chain, rope or webbing not forming part of an assembly must bear a mark or, where this is not possible, a plate or irremovable ring bearing the name and address of the responsible person and the identifying reference of the relevant certificate.
2
The information requirements do not apply to a person who—
a
falls within paragraph (b) of the definition of manufacturer in regulation 2(2);
b
c
before placing the machinery on the market, sets out the information referred to in sections 1.7.3 and 4.3.1 of Schedule 2, Part 1 in a document accompanying the machinery.
Amendment to Schedule 2 Part 1 Annex 1I8723
1
Schedule 2 Part 1 Annex 1 (essential health and safety requirements relating to the design and construction of machinery) is amended as follows.
2
In point 1 (general Principles) in the fourth indent omit “in accordance with the objective of the Directive”.
3
In section 1.5.1 (electricity Supply)—
a
for “Directive 2006/95/EC” substitute “
Electrical Equipment (Safety) Regulations 2016
”
M43
;
b
for “the Directive” substitute “
these Regulations
”
.
4
In section 1.5.7 (explosion) “EU directives” substitute “enactments”.
5
In section 1.7.1 (information and warnings on machinery) for the paragraph under the heading substitute—
Information and warnings on the machinery should preferably be provided in the form of readily understandable symbols or pictograms. Any written or verbal information and warnings must be expressed in English and may be accompanied on request by versions in any other language or languages understood by the operators.
6
In section 1.7.1.2 (warning devices) for “EU Directives” substitute “
enactments
”
.
7
In section 1.7.3 (marking of machinery) in both places in which it occurs for “CE” substitute “
UK
”
.
8
In section 1.7.4 (instructions)—
a
in the first paragraph, for “the official Community language or languages of the EEA state in which it is placed on the market and/or put into service” substitute “
English
”
;
b
in the third paragraph omit “official Community”.
9
In section 1.7.4.1 (general principles for the drafting of instructions)—
a
at subsection (a) for “one or more official Community languages” substitute “
English
”
;
b
at subsection (b)—
i
for “the official language(s) of the country where the machinery is to be used” substitute “
English
”
;
ii
omit “into that/those language(s)”;
iii
for “language area in question” substitute “
United Kingdom
”
.
10
In section 1.7.4.2 (contents of the instructions)—
a
in subsection (c) in both places in which it occurs omit “EC”;
b
in subsection (u)—
i
for “harmonised” substitute “
designated
”
;
ii
in both places in which it occurs for “EU directives” substitute “
enactments
”
.
11
In section 2.1.1 (general) in subsection (a) for “Directives” substitute “
enactments
”
.
12
In section 2.2.1.1 (instructions), in both places in which it occurs, for “harmonised” substitute “
designated
”
.
13
For section 2.4.10(i) substitute—
i
an indication that the machinery may be subject to requirements for regular inspection by designated bodies, as provided for in the Plant Protection Products (Sustainable Use) Regulations 2012 M44
14
In section 3.6.3.1 (vibrations) for “harmonised” substitute “
designated
”
.
Amendment to Schedule 2 Part 2 Annex III8824
1
Schedule 2 Part 2 Annex II (Declarations) is amended as follows.
2
In Section 1 Part A (EC Declaration of conformity of the machinery)—
a
in the heading and in the third paragraph omit “EC”;
b
in point 2 omit “, who must be established in an EEA state”;
c
in point 4—
i
for “the Directive” substitute “
these Regulations
”
;
ii
for “Directives and/or” substitute “
enactments or
”
;
F97d
in point 5—
i
for “notified” substitute “
approved
”
;
ii
for “EC type-” substitute “
Type-
”
in both places in which it occurs;
F96da
in point 6 for “notified” substitute “
approved
”
;
e
in point 7 for “published harmonised” substitute “
designated
”
.
3
in Section 1 Part B (declaration of incorporation of partly completely machinery)—
a
in point 2 omit “, who must be established in an EEA state”;
b
in point 4—
i
for “Directives” substitute “
enactments
”
;
ii
omit “These references must be those of the texts published in the Official Journal of the European Union”;
c
in point 6 for “the Directive” substitute “
these Regulations
”
.
4
In Section 2 (Custody) omit “EC” from the first paragraph.
Amendment to Schedule 2 Part 3 Annex IIII8925
For Schedule 2 Part 3 Annex III (CE marking) substitute—
Part 3Annex III UK marking
The UK marking must be affixed in the immediate vicinity of the name of the responsible person, using the same technique.
Where the full quality assurance procedure prescribed in Annex X (Part 10 of this Schedule) has been applied, the UK marking must be followed by the identification number of the approved body.
Amendment to Schedule 2 Part 6 Annex VII9026
In Schedule 2 Part 6 Annex VI (assembly instructions for partly completed machinery) for “an official Community” substitute “
English or a
”
.
Amendment to Schedule 2 Part 7 Annex VIII9127
1
Schedule 2 Part 7 Annex VII (technical files) is amended as follows.
2
In Part A (technical file for machinery)—
a
in the introductory paragraph—
i
for “the Directive” substitute “
these Regulations
”
;
ii
for “one or more official Community languages” substitute “
English
”
;
b
in point 1(a)(ii) in both places in which it occurs omit “EC”;
c
in point 1(b) for “the Directive” substitute “
these Regulations
”
;
d
in point 2—
i
in the first paragraph omit “and the competent authorities of any other EEA state”;
ii
in the second paragraph—
aa
for “territory of an EEA state” substitute “
United Kingdom
”
;
bb
omit “EC”;
e
in point 3 for “competent national authorities” substitute “
enforcement authorities
”
.
3
In Part B (relevant technical documentation for partly completed machinery)—
a
in the introductory paragraph—
i
for “the Directive” substitute “
these Regulations
”
;
ii
for “one or more official Community languages” substitute “
English
”
;
b
in point (b)—
i
in the second paragraph for “territory of an EEA state” substitute “
United Kingdom
”
;
ii
in both places in which it occurs in the second and third paragraphs, omit “or a competent authority of any other EEA state”.
Amendment to Schedule 2 Part 8 Annex VIIII9228
In Schedule 2 Part 8 Annex VIII (assessment of conformity with internal checks on the manufacture of machinery) in points 1 and 3 for “the Directive” substitute “
these Regulations
”
.
Amendment to Schedule 2 Part 9 Annex IXI9329
1
Schedule 2 Part 9 (EC type-examinations) is amended as follows.
2
In the heading for “EC type-” substitute “
Type-
”
.
3
In the introductory paragraph—
a
for “EC type-” substitute “
Type-
”
;
F98aa
for “a notified” substitute “
an approved
”
;
b
for “the Directive” substitute “
these Regulations
”
.
4
In point 2—
a
for “an EC type-” substitute “
a Type-
”
;
b
for “a notified” substitute “
an approved
”
;
c
in each place in which it occurs (other than that referred to in sub-paragraph (b)) for “notified” substitute “
approved
”
.
5
In point 3 for “notified” substitute “
approved
”
.
6
In points 3.2 and 3.3 for “published harmonised” substitute “
designated
”
.
7
In point 4—
a
for “the Directive” substitute “
these Regulations
”
;
b
in both places in which it occurs for “notified” substitute “
approved
”
;
c
for “an EC type-” substitute “
a Type-
”
.
8
For point 5 substitute—
5
If the type does not satisfy the provisions of these Regulations, the approved body shall refuse to issue the applicant with a Type-examination certificate, giving detailed reasons for its refusal. It shall inform the applicant, the other approved bodies and the Secretary of State. An appeal procedure must be available.
9
In point 6—
a
in both places in which it occurs for “notified” substitute “
approved
”
;
b
in both places in which it occurs for “EC type-” substitute “
Type-
”
.
10
In point 7—
a
for “Commission, the Member States” substitute “
Secretary of State
”
;
b
for “Commission and the Member States” substitute “
Secretary of State
”
;
c
in both places in which it occurs for “notified” substitute “
approved
”
;
d
for “EC type-” substitute “
Type-
”
.
11
For the text in point 8 substitute—
8
Files and correspondence referring to the Type-examination procedures shall be written in English or any other language acceptable to the approved body.
12
In point 9, 9.1, the first paragraph of 9.3 and 9.4 for “EC type-” substitute “
Type-
”
.
13
Amendment to Schedule 2 Part 10 Annex XI9430
In Schedule 2 Part 10 Annex X (full quality assurance)—
a
in the introductory paragraph and in the first paragraph in point 2.1 for “a notified” substitute “
an approved
”
;
b
for “notified” substitute “
approved
”
in—
i
the fourth indent to point 2.1;
ii
the first paragraph of point 2.3;
iii
the F101second paragraph and the third paragraph of point 2.4;
iv
point 3;
v
the first paragraph of point 3.2;
vi
point 3.3;
vii
the first paragraph F102in both places in which it occurs and in the final paragraph of point 3.4;
viii
the second indent to point 4;
c
in both places in which it occurs in point 2.2 for “the Directive” substitute “
these Regulations
”
;
d
in the second indent to point 2.2 and in the second paragraph to point 2.3 for “harmonised” substitute “
designated
”
.
Amendment to Schedule 2 Part 11 Annex XII9531
In Schedule 2 Part 11 Annex XI (minimum criteria to be taken into account by Member States for the notification of bodies)—
a
in the heading for “Member States for the notification” substitute “
the Secretary of State for approval
”
;
F103aa
in point 3 for “notified” substitute “
approved
”
;
b
in point 8—
i
for “notified” substitute “
approved
”
;
ii
omit “take part directly or be represented in European standardisation, or”.
Amendment to Schedule 3I9632
In Schedule 3—
F1a
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
b
in paragraph 1(k) for “Council Directive 2006/95/EC of 12 December 2006 on the harmonisation of the laws of Member States” substitute “
The Electrical Equipment (Safety) Regulations 2016 M45
”
;
F2c
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Amendment to Schedule 4I9733
In Schedule 4 (appeals against notified body decisions)—
a
in the heading for “notified” substitute “
approved
”
;
b
in paragraph 1—
i
for “a UK notified” substitute “
an approved
”
;
ii
for “UK notified” substitute “
approved
”
.
Amendment to Schedule 5I9834
In Schedule 5 (enforcement)—
a
in points (8)(f)(i), (10)(e)(i) and in paragraph 16 for “CE” substitute “
UK
”
;
b
in paragraph 16 omit “with a view to that information being passed by the Secretary of State to the Commission”.
SCHEDULE 13Amendment of the Aerosol Dispensers Regulations 2009
IntroductionI8961
The Aerosol Dispensers Regulations 2009 are amended in accordance with paragraphs 2 to 8.
Amendment to regulation 2I992
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “compliance mark”;
b
for the definition of “relevant aerosol dispenser” substitute—
“relevant aerosol dispenser” means an aerosol dispenser which does not have a maximum capacity of—
a
less than 50ml; or
b
greater than that specified—
i
in point 3.1 of Schedule 1A, in relation to metal aerosol dispensers (1000ml);
ii
in point 4.1.1 of Schedule 1A, in relation to aerosol dispensers with plastic coated or permanently protected glass containers or plastic aerosol dispensers which cannot splinter on bursting (220ml); or
iii
in point 4.2.1 of Schedule 1A, in relation to aerosol dispensers with unprotected glass containers or plastic aerosol dispensers which may splinter on bursting (150ml);
c
after the definition of “relevant aerosol dispenser” insert—
“UK marking” means the marking in the form set out in Annex 2 of Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9 July 2008 setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93.
3
After paragraph (1) insert—
1A
Schedule 1A reproduces the provisions of the Annex to the Directive with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedule 1A is a reference to the equivalent provision of the Annex to the Directive as set out in that Schedule.
4
In paragraph (2)—
a
for “the Annex to the Directive” substitute “
Schedule 1A
”
;
b
after “is a reference to” insert “
a test method specified in point 6.1.4.1(b) or (c) which is
”
;
c
in subparagraph (a)—
i
for “that Annex” substitute “
the Annex to the Directive
”
;
ii
after “point 6.1.4.3” insert “
of that Annex
”
;
d
in subparagraph (b) after “6.1.4.3” insert “
of Schedule 1A
”
.
Amendment to regulation 3I1003
In regulation 3 (requirements for the marking of aerosol dispensers)—
F105a
in paragraph (1)—
i
after “dispenser” insert “
or where paragraph (1A) applies, on a label affixed to, or a document accompanying, the aerosol dispenser
”
;
ii
for “a compliance mark” substitute “
the UK marking
”
;
F104aa
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the aerosol dispenser; or
b
a document accompanying the aerosol dispenser.
b
in each place in which it occurs, for “the Annex to the Directive” substitute “
Schedule 1A
”
;
c
in paragraph (7)(b) after “6.1.3” insert “
of Schedule 1A
”
;
d
in paragraph (7)(c)—
i
after “6.1.4.1(a)”, “6.1.4.1(b)” and “6.1.4.1(c)” (in paragraphs (i), (ii) and (iii) respectively), insert “
of Schedule 1A
”
;
ii
after “6.1.4.2” in both places in which it occurs insert “
of that Schedule
”
;
e
for paragraph (8)(a) substitute—
a
the following information—
i
the name and address or trade mark of the person responsible for marketing the aerosol dispenser;
ii
code markings enabling the filling batch to be identified;
iii
the details referred to in point 2.2 of Schedule 1A; and
iv
where an aerosol dispenser—
aa
contains flammable components as defined in point 1.8 of Schedule 1A; and
bb
is not classified as extremely flammable or flammable in accordance with the classifications set out in point 1.9 of Schedule 1A;
the quantity of flammable material contained in the aerosol dispenser must be clearly stated on the label in legible and indelible wording in the following form—“X % by mass of the contents are flammable”;
Insertion of regulation 3AI1014
After regulation 3, insert—
Power to amend Schedule 1A3A
1
The Secretary of State may by regulations amend Schedule 1A where the Secretary of State considers it necessary to do so in order to take technical progress into account.
2
The power to make regulations made under paragraph (1) includes power—
a
to make different provisions for different cases; and
b
to make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
3
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 4I102F1065
For regulation 4 substitute—
4
1
Subject to paragraph (2), a person shall not supply or have in his possession for supply a relevant aerosol dispenser which is not marked with the UK marking or the symbol “3” (inverted epsilon).
2
Where regulation 3(1A) applies, paragraph (1) does not apply where the UK marking is affixed to—
a
a label affixed to the aerosol dispenser; or
b
a document accompanying the aerosol dispenser.
Amendment to regulation 5I1036
In regulation 5 (prohibition of sale or supply of non-compliant marked aerosol dispensers)—
a
for paragraph (a), substitute—
a
an aerosol dispenser which—
i
is marked with the UK marking where all the requirements referred to in regulation 3 relating to that aerosol dispenser are not complied with; F107...
F108ia
has a label affixed to it, or a document accompanying it, which is marked with the UK marking, where—
aa
the requirements of regulation 3 are not complied with; or
bb
the requirements of regulation 3 are complied with but the period referred to in regulation 3(1A) has elapsed; or
F109ii
is marked with the symbol “3” (inverted epsilon) where either—
aa
all the requirements of regulation 5A(3) are not complied with; or
bb
all the requirements of regulation 5B are not complied with; or
F110b
for paragraph (b) substitute—
b
an aerosol dispenser which—
i
is marked with a mark so closely resembling the UK marking or the symbol “3” (inverted epsilon) as to be likely to deceive; or
ii
has a label affixed to it or a document accompanying it which is marked with a mark so closely resembling the UK marking as to be likely to deceive.
Insertion of regulation 5AI1047
After regulation 5 insert—
Obligations which are met by complying with obligations in the Directive5A
1
In this regulation any reference to an Article or an Annex is a reference to an Article of or the Annex to the Directive.
2
Paragraph (3) sets out the requirements which must be complied with under regulation 5F111(a)(ii)(aa) in order to mark an aerosol dispenser with the symbol “3” (inverted epsilon).
3
The requirements referred to in paragraph (2) are that—
a
the aerosol dispenser is a relevant aerosol dispenser; F112...
F113aa
the aerosol dispenser is supplied within a period of 12 months beginning with IP completion day; and
b
the relevant aerosol dispenser—
i
complies with the obligations of Article 8(1) (or where permitted by that Article, where its label complies with those requirements);
ii
complies with the requirements of Article 8(1a);
iii
bears or, where permitted by Article 8(1), has on its label the information required by Article 8(1)(d) and 8(1a) prepared in or translated into English (unless it is unlikely that the aerosol dispenser will be used in the United Kingdom);
iv
complies with the general provisions relating to construction set out in point 2.1 of the Annex;
v
complies with the provisions relating to the volume of the liquid phase set out in point 2.3 of the Annex;
vi
complies with the special provision set out in points 3, 4 and 5 of the Annex in the case of metal, glass and plastic dispensers respectively;
vii
meets the tests specified in point 6.1.1 of the Annex in the case of empty containers;
viii
meets the test specified in point 6.1.2 and 6.1.3 of the Annex in the case of empty metal and protected glass dispensers respectively; and
ix
satisfies one of the following paragraphs—
aa
it meets the test methods specified in point 6.1.4.1(a) of the Annex and the dispenser is not of a type referred to in point 6.1.4.2 of the Annex;
bb
it meets the test method specified in point 6.1.4.1(b) of the Annex and the dispenser is not of a type referred to in point 6.1.4.2 of the Annex;
cc
it meets the test method specified in point 6.1.4.1(c) of the Annex; and
c
the person responsible for the marketing of the relevant aerosol dispenser—
i
has affixed the symbol “3” (inverted epsilon) in accordance with Article 3; and
ii
has complied with the obligations set out after the heading to point 2 (general provisions) and immediately before point 2.1 of the Annex.
F114Qualifying Northern Ireland Goods5B
1
Paragraph (2) sets out the requirements that must be complied with under regulation 5(a)(ii)(bb) to mark an aerosol dispenser with the symbol “3” (inverted epsilon);
2
The requirements referred to in paragraph (1) are that—
a
the requirements of regulation 3 as it applies in Northern Ireland have been complied with in relation to the aerosol dispenser; and
b
the aerosol dispenser is qualifying Northern Ireland goods.
3
In this regulation “qualifying Northern Ireland goods” has the meaning given to it in in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018.
Insertion of Schedule 1AI1058
After the Schedule, insert—
SCHEDULE 1A(Annex to the Directive)
1
DEFINITIONS
1
Regulation (EC) No 1272/2008
“Regulation (EC) No 1272/2008” means Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on the classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006.
Pressures1
“Pressures” means the internal pressures expressed in bars (relative pressures).
Test pressure1
“Test pressure” means the pressure to which an unfilled aerosol dispenser container may be subjected for 25 seconds without any leakage being caused or, in the case of metal or plastic containers, any visible or permanent distortion except as allowed under 6.1.1.2.
Bursting pressure1
“Bursting pressure” means the minimum pressure which causes the aerosol dispenser container to burst or rupture.
Total capacity of the container1
“Total capacity of the container” means the volume in millilitres of an open container up to the rim of the opening.
Net capacity1
“Net capacity” means the volume in millilitres of a filled and closed aerosol dispenser.
Volume of liquid phase1
“Volume of liquid phase” means the volume of the non-gaseous phases in the filled and closed aerosol dispenser.
Test conditions1
“Test conditions” means the values of test and bursting pressures exerted hydraulically at 20° C (± 5°C).
Substance1
“Substance” means substance as defined in Article 2(7) of Regulation (EC) No 1272/2008.
Mixture1
“Mixture” means mixture as defined in Article 2(8) of Regulation (EC) No 1272/2008.
Flammable contents1
Contents of aerosols shall be considered as flammable if they contain any component which is classified as flammable:
a
flammable liquid means a liquid having a flashpoint of not more than 93°C.
b
flammable solid means a solid substance or mixture which is readily combustible or may cause or contribute to fire as a result of friction; readily combustible solids are powdered, granular, or pasty substances or mixtures which are dangerous if they can be easily ignited by brief contact with an ignition source, such as a burning match, and if the flame spreads rapidly.
c
flammable gas means a gas or gas mixture having a flammable range with air at 20°C and a standard pressure of 1.013 bar.
This definition does not cover pyrophoric, self—heating or water—reactive substances and mixtures, which shall never be components of aerosol contents.
Flammable aerosols1
For the purpose of these Regulations, an aerosol is considered as “non-flammable”, “flammable” or “extremely flammable” according to its chemical heat of combustion and mass content of flammable components, as follows:
a
the aerosol is classified as “extremely flammable” if it contains 85% or more flammable components and the chemical heat of combustion exceeds or is equal to 30 kJ/g;
b
the aerosol is classified as “non-flammable” if it contains 1% or less flammable components and the chemical heat of combustion is less than 20 kJ/g;
c
all other aerosols will be submitted to the flammability classification procedures set out in 1.9.1. to 1.10. or shall be classified as “extremely flammable”. The ignition distance test, the enclosed space test and the foam flammability test shall comply with point 6.3.
Flammable spray aerosols1
In the case of spray aerosols, the classification shall be made taking into account the chemical heat of combustion and on the basis of the results of the ignition distance test, as follows:
a
if the chemical heat of combustion is less than 20kJ/g:
i
the aerosol is classified as “flammable” if ignition occurs at a distance equal or greater than 15 cm but less than 75 cm;
ii
the aerosol is classified as “extremely flammable” if ignition occurs at a distance of 75 cm or more;
iii
if no ignition occurs in the ignition distance test, the enclosed space test shall be performed and in this case, the aerosol is classified as “flammable” if the time equivalent is less than or equal to 300 s/m3 or the deflagration density is less than or equal to 300 g/m3; otherwise the aerosol is classified as “non-flammable”;
b
if the chemical heat of combustion is equal to or more than 20 kJ/g, the aerosol is classified as “extremely flammable” if ignition occurs at a distance of 75 cm or more; otherwise the aerosol is classified as “flammable”.
Flammable foam aerosols1
In the case of foam aerosols, the classification shall be made on the basis of the results of the foam flammability test.
a
The aerosol product shall be classified as “extremely flammable” if:
i
the flame height is 20 cm or more and the flame duration is two seconds or more; or
ii
the flame height is 4 cm or more and the flame duration is seven seconds or more.
b
The aerosol product which does not meet the criteria in (a) is classified as “flammable” if the flame height is 4 cm or more and the flame duration is two seconds or more.
Chemical Heat of Combustion1
The chemical heat of combustion ΔHc shall be either determined by:
a
acknowledged rules of technology, described e.g. in standards such as ASTM D 240, ISO 13943 86.1 to 86.3 and NFPA 30B, or found in the scientifically established literature; or
b
applying the following calculation method:
The chemical heat of combustion (ΔHc), in kilojoules per gram (kJ/g), can be calculated as the product of the theoretical heat of combustion (ΔHcomb), and a combustion efficiency, usually less than 1,0 (a typical combustion efficiency is 0,95 or 95%).
For a composite aerosol formulation, the chemical heat of combustion is the summation of the weighted heats of combustion for the individual components, as follows:
where:
ΔHc = chemical heat of combustion (kJ/g) of the product;
wi% = mass fraction of component i in the product;
ΔHc(i) = specific heat of combustion (kJ/g) of component i in the product.
The person responsible for the marketing of the aerosol dispenser has to describe the method used for determining the chemical heat of combustion in a document to be made readily available in English at the address specified on the label in accordance with regulation 3(8)(a)(i), if the chemical heat of combustion is used as a parameter for assessing the flammability of aerosols, according to the provisions of these Regulations.
GENERAL PROVISIONS2
Without prejudice to specific provisions of this Schedule on requirements related to the flammability and pressure hazard, the person responsible for the marketing of aerosol dispensers is under an obligation to analyse the hazards in order to identify those which apply to their aerosol dispensers. Where appropriate, this analysis shall include a consideration of the risks resulting from the inhalation of the spray ejected by the aerosol dispenser under normal and reasonably foreseeable conditions of use, taking into account droplet size distribution in conjunction with physical and chemical properties of the contents. The person responsible for the marketing of the aerosol dispenser must then design, construct and test it and, if applicable, draft special statements concerning its use, taking account of this analysis.
Construction and equipment2
2
The filled aerosol dispenser must be such that, under normal conditions of use and storage, it complies with the provisions of this Schedule.
2
The valve must enable the aerosol dispenser to be virtually hermetically sealed under normal conditions of storage or transport and must be protected, for example by means of a protective cap, against any unintentional opening and any deterioration.
2
There must be no possibility that the mechanical resistance of the aerosol dispenser can be impaired by the action of the substances contained in it, even during prolonged storage.
Labelling2
Without prejudice to Regulation (EC) No 1272/2008, each aerosol dispenser must visibly bear the following and indelible marking:
a
where the aerosol is classified as “non-flammable” according to the criteria of point 1.9, the signal word “Warning” and the other label elements for Aerosols Category 3 provided for in Table 2.3.1 of Annex I to Regulation (EC) No 1272/2008;
b
where the aerosol is classified as “flammable” according to the criteria of point 1.9, the signal word “Warning” and the other label elements for Aerosols Category 2 provided for in Table 2.3.1 of Annex I to Regulation (EC) No 1272/2008;
c
where the aerosol is classified as “extremely flammable” according to the criteria of point 1.9, the signal word “Danger” and other label elements for Aerosols Category 1 provided for in Table 2.3.1 of Annex I to Regulation (EC) No 1272/2008;
d
where the aerosol dispenser is a consumer product, the precautionary statement P102 provided for in Part 1, Table 6.1 of Annex IV to Regulation (EC) No 1272/2008;
e
any additional operating precautions which alert consumers to the specific dangers of the product; if the aerosol dispenser is accompanied by separate instructions for use, the latter must also reflect such operating precautions.
Volume of the liquid phase2
The volume of the liquid phase at 50°C must not exceed 90% of the net capacity.
SPECIAL PROVISIONS FOR METAL AEROSOL DISPENSERS3
Capacity3
The total capacity of these containers may not exceed 1000 ml.
Test pressure of the container3
a
For containers filled at a pressure of less than 6.7 bars at 50°C, the test pressure must be equal to at least 10 bars.
b
For containers filled at a pressure equal to or greater than 6.7 bars at 50°C, the test pressure must be 50% higher than the internal pressure at 50° C.
3
The pressure at 50°C in the aerosol dispenser must not exceed the values provided for in the following table, depending upon the content of gases in the aerosol dispenser:
Content of gases
Pressure at 50°C
Liquified gas or mixture of gases having a flammable range with air at 20°C and a standard pressure of 1,013 bar
12 bar
Liquified gas or mixture of gases not having a flammable range with air at 20°C and a standard pressure of 1,013 bar
13.2 bar
Compressed gases or gases dissolved under pressure not having a flammable range with air at 20°C and a standard pressure of 1.013 bar
15 bar
SPECIAL PROVISIONS FOR GLASS AEROSOL DISPENSERS4
Plastic coated or permanently protected containers4
Containers of this type may be used for filling with compressed, liquefied or dissolved gas.
Capacity4
The total capacity of these containers may not exceed 220 ml.
Coating4
The coating must be a protective envelope of plastic or other suitable material, intended to prevent the risk of flying particles of glass if the container is accidently broken, and must be so designed that there are no flying particles of glass if the filled aerosol dispenser, brought to a temperature of 20° C, is dropped from a height of 1.8 m onto a concrete floor.
Test pressure of the container4
a
Containers used for filling with compressed or dissolved gas must resist a test pressure equal to at least 12 bars.
b
Containers used for filling with liquefied gas must resist a test pressure equal to at least 10 bars.
Filling4
a
Aerosol dispensers filled with compressed gas shall not be required to withstand a pressure of more than 9 bars at 50°C.
b
Aerosol dispensers filled with dissolved gas shall not be required to withstand a pressure of more than 8 bars at 50°C.
c
Aerosol dispensers containing liquefied gas or mixtures of liquefied gas shall not be required to withstand, at 20°C, pressures higher than those shown in the following table:
Total capacity
Percentage by weight of liquefied gas in the total mixture
20 %
50 %
80 %
50 to 80 ml
3.5 bars
2.8 bars
2.5 bars
< 80 to 160 ml
3.2 bars
2.5 bars
2.2 bars
< 160 to 220 ml
2.8 bars
2.1 bars
1.8 bars
This table shows the pressure limits permitted at 20°C in relation to the percentage of gas.
Pressure limits for percentages of gas not shown in the table shall be extrapolated from it.
Unprotected glass containers4
Aerosol dispensers using unprotected glass containers shall be filled exclusively with liquefied or dissolved gases.
Capacity4
The total capacity of these containers may not exceed 150ml.
Test pressure of the container4
The test pressure of the container must be equal to at least 12 bars.
Filling4
a
Aerosol dispensers filled with dissolved gas shall not be required to withstand a pressure of more than 8 bars at 50°C.
b
Aerosol dispensers containing liquefied gas shall not be required to withstand, at 20° C, pressures in excess of those shown in the following table:
Total capacity
Percentage by weight of liquefied gas in the total mixture
20 %
50%
80%
50 to 70 ml
1.5 bar
1.5 bar
1.25 bar
< 70 to 150 ml
1.5 bar
1.5 bar
1 bar
This table shows the pressure limits permitted at 20°C in relation to the percentage of liquefied gas.
Pressure limits for percentages of gas not shown in the table shall be extrapolated from it.
SPECIAL PROVISIONS APPLYING TO PLASTIC AEROSOL DISPENSERS5
5
Plastic aerosol dispensers which may splinter on bursting shall be treated in the same way as unprotected glass aerosol dispensers.
5
Plastic aerosol dispensers which cannot splinter on bursting shall be treated in the same way as glass aerosol dispensers with a protective coating.
TESTS6
Test requirements to be guaranteed by the person responsible for marketing the aerosol dispenser6
Hydraulic test on empty containers6
6
Metal, glass or plastic aerosol dispensers must be able to withstand a hydraulic pressure test as laid down in 3.1.1, 4.1.3 and 4.2.2.
6
Metal containers showing assymetrical or major distortions or other similar faults shall be rejected. A slight symmetrical distortion of the base or one affecting the profile of the upper casing shall be allowed provided that the container passes the bursting test.
Bursting test for empty metal containers6
The person responsible for marketing the aerosol dispenser must ensure that the bursting pressure of containers is at least 20% higher than the test pressure laid down.
Dropping test for protected glass containers6
The manufacturer must ensure that the containers satisfy the test requirements laid down in 4.1.2.
Final inspection of filled aerosol dispensers6
6
Aerosol dispensers shall be subject to one of the following final test methods.
a
Hot water bath test
Each filled aerosol dispenser shall be immersed in a hot water bath.
i
The temperature of the water bath and the duration of the test shall be such that the internal pressure reaches that which would be exerted by its contents at a uniform temperature of 50°C.
ii
Any aerosol dispenser showing visible permanent distortion or a leak must be rejected.
b
Hot final test methods
Other methods for heating the contents of aerosol dispensers may be used if they guarantee that the pressure and temperature in each filled aerosol dispenser reach the values required for the hot water bath test and distortions and leaks are detected with same precision as in the case of the hot water bath test.
c
Cold final test methods
An alternative cold final test method may be used if it is in accordance with the provisions of an alternative method to the hot water bath test for aerosol dispensers in paragraph 6.2.6.3.2 of Chapter 6.2 of Part 6 of Annex A to the European Agreement on the international carriage of dangerous goods by road M46.
6
For aerosol dispensers the contents of which undergo a physical or chemical transformation changing their pressure characteristics after filling and before first use, cold final test methods according to point 6.1.4.1(c) should be applied.
6
In case of test methods according to points 6.1.4.1(b) and 6.1.4.1(c):
a
the test method must be approved by the Secretary of State;
b
the person responsible for the marketing of aerosol dispensers must submit an application for approval to the Secretary of State. The application must be accompanied by the technical file describing the method;
c
the person responsible for the marketing of aerosol dispensers must, for surveillance purposes, keep the approval of the Secretary of State, the technical file describing the method and, if applicable, control reports readily available at the address specified on the label in accordance with regulation 3(8)(a)(i); and
d
the technical file must be in English.
Examples of inspection tests which may be carried out6
Test on unfilled containers6
The test pressure shall be applied for 25 seconds on five containers selected at random from a homogeneous batch of 2500 unfilled containers, that is, manufactured from the same materials by the same continuous batch manufacturing process, or from a batch constituting one hour's production.
If any one of these containers does not pass the test, ten additional containers shall be drawn at random from the same batch and put through the same test.
If any one of these aerosol containers does not pass the test, the whole batch shall be unsuitable for use.
Tests on filled aerosol dispensers6
Air and water-tightness inspection tests shall be carried out by immersing a representative number of filled aerosol dispensers in a bath of water. The temperature of the bath and the period of immersion must be such as to enable the contents of the aerosol dispenser to attain a uniform temperature of 50°C during the time required to ensure that there is no bursting or rupture.
Any batch of aerosol dispensers which does not pass these tests must be considered unsuitable for use.
Tests on the flammability of aerosols6
Ignition distance test for spray aerosols6
Introduction6
6
This test standard describes the method to determine the ignition distance of an aerosol spray in order to assess the associated flame risk. The aerosol is sprayed in the direction of an ignition source at intervals of 15cm to observe if ignition and sustained combustion of the spray takes place. Ignition and sustained combustion is defined as when a stable flame is maintained for at least five seconds. The ignition source is defined as a gas burner with a blue, non-luminous flame 4-5cm in height.
6
This test is applicable to aerosol products with a spray distance of 15 cm or more. Aerosol products with a spray distance of less than 15 cm such as dispensing foams, mousses, gels and pastes or fitted with a metering valve, are excluded from this test. Aerosol products that dispense foams, mousses, gels or pastes are subject to testing under the aerosol foam flammability test.
Apparatus and material6
6
The following apparatus is required:
Water bath maintained at 20°C
accurate to ± 1°C
Calibrated laboratory scales (balance)
accurate to ± 0.1 g
Chronometer (stopwatch)
accurate to ± 0.2 s
Graduated scale, support and clamp
graduations in cm
Gas burner with support and clamp
Thermometer
accurate to ± 1°C
Hygrometer
accurate to ± 5%
Pressure gauge
accurate to ± 0.1 bar
Procedure6
General requirements6
6
Before testing, each aerosol dispenser shall be conditioned and then primed by discharging for approximately one second. The purpose of this action is to remove non-homogeneous material from the diptube.
6
The instructions of use shall be strictly followed, including whether the dispenser is intended to be used in the upright or inverted position. When shaking is required, shake immediately before testing.
6
The test shall be carried out in a draught-free environment capable of ventilation, with the temperature controlled at 20°C ± 5°C and relative humidity in the range 30-80%.
6
Each aerosol dispenser is to be tested:
a
when full according to the complete procedure, with the gas burner in the range of 15-90cm distance from the actuator of the aerosol can;
b
when 10-12% full nominal (% by mass) only one test, either at 15cm distance from the actuator when the spray from a full can did not ignite at all, or at the flame ignition distance of the spray of a full can plus 15cm.
6
During the test, the can shall be positioned as indicated by label instructions. The ignition source shall be positioned accordingly.
6
The following procedure requires testing the spray at intervals of 15cm between the burner flame and the aerosol actuator, in the range of 15-90cm. It is efficient to start at 60cm distance between burner flame and aerosol actuator. The distance between burner flame and aerosol actuator shall be increased by 15cm in the case of an ignition of the spray at 60cm distance. The distance shall be decreased by 15cm in the case of no ignition at 60cm distance between burner flame and aerosol actuator. The aim of the procedure is to determine the maximum distance between aerosol actuator and burner flame that leads to sustained combustion of the spray or to determine that ignition could not be obtained at 15cm distance between the burner flame and the aerosol's actuator.
Test procedure6
a
a minimum of 3 full aerosol dispensers per product shall be conditioned to 20°C ± 1 °C with at least 95 % of the dispenser immersed in the water for at least 30 minutes before each test (if the aerosol is fully immersed, 30 minutes conditioning is sufficient);
b
comply with general requirements; record the temperature and relative humidity of the environment;
c
weigh an aerosol dispenser and note its mass;
d
determine the internal pressure and initial discharge rate at 20°C ± 1°C (to eliminate faulty or partly filled aerosol dispensers);
e
support the gas burner on a flat horizontal surface or fix the burner to a support by means of a clamp;
f
ignite the gas burner; the flame shall be non-luminous and approximately 4-5cm high;
g
place the actuator's exit orifice at the required distance from the flame; the aerosol shall be tested in the position it is designed to be used, e.g. upright or inverted;
h
level the actuator's orifice and burner flame, ensuring that the orifice is properly directed towards and aligned with the flame (see Figure below); the spray shall be expelled through the top half of the flame;
i
comply with the general requirements regarding shaking of the dispenser;
j
actuate the valve of the aerosol dispenser, to discharge its contents for five seconds, unless ignition occurs; if ignition occurs, continue discharging and time the duration of the flame for five seconds, from the start of ignition;
k
note the ignition results for the distance between the gas burner and the aerosol dispenser in the table provided;
l
if no ignition occurs during step (j), the aerosol shall be tested in alternative orientations, e.g. inverted for upright use products, to check if ignition is obtained;
m
repeat steps (g) to (l) twice more (a total of 3) for the same can at the same distance between the gas burner and the aerosol actuator;
n
repeat the test procedure for another two aerosol cans of the same product at the same distance between gas burner and aerosol actuator;
o
repeat steps (g) to (n) of the test procedure at a distance between 15 and 90 cm between the actuator of the aerosol can and the burner flame depending on the outcome of each test (see also 6.3.1.3.1.4 and 6.3.1.3.1.5);
p
if no ignition occurs at 15cm, the procedure is finished for initially full cans; the procedure is also finished when ignition and sustained combustion is obtained at a distance of 90cm; if ignition could not be obtained at 15cm distance, record that ignition did not occur; the maximum distance between burner flame and the aerosol's actuator for which an ignition and sustained combustion was observed is noted as the “ignition distance”, in all other circumstances;
q
one test shall also be conducted on three cans of 10-12% nominal fill level; these cans shall be tested at a distance between the aerosol's actuator and the burner flame of the “flame ignition distance of full cans + 15cm”;
r
discharge an aerosol can to a 10-12% nominal fill level (by mass) in bursts of 30 seconds maximum; observe a 300 seconds minimum time period between bursts; during this interim period dispensers shall be placed in the water bath for conditioning;
s
repeat steps (g) to (n) for 10-12% nominal fill aerosol cans, omitting steps (l) and (m); this test shall only be performed with the aerosol in one position, e.g. upright or inverted, corresponding with that which produced the ignition (if any) for filled cans;
t
record all results in the Table 6.3.1.1 as shown below.
6
All experiments shall be performed in a fume hood in a room that may be well ventilated. Ventilation of the fume hood and room can be applied for at least three minutes after each test. Take all necessary safety precautions to prevent the inhalation of combustion products.
6
The cans with a 10-12 % nominal fill level shall be tested only once. The result tables need only one result per can indicated.
6
When the test in the position in which the dispenser is designed to be used gives a negative result, the test shall be repeated in the position of the dispenser most likely to result in a positive result.
Method of assessing results6
6
All the results shall be recorded. Table 6.3.1.1 below shows the model of “result table” to be used.
Table 6.3.1.1
Date
Temperature … °C Relative humidity … %
Name of product
Net volume
Can 1
Can 2
Can 3
Initial level of filling
%
%
%
Dispenser distance
Test
1
2
3
1
2
3
1
2
3
15 cm
Ignition?
Y or N
30 cm
Ignition?
Y or N
45 cm
Ignition?
Y or N
60 cm
Ignition?
Y or N
75 cm
Ignition?
Y or N
90 cm
Ignition?
Y or N
Observations — including can position
Enclosed space ignition test6
Introduction6
This test standard describes the method to assess the flammability of products emerging from aerosol dispensers due to their propensity to ignite in an enclosed or confined space. The contents of an aerosol dispenser are sprayed into a cylindrical test vessel containing a burning candle. If an observable ignition occurs, the elapsed time and amount discharged is noted.
Apparatus and material6
6
The following apparatus is required:
Chronometer (stopwatch)
accurate to ± 0,2 s
Water bath maintained at 20 °C
accurate to ± 1 °C
Calibrated laboratory scales (balance)
accurate to ± 0,1 g
Thermometer
accurate to ± 1 °C
Hygrometer
accurate to ± 5 %
Pressure gauge
accurate to ± 0.1 bar
Cylindrical test vessel
as detailed below
Preparation of test apparatus6
6
A cylindrical vessel approximately 200 dm3 volume, approximately 600mm in diameter and approximately 720mm long and open at one end shall be modified as follows:
a
a closure system consisting of a hinged cover shall be matched to the open end of the receptacle; or
b
a plastic film 0.01 to 0.02 mm thick may be used as a closure system; if the test is carried out with a plastic film this must be used as described below: stretch the film over the open end of the drum and hold it in place with an elastic band; the strength of the band shall be such that when placed around the drum resting on its side, it stretches by only 25mm when a mass of 0.45 kg is attached to its lowest point; cut a 25 mm slit in the film, starting 50 mm from the edge of the drum. Ensure that the film is taut;
c
at the other end of the drum drill a 50mm diameter hole 100mm from the edge in such a way that the orifice is uppermost when the receptacle is laid down and ready for the test (Figure below);
d
on a 200 × 200 mm metal support place a paraffin wax candle 20 to 40 mm in diameter and 100 mm high; the candle shall be replaced when having a height of less than 80 mm; the candle's flame is protected from the action of the spray by a 150 mm wide, 200 mm high deflector; this includes the plane inclined at 45° produced 150 mm from the base of the deflector (see Figure below);
e
the candle placed on the metal support shall be positioned midway between the two ends of the drum (see Figure below);
f
the drum is laid on the ground or on a support at a spot where the temperature is between 15 °C and 25 °C; the product to be tested will be sprayed within the drum of roughly 200 dm3 in which there will be a source of ignition.
6
Usually, the product leaves the aerosol can at an angle of 90° relevant to the vertical axis of the can. The layout and procedure described refers to this kind of aerosol product. In the case of unusually operating aerosols (e.g. vertical-spray aerosol dispensers) it will be necessary to record changes to equipment and procedures in accordance with good laboratory practice, such as International Standard ISO/IEC 17025:2007 General requirements for the competence of testing and calibration laboratories.
Procedure6
General requirements6
6
Before testing, each aerosol dispenser shall be conditioned and then primed by discharging for approximately 1 second. The purpose of this action is to remove non-homogeneous material from the diptube.
6
The instructions of use shall be strictly followed, including whether the dispenser is intended to be used in the upright or inverted position. When shaking is required, shake immediately before testing.
6
The tests shall be carried out in a draught-free environment capable of ventilation, with the temperature controlled at 20°C ± 5°C and relative humidity in the range 30-80%.
Test procedure6
a
a minimum of 3 full aerosol dispensers per product shall be conditioned to 20 °C ± 1 °C in a water bath with at least 95 % of the dispenser immersed in the water for at least 30 min (if the aerosol is fully immersed, 30 min conditioning is sufficient);
b
measure or calculate the actual volume of the drum in dm3;
c
comply with general requirements; record the temperature and relative humidity of the environment;
d
determine the internal pressure and initial discharge rate at 20 °C ± 1 °C (to eliminate faulty or partly filled aerosol dispensers);
e
weigh one of the aerosol dispensers and note its mass;
f
light the candle and apply the closure system (cover or plastic film);
g
place the aerosol dispenser actuator orifice 35 mm or closer for a wide spray product, from the centre of the entrance hole in the drum; start the chronometer (stopwatch) and following the instructions for use of the product; direct the spray towards the centre of the opposite extremity (cover or plastic film); the aerosol shall be tested in the position it is designed to be used, e.g. upright or inverted;
h
spray until ignition occurs; stop the chronometer and note the time elapsed; re-weigh the aerosol dispenser and note its mass;
i
ventilate and clean the drum removing any residue likely to affect subsequent tests; allow the drum to cool if necessary;
j
repeat the test procedure steps (d) to (i) for another two aerosol dispensers of the same product (three in total, note: each dispenser is only tested once).
Method of assessing results6
6
A test report containing the following information shall be drawn up:
a
the product tested and its references;
b
the internal pressure and discharge rate of the aerosol dispenser;
c
the temperature and relative air humidity of the room;
d
for each test, the discharge time (s) needed to achieve ignition (if the product does not ignite, state this);
e
the mass of the product sprayed during each test (in g);
f
the actual volume of the drum (in dm3).
6
The time equivalent (teq) needed to achieve ignition in one cubic metre can be calculated as follows:
6
The deflagration density (Ddef) needed to achieve ignition during the test may also be calculated as follows:
Aerosol foam flammability test6
Introduction6
6
This test standard describes the method to determine the flammability of an aerosol spray emitted in the form of a foam, mousse, gel or paste. An aerosol, which emits a foam, mousse, gel or paste is sprayed (approximately 5 g) on a watchglass and an ignition source (candle, wax taper, match or lighter) is placed at the base of the watchglass to observe if ignition and sustained combustion of the foam, mousse, gel or paste occurs. Ignition is defined as a stable flame maintained for at least two seconds and a minimum 4 cm in height.
Apparatus and material6
6
The following apparatus is required:
Graduated scale, support and clamp
gradations in cm
Fire-resistant watchglass roughly 150 mm in diameter
Chronometer (stopwatch)
accurate to ± 0,2 s
Candle, wax taper, match or lighter
Calibrated laboratory scales (balance)
accurate to ± 0,1 g
Water bath maintained at 20°C
accurate to ± 1 °C
Thermometer
accurate to ± 1 °C
Hygrometer
accurate to ± 5 %
Pressure gauge
accurate to ± 0.1 bar
6
The watch-glass is placed on a fire-resistant surface within a draught-free area that may be ventilated after each test. The graduated scale is positioned exactly behind the watch-glass and held vertically by means of a support and clamp.
6
The scale is positioned in such a way that its origin is on a level with the watch-glass base in a horizontal plane.
Procedure6
General requirements6
6
Before testing, each aerosol dispenser shall be conditioned and then primed by discharging for approximately 1 second. The purpose of this action is to remove non-homogeneous material from the diptube.
6
The instructions of use shall be strictly followed, including whether the dispenser is intended to be used in the upright or inverted position. When shaking is required, shake immediately before testing.
6
The tests shall be carried out in a draught-free environment capable of ventilation, with the temperature controlled at 20°C ± 5°C and relative humidity in the range of 30-80%.
Test procedure6
a
a minimum of four full aerosol dispensers per product shall be conditioned to 20 °C ± 1°C with at least 95% of the dispenser immersed in the water for at least 30 min before each test (if the aerosol is fully immersed, 30 minutes conditioning is sufficient);
b
comply with general requirements. Record the temperature and relative humidity of the environment;
c
determine the internal pressure at 20°C ± 1 °C (to eliminate faulty or partly filled aerosol dispensers);
d
measure the discharge or flow rate of the aerosol product to be examined, so that the amount of test product dispensed can be more accurately gauged;
e
weigh one of the aerosol dispensers and note its mass;
f
on the basis of the measured discharge or flow rate and following the manufacturer's instructions, release approximately 5 g of the product onto the centre of the clean watch glass with the aim of producing a mound no higher than 25 mm;
g
within five seconds of completion of discharge, apply the source of ignition to the edge of the sample at its base and at the same time start the chronometer (stopwatch); if necessary, the ignition source shall be removed from the edge of the sample after approximately two seconds, in order to clearly observe if ignition has occurred; if no ignition of the sample is apparent, the ignition source shall be reapplied to the edge of the sample;
h
if ignition occurs note the following points:
i
the maximum height of the flame in cm above the base of the watch-glass;
ii
the flame duration in s;
iii
dry and re-weigh the aerosol dispenser and calculate the mass of the released product;
i
ventilate the test area immediately after each test;
j
if ignition is not obtained and the released product remains in the form of a foam or paste throughout its period of use, steps (e) to (i) shall be repeated; allow the product to stand for 30 seconds, 1 minute, 2 minutes or 4 minutes before applying the ignition source;
k
repeat the test procedure steps (e) to (j) twice more (a total of 3) for the same can;
l
repeat the test procedure steps (e) to (k) for another two aerosol cans (3 cans in total) of the same product.
Method of assessing results6
6
A test report containing the following information shall be drawn up:
a
whether the product ignites;
b
the maximum flame height in cm;
c
the duration of flame in seconds;
d
the mass of the product tested.
SCHEDULE 14Amendment of the Accreditation Regulations 2009
GeneralI8971
The Accreditation Regulations 2009 are amended in accordance with paragraphs 2 to 4.
I1062
In regulation 2—
a
in paragraph (1)—
i
for the definition of “the EC Regulation” substitute—
“RAMS” means Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9th July 2008 setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93;
b
in paragraph (2) for “the EC Regulation” in both places in which it occurs substitute “
RAMS
”
.
I1073
In regulation 3—
a
for “the EC Regulation” substitute “
RAMS
”
;
b
for “national accreditation body” substitute “
UK national accreditation body
”
.
I1084
In regulations 4(1), 5 and 6 and paragraph (1) of the Schedule for “the EC Regulation” substitute “
RAMS
”
.
SCHEDULE 15Amendment of the Toys (Safety) Regulations 2011
InterpretationI8981
The Toys (Safety) Regulations 2011are amended in accordance with paragraphs 2 to 43.
Amendment to regulation 2I1092
1
In regulation 2(2) (revocation, saving and amendment)—
a
before “as if” insert “subject to the modifications in paragraph (2A);
b
after paragraph (2) insert—
2A
The modifications referred to in paragraph (2) are—
a
that references to “the Community” are to be read as including the United Kingdom; and
b
paragraph (5) of regulation 9 is to be read as if “, the Commission of the Communities, the other member States and other approved bodies” were omitted.
Insertion of regulation 2AI1103
After regulation 2 insert—
Transitional provision in relation to EU Exit2A
1
In this regulation—
“pre-exit period” means the period beginning with 19th August 2011 and ending immediately before F115IP completion day;
“product” means a toy to which these Regulations apply.
2
Subject to paragraphs (3) and (4), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 15 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M47, any obligation or prohibition to which a person was subject under these Regulations as they had effect immediately before F115IP completion day, continues to have effect as it did immediately before F115IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcement authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
4
Where an EC-type examination was issued in relation to a product to which paragraph (2) applies references to “Type examination” in regulations 22 and 45 are to be read as referring to an EC-type examination referred to in regulation 44 as it had effect immediately before F115IP completion day.
5
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulations 11 to 13 as they had effect immediately before F115IP completion day in relation to that product,
that action has effect as if it had been done under regulations 11 to 13 as they have effect on and after F115IP completion day.
Amendment to regulation 3I1114
In regulation 3 (interpretation)—
a
in the definition of “the Directive” at the end insert “
(as it has effect immediately before F116 IP completion day)
”
;
b
after the definition of “the GPSR” insert—
“approved body requirements” has the meaning given to it in regulation 40A;
F117c
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
d
omit the definition of “CE marking”;
e
before the definition of “distributor” insert—
“designated standard” has the meaning given to it in regulation 3A;
f
omit the definition of “harmonised standard”;
F118g
for the definition of “importer” substitute—
“importer” means a person who—
a
is established in the United Kingdom and places a toy from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a toy on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F119h
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
i
for the definition of “Module” substitute—
“Module” means a Module set out in Schedule 6 and Module A, B or C is to be construed accordingly;
j
omit the definition of “notified body designation”;
F120k
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
l
after the definition of “place on the market” insert—
“RAMS” means Regulation (EC) No 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93;
m
after the definition of “toy” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
n
omit the definition of “UK notified body”.
Insertion of Regulations 3A and 3BI1125
After regulation 3, insert—
Designated standard3A
1
Subject to paragraphs (6) and (7) in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate;
2
For the purposes of subparagraph (a), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one of more of the following—
a
the characteristics required of a product including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; or
b
production methods and processes relating to the products, where these have an effect on their characteristics;
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI);
4
When considering whether the manner of publication of a reference in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard;
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation organisations;
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation a reference to a “product” is a reference to a toy to which these Regulations apply
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Annexes to EU legislation as Schedules3B
1
Schedules 1, 2, 4 and 5 reproduce provisions of the Annexes I, II, IV and V (respectively) to the Directive with amendments to correct deficiencies in retained EU law.
2
A reference to a provision of Schedules 1, 2, 4, 5 is a reference to the equivalent provision of the relevant Annex to the Directive as set out in the relevant Schedule.
3
Schedule 6 reproduces provisions of Annex II to Decision No 768/2008/EC of the European Parliament and of the Council of 9 July 2008 on a common framework for the marketing of products, and repealing Council Decision 93/465/EECM48 (“Decision No 768/2008/EC”) which are relevant to these Regulations, as it has effect immediately before F121IP completion day, with amendments to correct deficiencies in retained EU law.
4
A reference to a provision of Schedule 6 is a reference to the equivalent provision of Annex II of Decision No 768/2008/EC as set out in that Schedule.
Amendment to regulation 4I1136
In regulation 4 (toys to which these Regulations apply) in paragraph (3)(f) for “Annex I to the Directive” substitute “
Schedule 1
”
.
Amendment to regulation 5I1147
In regulation 5(1)(b) (essential safety requirement) for “Annex II to the Directive (as amended from time to time)” substitute “
Schedule 2
”
.
Omission of regulation 6I1158
Regulation 6 (toys placed on the market before 20th July 2013) is omitted.
Amendment to regulation 7I1169
In regulation 7 (presumption of conformity) for “harmonised” substitute “
designated
”
.
Amendment to regulation 8I11710
In regulation 8 (exception for trade fairs or exhibitions)—
a
in paragraph (1) for “CE” substitute “
UK
”
;
b
in paragraph (2) in both places in which it occurs for “the Directive” substitute “
these Regulations
”
;
c
in paragraph (2)(b) for “EU” substitute “
United Kingdom
”
.
Amendment to regulation 10I11811
In regulation 10 (prohibitions on placing toys on the market) in paragraph (2)(d)—
a
omit “EC”;
b
for “CE” substitute “
UK
”
.
Amendment to regulation 13I11912
In regulation 13 (applicable conformity assessment procedures)—
a
in paragraphs (2) and (3)(a) to (c) for “harmonised” substitute “
designated
”
;
b
in paragraph (3) for “EC-type” substitute “
Type
”
.
Amendment to regulation 14I12013
In regulation 14 (application for EC-type examination)—
a
in the heading and in the regulation for “EC-type” substitute “
Type
”
b
in paragraph (a) for “a notified” substitute “
an approved
”
;
c
in paragraph (e)—
i
omit the words beginning with “if” and ending with “UK notified body,”;
ii
for “by UK notified” substitute “
by approved
”
.
Amendment to regulation 15I12114
In regulation 15 (EC declaration of conformity and CE marking)—
a
in the heading—
i
for “EC declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (a) for “an EC” substitute “
a
”
;
c
in paragraph (b) for “CE” substitute “
UK
”
.
Amendment to regulation 16I12215
In regulation 16—
a
omit “EC” in each place in which it occurs;
b
in paragraph 2(a) for “Annex III to the Directive” substitute “
Schedule 3
”
;
c
omit paragraph (5).
Amendment to regulation 17I12316
In regulation 17 (technical documentation and correspondence)—
a
in the heading for “EC-type” substitute “
Type
”
;
b
for paragraph (2) substitute—
2
The technical documentation must be drawn up in English.
c
in paragraph (3) for “Annex IV of the Directive” substitute “
Schedule 4
”
;
d
for paragraph (4) substitute—
4
Any correspondence relating to the Type examination of a toy must be drawn up in English.
e
in paragraph (5) omit “EC”;
f
in paragraph (10)—
i
for “a notified” substitute “
an approved
”
;
ii
for “the notified” substitute “
the approved
”
in both places in which it occurs;
iii
for “harmonised” substitute “
designated
”
.
Amendment to regulation 18I124F12217
In regulation 18 (Toys to bear CE marking)—
a
in the heading and in each place in which it occurs for “CE” substitute “
UK
”
;
b
after paragraph (3) insert—
3A
For a period of 24 months beginning with IP completion day, the manufacturer may, in place of affixing the UK marking in accordance with paragraph (3) affix the UK marking to a document accompanying the toy.
Amendment to regulation 20I12518
In regulation 20 (instructions for use, safety information and warnings)—
a
omit paragraph (10);
b
in each place in which it occurs for “Annex V to the Directive” substitute “
Schedule 5
”
.
Amendment to regulation 21I12619
In regulation 21(2)(b) (compliance procedures for series production)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EC”.
Amendment to regulation 22I12720
In regulation 22 (submission of EC-type examination certificate for review)—
a
in the heading for “EC-type” substitute “
Type
”
;
b
for “An EC-type” substitute “
A Type
”
;
c
for “a notified” in each place in which it occurs substitute “
an approved
”
.
Amendment to regulation 25I12821
In regulation 25 (manufacturer's authorised representative)—
a
in paragraph (1) for “within the EU” substitute “
in the United Kingdom
”
;
b
in paragraph (2)(a) omit “or translation”.
Amendment to regulation 26I12922
Regulation 26 (prohibitions on placing toys on the market) in paragraph 2(a)(iii) for “CE” substitute “
UK
”
.
Amendment to regulation 27I13023
In regulation 27 (information identifying importer) for paragraph (2) substitute—
2
Paragraph (1) does not apply where—
a
either—
i
the size or nature of the toy precludes the information from being marked on the toy;
ii
the importer would have to open the toy's packaging in order to mark the information on the toy; or
b
the importer ensures that the information referred to in paragraph (1) is set out F124on the toy's packaging or in a document accompanying the toy.
Amendment to regulation 31I13124
In regulation 31 (duties to retain and provide information) omit “EC”.
Amendment to regulation 33I13225
In regulation 33 (duty to act with due care and prohibitions) in paragraph (3)(a)(i) for “CE” substitute “
UK
”
.
Amendment to regulation 39I13326
In regulation 39 (protection of CE marking)—
a
in the heading and in each place in which it occurs for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)(ii) omit “in accordance with regulation 25(1)”.
Insertion of regulation 39A and Part 2AI13427
After regulation 39 insert—
Obligations which are met by complying with obligations in the Directive39A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article of or an Annex to the Directive;
b
“CE marking” has the meaning given to it in Article 3(16);
c
“harmonised standard” has the meaning given to it in Article 3(8);
2
Subject to paragraphs (6) and (7) paragraph (3) applies where, before placing a toy on the UK market, a manufacturer—
a
ensures that the toy has been designed and manufactured in accordance with the requirements set out in—
i
in Article 10 (essential safety requirements); and
ii
Annex II (particular safety requirements);
b
carries out the safety assessment in accordance with Article 18;
c
ensures that the relevant conformity assessment procedure has been carried out in accordance with Article 19;
d
in cases where the manufacturer considers that Article 19(3) applies, ensures that the provisions of Article 20 are complied with;
e
draws up the technical documentation in accordance with Article 21(1);
f
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
g
affixes the CE marking in accordance with Articles 16 and 17;
h
draws up an EC declaration of conformity, in accordance with Article 15; and
i
ensures that the EC declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 10 to 15, 16(1) to (2), 17(1) to (4) and 18, are to be treated as being satisfied;
b
regulations 16(4) to (5), 17(5) and (10), 21, 22, 39 and 44 are to be read subject to the modifications in paragraph (10);
c
regulations 42 to 44 do not apply; and
d
regulation 52 does not apply.
4
Subject to paragraphs (6) and (7), paragraph (5) applies, where before placing a toy on the market, the importer ensures that—
a
the relevant conformity assessment procedure that applies to that toy has been carried out in accordance with Article 19;
b
the manufacturer has drawn up the technical documentation in accordance with Article 21(1); and
c
the toy bears the CE marking affixed in accordance with Articles 16 and 17.
5
Where this paragraph applies—
a
the requirements in regulation 26(a)(i) to (iii) are to be treated as being satisfied; and
b
regulations 26(1), 28 and 30 to 32 are to be read subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard.
7
Where paragraph (6) applies paragraphs (2)(c) and (4)(a) are to be treated as requiring the manufacturer to carry out the conformity assessment procedure referred to in Article 19(3).
8
Paragraph (9) applies where before making a toy available on the market, a distributor ensures that the manufacturer has affixed the CE marking in accordance with Articles 16 and 17.
9
Where this paragraph applies—
a
regulation 33(3)(a)(i) is to be treated as being satisfied;
b
regulation 33(2), 34, 35 and 37 are to be read subject to the modifications in paragraph (10).
10
The modifications referred to in paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EC declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the requirements set out in—
i
in Article 10 (essential safety requirements); and
ii
Annex II (particular safety requirements);
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “applicable conformity assessment procedure” is to be read as a reference to the applicable conformity assessment procedures referred to in Article 19;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Article 21(1);
g
any reference to “authorised representative” is a reference to a person appointed in accordance with Article 5; and
h
any reference to “Type examination” is a reference to “EC-type examination”.
F128Expiry of regulation 39A39AA
1
Subject to paragraph (2), regulation 39A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 39A—
a
any toy which was placed on the market pursuant to regulation 39A may continue to be made available on the market on or after the expiry of regulation 39A;
b
any obligation to which a person was subject under regulation 39A in respect of a toy placed on the market pursuant to regulation 39A continues to have effect after the expiry of regulation 39A, in respect of that toy.
Qualifying Northern Ireland Goods39AB
1
Where paragraph (2) applies—
a
a toy is to be treated as being in conformity with the essential safety requirements; and
b
each relevant economic operator is to be treated as having complied or as complying with the obligations imposed on them under Part 2.
2
This paragraph applies where—
a
a toy is—
i
in conformity with the essential safety requirements, within the meaning of regulation 3 as it applies in Northern Ireland; and
ii
qualifying Northern Ireland goods;
b
each relevant economic operator has complied or is complying with the obligations imposed on them under Part 2, as that Part applies in Northern Ireland; and
c
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(c) are that, before placing the toy on the market, the importer—
a
complies with regulation 27;
b
ensures that—
i
the applicable conformity assessment procedure has been carried out;
ii
the manufacturer has drawn up the technical documentation; and
iii
the toy bears the CE marking.
4
In this regulation—
“applicable conformity assessment procedure” means the conformity assessment procedure applicable to the toy under regulation 15, as it applies in Northern Ireland;
“CE marking” has the meaning given to it in regulation 3, as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” means the technical documentation that a manufacturer has to draw up in accordance with regulation 17, as it applies in Northern Ireland.
PART 2APowers and duties of the Secretary of State in relation to toys
Power to amend Schedules 1, 2 and 539B
1
The Secretary of State may by regulations amend the provision of the Schedules referred to in paragraph (2) where the Secretary of State considers it necessary to do so in order to take technical progress and scientific developments into account.
2
The provisions referred to in paragraph (1) are—
a
any provision in Schedule 1;
b
points 11 and 13 of Part 3 of Schedule 2; and
c
any provision of Schedule 5.
3
The power to make regulations made under paragraph (1) includes power—
a
to make different provisions for different cases; and
b
to make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
4
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Power to amend Appendix C to Schedule 239C
1
The Secretary of State may by regulations amend Appendix C to Schedule 2 to add specific values for chemicals used in toys intended for use by children under 36 months or in other toys intended to be placed in the mouth.
2
Regulations made under paragraph (1) may—
a
make different provisions for different cases; and
b
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
3
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Powers to amend Appendix A to Schedule 239D
1
Where the conditions set out in paragraph (3)(a) and (b) are met, the Secretary of State may by regulations amend Appendix A to Schedule 2 to allow substances or mixtures classified as carcinogenic, mutagenic or toxic for reproduction of the categories laid down in Section 4 of Appendix B of Schedule 2 to be used in toys, in components of toys or micro-structurally distinct parts of toys.
2
Where the conditions set out in paragraphs (3)(a), (b) and (c) are met, the Secretary of State may by regulations amend Appendix A to Schedule 2 to allow substances or mixtures classified as carcinogenic, mutagenic or toxic for reproduction of the categories laid down Section 3 of Appendix B of Schedule 2 to be used in toys, in components of toys or micro-structurally distinct parts of toys.
3
The conditions referred to in paragraphs (1) and (2) are—
a
the Secretary of State considers that there is sufficient scientific evidence to demonstrate that the use of substances or mixtures that are classified as carcinogenic, mutagenic or toxic for reproduction of the categories laid down in Section 5 of Appendix B to Schedule 2 are safe for use in toys, particularly in view of exposure;
b
the substance or mixture is not prohibited for use in consumer articles by Regulation (EC) 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) establishing a European Chemicals Agency, amending Directive 19999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/105/EC and 2000/21/EC.
c
there are no suitable alternative substances or mixtures available, as documented in an analysis of alternatives; and
4
Regulations made under paragraph (1) or (2) may—
a
make different provisions for different cases; and
b
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
5
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
6
The Secretary of State must—
a
carry out a review of regulations made under paragraph (1) or (2);
b
set out the conclusions of the review in a report; and
c
publish the report.
7
A review to which paragraph (6) refers must be made—
a
as soon as any safety concerns arise; and
b
at intervals not exceeding five years beginning with the date regulations made under paragraph (1) or (2) come into force.
Duty of the Secretary of State to evaluate use of hazardous substances39E
1
The Secretary of State must—
a
evaluate the occurrence of hazardous substances of materials in toys;
b
set out the conclusions of the evaluation in a report; and
c
publish the report.
2
During the evaluation the Secretary of State must consult—
a
any enforcement authority which is not the Secretary of State; and
b
any person that the Secretary of State considers appropriate.
3
The first report must be published before the end of the period of five years beginning on F127IP completion day.
4
Subsequent reports are to be published at intervals not exceeding five years.
Substitution of Part 3I13528
For Part 3, substitute—
PART 3Approval of Conformity Assessment Bodies
Approved bodies40A
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 40B (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 40E (restriction, suspension or withdrawal of approval).
3
In this Part—
“UK notified body” means a body—
- a
which the Secretary of State had before F129 IP completion day notified to the European Commission and the member States of the European Union, in accordance with Article 31 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 40(2), as it had effect immediately before F129 IP completion day;
“approved body requirements” means the requirements set out in Schedule 7.
Approval of conformity assessment bodies40B
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment module in respect of which the conformity assessment body claims to be competent;
iii
the category of toys in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate issued by the UK national accreditation body attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies40C
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring40D
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 40B(6)(b); or
ii
by the Secretary of State before F130 IP completion day in that body's capacity as a UK notified body; and
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval40E
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement; or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 40D(b), the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 40A (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 40D(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 40A.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and other enforcement authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a UK notified body.
Operational matters in relation to approved bodies40F
1
Subject to the terms of its appointment and to regulation 44, an approved body must carry out the conformity assessment activities and modules—
a
in respect of which the body's approval was given under regulation 40B; or
b
in respect of which body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 6.
Subsidiaries and contractors40G
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M49.
Register of approved bodies40H
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body41
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 40D; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 40H.
Amendment to Part 4I13629
In the heading of Part 4 (Functions of UK Authorised Bodies) for “UK Notified” substitute “
Approved
”
.
Amendment to regulation 42I13730
In regulation 42 (duty to perform EC-type examinations)—
a
in the heading and in paragraph (1) in both places in which it occurs for “EC-type” substitute “
Type
”
;
b
in paragraph (1) for “A UK notified” substitute “
An approved
”
;
c
in paragraph (2) for “a UK notified” substitute “
an approved
”
;
d
in paragraph (2)(d)—
i
for “designation” substitute “
approval
”
;
ii
for “notified” substitute “
approved
”
.
Amendment to regulation 43I13831
In regulation 43 (performance of EC-type examinations)—
a
in the heading and in paragraph (2) for “EC-type” substitute “
Type
”
;
b
in paragraph (1)—
i
for “A UK notified” substitute “
An approved
”
;
ii
for “an EC-type” substitute “
a Type
”
.
Amendment of regulation 44I13932
In regulation 44 (issue and content of EC-type examination certificate)—
a
in the heading and in paragraph (2)(d) for “EC-type” substitute “
Type
”
;
b
in each place in which it occurs for “an EC-type” substitute “
a Type
”
;
c
in paragraphs (1) and (3) for “A UK notified” substitute “
An approved
”
;
d
in paragraph (2)(a) for “the Directive” substitute “
these Regulations
”
;
e
in paragraph (3)(b) for “notified” substitute “
approved
”
;
f
in paragraph (3)(c) for “a notified” substitute “
an approved
”
;
g
in paragraphs (4), (5) and (6) for “a UK notified body” substitute “
an approved body
”
.
Amendment of regulation 45I14033
In regulation 45 (action after issue of EC-type examination certificate)—
a
in the heading and in paragraphs (2)(a)(ii), (3) and (3)(a) for “EC-type” substitute “
Type
”
;
b
in paragraph (1)(a) and (4) for “an EC-type” substitute “
a Type
”
;
c
in paragraph (1)(b) for “a UK notified” substitute “
an approved
”
;
d
for “the UK notified” in each place in which it occurs substitute “
the approved
”
.
Amendment to regulation 46I14134
In regulation 46 (provision of information by UK notified bodies)—
a
in the heading—
i
for “UK notified” substitute “
approved
”
;
ii
for “other notified” substitute “
other approved
”
;
b
for “A UK notified” substitute “
An approved
”
;
c
for “other notified” substitute “
other approved
”
.
Amendment of regulation 47I14235
In regulation 47 (instructions to UK notified bodies)—
a
in the heading, in paragraph (3) and paragraph (7) for “UK notified” substitute “
approved
”
;
b
in the title for “EC-type” substitute “
Type
”
;
c
for “a notified” in each place in which it occurs substitute “
an approved
”
;
d
for “a UK notified” in each place in which it occurs substitute “
an approved
”
;
e
for “an EC-type” in each place in which it occurs substitute “
a Type
”
.
Omission of regulations 48 and 49I14336
Regulations 48 (participation by UK notified bodies) and 49 (subcontracting by a UK notified body) are omitted.
Amendment to regulation 50I14437
In regulation 50 (charging of fees by UK notified body)—
a
in the heading for “UK notified” substitute “
approved
”
;
b
for “A UK notified” in both places in which it occurs substitute “
An approved
”
.
Amendment to regulation 51I14538
In regulation 51 (provision of information by UK notified bodies)—
a
in the heading and in paragraph (3) for “UK notified” substitute “
approved
”
;
b
for “a UK notified” in both places in which it occurs substitute “
an approved
”
;
c
for “an EC-type” in both places in which it occurs substitute “
a Type
”
;
d
for “designation” in each place in which it occurs substitute “
approval
”
;
e
in paragraph (2)(c) for the words beginning with “paragraphs” and ending in “bodies)” substitute “
the approved body requirements
”
.
Amendment to regulation 52I14639
In regulation 52 (enforcement action in cases of formal non-compliance)—
a
for “CE” in both places in which it occurs substitute “
UK
”
;
b
for “an EC” in both places in which it occurs substitute “
a
”
.
Amendment to regulation 53I14740
In regulation 53 (enforcement action in cases of toys presenting a risk) omit paragraph (5).
Amendment to regulation 54I14841
In regulation 54 (notification of enforcement action)—
a
for paragraph (1) substitute—
1
Where a person or an enforcing authority is not the Secretary of State and it has taken action under regulation 53, it must notify the Secretary of State of—
a
the results of the evaluation; and
b
the corrective actions which it requires the relevant economic operator to take.
b
in paragraph (2) for “notified” substitute “
approved
”
.
Amendment to regulation 55I14942
In regulation 55 omit paragraph (4).
Insertion of SchedulesI15043
At the end of the Regulations insert—
SCHEDULE 1PRODUCTS THAT ARE NOT TOYS (Annex I to the Directive)
1
Products listed in paragraphs 2 to 20 are not to be considered as toys.
2
Decorative objects for festivities and celebrations.
3
Products for collectors, provided that the product or its packaging bears a visible and legible indication that it is intended for collectors of 14 years of age and aboveExamples of this category are—
a
detailed and faithful scale models;
b
kits for the assembly of detailed scale models;
c
folk dolls and decorative dolls and other similar articles;
d
historical replicas of toys; and
e
reproductions of real fire arms.
4
Sports equipment, including roller skates, inline skates, and skateboards intended for children with a body mass of more than 20 kg.
5
Bicycles with a maximum saddle height of more than 435 mm, measured as the vertical distance from the ground to the top of the seat surface, with the seat in a horizontal position and with the seat pillar set to the minimum insertion mark.
6
Scooters and other means of transport designed for sport or which are intended to be used for travel on public roads or public pathways.
7
Electrically driven vehicles which are intended to be used for travel on public roads, public pathways, or the pavement thereof.
8
Aquatic equipment intended to be used in deep water, and swimming learning devices for children, such as swim seats and swimming aids.
9
Puzzles with more than 500 pieces.
10
Guns and pistols using compressed gas, with the exception of water guns and water pistols, and bows for archery over 120 cm long.
11
Fireworks, including percussion caps which are not specifically designed for toys.
12
Products and games using sharp-pointed missiles, such as sets of darts with metallic points.
13
Functional educational products, such as electric ovens, irons or other functional products operated at a nominal voltage exceeding 24 volts which are sold exclusively for teaching purposes under adult supervision.
14
Products intended for use for educational purposes in schools and other pedagogical contexts under the surveillance of an adult instructor, such as science equipment.
15
Electronic equipment, such as personal computers and game consoles, used to access interactive software and their associated peripherals, unless the electronic equipment or the associated peripherals are specifically designed for and targeted at children and have a play value on their own, such as specially designed personal computers, key boards, joy sticks or steering wheels.
16
Interactive software, intended for leisure and entertainment, such as computer games, and their storage media, such as compact disks.
17
Babies' soothers.
18
Child-appealing luminaires.
19
Electrical transformers for toys.
20
Fashion accessories for children which are not for use in play.
SCHEDULE 2PARTICULAR SAFETY REQUIREMENTS (Annex II to the Directive)
Part 1 Physical and Mechanical Properties1
Toys and their parts and, in the case of fixed toys, their anchorages, must have the requisite mechanical strength and, where appropriate, stability to withstand the stresses to which they are subjected during use without breaking or becoming liable to distortion at the risk of causing physical injury.
2
Accessible edges, protrusions, cords, cables and fastenings on toys must be designed and manufactured in such a way that the risks of physical injury from contact with them are reduced as far as possible.
3
Toys must be designed and manufactured in such a way as not to present any risk or only the minimum risk inherent to their use which could be caused by the movement of their parts.
4
a
Toys and their parts must not present a risk of strangulation.
b
Toys and their parts must not present a risk of asphyxiation by closing off the flow of air as a result of airway obstruction external to the mouth and nose.
c
Toys and their parts must be of such dimensions as to not present a risk of asphyxiation by closing off the flow of air as a result of internal airway obstruction by objects wedged in the mouth or pharynx or lodged over the entrance to the lower airways.
d
Toys, which are clearly intended for use by children under 36 months, and their component parts and any of their detachable parts must be of such dimensions as to prevent their being swallowed or inhaled. This also applies to other toys which are intended to be put in the mouth, and to their component parts and any of their detachable parts.
e
The packaging in which toys are contained for retail sale must not present a risk of strangulation or asphyxiation caused by airway obstruction external to the mouth and nose.
f
Toys contained within food or co-mingled with food must have their own packaging. This packaging, as it is supplied, must be of such dimensions as to prevent its being swallowed and/or inhaled.
g
Toy packaging, as referred to in points (e) and (f), which is spherical, egg-shaped or ellipsoidal, and any detachable parts of this or of cylindrical toy packaging with rounded ends, must be of such dimensions as to prevent it from causing airway obstruction by being wedged in the mouth or pharynx or lodged over the entrance to the lower airways.
h
Toys firmly attached to a food product at the moment of consumption, in such a way that the food product needs to be consumed in order to get direct access to the toy, are prohibited. Parts of toys otherwise directly attached to a food product must fulfil the requirements set out in points (c) and (d).
5
Aquatic toys must be designed and manufactured so as to reduce as far as possible, taking into account the recommended use of the toy, any risk of loss of buoyancy of the toy and loss of support afforded to the child.
6
Toys which it is possible to get inside and which thereby constitute an enclosed space for occupants must have a means of exit which the intended user can open easily from the inside.
7
Toys conferring mobility on their users must, as far as possible, incorporate a braking system which is suited to the type of toy and is commensurate with the kinetic energy generated by it. Such a system must be easy for the user to operate without risk of ejection or physical injury for the user or for third parties.
The maximum design speed of electrically driven ride-on toys must be limited so as to minimise the risk of injury.
8
The form and composition of projectiles and the kinetic energy they may generate when fired from a toy designed for that purpose must be such that, taking into account the nature of the toy, there is no risk of physical injury to the user or to third parties.
9
Toys must be manufactured so as to ensure that:
a
the maximum and minimum temperature of any accessible surfaces does not cause injury when touched; and
b
liquids and gases contained within the toy do not reach temperatures or pressures which are such that their escape from the toy, other than for reasons essential to the proper functioning of the toy, might cause burns, scalds or other physical injury.
10
Toys which are designed to emit a sound must be designed and manufactured in such a way in terms of the maximum values for impulse noise and continuous noise that the sound from them is not able to impair children's hearing.
11
Activity toys must be manufactured so as to reduce the risk of crushing or trapping of body parts or trapping of clothing and of falls, impacts and drowning as far as possible. In particular, any surface of such a toy accessible for one or more children to play on must be designed to bear their load.
Part 2 Flammability1
Toys must not constitute a dangerous flammable element in the child's environment. They must therefore be composed of materials which fulfil one or more of the following conditions:
a
they do not burn if directly exposed to a flame or spark or other potential source of fire;
b
they are not readily flammable (the flame goes out as soon as the fire cause disappears);
c
if they do ignite, they burn slowly and present a low rate of spread of the flame;
d
irrespective of the toy's chemical composition, they are designed so as to mechanically delay the combustion process.
Such combustible materials must not constitute a risk of ignition for other materials used in the toy.
2
Toys which, for reasons essential to their functioning, contain substances or mixtures that meet the classification criteria laid down in Section 1 of Appendix B, in particular materials and equipment for chemistry experiments, model assembly, plastic or ceramic moulding, enamelling, photography or similar activities, must not contain, as such, substances or mixtures which may become flammable due to the loss of non-flammable volatile components.
3
Toys other than toy percussion caps must not be explosive or contain elements or substances likely to explode when used as intended or in a foreseeable way, bearing in mind the behaviour of children.
4
Toys and, in particular, chemical games and toys, must not contain as such substances or mixtures:
a
which, when mixed together, may explode through chemical reaction or through heating;
b
which may explode when mixed with oxidizing substances; or
c
which contain volatile components which are flammable in air and liable to form a flammable or explosive vapour/air mixture.
Part 3 Chemical Properties1
Toys must be designed and manufactured in such a way that there are no risks of adverse effects on human health due to exposure to the chemical substances or mixtures of which the toys are composed or which they contain when the toys are used as intended or in a foreseeable way, bearing in mind the behaviour of children.
2
Toys that are themselves substances or mixtures must comply also with Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, as applicable, relating to the classification, packaging and labelling of certain substances and mixtures (“Regulation 1272/2008”).
3
Without prejudice to the restrictions referred to in the second paragraph of point 1, substances that are classified as carcinogenic, mutagenic or toxic for reproduction (CMR) of category 1A, 1B or 2 under Regulation 1272/2008 must not be used in toys, in components of toys or in micro-structurally distinct parts of toys.
4
By way of derogation from point 3, substances or mixtures classified as CMR of the categories laid down in Section 3 of Appendix B may be used in toys, in components of toys or micro-structurally distinct parts of toys provided that one or more of the following conditions is met:
a
these substances and mixtures are contained in individual concentrations equal to or smaller than the relevant concentrations established in the Community legal acts referred to in Section 2 of Appendix B for the classification of mixtures containing these substances;
b
these substances and mixtures are inaccessible to children in any form, including inhalation, when the toy is used as intended or in a foreseeable way, bearing in mind the behaviour of children;
c
regulations have been made under regulation 39D.
5
By way of derogation from point 3, substances or mixtures classified as CMR of the categories laid down in Section 4 of Appendix B may be used in toys, in components of toys or micro-structurally distinct parts of toys provided that one of the following conditions is met:
a
these substances and mixtures are contained in individual concentrations equal to or smaller than the relevant concentrations established in the Community legal acts referred to in Section 2 of Appendix B for the classification of mixtures containing these substances;
b
these substances and mixtures are inaccessible to children in any form, including inhalation, when the toy is used as intended or in a foreseeable way, bearing in mind the behaviour of children; or
c
regulations have been made under regulation 39D.
6
Points 3, 4 and 5 do not apply to nickel in stainless steel.
7
Points 3, 4 and 5 do not apply to materials that comply with the specific limit values set out in Appendix C.
8
Without prejudice to the application of points 3 and 4, nitrosamines and nitrosable substances are be prohibited for use in toys intended for use by children under 36 months or in other toys intended to be placed in the mouth if the migration of the substances is equal to or higher than 0,05 mg/kg for nitrosamines and 1 mg/kg for nitrosable substances.
9
Not applicable.
10
Cosmetic toys, such as play cosmetics for dolls, must comply with the compositional and labelling requirements laid down in Regulation (EC) 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products.
11
Toys must not contain the following allergenic fragrances:
No
Name of the allergenic fragrance
CAS number
(1)
Alanroot oil (Inula helenium)
97676-35-2
(2)
Allylisothiocyanate
57-06-7
(3)
Benzyl cyanide
140-29-4
(4)
4 tert-Butylphenol
98-54-4
(5)
Chenopodium oil
8006-99-3
(6)
Cyclamen alcohol
4756-19-8
(7)
Diethyl maleate
141-05-9
(8)
Dihydrocoumarin
119-84-6
(9)
2,4-Dihydroxy-3-methylbenzaldehyde
6248-20-0
(10)
3,7-Dimethyl-2-octen-1-ol (6,7-Dihydrogeraniol)
40607-48-5
(11)
4,6-Dimethyl-8-tert-butylcoumarin
17874-34-9
(12)
Dimethyl citraconate
617-54-9
(13)
7,11-Dimethyl-4.6,10-dodecatrien-3-one
26651-96-7
(14)
6,10-Dimethyl-3.5,9-undecatrien-2-one
141-10-6
(15)
Diphenylamine
122-39-4
(16)
Ethyl acrylate
140-88-5
(17)
Fig leaf, fresh and preparations
68916-52-9
(18)
trans-2-Heptenal
18829-55-5
(19)
trans-2-Hexenal diethyl acetal
67746-30-9
(20)
trans-2-Hexenal dimethyl acetal
18318-83-7
(21)
Hydroabietyl alcohol
13393-93-6
(22)
4-Ethoxy-phenol
622-62-8
(23)
6-lsopropyl-2-decahydronaphthalenol
34131-99-2
(24)
7-Methoxycoumarin
531-59-9
(25)
4-Methoxyphenol
150-76-5
(26)
4-(p-Methoxyphenyl)-3-butene-2-one
943-88-4
(27)
1-(p-Methoxyphenyl)-1-penten-3-one
104-27-8
(28)
Methyl trans-2-butenoate
623-43-8
(29)
6-Methylcoumarin
92-48-8
(30)
7-Methylcoumarin
2445-83-2
(31)
5-Methyl-2,3-hexanedione
13706-86-0
(32)
Costus root oil (Saussurea lappa Clarke)
8023-88-9
(33)
7-Ethoxy-4-methylcoumarin
87-05-8
(34)
Hexahydrocoumarin
700-82-3
(35)
Peru balsam, crude (Exudation of Myroxylon pereirae (Royle) Klotzsch)
8007-00-9
(36)
2-Pentylidene-cyclohexanone
25677-40-1
(37)
3.6,10-Trimethyl-3.5,9-undecatrien-2-one
1117-41-5
(38)
Verbena oil (Lippia citriodora Kunth)
8024-12-2
(39)
Musk ambrette (4-tert-Butyl-3-methoxy-2,6-dinitrotoluene)
83-66-9
(40)
4-Phenyl-3-buten-2-one
122-57-6
(41)
Amyl cinnamal
122-40-7
(42)
Amylcinnamyl alcohol
101-85-9
(43)
Benzyl alcohol
100-51-6
(44)
Benzyl salicylate
118-58-1
(45)
Cinnamyl alcohol
104-54-1
(46)
Cinnamal
104-55-2
(47)
Citral
5392-40-5
(48)
Coumarin
91-64-5
(49)
Eugenol
97-53-0
(50)
Geraniol
106-24-1
(51)
Hydroxy-citronellal
107-75-5
(52)
Hydroxy-methylpentylcyclohexenecarboxaldehyde
31906-04-4
(53)
Isoeugenol
97-54-1
(54)
Oakmoss extracts
90028-68-5
(55)
Treemoss extracts
90028-67-4
However, the presence of traces of these fragrances is allowed provided that such presence is technically unavoidable under good manufacturing practice and does not exceed 100 mg/kg.
In addition, the names of the following allergenic fragrances must be listed on the toy, on an affixed label, on the packaging or in an accompanying leaflet, if added to a toy, as such, at concentrations exceeding 100 mg/kg in the toy or components thereof:
No
Name of the allergenic fragrance
CAS number
(1)
Anisyl alcohol
105-13-5
(2)
Benzyl benzoate
120-51-4
(3)
Benzyl cinnamate
103-41-3
(4)
Citronellol
106-22-9
(5)
Farnesol
4602-84-0
(6)
Hexyl cinnamaldehyde
101-86-0
(7)
Lilial
80-54-6
(8)
d-Limonene
5989-27-5
(9)
Linalool
78-70-6
(10)
Methyl heptine carbonate
111-12-6
(11)
3-methyl-4-(2.6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one
127-51-5
12
The use of the fragrances set out in points 41 to 55 of the list set out in the first paragraph of point 11 and of the fragrances set out in points 1 to 11 of the list set out in the third paragraph of that point are allowed in olfactory board games, cosmetic kits and gustative games, provided that
i
those fragrances are clearly labelled on the packaging, and the packaging contains the warning set out in point 10 of Part B of Annex V;
ii
if applicable, the resulting products made by the child in accordance with the instructions comply with the requirements of Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products; and
iii
if applicable, those fragrances comply with the relevant legislation on food.
Such olfactory board games, cosmetic kits and gustative games must not be used by children under 36 months and must comply with point 1 of Part B of Schedule 5.
13
Without prejudice to points 3, 4 and 5, the following migration limits, from toys or components of toys, must not be exceeded:
Element
mg/kg
mg/kg
mg/kg
in dry, brittle, powder-like or pliable toy material
in liquid or sticky toy material
in scraped-off toy material
Aluminium
5 625
1 406
70 000
Antimony
45
11,3
560
Arsenic
3,8
0,9
47
Barium
1 500
375
18 570
Boron
1 200
300
15 000
Cadmium
1,3
0,3
17
Chromium (III)
37,5
9,4
460
Chromium (VI)
0,02
0,005
F131 0,053
Cobalt
10,5
2,6
130
Copper
622,5
156
7 700
Lead
2,0
0,5
23
Manganese
1 200
300
15 000
Mercury
7,5
1,9
94
Nickel
75
18,8
930
Selenium
37,5
9,4
460
Strontium
4 500
1 125
56 000
Tin
15 000
3 750
180 000
Organic tin
0,9
0,2
12
Zinc
3 750
938
46 000
These limit values do not apply to toys or components of toys which, due to their accessibility, function, volume or mass, clearly exclude any hazard due to sucking, licking, swallowing or prolonged contact with skin when used as intended or in a foreseeable way, bearing in mind the behaviour of children.
Part 4 Electrical Properties1
Toys must not be powered by electricity of a nominal voltage exceeding 24 volts direct current (DC) or the equivalent alternating current (AC) voltage, and their accessible parts must not exceed 24 volts DC or the equivalent AC voltage.
Internal voltages must not exceed 24 volts DC or the equivalent AC voltage unless it is ensured that the voltage and the current combination generated do not lead to any risk or harmful electric shock, even when the toy is broken.
2
Parts of toys which are connected to, or liable to come into contact with, a source of electricity capable of causing electric shock, together with the cables or other conductors through which electricity is conveyed to such parts, must be properly insulated and mechanically protected so as to prevent the risk of such shock.
3
Electric toys must be designed and manufactured in such a way as to ensure that the maximum temperatures reached by all directly accessible surfaces are not such as to cause burns when touched.
4
Under foreseeable fault conditions, toys must provide protection against electrical hazards arising from an electrical power source.
5
Electric toys must provide adequate protection against fire hazards.
6
Electric toys must be designed and manufactured in such a way that electric, magnetic and electromagnetic fields and other radiations generated by the equipment are limited to the extent necessary for the operation of the toy and must operate at a safe level in compliance with the generally acknowledged state of the art, taking account of specific Community measures.
7
Toys which have an electronic control system must be designed and manufactured in such a way that the toy operates safely even when the electronic system starts malfunctioning or fails due to failure of the system itself or an outside factor.
8
Toys must be designed and manufactured in such a way that they do not present any health hazards or risk of injury to eyes or skin from lasers, light-emitting diodes (LEDs) or any other type of radiation.
9
The electrical transformer of a toy must not be an integral part of the toy.
Part 5 Hygiene1
Toys must be designed and manufactured in such a way as to meet hygiene and cleanliness requirements in order to avoid any risk of infection, sickness or contamination.
2
A toy intended for use by children under 36 months must be designed and manufactured in such a way that it can be cleaned. A textile toy must, to this end, be washable, except if it contains a mechanism that may be damaged if soak washed. The toy must fulfil the safety requirements also after having been cleaned in accordance with this point and the manufacturer's instructions.
Part 6 RadioactivityToys must comply with all retained EU law that was adopted for the purposes of implementing Chapter 3 of Euratom.
Appendix AList of CMR substances and their permitted uses in accordance with points 4, 5 and 6 of Part III
Substance
Classification
Permitted use
Nickel
CMR 2
In toys and toy components made of stainless steel.
In toy components which are intended to conduct an electric current
Appendix BClassification of Substances and Mixtures
A1
In this Appendix—
“Regulation (EC) No 1272/2008” means Regulation (EC) 1272/2008 of the European Parliament and of the Council of 16th December 2008 on classifications, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) 1907/2006.
1
Criteria for classifying substances and mixtures for the purposes of point 2 of Part 2
The substance or mixture fulfils the criteria for any of the following hazard classes or categories set out in Annex I to Regulation (EC) 1272/2008:
a
hazard classes 2.1 to 2.4, 2.6 and 2.7, 2.8 types A and B, 2.9, 2.10, 2.12, 2.13 categories 1 and 2, 2.14 categories 1 and 2, 2.15 types A to F;
b
hazard classes 3.1 to 3.6, 3.7 adverse effects on sexual function and fertility or on development, 3.8 effects other than narcotic effects, 3.9 and 3.10;
c
hazard class 4.1;
d
hazard class 5.1.
2
Enactments governing the use of certain substances for the purposes of points 4(a) and 5(a) of Part 3
The relevant concentrations for the classification of mixtures containing the substances are those established in accordance with Regulation (EC) No 1272/2008.
3
Categories of substances and mixtures classified as carcinogenic, mutagenic or toxic for reproduction (CMR) for the purposes of point 4 of Part 3.
Substances Point 4 of Part 3 concerns substances classified as CMR category 1A and 1B under Regulation (EC) No 1272/2008.
Mixtures Point 4 of Part 3 concerns mixtures classified as CMR category 1A and 1B under Regulation (EC) No 1272/2008.
4
Categories of substances and mixtures classified as carcinogenic, mutagenic or toxic for reproduction (CMR) for the purposes of point 5 of Part III
Substances Point 5 of Part 3 concerns substances classified as CMR category 2 under Regulation (EC) No 1272/2008.
Mixtures Point 5 of Part 3 concerns mixtures classified as CMR category 2 under Regulation (EC) No 1272/2008.
5
Categories of substances and mixtures classified as carcinogenic, mutagenic or toxic for reproduction (CMR) for the purposes of regulation 39D(3)(a).
Substances Regulation 39D(3)(a) concerns substances classified as CMR category 1A, 1B and 2 under Regulation (EC) No 1272/2008.
Mixtures Regulation 39D(3)(a) concerns mixtures classified as CMR category 1A, 1B and 2 under Regulation (EC) No 1272/2008.
Appendix C
Specific limit values for chemicals used in toys intended for use by children under 36 months or in other toys intended to be placed in the mouth adopted by the Secretary of State.
Substance
CAS No
Limit value
TCEP
115-96-8
5 mg/kg (content limit)
TCPP
13674-84-5
5 mg/kg (content limit)
TDCP
13674-87-8
5 mg/kg (content limit)
Bisphenol A
80-05-7
0,04 mg/l (migration limit) in accordance with the methods laid down in EN 71-10:2005 and EN 71-11:2005.
Formamide
75-12-7
20μg/m3 (emission limit) after a maximum of 28 days from commencement of the emission testing of foam toy materials containing more than 200 mg/kg (cut-off limit based on content)
1,2-benzisothiazol-3(2H)-one
2634-33-5
5 mg/kg (content limit) in aqueous toy materials, in accordance with the methods laid down in EN 71-10:2005 and EN 71-11:2005
Reaction mass of: 5-chloro-2- methyl-4-isothiazolin-3-one (EC no. 247-500-7) and 2-methyl-2H -isothiazol-3-one (EC no. 220-239-6) (3:1)
55965-84-9
1 mg/kg (content limit) in aqueous toy materials
5-Chloro-2-methyl-isothiazolin-3(2H)-one
26172-55-4
0,75 mg/kg (content limit) in aqueous toy materials
2-methylisothiazolin-3(2H)-one
2682-20-4
0,25 mg/kg (content limit) in aqueous toy materials
Phenol
108-95-2
5mg/l (migration limit) in polymeric materials in accordance with the methods laid down in EN 71-10:2005 and EN 71-11:2005
10mg/kg (content limit) as a preservative in accordance with the methods laid down in EN 71-10: 2005 and EN 71-11:2005.
SCHEDULE 3DECLARATION OF CONFORMITY
1
No (unique identification of the toy(s))
2
Name and address of the manufacturer or the manufacturer's authorised representative:
3
This declaration of conformity is issued under the sole responsibility of the manufacturer:
4
Object of the declaration (identification of toy allowing traceability). It must include a colour image of sufficient clarity to enable the identification of the toy.
5
The object of the declaration described in point 4 is in conformity with the following enactments:
6
References to the relevant designated standards used, or references to the specifications in relation to which conformity is declared:
7
Where applicable: the approved body … (name, number) … performed … (description of intervention) … and issued the certificate:
8
Additional information:
Signed for and on behalf of: (place and date of issue) (name, function) (signature)
SCHEDULE 4TECHNICAL DOCUMENTATION (Annex IV to the Directive)
The technical documentation referred to in regulation 17(3) must contain so far as relevant for assessment:
a
a detailed description of the design and manufacture, including a list of components and materials used in the toy as well as the safety data sheets on chemicals used, to be obtained from the chemical suppliers;
b
the safety assessment(s) carried out in accordance with regulation 12.
c
a description of the conformity assessment procedure followed;
d
a copy of the declaration of conformity;
e
the addresses of the places of manufacture and storage;
f
copies of documents that the manufacturer has submitted to an approved body, if involved;
g
test reports and description of the means whereby the manufacturer ensured conformity of production with designated standards, if the manufacturer followed the internal production control procedure set out in Module A; and
h
a copy of the Type examination certificate, a description of the means whereby the manufacturer ensured conformity of the production with the product type as described in the Type examination certificate, and copies of the documents that the manufacturer submitted to the approved body, if the manufacturer submitted the toy to Type examination and followed the conformity to type procedure set out in Module C.
SCHEDULE 5WARNINGS (Annex V to the Directive)
PART AGENERAL WARNINGS
The user limitations referred to in regulation 20(3) must include at least the minimum or maximum age of the user and, where appropriate, the abilities of the user, the maximum or minimum weight of the user and the need to ensure that the toy is used only under adult supervision.
PART BSPECIFIC WARNINGS AND INDICATIONS OF PRECAUTIONS TO BE TAKEN WHEN USING CERTAIN CATEGORIES OF TOYS
1
Toys not intended for use by children under 36 months
Toys which might be dangerous for children under 36 months of age must bear a warning such as ‘Not suitable for children under 36 months’ or ‘Not suitable for children under three years’ or a warning in the form of the following graphic:
These warnings must be accompanied by a brief indication, which may appear in the instructions for use, of the specific hazard calling for this precaution.
This point does not apply to toys which, on account of their function, dimensions, characteristics or properties, or on other cogent grounds, are manifestly unsuitable for children under 36 months.
2
Activity toys
Activity toys must bear the following warning:‘Only for domestic use’.
Activity toys attached to a crossbeam as well as other activity toys, where appropriate, must be accompanied by instructions drawing attention to the need to carry out checks and maintenance of the main parts (suspensions, fixings, anchorages, etc.) at intervals, and pointing out that, if these checks are not carried out, the toy may cause a fall or overturn.
Instructions must also be given as to the correct assembly of the toy, indicating those parts which can present a danger if incorrectly assembled. Specific information regarding a suitable surface on which to place the toy must be given.
3
Functional toys
Functional toys must bear the following warning:‘To be used under the direct supervision of an adult’.
In addition, these toys must be accompanied by directions giving working instructions as well as the precautions to be taken by the user, with the warning that failure to take these precautions will expose the user to the hazards – to be specified – normally associated with the appliance or product of which the toy is a scale model or imitation. It must also be indicated that the toy must be kept out of the reach of children under a certain age, which must be specified by the manufacturer.
4
Chemical toys
Without prejudice to the application of the provisions laid down in applicable enactments on the classification, packaging and labelling of certain substances or mixtures, the instructions for use of toys containing inherently dangerous substances or mixtures must bear a warning of the dangerous nature of these substances or mixtures and an indication of the precautions to be taken by the user in order to avoid hazards associated with them, which must be specified concisely according to the type of toy. The first aid to be given in the event of serious accidents resulting from the use of this type of toy must also be set out. It must also be stated that the toy must be kept out of reach of children under a certain age, which must be specified by the manufacturer.
In addition to the instructions provided for in the first subparagraph, chemical toys must bear the following warning on their packaging:‘Not suitable for children under (insert appropriate age) years. For use under adult supervision’.
In particular, the following are regarded as chemical toys: chemistry sets, plastic embedding sets, miniature workshops for ceramics, enamelling or photography and similar toys which lead to a chemical reaction or similar substance alteration during use.
5
Skates, roller skates, online skates, skateboards, scooters and toy bicycles for children
Where these toys are offered for sale as toys, they must bear the following warning:‘Protective equipment should be worn. Not to be used in traffic’.
Moreover, the instructions for use must contain a reminder that the toy must be used with caution, since it requires great skill, so as to avoid falls or collisions causing injury to the user or third parties. Some indication must also be given as to recommended protective equipment (helmets, gloves, knee-pads, elbow-pads, etc.).
6
Aquatic toys
Aquatic toys must bear the following warning:‘Only to be used in water in which the child is within its depth and under adult supervision’.
7
Toys in food
Toys contained in food or co-mingled with food must bear the following warning:‘Toy inside. Adult supervision recommended’.
8
Imitations of protective masks and helmets
Imitations of protective masks and helmets must bear the following warning:‘This toy does not provide protection’.
9
Toys intended to be strung across a cradle, cot or perambulator by means of strings, cords, elastics or straps
Toys intended to be strung across a cradle, cot or perambulator by means of strings, cords, elastics or straps must carry the following warning on the packaging, which must also be permanently marked on the toy:‘To prevent possible injury by entanglement, remove this toy when the child starts trying to get up on its hands and knees in a crawling position’.
10
Packaging for fragrances in olfactory board games, cosmetic kits and gustative games
Packaging for fragrances in olfactory board games, cosmetic kits and gustative games that contain the fragrances set out in points 41 to 55 of the list set out in the first paragraph of point 11 of Part 3 of Schedule 2 and of the fragrances set out in points 1 to 11 of the list set out in third paragraph of that point must contain the following warning:‘Contains fragrances that may cause allergies’.
SCHEDULE 6CONFORMITY ASSESSMENT PROCEDURES (Annex II to Decision No 768/2008/EC)
MODULE AInternal production control
1
Internal production control is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2, 3 and 4, and ensures and declares on the manufacturer's sole responsibility that the products concerned satisfy the requirements of the legislative instrument that apply to them.
Technical documentation2
The manufacturer must establish the technical documentation. The documentation must make it possible to assess the product's conformity to the relevant requirements, and must include an adequate analysis and assessment of the risk(s). The technical documentation must specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation must, wherever applicable, contain at least the following elements:
— a general description of the product,
— conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.
— descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
— a list of the designated standards and/or other relevant technical specifications applied in full or in part, and descriptions of the solutions adopted to meet the essential requirements of the enactments where those designated standards have not been applied. In the event of partly applied designated standards, the technical documentation must specify the parts which have been applied,
— results of design calculations made, examinations carried out, etc., and
— test reports.
Manufacturing3
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured products with the technical documentation referred to in point 2 and with the requirements of the legislative instruments that apply to them.
UK marking and declaration of conformity4
The manufacturer must affix the UK marking to each individual product in accordance with regulation 18.
4
The manufacturer must draw up a written declaration of conformity for a product model and keep it together with the technical documentation at the disposal of the national authorities for 10 years after the product has been placed on the market. The declaration of conformity must identify the product for which it has been drawn up.
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
Authorised representative5
The manufacturer's obligations set out in point 4 may be fulfilled by the manufacturer's authorised representative, on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate.
MODULE BType examination
1
Type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of a product and verifies and attests that the technical design of the product meets the requirements of these Regulations.
2
Type examination may be carried out in either of the following manners:
— examination of a specimen, representative of the production envisaged, of the complete product (production type),
— assessment of the adequacy of the technical design of the product through examination of the technical documentation and supporting evidence referred to in point 3, plus examination of specimens, representative of the production envisaged, of one or more critical parts of the product (combination of production type and design type),
— assessment of the adequacy of the technical design of the product through examination of the technical documentation and supporting evidence referred to in point 3, without examination of a specimen (design type).
3
The manufacturer must lodge an application for Type examination with a single approved body of the manufacturer's choice.
The application must include:
— the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative as well,
— a written declaration that the same application has not been lodged with any other approved body,
— the technical documentation. The technical documentation must make it possible to assess the product's conformity with the applicable requirements of these Regulations and must include an adequate analysis and assessment of the risk(s). The technical documentation must specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation must contain, wherever applicable, at least the following elements:
— a general description of the product,
— conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.,
— descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
— a list of the designated standards and/or other relevant technical specifications applied in full or in part, and descriptions of the solutions adopted to meet the essential safety requirements where those designated standards have not been applied. In the event of partly applied designated standards, the technical documentation must specify the parts which have been applied,
— results of design calculations made, examinations carried out, etc., and
— test reports,
— the specimens representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme,
— the supporting evidence for the adequacy of the technical design solution. This supporting evidence must mention any documents that have been used, in particular where the relevant designated standards and/or technical specifications have not been applied in full. The supporting evidence must include, where necessary, the results of tests carried out by the appropriate laboratory of the manufacturer, or by another testing laboratory on the manufacturer's behalf and under the manufacturer's responsibility.
4
The approved body must:
For the product:
4
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the product;
For the specimen(s):
4
verify that the specimen(s) have been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards and/or technical specifications, as well as the elements which have been designed without applying the relevant provisions of those standards;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards and/or technical specifications, these have been applied correctly;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards and/or technical specifications have not been applied, the solutions adopted by the manufacturer meet the corresponding essential requirements of the legislative instrument;
4
agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body must draw up an evaluation report that records the activities undertaken in accordance with point 4 and their outcomes. Without prejudice to its obligations set out in paragraph 8, the approved body must release the content of that report, in full or in part, only with the agreement of the manufacturer.
6
Where the type meets the requirements of the specific legislative instrument that apply to the product concerned, the approved body must issue a Type examination certificate to the manufacturer. The certificate must contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The certificate may have one or more annexes attached.
The certificate and its annexes must contain all relevant information to allow the conformity of manufactured products with the examined type to be evaluated and to allow for in-service control.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body must refuse to issue a Type examination certificate and must inform the applicant accordingly, giving detailed reasons for its refusal.
7
The approved body must keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of the legislative instrument, and must determine whether such changes require further investigation. If so, the approved body must inform the manufacturer accordingly.
The manufacturer must inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the product with the essential safety requirements or the conditions for validity of the certificate. Such modifications must require additional approval in the form of an addition to the original Type examination certificate.
8
Each approved body must inform the Secretary of State concerning the Type examination certificates and/or any additions thereto which it has issued or withdrawn, and must, periodically or upon request, make available to the Secretary of State the list of certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body must inform the other approved bodies concerning the Type examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning the certificates and/or additions thereto which it has issued.
The authorised body must keep a copy of the Type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of the certificate.
9
The manufacturer must keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the product has been placed on the market.
10
The manufacturer's authorised representative may lodge the application referred to in point 3 and fulfil the obligations set out in points 7 and 9, provided that they are specified in the mandate.
MODULE CConformity to type based on internal production control
1
Conformity to type based on internal production control is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2 and 3, and ensures and declares that the products concerned are in conformity with the type described in the type examination certificate and satisfy the requirements of the legislative instrument that apply to them.
Manufacturing2
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the approved type described in the Type examination certificate and with the requirements of these Regulations.
Conformity marking and declaration of conformity3
The manufacturer must affix the UK marking to each individual product that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
3
The manufacturer must draw up a written declaration of conformity for a product model and keep it at the disposal of the enforcement authorities for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
A copy of the declaration of conformity must be made available to the enforcement authorities upon request.
Authorised representative4
The manufacturer's obligations set out in point 3 may be fulfilled by the manufacturer's authorised representative, on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate.
SCHEDULE 7Approved body requirements
1
A conformity assessment body must be established in the United Kingdom and must have legal personality.
2
1
A conformity assessment body must be a third-party body independent of the organisation or the toy it assesses.
2
A body belonging to a business association or professional federation representing undertakings involved in the design, manufacturing, provision, assembly, use or maintenance of toys which it assesses may, on condition that its independence and the absence of any conflict of interest are demonstrated, be considered such a body.
3
1
A conformity assessment body, its top level management and the personnel responsible for carrying out the conformity assessment activities must not be the designer, manufacturer, supplier, installer, purchaser, owner, user or maintainer of the toys which they assess, not the authorised representative of any of those parties.
2
Sub-paragraph (1) does not preclude the use of assessed toys that are necessary for the operations of the conformity assessment body or the use of such toys for personal purposes.
4
A conformity assessment body, its top level management and the personnel responsible for carrying out the conformity assessment tasks must not be directly involved in the design or manufacture, the marketing, installation, use or maintenance of toys it assesses, or represent parties involved in those activities.
5
A conformity assessment body must not engage in any activity, including consultancy services, that may conflict with their independence of judgement or integrity in relation to conformity assessment activities for which they are approved.
6
Conformity assessment bodies must ensure that the activities of their subsidiaries and subcontractors do not affect the confidentiality, objectivity or impartiality of their conformity assessment activities.
7
Conformity assessment bodies and their personnel must carry out the conformity assessment activities with the highest degree of professional integrity and the requisite technical competence in the specific field and must be free from all pressures and inducements, particularly financial, which might influence their judgement or the results of their conformity assessment activities, especially as regards persons or groups of persons with an interest in the results of those activities.
8
Conformity assessment bodies must be capable of carrying out the conformity assessment tasks assigned to them by the provisions of regulation 14 or by their approval whether those tasks are carried out by the conformity assessment body itself or on its behalf and under its responsibility.
9
At all times and for each conformity assessment procedure and each kind or category of toy in relation to which it has been approved, a conformity assessment body must have at its disposal—
a
personnel with technical knowledge and sufficient and appropriate experience to perform the conformity assessment tasks;
b
descriptions of procedures in accordance with which conformity assessment is carried out ensuring the transparency and ability of reproduction of those procedures. It must have appropriate policies and procedures in place that distinguish between tasks it carries out as an approved body and other activities.
c
procedures for the performance of activities which take due account of the size of an undertaking, the sector in which it operates, its structure, the degree of complexity of the technology of the toy in question and the mass or serial nature of the production process.
10
A conformity assessment body must have the means necessary to perform the technical and administrative tasks connected with the conformity assessment activities in an appropriate manner and must have access to all necessary equipment or facilities.
11
The personnel responsible for carrying out the conformity assessment activities must have—
a
sound technical and vocational training covering all the conformity assessment activities in relation to which the conformity assessment body has been approved;
b
satisfactory knowledge of the requirements of the assessments they carry out and adequate authority to carry out those assessments;
c
appropriate knowledge and understanding of the essential safety requirements;
d
the ability to draw up certificates, records and reports demonstrating that assessments have been carried out.
12
A conformity assessment body must be able to demonstrate the impartiality of their top level management and personnel responsible for assessment.
13
The renumeration of the top level management and personnel responsible for assessment of a conformity assessment body must not depend on the number of assessments carried out or on the results of those assessments.
14
A conformity assessment body must have, and must satisfy the Secretary of State that it has, adequate civil liability insurance in respect of its activities.
15
A conformity assessment body must ensure that its personnel observe professional secrecy with regard to all information obtained in carrying out their tasks in accordance with these Regulations and that proprietary rights are protected.
16
Paragraph 15 does not prevent the personnel from providing information to the Secretary of State or an enforcement authority.
17
A conformity assessment body must participate in, or ensure that its personnel who are responsible for carrying out the conformity assessment activities are informed of, the relevant standardisation activities and the activities of any approved body coordination group established by the Secretary of State and must apply as general guidance the administrative decisions and documents produced as a result of the work of that group.
SCHEDULE 16Amendment of the Explosives Regulations 2014
PART 1Amendments to the Explosives Regulations 2014
IntroductionI8991
The Explosives Regulations 2014 are amended in accordance with paragraphs 2 to 35.
Amendment to regulation 2I1512
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “ammonium nitrate blasting intermediate” insert—
“approved body” has the meaning given to it in regulation 69 (approved bodies);
F132d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
after the definition of “conformity assessment body” insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 41;
h
after the definition of “desensitised explosive” insert—
“designated standard” has the meaning given to it in regulation 2A;
i
in the definition of “the Directive” at the end insert “
(as it has effect immediately before F133IP completion day)
”
;
j
omit the definition of “EU declaration of conformity”;
k
omit the definition of “harmonised standard”;
l
for the definition of “importer” substitute—
F134“importer”, in relation to civil explosives, means any person who—
a
is established in the United Kingdom and places a civil explosive from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a civil explosive on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F135m
in the definition of “making available on the market” for “in an EEA state” substitute “of Great Britain”;
n
omit the definition of “notified body requirements”;
F136o
in the definition of “place on the market” for “in an EEA state” substitute “of Great Britain”;
p
after the definition of “recipient competent authority document” insert—
“relevant authority” means any public authority which has a function under these Regulations or a function under another enactment in relation to the security or traceability of civil explosives;
q
after the definition of “transfer” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
Insertion of regulation 2AI1533
After regulation 2 insert—
Interpretation: designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means technical specification which is—
a
adopted by a recognised standardisation body for repeated or continuous application with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures;
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing a reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a civil explosive.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 8I1544
1
Regulation 8 (authorisation to transfer civil explosives) is amended as follows.
2
In paragraph (1) omit “for the place where the transfer will terminate”.
3
In paragraph (2) for “relevant authority” substitute “
relevant competent authority
”
.
4
In paragraph (5), in both places where it appears, for “the area of the EEA States” substitute “
the United Kingdom
”
.
5
After paragraph (8) insert—
8A
A recipient competent authority document issued under this regulation may be granted for such period as the competent authority determines and may be revoked by notice in writing by that authority on grounds of safety or security.
6
For paragraph (9) substitute—
9
In this regulation—
a
“competent authority” means the Executive; and
b
“recipient competent authority document” means a document issued in accordance with this regulation by the competent authority;
c
“relevant competent authority” means—
i
in respect of a transfer or part of a transfer which takes place within Great Britain, the Executive; and
ii
in respect of a transfer or part of a transfer which takes place in Northern Ireland, the body which discharges in Northern Ireland similar functions to those discharged by the Executive under these Regulations in relation to Great Britain.
7
After paragraph (9) insert—
10
A transfer document issued under the Directive, which was valid immediately before F137IP completion day is deemed to be a valid recipient competent authority document for the purposes of this regulation after F137IP completion day, until such time as it expires or is withdrawn by a relevant competent authority.
Amendment to regulation 34I1555
Regulation 34 (Attribution of manufacturing site codes for civil explosives) is amended as follows—
a
in paragraph (3)—
i
for “that is not an EEA State” substitute “
other than the United Kingdom
”
;
ii
in sub-paragraph (a) for “an EEA State” substitute “
the United Kingdom
”
;
iii
in sub-paragraph (b) for “an EEA State” substitute “
the United Kingdom
”
;
iv
in sub-paragraph (c) after “import of the civil explosives is” omit “either”;
v
in sub-paragraph (c) after “Northern Ireland” omit “or an EEA State other than the United Kingdom”;
b
in paragraph (4)(b), after “Northern Ireland” omit “or an EEA State other than the United Kingdom”;
c
in paragraph (5)—
i
in sub-paragraph (a), at the end omit “and”;
ii
for sub-paragraph (b) substitute—
b
the importer must at the time of its application provide the Executive with the details of any site code previously attributed to those explosives; and
c
the Executive must attribute the code (which may be the same as the code previously attributed to the explosives) and inform the importer accordingly.
d
for paragraph (6) substitute—
6
Where this paragraph applies, the manufacturer must apply to the Secretary of State for Northern Ireland for the Secretary of State to attribute a code for the site where the civil explosives are manufactured.
Amendment to regulation 40I1566
Regulation 40 (technical documentation and conformity assessment) is amended as follows—
a
in paragraph (b)(i)—
i
for “66(a)” substitute “
66(2)(a)
”
;
ii
for “point 3(c) of Module B of Annex III to the Directive as amended from time to time” substitute “
paragraph 2(2)(c) of Part 1 (Module B) of Schedule 17
”
;
b
in paragraph (b)(ii)—
i
for “66(b)” substitute “
66(2)(b)
”
;
ii
for “point 2 of Module G of Annex III to the Directive as amended from time to time” substitute “
paragraph 46 of Part 6 (Module G) of Schedule 17
”
.
Amendment to regulation 41I1577
Regulation 41 (EU declaration of conformity and CE marking) is amended as follows—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
;
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a) omit “(EU declaration of conformity)”;
c
in paragraph (1)(b)—
i
for “CE” substitute “
UK
”
;
ii
omit “(CE Marking)”;
d
for paragraph (3) substitute—
3
Where a civil explosive is subject to more than one enactment requiring a declaration of conformity to be drawn up, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 42I1588
In regulation 42 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 43I1599
In regulation 43 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Amendment to regulation 44I16010
In regulation 44 (traceability of certain civil explosives excluded from the scope of regulations 33, 34 and 36) for paragraph (4) substitute—
4
For a civil explosive that is to be made available on the market in Great Britain the contact details referred to in paragraph (1) must be provided in English.
Amendment to regulation 45I16111
For regulation 45 (instructions and safety information), substitute—
Instructions and safety information45
1
When placing a civil explosive on the market, a manufacturer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
2
Any labelling on the civil explosive must be clear, legible and in easily understandable English.
Amendment to regulation 46I16212
Regulation 46 (appointment of authorised representative by written mandate) is amended as follows—
a
in paragraph (1) after “appoint a person” insert “
established in the United Kingdom
”
;
b
in paragraph (2)(a) omit “EU”.
Amendment to regulation 48I16313
In regulation 48 (requirements which must be satisfied before an importer places a civil explosive on the market), in paragraph (1)(c)(i) for “CE” substitute “
UK
”
.
Amendment to regulation 50I16414
In regulation 50 (information identifying importer)—
a
after paragraph (1) insert—
1A
Paragraph (1) does not apply where the importer has imported the civil explosive from an EEA state F138or Switzerland and places it on the market within the period of F13924 months beginning with F140IP completion day, and before placing the civil explosive on the market, the importer sets out the information referred to in paragraph (1) in a document accompanying the civil explosive.
b
in paragraph (2) for “the competent national authority in the EEA state in which the civil explosive is to be made available to such end users” substitute “
a relevant authority
”
.
Amendment to regulation 51I16515
For regulation 51 (instructions and safety information) substitute—
Instructions and safety information51
When placing a civil explosive on the market, an importer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 52I16616
In regulation 52 (retention of technical documentation and EU declaration of conformity), in the heading to that regulation and in paragraph (a), omit “EU”.
Amendment to regulation 53I16717
In regulation 53 (duty to take action in respect of civil explosives placed on the market which are considered not to be in conformity), in paragraph (2) omit “, and the competent national authorities of any other EEA state in which the manufacturer or importer made the civil explosive available on the market,”.
Amendment to regulation 54I16818
In regulation 54 (provision of information and cooperation), for paragraph (1)(b) substitute—
b
in clear, legible and easily understandable English.
Amendment to regulation 56I16919
In regulation 56 (requirements which must be satisfied before a distributor makes a civil explosive available on the market), paragraph (1)(a) is amended as follows—
a
in sub-paragraph (i) for “CE” substitute “
UK
”
; and
b
for sub-paragraph (iii) substitute—
iii
is accompanied by instructions and safety information that are clear, legible and in easily understandable English;
Amendment to regulation 58I17020
In regulation 58 (duty to take action in respect of civil explosives made available on the market which are not in conformity), in paragraph (2) omit “, and the competent national authorities of the other EEA states in which the distributor has made the civil explosive available on the market,”.
Revocation of regulation 62I17121
Omit regulation 62 (translation of declaration of conformity).
Amendment to regulation 64I17222
In regulation 64 (prohibition on improper use of CE marking) in the heading, and in each place in which it occurs, for “CE” substitute “
UK
”
.
Insertion of regulation F141regulations 64A, 64B, 64C and 64DI17323
After regulation 64 insert—
Obligations which are met by complying with obligations in the Directive64A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(24);
c
“harmonised standard” has the meaning given to it in Article 2(16).
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing a civil explosive on the market, the manufacturer—
a
ensures that the civil explosive has been designed and manufactured in accordance with the essential safety requirements set out in Annex II;
b
ensures that the relevant conformity assessment procedures that apply to that civil explosive in accordance with Article 20 have been carried out;
c
draws up the technical documentation referred to in Annex III;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking, in accordance with Articles 22 and 23(1) to (5);
f
draws up an EU declaration of conformity, in accordance with Article 21; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 39, 40, 41(1) and 41(3) are to be treated as being satisfied;
b
regulations 41(2), 42, 43(2), 46(2) and 64 apply subject to the modifications in paragraph (10); and
c
Schedule 12 paragraph 12 does not apply.
4
Subject to paragraphs (6) and (7), paragraph (5) applies where, before placing a civil explosive on the market, the importer ensures that—
a
the relevant conformity assessment procedures that apply to that explosive in accordance with Article 20 have been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III; and
c
the civil explosive bears the CE marking referred to in Article 23.
5
Where this paragraph applies—
a
the requirements of regulation 48(1)(a) to (c) are to be treated as being satisfied; and
b
regulations 47, 49(1), 52 and 60 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 19.
7
Where paragraph (6) applies paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to carry out one of the conformity assessment procedures set out in Article 20.
8
Paragraph (9) applies where, before making a civil explosive available on the market, a distributor ensures that the civil explosive bears the CE marking referred to in Article 23.
9
Where this paragraph applies—
a
regulation 56(1)(a)(i) is to be treated as being satisfied; and
b
regulations 57(1) and 60 apply subject to the modifications in paragraph (10).
10
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex II;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 20;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III.
Conformity assessment procedure obligation which is met by complying with the Directive64B
1
In this regulation any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive.
2
Paragraph (3) applies where, prior to the manufacture of a civil explosive, the manufacturer ensures that the conformity assessment procedure that applies to that explosive in accordance with Article 20(a) has been carried out.
3
Where this paragraph applies—
a
any reference to “relevant conformity assessment procedure” in regulations 40(a), 41(1), 48(1)(a), 64(1)(b), 67(b) and 68(3) are to be read as including the conformity assessment procedure referred to in Article 20(a) of the Directive; and
b
any reference to “technical documentation” in regulations 40(b), 42, 48(1)(b), 52(b), and in paragraph 12(1)(d) of Part 1 of Schedule 12 and Schedule 17 is to be read as including the technical documentation relating to the design of the civil explosive referred to in Annex III.
F142Expiry of regulations 64A and 64B64C
1
Subject to paragraph (2), regulation 64A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 64A—
a
any civil explosive which was placed on the market pursuant to regulation 64A may continue to be made available on the market on or after the expiry of regulation 64A;
b
any obligation to which a person was subject under regulation 64A in respect of a civil explosive placed on the market pursuant to regulation 64A continues to have effect after the expiry of regulation 64A, in respect of that civil explosive.
3
Subject to paragraph (4), regulation 64B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 64B in relation to a civil explosive prior to the expiry of regulation 64B, regulation 64B continues to apply in respect of that civil explosive where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after an EU-Type examination has been carried out in accordance with a conformity assessment procedure set out in point 1 of Annex III of the Directive.
Qualifying Northern Ireland Goods64D
1
In this regulation—
“the 2016 Regulations” means the Making Available on the Market and Supervision of Transfers of Explosives Regulations (Northern Ireland) 2016;
“CE marking” has the meaning given to it in regulation 2(1) of the 2016 Regulations;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1) of the 2016 Regulations;
“technical documentation” has the meaning given to it in regulation 2(1) of the 2016 Regulations.
2
Where paragraph (3) applies, a civil explosive is to be treated as being in conformity with Part 13 Sub-Part A.
3
This paragraph applies where—
a
a civil explosive—
i
is in conformity with Part 3 Sub-Part A of the 2016 Regulations;
ii
is qualifying Northern Ireland goods; and
b
an importer has met the obligations set out in paragraph (4).
4
The obligations referred to in paragraph (3)(b) are that, before placing the civil explosive on the market, the importer—
a
complies with regulation 50;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in in relation to the civil explosive;
ii
the manufacturer has drawn up the technical documentation; and
iii
the civil explosive bears the CE marking.
Amendment to regulation 65I17424
In regulation 65 (presumption of conformity), paragraph (1) is amended as follows—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal of the European Union”.
Amendment to regulation 66I17525
For regulation 66 (conformity assessment procedures) substitute—
Conformity assessment procedures66
1
Assessment of conformity of a civil explosive is carried out by an approved body in accordance with the procedures set out in Schedule 17.
2
For the assessment of conformity of a civil explosive, the manufacturer must follow one of the following procedures set out in Schedule 17—
a
in Part 1 of Schedule 17, Type examination carried out by an approved body (Module B), and, at the choice of the manufacturer, one of the following procedures—
i
in Part 2 of Schedule 17, conformity to type based on internal production control plus supervised product checks at random intervals (Module C2);
ii
in Part 3 of Schedule 17, conformity to type based on quality assurance of the production process (Module D);
iii
in Part 4 of Schedule 17, conformity to type based on product quality assurance (Module E);
iv
in Part 5 of Schedule 17, conformity to type based on product verification (Module F);
b
in Part 6 of Schedule 17, conformity based on unit verification (Module G).
Amendment to regulation 67I17626
Regulation 67 (EU declaration of conformity) is amended as follows—
a
in the heading for “EU declaration” substitute “
Declaration
”
;
b
in the opening words omit “EU”;
c
in paragraph (b) for “Annex III to the Directive, as amended from time to time,” substitute “
Schedule 17,
”
;
d
in paragraph (c) for “Annex IV to the Directive, as amended from time to time” substitute “
Schedule 18.
”
.
Amendment to regulation 68I17727
Regulation 68 (CE Marking) is amended as follows—
a
in the heading for “CE” substitute “
UK
”
;
F144b
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly—
a
to the civil explosive; or
b
where paragraph (1A) applies, to—
i
a label affixed to the civil explosive; or
ii
the accompanying documents.
F143ba
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the civil explosive; or
b
the accompanying documents.
F145c
in paragraph (2)—
i
after “Where” insert “
paragraph (1A) does not apply and
”
;
ii
for “CE” substitute “
UK
”
(twice);
d
in paragraph (3)—
i
for “CE” substitute “
UK
”
;
ii
for “notified” substitute “
approved
”
;
e
in paragraph (4) for “notified” in each place it appears, substitute “
approved
”
;
f
in paragraph (5) for “CE” substitute “
UK
”
.
Amendment to Part 13, Sub-Part CI17828
For Part 13, Sub-Part C (notification of conformity assessment bodies) substitute—
SUB-PART CApproval of Conformity Assessment Bodies
Approved bodies69
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 70 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 73 (restriction, suspension or withdrawal of approval).
3
In this Sub-Part—
“notified body” means a body—
- a
which the Secretary of State had before F146IP completion day notified to the European Commission and to the other EEA states, in accordance with Article 24 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 69(1)(b);
“approved body requirements” means the requirements set out in Schedule 15.
Approval of conformity assessment bodies70
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the civil explosives in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies71
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring72
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 70(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval73
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 72(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 69 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 72(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 69.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies74
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 70; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 16 (operational obligations of approved bodies).
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Schedule 17 (conformity assessment procedures); or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 68 (UK marking).
Subsidiaries and contractors75
1
An approved body may subcontract specific conformity assessment activities or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M50.
Register of approved bodies76
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body77
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 72; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 76.
Transitional provision in relation to EU ExitI15229
After regulation 82 (savings and transitional provisions) insert—
Transitional provision in relation to EU Exit82A
1
In this regulation—
“pre-exit period” means the period beginning with 20th April 2016 and ending immediately before F148IP completion day;
“product” means a civil explosive to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 16 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M51, any obligation to which a person was subject under these Regulations as they had effect immediately before F148IP completion day, continues to have effect as it did immediately before F148IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 40 as it had effect immediately beforeF148IP completion day in relation to that product
that action has effect as if it had been done under regulation 40 as it had effect on and after F148IP completion day.
Amendment to Schedule 6I17930
In Schedule 6 in paragraph 1(a)(ii)(aa) for “the EEA State (place of production or import onto the market of the EEA States)” substitute “
Great Britain, Northern Ireland or the EEA state (place of production or import)
”
.
Amendment to Schedule 9I18031
In Schedule 9 at the beginning omit “(This Schedule reproduces, with minor modifications, the provisions of Annex II to the Directive)”.
Amendment to Schedule 12I18132
Schedule 12 is amended as follows—
a
in paragraph 2 for “the Directive” substitute “
these Regulations
”
;
b
omit paragraph 4(c);
c
in paragraph 9(2) for “notified body” substitute “
approved body
”
;
d
omit paragraph 9(4);
e
omit paragraph 9(7);
f
in paragraph 9(8)—
i
for “The notices in sub-paragraphs (6) and (7)” substitute “
The notice in sub-paragraph (6)
”
;
ii
in sub-paragraph (f)(ii) for “harmonised” substitute “
designated
”
;
g
in paragraph 9(10) for “competent national authority” substitute “
relevant authority
”
;
h
omit paragraph 10 (EU safeguarding procedure);
i
omit paragraph 11(3);
j
in paragraph 11(4) for “The notices referred to in sub-paragraphs (2) and (3)” substitute “
The notice referred to in sub-paragraph (2)
”
;
k
in paragraph 12—
i
in sub-paragraphs (1)(a) and 1(c), for “CE marking” substitute “
UK marking
”
in each place it appears;
ii
in sub-paragraph (1)(b) for “a notified body” substitute “
an approved body
”
;
iii
in sub-paragraph (1)(b) for “the notified body” substitute “
the approved body
”
; and
iv
in sub-paragraph (1)(c) for “EU declaration of conformity” substitute “
declaration of conformity
”
in each place it appears.
Amendment to Schedule 15I18233
Schedule 15 is amended as follows—
a
in the heading, for “Notified” substitute “
Approved
”
;
b
in paragraph 8 F149and paragraph 9(b), for “notified” substitute “
approved
”
;
c
in paragraph 11(c)—
i
for “harmonised” substitute “
designated
”
;
ii
omit “of the Directive and Part 13”;
d
in paragraph 17—
i
for “notified” substitute “
approved
”
;
ii
for “under the Directive” substitute “
by the Secretary of State
”
.
Amendment to Schedule 16I18334
Schedule 16 is amended as follows—
a
in the heading, for “Notified” substitute “
Approved
”
;
b
in paragraph 1, for “A notified” substitute “
An approved
”
;
c
in paragraph 2, for “A notified” substitute “
An approved
”
;
d
in paragraph 3, for “A notified” substitute “
An approved
”
;
e
in paragraph 4, for “A notified” substitute “
An approved
”
;
f
in paragraph 5—
i
for “a notified” substitute “
an approved
”
;
ii
for “harmonised” substitute “
designated
”
;
g
in paragraph 6, for “a notified” substitute “
an approved
”
;
h
in paragraph 7, for “notified” substitute “
approved
”
in both places it appears;
i
in paragraph 8, for “a notified” substitute “
an approved
”
;
j
in paragraph 9, for “notified” substitute “
approved
”
;
k
in paragraph 10—
i
for “A notified” substitute “
An approved
”
;
ii
in sub-paragraph (b)—
aa
for “notification” in the first place it appears substitute “
approval
”
;
bb
in the second place it appears omit “(notification)”;
iii
in sub-paragraph (d) for “notification” substitute “
approval
”
;
l
in paragraph 11, for “A notified” substitute “
An approved
”
;
m
in paragraph 12—
i
for “A notified” substitute “
An approved
”
;
ii
for “notified under the Directive” substitute “
approved under these Regulations
”
;
n
in paragraph 13—
i
for “A notified” substitute “
An approved
”
;
ii
for “any notified body” substitute “
any approved body
”
;
iii
for “under the Directive” substitute “
by the Secretary of State
”
.
Insertion of Schedule 17 and Schedule 18I18435
After Schedule 16 insert—
SCHEDULE 17CONFORMITY ASSESSMENT PROCEDURES
PART 1TYPE EXAMINATION (MODULE B)
1
1
Type examination (Module B) is a conformity assessment procedure in which an approved body examines the technical design of an explosive and verifies and attests that the technical design of the explosive meets the requirements of these Regulations that apply to it.
2
Type examination must be carried out as an assessment of the adequacy of the technical design of the explosive through—
a
examination of the technical documentation and supporting evidence referred to in paragraph 2; and
b
examination of a specimen of the production envisaged which is representative of the complete product (combination of production type and design type).
2
1
A manufacturer must lodge an application for Type examination (Module B) with an approved body of the manufacturer's choice.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation;
d
the specimens representative of the production envisaged, and any further specimens requested by the approved body if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution; this supporting evidence must—
i
mention any documents that have been used, in particular where the relevant designated standards have not been applied in full;
ii
include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer, or by another testing laboratory on the manufacturer's behalf and under the manufacturer's responsibility.
3
The technical documentation referred to in paragraph 2(2)(c) must—
a
make it possible to assess the explosive's conformity with the applicable requirements of these Regulations and must include an adequate analysis and assessment of any risks;
b
specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the explosive;
c
contain, wherever applicable, at least the following elements—
i
a general description of the explosive;
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the explosive;
iv
a list of the designated standards applied in full or in part (where applicable specifying the parts which have been applied);
v
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements, including a list of other relevant technical specifications applied to meet the essential safety requirements;
vi
the results of design calculations made and examinations carried out;
vii
test reports.
4
1
The approved body must examine the technical documentation and supporting evidence in respect of an explosive to assess the adequacy of the technical design of the explosive.
2
For each of the specimens examined, the approved body must—
a
verify that the specimen—
i
has been manufactured in conformity with the technical documentation;
ii
identifies the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
b
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
c
carry out, or arrange the carrying out of, appropriate examinations and tests to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential safety requirements;
d
agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body must draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes and, without prejudice to the approved body's obligations in relation to the Secretary of State, the approved body may disclose the content of that report, in full or in part, only with the agreement of the manufacturer.
6
1
Where the type meets the applicable requirements of these Regulations, the approved body must issue a Type examination certificate to the manufacturer, which must contain—
a
the name and address of the manufacturer;
b
the conclusions of the examination;
c
the conditions (if any) for its validity;
d
the necessary data for the identification of the approved type;
e
all relevant information to allow the conformity of manufactured explosives with the examined type to be evaluated and to allow for in-service control.
2
The Type examination certificate referred to in sub-paragraph (1)—
a
may have one or more annexes attached;
b
must be accompanied by the descriptions and drawings necessary for identification of the approved type.
3
Where the type does not satisfy the applicable requirements of these Regulations, the approved body must refuse to issue a Type examination certificate and must inform the applicant accordingly, giving detailed reasons for its refusal.
7
An approved body must keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations, and must determine whether such changes require further investigation and, if so, the approved body must inform the manufacturer accordingly.
8
A manufacturer must inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the explosive with the essential safety requirements or the conditions for validity of that certificate; such modifications require additional approval in the form of an addition to the original Type examination certificate.
9
1
Each approved body must inform the Secretary of State of all Type examination certificates and any additions thereto which it has issued or withdrawn, and must, periodically or upon request, make available to the Secretary of State the list of such certificates and any additions thereto refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of all Type examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and must, upon request, inform the other approved bodies of such certificates and additions thereto which it has issued.
3
The other approved bodies and the Secretary of State may obtain from the approved body a copy of—
a
the Type examination certificates and additions thereto;
b
the technical documentation and the results of the examinations carried out by the approved body.
4
An approved body must keep a copy of the Type examination certificate, its annexes and additions, as well as the file containing the technical documentation including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
5
A manufacturer must keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
10
A manufacturer's authorised representative (if any) may lodge the application referred to in paragraph 2 and fulfil the obligations set out in paragraphs 8 and 9(5), provided that they are specified in the mandate by which they were appointed under regulation 46.
PART 2CONFORMITY TO TYPE BASED ON INTERNAL PRODUCTION CONTROL PLUS SUPERVISED PRODUCT CHECKS AT RANDOM INTERVALS (MODULE C2)
11
Conformity to type based on internal production control plus supervised product checks at random intervals (Module C2) is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 12 to 14, and it is solely the manufacturer's responsibility to ensure and declare that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing12
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosives with the type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Product checks13
1
The approved body chosen by the manufacturer must carry out product checks or have them carried out at random intervals determined by that body, in order to verify the quality of the internal checks on the explosive, taking into account, amongst other things, the technological complexity of the explosives and the quantity of production.
2
The approved body must ensure that—
a
it takes an adequate sample of the final product on site before its placing on the market; and
b
the sample is examined and appropriate tests as identified by the relevant parts of the designated standards, or equivalent tests set out in other relevant technical specifications, are carried out to check the conformity of the explosive with the type described in the Type examination certificate and with the relevant requirements of these Regulations.
3
Where a sample does not conform to the acceptable quality level, the approved body must take appropriate measures.
4
The acceptance sampling procedure to be applied is intended to determine whether the manufacturing process of the explosive performs within acceptable limits, with a view to ensuring conformity of the explosive.
5
The manufacturer must, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking and declaration of conformity14
1
A manufacturer must affix the UK marking to each individual explosive that is in conformity with the type described in the Type examination certificate and which satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market; the declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
Authorised representative15
A manufacturer's obligations set out in paragraph 14 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 46.
PART 3CONFORMITY TO TYPE BASED ON QUALITY ASSURANCE OF THE PRODUCTION PROCESS (MODULE D)
16
Conformity to type based on quality assurance of the production process (Module D) is a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 17 and 23, and it is solely the manufacturer's responsibility to ensure and declare that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing17
A manufacturer must operate an approved quality system for production, final product inspection and testing of the explosives specified in paragraph 18, and which is subject to surveillance as specified in paragraph 22.
Quality system18
1
A manufacturer must lodge an application for assessment of the manufacturer's quality system with an approved body of the manufacturer's choice.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the explosive category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
19
1
The quality system must ensure that the explosives are in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations that apply to them.
2
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions.
3
The quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
quality records, such as inspection reports and test data, calibration data, and qualification reports on the personnel concerned;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
20
1
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in paragraph 19 and, where applicable, it must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
2
The audit team appointed by the approved body to carry out the audit in paragraph 20(1) (“the audit”) must have experience in quality management systems, with at least one member of the team having experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations.
3
The audit must include an assessment visit to the manufacturer's premises.
4
The audit team must review the technical documentation referred to in paragraph 18(2)(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the explosive with those requirements.
5
The decision of the approved body must be notified to the manufacturer and must contain the conclusions of the audit and a reasoned assessment of the decision.
21
1
A manufacturer must—
a
fulfil the obligations arising out of the quality system as approved and maintain it in an adequate and efficient state; and
b
keep the approved body that has approved the quality system informed of any intended change to the quality system.
2
Where the approved body is notified by a manufacturer of any proposed change to the quality system the approved body must—
a
evaluate such proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 19 or whether a reassessment is necessary; and
b
notify the manufacturer of its decision and, that notification must contain the conclusions of the examination and a reasoned assessment of the decision.
Surveillance under the responsibility of the approved body22
1
The approved body must carry out surveillance, the purpose of which is to ensure that a manufacturer fulfils the obligations arising out of the approved quality system.
2
A manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide the approved body with all necessary information including, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to ensure that a manufacturer maintains and applies the quality system and, following each audit, must provide the manufacturer with an audit report.
4
The approved body may pay unexpected visits to a manufacturer; during such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly; and following such a visit the approved body must provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity23
1
A manufacturer must affix the UK marking, and, under the responsibility of the approved body referred to in paragraph 18(1), the latter's identification number to each individual explosive that is in conformity with the type described in the Type examination certificate and which satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market; the declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
24
A manufacturer must, for a period of 10 years beginning on the day on which the explosive has been placed on the market, keep at the disposal of the relevant authorities—
a
the documentation referred to in paragraph 18(2);
b
any information relating to the change referred to in paragraph 21(1)(b) and 21(2), as approved;
c
the decisions and reports of the approved body referred to in paragraphs 21, 22(3) and 22(4).
25
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
26
Each approved body must inform other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative27
A manufacturer's obligations set out in paragraphs 18(1), 18(2), 21(1)(b), 21(2), 23 and 24 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 46.
PART 4CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE (MODULE E)
28
Conformity to type based on product quality assurance (Module E) is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 29 and 34, and it is solely the responsibility of the manufacturer that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing29
A manufacturer must operate an approved quality system for final product inspection and testing of the explosives concerned as specified in paragraphs 30 and 31 and which must be subject to surveillance as specified in paragraph 33.
Quality system30
1
A manufacturer must lodge an application for assessment of the manufacturer's quality system with an approved body of the manufacturer's choice for the explosives concerned.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the explosive category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system must ensure compliance of the explosives with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
4
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions; this quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and, it must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned;
d
the means of monitoring the effective operation of the quality system.
31
1
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in paragraph 30(3) and (4) and, where applicable, it must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of a relevant designated standard.
2
The audit team appointed by the approved body to carry out the audit under paragraph 31(1) (“the audit”) must have experience in quality management systems and have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations.
3
The audit must include an assessment visit to the manufacturer's premises.
4
The audit team must review the technical documentation referred to in paragraph 30(2)(e), in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the explosive with those requirements.
5
The decision of the approved body must be notified to the manufacturer and the notification must contain the conclusions of the audit and the reasoned assessment for the decision.
32
1
A manufacturer must—
a
fulfil the obligations arising out of the quality system as approved and maintain it in an adequate and efficient state; and
b
keep the approved body that has approved the quality system informed of any intended change to the quality system.
2
Where the approved body is notified by a manufacturer of any proposed change to the quality system the approved body must—
a
evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 30(3) and (4) or whether a reassessment is necessary; and
b
notify the manufacturer of its decision and, that notification must contain the conclusions of the examination and the reasoned assessment for the decision.
Surveillance under the responsibility of the approved body33
1
The approved body must carry out surveillance, the purpose of which is to ensure that a manufacturer fulfils the obligations arising out of the approved quality system.
2
A manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to ensure that a manufacturer maintains and applies the quality system and, following each audit, must provide the manufacturer with an audit report.
4
The approved body may pay unexpected visits to the manufacturer; during such visits the approved body may carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly and, following such a visit, the approved body must provide the manufacturer with a visit report and, if tests have been carried out, a test report.
UK marking and declaration of conformity34
1
A manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 30(1), the latter's identification number to each individual explosive that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
3
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
35
A manufacturer must, for a period of 10 years, beginning on the day on which the explosive has been placed on the market, keep at the disposal of the relevant authorities—
a
the documentation referred to in paragraph 30(1) and 30(2);
b
the information relating to the change referred to in paragraph 32(1)(b) and 32(2), as approved;
c
the decisions and reports of the approved body referred to in paragraphs 32(2), 33(3) and 33(4).
36
1
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
Authorised representative37
A manufacturer's obligations set out in paragraphs 30(1), 30(2), 32(1)(b), 34 and 35 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 46.
PART 5CONFORMITY TO TYPE BASED ON PRODUCT VERIFICATION (MODULE F)
38
Conformity to type based on product verification (Module F) is the part of a conformity assessment procedure whereby a manufacturer fulfils the obligations laid down in paragraphs 39, 42(1) and 43, and it is solely the responsibility of the manufacturer to ensure and declare that the explosives concerned, which have been subject to examinations and tests under paragraph 40, are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing39
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosives with the approved type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Verification40
1
An approved body chosen by the manufacturer must carry out appropriate examinations and tests in order to check the conformity of the explosives with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
2
The examinations and tests to check the conformity of the explosives with the appropriate requirements must be carried out, at the choice of the manufacturer, either—
a
by examination and testing of every product as specified in paragraph 41; or
b
by examination and testing of the explosives on a statistical basis as specified in paragraph 42.
Verification of conformity by examination and testing of every product41
1
All explosives must be individually examined and appropriate tests in the relevant designated standard or equivalent tests in other relevant technical specifications must be carried out in order to verify conformity with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out, and must affix its identification number to each approved explosive or have it affixed under its responsibility.
3
A manufacturer must keep the certificates of conformity available for inspection by the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
Statistical verification of conformity42
1
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure the homogeneity of each lot produced, and must present the manufacturer's explosives for verification in the form of homogeneous lots.
2
The approved body must take a random sample from each lot; all explosives in a sample must be individually examined and appropriate tests set out in the relevant designated standards, or equivalent tests set out in other relevant technical specifications, must be carried out in order to verify their conformity with the approved type described in the Type examination certificate and with the applicable requirements of these Regulations and to determine whether the lot is accepted or rejected; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
3
If a lot is accepted, all explosives of the lot must be considered approved, except for those explosives from the sample that have been found not to satisfy the tests.
4
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out, and must affix its identification number to each approved explosive or have it affixed under its responsibility.
5
A manufacturer must keep the certificates of conformity at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
6
If a lot is rejected, the approved body, or enforcing authority, must take appropriate measures to prevent the placing on the market of that lot and, in the event of the frequent rejection of lots the approved body may suspend statistical verification and take appropriate measures.
UK marking and declaration of conformity43
1
A manufacturer must affix the UK marking, and, under the responsibility of the approved body referred to in paragraph 40(1), the latter's identification number to each individual explosive confirming that the explosive is in conformity with the approved type described in the Type examination certificate and that it satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market and, such a declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
4
If the approved body referred to in paragraph 40(1) agrees, and under its responsibility, the manufacturer may affix the approved body's identification number to the explosives.
5
If the approved body referred to in paragraph 40(1) agrees and under its responsibility, a manufacturer may affix the approved body's identification number to the explosives during the manufacturing process.
Authorised representative44
A manufacturer's obligations under this Part of this Schedule may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 46, but an authorised representative may not fulfil the manufacturer's obligations set out in paragraphs 39 and 42(1).
PART 6CONFORMITY BASED ON UNIT VERIFICATION (MODULE G)
45
Conformity based on unit verification (Module G) is the conformity assessment procedure whereby a manufacturer fulfils the obligations laid down in paragraphs 46, 47 and 49, and it is solely the responsibility of the manufacturer to ensure and declare that the explosive concerned, which has been subject to the provisions of paragraph 48, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation46
1
A manufacturer must establish the technical documentation and make it available to the approved body referred to in paragraph 48; the documentation must make it possible to assess the explosive's conformity with the relevant requirements and must include an adequate analysis and assessment of any risks.
2
The technical documentation must specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the explosive and, wherever applicable, the technical documentation must contain at least the following elements—
a
a general description of the explosive;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of the drawings and schemes and the operation of the explosive;
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements of these Regulations, including a list of other relevant technical specifications applied; and in the case of partly applied designated standards, the technical documentation must specify the parts which have been applied;
e
results of design calculations made and examinations carried out; and
f
test reports.
3
A manufacturer must keep the technical documentation at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
Manufacturing47
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosive with the applicable requirements of these Regulations.
Verification48
1
An approved body chosen by the manufacturer must carry out, or have carried out, appropriate examinations and tests set out in the relevant designated standards, or equivalent tests set out in other relevant technical specifications, to check the conformity of the explosive with the applicable requirements of these Regulations; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out and must affix its identification number to the approved explosive, or have it affixed under its responsibility.
3
A manufacturer must keep the certificates of conformity at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market.
UK marking and declaration of conformity49
1
A manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 48, the latter's identification number to each explosive that satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity and keep it at the disposal of the relevant authorities for 10 years beginning on the day on which the explosive has been placed on the market and, the declaration of conformity must identify the explosive for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authorities upon request.
Authorised representative50
A manufacturer's obligations set out in paragraphs 46(3) and 49 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 46.
SCHEDULE 18DECLARATION OF CONFORMITY
Declaration of conformity (No XXXX) M52
1
No … (product, type, batch or serial number):
2
Name and address of the manufacturer and, where applicable, the manufacturer's authorised representative:
3
This declaration of conformity is issued under the sole responsibility of the manufacturer.
4
Object of the declaration (identification of product allowing traceability):
5
The object of the declaration described above is in conformity with the relevant statutory requirements:
6
References to the relevant designated standards used or references to the other technical specifications in relation to which conformity is declared:
7
The approved body … (name, number) performed … (description of intervention) and issued the certificate:
8
Additional information:
Signed for and on behalf of: (place and date of issue): (name, function) (signature):
PART 2Revocations of EU instruments
GeneralI18536
The following EU instruments are revoked—
a
Commission Decision 2004/388/EC on an Intra-Community transfer of explosives document; and
b
Commission Decision 2010/347/EU amending Commission Decision 2004/388/EC on an Intra-Community transfer of explosives document.
SCHEDULE 17Amendment of the Weights and Measures (Revocations) Regulations 2015
GeneralI1861
1
The Schedule to the Weights and Measures (Revocations) Regulations 2015 is amended as follows.
2
In paragraph 4—
a
the existing text becomes sub-paragraph (1);
3
In paragraph 5—
a
in sub-paragraph (3) at the beginning insert “
Subject to sub-paragraph (5),
”
;
b
in sub-paragraph (4) at the beginning insert “
Subject to sub-paragraph (5),
”
;
c
after sub-paragraph (4) insert—
SCHEDULE 18Amendment of the Offshore Installations (Offshore Safety Directive) (Safety Case etc.) Regulations 2015
GeneralI1871
1
The Offshore Installations (Offshore Safety Directive) (Safety Case etc.) Regulations 2015 are amended as follows.
2
For regulation 9(2)(b) substitute—
b
examination of any design, specification, certificate, marking or other document, or standard relating to those elements or that plant by a verifier;
SCHEDULE 19Amendment of the Pyrotechnic Articles (Safety) Regulations 2015
IntroductionI9001
The Pyrotechnic Articles (Safety) Regulations 2015 are amended in accordance with paragraphs 2 to 43.
Amendment to regulation 2I1882
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “aerial wheel” insert—
“approved body” has the meaning given to it in regulation 43;
d
omit the definition of “CE marking”;
e
omit the definition of “competent national authority”;
f
after the definition of “conformity assessment body” insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 9(1)(a) (declaration of conformity and UK marking);
“designated standard” has the meaning given to it in regulation 2A;
g
in the definition of the “Directive”, at the end insert “
(as it has effect immediately before F154IP completion day
)
”
;
h
omit the definition of “EU declaration of conformity”;
i
omit the definition of “harmonised standard”;
j
for the definition of “importer” substitute—
F155“importer” means a person who—
a
is established in the United Kingdom and places a pyrotechnic article from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a pyrotechnic article on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F156k
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
l
omit the definition of “national accreditation body”;
m
omit the definition of “notified body requirements”;
F157n
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
o
omit the definition of “registration number”;
p
after the definition of “theatrical pyrotechnic article” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK registration number” means the number assigned to a pyrotechnic article by an approved body under paragraph 5(a) of Schedule 6 (operational obligations of approved bodies);
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
After paragraph (1) insert—
1A
Schedules 2A and 3A reproduce the provisions of Annexes II and III (respectively) to the Directive with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedule 2A and 3A is a reference to the equivalent provision of the Annex to the Directive as set out in that Schedule.
4
Omit paragraph (3).
Insertion of regulation 2AI1903
After regulation 2 insert—
Interpretation: designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions; and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any persons who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a pyrotechnic article to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 6I1914
In regulation 6 (categorisation), in paragraph (b) for “a notified body” substitute “
an approved body
”
.
Amendment to regulation 8I1925
In regulation 8 (technical documentation and conformity assessment), in paragraph (b) for “Annex II to the Directive (as amended from time to time)”, in each place it occurs, substitute “
Schedule 2A
”
.
Amendment to regulation 9I1936
1
Regulation 9 (EU declaration of conformity and CE marking) is amended as follows.
2
In the heading—
a
omit “EU”; and
b
for “CE” substitute “
UK
”
.
3
In paragraph (1)(a) omit “(EU declaration of conformity)”.
4
In paragraph (1)(b)—
a
for “CE” where it first appears substitute “
UK
”
;
b
omit “(CE marking)”.
5
In paragraph (2) omit “EU”.
6
For paragraph (3) substitute—
3
Where a pyrotechnic article is subject to more than one enactment requiring a declaration of conformity to be drawn up, the manufacturer must draw up a single declaration of conformity, which identifies each enactment by its title.
Amendment to regulation 10I1947
In regulation 10 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation, omit “EU”.
Amendment to regulation 11I1958
In regulation 11 (labelling of pyrotechnic articles other than pyrotechnic articles for vehicles)—
a
for paragraph (1)(c) substitute—
c
in English.
b
omit paragraphs (3) to (5).
Amendment to regulation 12I1969
In regulation 12 (labelling of pyrotechnic articles for vehicles)—
a
in paragraph (1)(c), before “registration number”, insert “
UK
”
; and
b
in paragraphs (3) and (4), before “safety data sheet”, insert “
UK
”
.
Amendment to regulation 13I19710
In regulation 13 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 15I19811
In regulation 15 (requirements which must be satisfied before an importer places a pyrotechnic article on the market)—
a
in paragraph (1)(c)(i) for “CE” substitute “
UK
”
; and
b
in paragraph (2)(b), before “safety data sheet”, insert “
UK
”
.
Amendment to regulation 17I19912
In regulation 17 (information identifying importer)—
a
in paragraph (2)—
i
for “competent national authority” substitute “
enforcing authority
”
;
ii
omit “in the member State in which it is to be made available to end users”;
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the pyrotechnic article; or
b
before placing the pyrotechnic article on the market, the importer sets out the information referred to in paragraph (1)—
i
on the packaging; or
ii
in a document accompanying the pyrotechnic article.
Amendment to regulation 18I20013
In Regulation 18 (instructions and safety information)—
a
in paragraph (1) for the words from “in a language” to the word “end-users” in the second place it occurs, substitute “
that are clear, legible and in easily understandable English
”
; and
b
omit paragraph (2).
Amendment to regulation 19I20114
In regulation 19 (retention of technical documentation and EU declaration of conformity) omit “EU” from the heading and from paragraph (a).
Amendment to regulation 20I20215
In regulation 20 (traceability), before “registration number”, in both places it occurs, insert “
UK
”
.
Amendment to regulation 22I20316
In regulation 22 (duty to take action in respect of pyrotechnic articles placed on the market which are considered not to be in conformity), in paragraph (2) omit the words from “and” to “market”.
Amendment to regulation 25I20417
In Regulation 25 (requirements which must be satisfied before a distributor makes a pyrotechnic article available on the market)—
a
in paragraph (1)(a)—
i
in sub-paragraph (i) for “CE” substitute “
UK
”
; and
ii
in sub-paragraph (iii) for the words from “in a language” to the words “on the market”, substitute “
that are clear, legible and in easily understandable English
”
; and
b
in paragraph (2)(c), before “safety data sheet”, insert “
UK
”
.
Amendment to regulation 27I20518
In regulation 27 (duty to take action in respect of pyrotechnic articles made available on the market which are not in conformity), in paragraph (2) omit the words from “and” to “market”.
Amendment to regulation 35I20619
In Regulation 35 (supply of safety data sheet)—
a
in the heading and in the body of the text, before “safety data sheet”, insert “
UK
”
; and
b
omit paragraph (a).
Revocation of regulation 36I20720
Omit regulation 36 (translation of declaration of conformity).
Amendment to regulation 38I20821
In regulation 38 (prohibition on improper use of CE marking) in each place in which it occurs and in the heading for “CE” substitute “
UK
”
.
Insertion of regulations 38A to DI20922
After regulation 38 insert—
Obligations which are met by complying with obligations in the Directive38A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex to the Directive; and
b
“CE marking” has the meaning given to it in Article 3(22); and
c
“harmonised standard” has the meaning given to it in Article 3(14).
2
Paragraph (3) applies where, before placing a pyrotechnic article on the market, the manufacturer—
a
ensures that the pyrotechnic article has been designed and manufactured in accordance with the essential safety requirements set out in Annex I;
b
ensures that the technical documentation referred to in Annex II has been drawn up;
c
ensures that the conformity assessment procedure that applies to that pyrotechnic article in accordance with Article 17 has been carried out;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and other markings, in accordance with Articles 19 and 20(1) to (4);
f
draws up an EU declaration of conformity, in accordance with Article 18; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulation 7, 8, 9(1) and 9(3) are to be treated as being satisfied;
b
regulations 2(2)(a), 9(2), 10, 13(2) and 38 apply subject to the modifications in paragraph (8);
c
Part 3 does not apply; and
d
regulations 60(1)(a), (c), (d) and (f) do not apply.
4
Paragraph (5) applies where, before placing a pyrotechnic article on the market, the importer ensures that—
a
the conformity assessment procedure that applies to that pyrotechnic article in accordance with Article 17 has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex II; and
c
the vessel bears the CE marking and other markings referred to in Article 20(3) to (4).
5
Where this paragraph applies—
a
the requirements of regulation 15(a) to (c) are to be treated as being satisfied; and
b
regulations 2(2)(a), 16(1) and 19, and regulation 29 insofar as it relates to importers, apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making a pyrotechnic article available on the market, a distributor ensures that the vessel bears the CE marking.
7
Where this paragraph applies—
a
regulation 25(1)(a)(i) is to be treated as being satisfied; and
b
regulations 2(2)(a), 26(1), and regulation 29 insofar as it relates to distributors, apply subject to the modifications in paragraph (8).
8
The modifications referred to in paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the conformity assessment procedure that applies to the pyrotechnic article in accordance with Article 17;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex II.
Obligations which are met by complying with obligations in the Directive that relate to the registration number38B
1
In this regulation—
a
any reference to an Article is a reference to an Article of the Directive; and
b
“registration number” means a number comprising the elements set out in Article 1 of Commission Implementing Directive 2014/58/EU of 16 April 2014 setting up a system for the traceability of pyrotechnic articles, as it has effect immediately before exit day M53.
2
Paragraph (3) applies where, before placing a pyrotechnic article on the market, the manufacturer labels the pyrotechnic article with a registration number in accordance with Article 10(2) or Article 11(1), as applicable.
3
Where this paragraph applies—
a
the requirements of regulations 11, 12 and 20 apply subject to the modifications in paragraph (8); and
b
regulation 60(1)(f) does not apply.
4
Paragraph (5) applies where, before placing a pyrotechnic article on the market, the importer ensures that the pyrotechnic article has been labelled with a registration number in accordance with Articles 10(2) or 11(1), as applicable.
5
Where this paragraph applies the requirements of regulation 15(1)(d) and 20 apply subject to the modifications at paragraph (8).
6
Paragraph (7) applies where, before making a pyrotechnic article available on the market, a distributor verifies that the pyrotechnic article has been labelled with a registration number in accordance with Articles 10(2) or 11(1), as applicable.
7
Where this paragraph applies the requirements of regulation 25(1)(b) apply subject to the modifications at paragraph (8).
8
The modifications referred to in paragraphs (3)(a), (5) and (7) are that—
a
any reference to “Schedule 3” in regulations 11 or 12 is to be read as a reference to Schedule 3 subject to the modification that, in paragraph 1(d) of that Schedule, the reference to “UK registration number” is to be read as a reference to the registration number; and
b
any reference to “UK registration number” is to be read as a reference to the registration number.
Obligations which are met by complying with obligations in the Directive that relate to safety data sheet38C
1
In this regulation “safety data sheet” means a document—
a
compiled in accordance with Annex II to Regulation No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency M54, as it has effect in EU law immediately before exit day; and
b
which takes into account the specific needs of professional users.
2
Paragraph (3) applies where, before placing a pyrotechnic article for vehicles on the market, the manufacturer draws up a safety data sheet in English.
3
Where this paragraph applies—
a
regulation 12 applies subject to the modification—
i
in paragraph (10); and
ii
that paragraph (4) of that regulation is omitted; and
b
regulation 60(1)(f) does not apply.
4
Paragraph (5) applies where, before placing a pyrotechnic article for vehicles on the market, the importer ensures that the article is accompanied by a safety data sheet in English.
5
Where this paragraph applies regulation 15 applies subject to the modification that paragraph (2) of that regulation is substituted by the following—
2
In paragraph (1)(c)(ii), “required documents” means—
a
the documents that are required to be provided with the pyrotechnic article pursuant to regulation 11(6); and
b
the safety data sheet.
6
Paragraph (7) applies where, before making a pyrotechnic article for vehicles available on the market, a distributor verifies that the article is accompanied by a safety data sheet in English.
7
Where this paragraph applies regulation 25 applies subject to the modification that paragraph (2) of that regulation is substituted by the following—
2
In paragraph (1)(a)(ii), “required documents” means—
a
the documents that are required to be provided with the pyrotechnic article pursuant to regulations 11(6) and 17(3)(b); and
b
the safety data sheet.
8
Paragraph (9) applies where, before making a pyrotechnic article for vehicles available on the market to a professional user, an economic operator supplies to the professional user a safety data sheet in respect of that article.
9
Where this paragraph applies regulation 35 applies subject to the modification at paragraph (10).
10
The modification referred to in sub-paragraphs (3) and (9) is that any reference to a “UK safety data sheet” is to be read as a reference to a safety data sheet.
Conformity assessment procedure obligation which is met by complying with the Directive38D
1
In this regulation any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive.
2
Paragraph (3) applies where, prior to the manufacture of a pyrotechnic article, the manufacturer ensures that the conformity assessment procedure that applies to that pyrotechnic article in accordance with Article 17(a), referred to as Module B and set out in Annex II, has been carried out.
3
Where this paragraph applies—
a
the requirement in regulation 40(a) to conform to Module B as set out in Schedule 2A is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 8(a), 9(1), 15(1)(a), 38(1)(b) and 41(b) is to be read as including the conformity assessment procedure referred to in Article 17(1)(a), referred to as Module B and set out in Annex II; and
c
any reference to “technical documentation” in regulations 8(b), 10, 15(1)(b) and 19(b) is to be read as including the technical documentation relating to the design of the pyrotechnic article referred to in Module B of Annex II.
F160Expiry of regulations 38A to D38E
1
Subject to paragraph (2), regulations 38A to 38C cease to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 38A—
a
any pyrotechnic article which was placed on the market pursuant to regulation 38A may continue to be made available on the market on or after the expiry of regulation 38A;
b
any obligation to which a person was subject under regulation 38A in respect of a pyrotechnic article placed on the market pursuant to regulation 38A continues to have effect after the expiry of regulation 38A, in respect of that article;
c
any obligation to which a person was subject under regulations 38B and 38C in respect of a pyrotechnic article pursuant to regulations 38B and 38C continues to have effect after the expiry of regulations 38B and 38C, in respect of that article.
3
Subject to paragraph (4), regulation 38D ceases to have effect at the end of the period of 24 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 38B in relation to a pyrotechnic article prior to the expiry of regulation 38D, regulation 38D continues to apply in respect of that article where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after an EU-Type examination has been carried out in accordance with a conformity assessment procedure set out in Annex II of the Directive.
Qualifying Northern Ireland Goods38F
1
Where paragraph (2) applies, a pyrotechnic article is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
a pyrotechnic article—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the pyrotechnic article on the market, the importer—
a
complies with regulation 17;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the pyrotechnic article;
ii
the manufacturer has drawn up the technical documentation; and
iii
the pyrotechnic article bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning give to it in regulation 2(1), as it applies in Northern Ireland;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 39I21023
In regulation 39 (presumption of conformity), in paragraph (1)—
a
for “harmonised” substitute “
designated
”
; and
b
omit the words from “the reference” to “Union”.
Amendment to regulation 40I21124
In Regulation 40 (conformity assessment procedures)—
a
for “Annex II to the Directive (as amended from time to time)” substitute “
Schedule 2A
”
;
b
in paragraph (a) for “EU-type” substitute “
Type
”
; and
c
for “a notified body”, in each place it occurs, substitute “
an approved body
”
.
Amendment to regulation 41I21225
In Regulation 41 (EU declaration of conformity)—
a
omit “EU” from the heading and from the body of the text;
b
for “Annex II to the Directive (as amended from time to time)” substitute “
Schedule 2A
”
; and
c
for “Annex III to the Directive (as amended from time to time)” substitute “
Schedule 3A
”
.
Amendment to regulation 42I21326
In Regulation 42 (CE Marking)—
a
for “CE”, in each place it occurs (including the heading), substitute “
UK
”
; and
b
for “notified body”, in each place it occurs, substitute “
approved
”
.
F161c
in paragraph (1) after “article” insert “
or, where paragraph (1A) applies, to a label affixed to the pyrotechnic article or to the accompanying documents
”
;
d
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the pyrotechnic article; or
b
the accompanying documents.
e
in paragraph (2) after “Where” insert “
paragraph (1A) does not apply and
”
.
Amendment to Part 4I21427
For Part 4 (notification of conformity assessment bodies), substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies43
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 44 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 47 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F162IP completion day notified to the European Commission and the member States, in accordance with Article 21 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 43(1)(b), as it had effect immediately before F162IP completion day
“approved body requirements” means the requirements set out in Schedule 5.
Approval of conformity assessment bodies44
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of–
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the relevant conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the pyrotechnic article in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies45
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring46
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 44(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval47
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement; or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 46(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 43.
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 46(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 43.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must—
a
on the request of the Secretary of State, transfer its files (including the register which it maintains under paragraph 5 of Schedule 6 (operational obligations of approved bodies)) to another approved body or to the Secretary of State; or
b
in the absence of a request under sub-paragraph (a), ensure that its files are kept available for the Secretary of State and each enforcing authority for a period equal to that specified in paragraphs 5 and 6 of Schedule 6.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies48
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 44; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 6.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type-examination certificate referred to in Schedule 2A; or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 42(4).
Subsidiaries and contractors49
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M55.
Register of approved bodies50
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body51
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 46; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 50.
Amendment to regulation 55I21528
In regulation 55 (exercise of enforcement powers), omit paragraph (c).
Amendment to regulation 57I21629
In Regulation 57 (enforcement action in respect of pyrotechnic articles which are not in conformity and which present a risk)—
a
in paragraph (2), for “notified” substitute “
approved
”
;
b
omit paragraphs (4) and (7); and
c
in paragraph (8)—
i
for “notices in paragraphs (6) and (7)” substitute “
notice in paragraph (6)
”
; and
ii
in sub-paragraph (f)(ii) for “harmonised” substitute “
designated
”
.
Amendment to regulation 58I21730
Omit regulation 58 (EU safeguard procedure).
Amendment to regulation 59I21831
In regulation 59 (enforcement action in respect of pyrotechnic articles which are in conformity, but present a risk)—
a
omit paragraph (3); and
b
in paragraph (4) for “notices referred to in paragraphs (2) and (3)” substitute “
notice referred to in paragraph (2)
”
.
Amendment to regulation 60I21932
In Regulation 60 (enforcement action in respect of formal non-compliance)—
a
in paragraph (1)(a) for “CE”, in each place it occurs, substitute “
UK
”
;
b
in paragraph (1)(b)—
i
for “a notified body” substitute “
an approved body
”
; and
ii
for “the notified body” substitute “
the approved body
”
; and
c
in paragraph (1)(c)—
i
omit “EU”, in each place it occurs; and
ii
for “CE” substitute “
UK
”
.
Amendment to regulation 61I22033
In regulation 61 (restrictive measures) omit “(as amended from time to time)”.
Amendment to regulation 62I22134
In Regulation 62 (offences)—
a
in paragraph (1)—
i
in sub-paragraphs (d) and (t) for “CE marking” substitute “
UK marking
”
;
ii
in sub-paragraphs (d), (e) and (r) omit “EU”; and
iii
in sub-paragraph (q), before “safety data sheet”, insert “
UK
”
;
b
in paragraph (2)(f) omit “EU”; and
c
in paragraph (6) omit “(as amended from time to time)”, in both places it occurs.
Amendment to regulation 74I22235
In Regulation 74 (transitional provisions) in paragraph (5), for “another” substitute “
a
”
.
Insertion of Transitional provision in relation to EU ExitI18936
After regulation 74 (transitional provisions) insert—
Transitional provision in relation to EU Exit74A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F164IP completion day; and
“product” means a pyrotechnic article to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 19 to the Product Safety and Metrology (Amendment etc.) (EU Exit) Regulations 2019 M56 any obligation to which a person was subject under these Regulations as they had effect immediately before F164IP completion day, continues to have effect as it did immediately before F164IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member state of any matter;
b
any obligation to take action outside the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 40 as it had effect immediately before F164IP completion day in relation to that product,
that action has effect as if it had been done under regulation 40 as it had effect on and after F164IP completion day.
Amendment to regulation 75I22337
1
Regulation 75 (Consequential revocations, savings and amendments) is amended as follows.
2
In paragraph (2) for “The” substitute “
Subject to the modifications made to the 2010 Regulations in paragraph (2A), the
”
.
3
After paragraph (2) insert—
2A
The modifications referred to in paragraph (2) are as follows—
a
in regulations 18(3) and 36(3), for “EU” substitute
“ UK ”;b
in regulation 13, omit paragraph (4);
c
in regulation 19—
i
omit paragraphs (9) and (11); and
ii
for paragraph (10) substitute—
10
Where the Secretary of State has sufficient reason to believe that a category 1,2 or 3 firework presents a serious risk to the health or safety of persons in the UK, the Secretary of State shall perform an appropriate evaluation.
d
in regulation 30 omit paragraph (4);
e
in regulation 37—
i
omit paragraphs (10) and (12); and
ii
for paragraph (11) substitute—
11
Where the Secretary of State has sufficient reason to believe that a pyrotechnic article presents a serious risk to the health or safety of persons in the UK, the Secretary of State shall perform an appropriate evaluation.
Amendment to Schedule 2I22438
In Schedule 2 (essential safety requirements) in paragraph 1(1) for “notified” substitute “
approved
”
.
Insertion of Schedule 2AI22539
After Schedule 2 insert—
SCHEDULE 2AConformity Assessment Procedures (Annex II to the Directive)
PART 1Module B: Type Examination
1
Type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of a pyrotechnic article and verifies and attests that the technical design of the pyrotechnic article meets the requirements of these Regulations that apply to it.
2
Type examination shall be carried out as an assessment of the adequacy of the technical design of the pyrotechnic article through examination of the technical documentation and supporting evidence referred to in point 3, plus examination of a specimen, representative of the production envisaged, of the complete product (combination of production type and design type).
3
The manufacturer shall lodge an application for a Type examination with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation. The technical documentation shall make it possible to assess the pyrotechnic article's conformity with the applicable requirements of these Regulations and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the pyrotechnic article. The technical documentation shall contain, wherever applicable, at least the following elements:
i
a general description of the pyrotechnic article;
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the pyrotechnic article;
iv
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements of these Regulations including a list of other relevant technical specifications applied. In the case of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
v
results of design calculations made, examinations carried out, etc.;
vi
test reports;
d
the specimens representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution. This supporting evidence shall mention any documents that have been used, in particular where the relevant designated standards have not been applied in full. The supporting evidence shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer, or by another testing laboratory on his behalf and under his responsibility.
4
The approved body shall:
For the pyrotechnic article:
4
Examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the pyrotechnic article.
For the specimen(s):
4
Verify that the specimen(s) have been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
4
Carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
4
Carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer, including those in other relevant technical specifications applied, meet the corresponding essential safety requirements of these Regulations;
4
Agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body shall draw up an evaluation report that records the activities undertaken in accordance with point 4 and their outcomes. Without prejudice to its obligations vis-à vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
6
Where the type meets the requirements of these Regulations that apply to the pyrotechnic article concerned, the approved body shall issue a Type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The Type examination certificate may have one or more annexes attached.
The Type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured pyrotechnic articles with the examined type to be evaluated and to allow for in-service control.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a Type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
7
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations, and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the pyrotechnic article with the essential safety requirements of these Regulations or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original Type examination certificate.
8
Each approved body shall inform the Secretary of State concerning the Type examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the Type examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and/or additions thereto which it has issued.
The Secretary of State and other approved bodies may, on request, obtain a copy of the Type examination certificates and/or additions thereto. On request the Secretary of State may obtain a copy of the technical documentation and the results of the examinations carried out by the approved body. The approved body shall keep a copy of the Type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
9
The manufacturer shall keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market.
PART 2Module C2: Conformity to type based on internal production control plus supervised product checks at random intervals
1
Conformity to type based on internal production control plus supervised product checks at random intervals is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2, 3 and 4, and ensures and declares on his sole responsibility that the pyrotechnic articles concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
2
Manufacturing
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured pyrotechnic articles with the type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
3
Product checks
An approved body, chosen by the manufacturer, shall carry out product checks or have them carried out at random intervals determined by the body, in order to verify the quality of the internal checks on the pyrotechnic article, taking into account, inter alia, the technological complexity of the pyrotechnic articles and the quantity of production. An adequate sample of the final products, taken on site by the approved body before the placing on the market, shall be examined and appropriate tests as identified by the relevant parts of the designated standards and/or equivalent tests set out in other relevant technical specifications, shall be carried out to check the conformity of the pyrotechnic article with the type described in the Type examination certificate and with the relevant requirements of these Regulations. Where a sample does not conform to the acceptable quality level, the body shall take appropriate measures.
The acceptance sampling procedure to be applied is intended to determine whether the manufacturing process of the pyrotechnic article performs within acceptable limits, with a view to ensuring conformity of the pyrotechnic article.
The manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
4
UK marking and declaration of conformity
4
The manufacturer shall affix the UK marking to each individual pyrotechnic article that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for each product model and keep it at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market. The declaration of conformity shall identify the pyrotechnic article for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
PART 3Module D: Conformity to type based on quality assurance of the production process
1
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2 and 5, and ensures and declares on his sole responsibility that the pyrotechnic articles concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
2
Manufacturing
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the pyrotechnic articles concerned as specified in point 3 and shall be subject to surveillance as specified in point 4.
3
Quality system
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice for the pyrotechnic articles concerned.
The application shall include:
a
the name and address of the manufacturer;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the pyrotechnic article category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure that the pyrotechnic articles are in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.; and
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in point 3.1(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the pyrotechnic article with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
4
Surveillance under the responsibility of the approved body
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
5
UK marking and declaration of conformity
5
The manufacturer shall affix the UK marking, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number to each individual pyrotechnic article that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model and keep it at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market. The declaration of conformity shall identify the pyrotechnic article for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
6
The manufacturer shall, for a period ending 10 years after the pyrotechnic article has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in point 3.1;
b
the information relating to the change referred to in point 3.5, as approved;
c
the decisions and reports of the approved body referred to in points 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
PART 4Module E: Conformity to type based on product quality assurance
1
Conformity to type based on product quality assurance is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2 and 5, and ensures and declares on his sole responsibility that the pyrotechnic articles concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
2
Manufacturing
The manufacturer shall operate an approved quality system for final product inspection and testing of the pyrotechnic articles concerned as specified in point 3 and shall be subject to surveillance as specified in point 4.
3
Quality system
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice for the pyrotechnic articles concerned.
The application shall include the following information:
a
the name and address of the manufacturer;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the pyrotechnic article category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure compliance of the pyrotechnic articles with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.;
d
the means of monitoring the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in point 3.1(e), in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the pyrotechnic article with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
4
Surveillance under the responsibility of the approved body
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
5
UK marking and declaration of conformity
5
The manufacturer shall affix the UK marking, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number to each individual pyrotechnic article that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model and keep it at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market. The declaration of conformity shall identify the pyrotechnic article for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
6
The manufacturer shall, for a period ending 10 years after the pyrotechnic article has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in point 3.1;
b
the information relating to the change referred to in point 3.5, as approved;
c
the decisions and reports of the approved body referred to in points 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
PART 5Module G: Conformity based on unit verification
1
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2, 3 and 5, and ensures and declares on his sole responsibility that the pyrotechnic article concerned, which has been subject to the provisions of point 4, is in conformity with the requirements of these Regulations that apply to it.
2
Technical documentation
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in point 4. The documentation shall make it possible to assess the pyrotechnic article's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the pyrotechnic article. The technical documentation shall, wherever applicable, contain at least the following elements:
a
a general description of the pyrotechnic article;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the pyrotechnic article;
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the case of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
e
results of design calculations made, examinations carried out, etc.;
f
test reports.
The manufacturer shall keep the technical documentation at the disposal of the relevant national authorities for 10 years after the pyrotechnic article has been placed on the market.
3
Manufacturing
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured pyrotechnic article with the applicable requirements of these Regulations.
4
Verification
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests, set out in the relevant designated standards and/or equivalent tests set out in other relevant technical specifications, to check the conformity of the pyrotechnic article with the applicable requirements of these Regulations, or have them carried out. In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to the approved pyrotechnic article, or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market.
5
UK marking and declaration of conformity
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in point 4, the latter's identification number to each pyrotechnic article that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity and keep it at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market. The declaration of conformity shall identify the pyrotechnic article for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
PART 6Module H: Conformity based on full quality assurance
1
Conformity based on full quality assurance is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2 and 5, and ensures and declares on his sole responsibility that the pyrotechnic articles concerned satisfy the requirements of these Regulations that apply to them.
2
Manufacturing
The manufacturer shall operate an approved quality system for design, manufacture and final product inspection and testing of the pyrotechnic articles concerned as specified in point 3 and shall be subject to surveillance as specified in point 4.
3
Quality system
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice for the pyrotechnic articles concerned.
The application shall include:
a
the name and address of the manufacturer;
b
the technical documentation for one model of each pyrotechnic article category intended to be manufactured. The technical documentation shall, wherever applicable, contain at least the following elements:
— a general description of the pyrotechnic article;
— conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
— descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the pyrotechnic article;
— a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
— results of design calculations made, examinations carried out, etc.;
— test reports;
c
the documentation concerning the quality system;
d
a written declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the pyrotechnic articles with the applicable requirements of these Regulations.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. That quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards that will be applied and, where the relevant designated standards will not be applied in full, the means that will be used to ensure that the essential safety requirements of these Regulations will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the pyrotechnic articles pertaining to the pyrotechnic article category covered;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.;
g
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member experienced as an assessor in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in point 3.1(b) to verify the manufacturer's ability to identify the applicable requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the pyrotechnic article with those requirements.
The manufacturer shall be notified of the decision.
The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
4
Surveillance under the responsibility of the approved body
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the design, manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records as provided for by the design part of the quality system such as the results of analyses, calculations, tests, etc.;
c
the quality records as provided for by the manufacturing part of the quality system such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits, the approved body may, if necessary, carry out product tests, or have them carried out, in order to check the proper functioning of the quality system. It shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
5
UK marking and declaration of conformity
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number to each individual pyrotechnic article that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model and keep it at the disposal of the national authorities for 10 years after the pyrotechnic article has been placed on the market. The declaration of conformity shall identify the pyrotechnic article for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
6
The manufacturer shall, for a period ending 10 years after the pyrotechnic article has been placed on the market, keep at the disposal of the national authorities:
a
the technical documentation referred to in point 3.1;
b
the documentation concerning the quality system referred to in point 3.1;
c
the information relating to the change referred to in point 3.5, as approved;
d
the decisions and reports of the approved body referred to in points 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
Amendment to Schedule 3I22640
In Schedule 3, in paragraph 1(d), before “registration number” insert “
UK
”
.
Insertion of Schedule 3AI22741
After Schedule 3 insert—
SCHEDULE 3ADeclaration of Conformity (Annex III to the Directive)
1
UK registration number:
2
Product, batch or serial number:
3
Name and address of the manufacturer:
4
This declaration of conformity is issued under the sole responsibility of the manufacturer.
5
Object of the declaration (identification of product allowing traceability):
6
The object of the declaration described above is in conformity with the relevant statutory requirements:
7
References to the designated standards used or references to the other technical specifications in relation to which conformity is declared:
8
The approved body (name, number) performed (description of intervention) and issued the certificate:
9
Additional information:
Signed for and on behalf of:
[place and date of issue]:
[name, function] [signature]:
Amendment to Schedule 5I22842
In Schedule 5—
a
in the heading for “Notified” substitute “
Approved
”
;
F165aa
in paragraph 1 for “in the United Kingdom” substitute “
under the national law of the country in which the body is established
”
;
ab
for paragraph 8 substitute—
8
A conformity assessment body must ensure that it, either on its own or with the assistance of its subcontractors or subsidiaries, is capable of carrying out the conformity assessment activities in relation to which the conformity assessment body has been, or is to be, approved.
b
in paragraph 9(b) for “a notified” substitute “
an approved
”
;
c
in paragraph 11(c)—
i
for “harmonised” substitute “
designated
”
; and
ii
omit “the Directive and of”; and
d
in paragraph 17—
i
for “notified” substitute “
approved
”
; and
ii
for “under the Directive” substitute “
by the Secretary of State
”
.
Amendment to Schedule 6I22943
1
Schedule 6 is amended as follows.
2
In the shoulder reference for “Regulation 50” substitute “
Regulation 48
”
.
3
In the heading for “notified” substitute “
approved
”
.
4
For “a notified body”, in each place it occurs, substitute “
an approved body
”
.
5
For “the notified body”, in each place it occurs, substitute “
the approved body
”
.
6
In paragraph 5—
a
before “registration number”, in both places it occurs, insert “
UK
”
; and
b
in sub-paragraph (a), for the words from “Article 1” to “time to time)” substitute “
paragraph 5A
”
;
7
After paragraph 5 insert—
5A
1
Pyrotechnic articles must be labelled with a UK registration number comprising the following:
a
the four-digit identification number of the approved body that has issued a Type-examination certificate (Schedule 2A, Module B), certificate of conformity (Schedule 2A, Module G) or quality system approval (Schedule 2A, Module H), as applicable;
b
the category of the pyrotechnic article for which conformity is certified in abbreviated format, in upper case—
i
F1, F2, F3 or F4 for fireworks of category 1, 2, 3 and 4 respectively;
ii
T1 or T2 for theatrical pyrotechnic articles of category T1 and T2 respectively; and
iii
P1 or P2 for other pyrotechnic articles of category P1 and P2 respectively; and
c
the processing number used by the approved body for the pyrotechnic article.
2
The UK registration number must be structured as follows: ‘XXXX — YY — ZZZZ…’, where XXXX refers to sub-paragraph (1)(a), YY refers to sub-paragraph (1)(b) and ZZZZ… refers to sub-paragraph (1)(c).
7
In paragraph 6—
a
for “After 16th October 2016” substitute “From F166IP completion day”
;
b
in sub-paragraph (a) for the words from “the Annex” to “time to time)” substitute “
paragraph 6A
”
;
c
in sub-paragraph (b) for “notified” substitute “
approved
”
.
8
After para 6 insert—
6A
1
Entries made in the register from F167IP completion day must contain at least the following information:
a
UK registration number;
b
date of issue of Type-examination certificate (where the conformity assessment procedure set out in Part 1 of Schedule 2A (Module B) has been undertaken), certificate of conformity (where the conformity assessment procedure set out in Part 5 of Schedule 2A (Module G) has been undertaken) or quality system approval (where the conformity assessment procedure set out in Part 6 of Schedule 2A (Module H) has been undertaken) as applicable, and date of expiry where applicable;
c
manufacturer;
d
type of product (generic) and subtype, if applicable;
e
where applicable, which conformity assessment procedure has been undertaken from those set out in Part 2 of Schedule 2A (Module C2), Part 3 of Schedule 2A (Module D) and Part 4 of Schedule 2A (Module E); and
f
where applicable, the approved body that undertook the conformity assessment procedure described in sub-paragraph (e).
2
Sub-paragraphs (1)(e) and (1)(f) apply where the register is maintained by an approved body carrying out the conformity assessment procedure set out in Part 1 of Schedule 2A (Module B), except where the relevant information is not known to that approved body.
9
In paragraph 7, for “harmonised” substitute “
designated
”
.
10
In paragraph 14 for “notified under the Directive” substitute “
approved under these Regulations
”
.
11
In paragraph 15—
a
for “notified”, in the second place it occurs, substitute “
approved
”
; and
b
omit “under the Directive”.
SCHEDULE 20Amendment of the Electromagnetic Compatibility Regulations 2016
GeneralI9011
The Electromagnetic Compatibility Regulations 2016 are amended in accordance with paragraphs 2 to 40.
Amendment to regulation 2I2302
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “the “2006 Regulations”” insert—
“approved body” has the meaning given in regulation 43 (approved bodies);
F168d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
after the definition of “conformity assessment body” insert—
“conformity assessment procedure” means a procedure referred to in regulation 40;
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 10(1)(a) (declaration of conformity and UK marking);
“designated standard” has the meaning given to it in regulation 2A;
h
in the definition of “the Directive” at the end insert “
(as it has effect immediately before F169 IP completion day)
”
;
i
omit the definition of “EU declaration of conformity”;
j
omit the definition of “EU harmonisation legislation”;
k
omit the definition of “harmonised standard”;
l
for the definition of “importer” substitute—
F170“importer” means a person who—
a
is established in the United Kingdom and places apparatus from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places apparatus on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F171m
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
n
omit the definition of “national accreditation body”;
o
omit the definition of “notified body requirements”;
p
omit the definition of “Official Journal”;
F172q
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
r
in the definition of “put into service”, for “EU” substitute “
United Kingdom market
”
; and
s
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
In paragraph (3) for “aspects of public interest protection” to the end substitute “
the protections against electromagnetic disturbance referred to in these Regulations
”
.
4
Omit paragraphs (4) and (5).
Insertion of regulation 2AI2323
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to apparatus to which these Regulations apply.
9
The Secretary of State may by regulation amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 3I2334
Regulation 3 (application) is amended as follows—
a
in paragraph (2)(a) for the words from “Directive” to “applies” substitute “
the Radio Equipment Regulations 2017 apply
”
;
b
in paragraph (4) for the words from “Directive” to the end substitute “
the Measuring Instruments Regulations 2016
”
;
c
for “EU law” in the third place in which it occurs, substitute “
that enactment
”
; and
i
omit “rules of” in both places which it occurs;
ii
for “EU law” in the first two places in which it occurs substitute “
any enactment
”
;
iii
for “EU law” in the third place in which it occurs, substitute “
that enactment
”
; and
iv
for “the Directive” substitute “
these Regulations
”
in both places in which it occurs.
Amendment to regulation 4I2345
In regulation 4 (application of safety legislation) for “EU or national legislation” substitute “
any enactment
”
.
Amendment to regulation 9I2356
In regulation 9 (technical documentation and conformity assessment) in paragraph (b)(i) omit “EU-”.
Amendment to regulation 10I2367
In regulation 10 (EU declaration of conformity and CE marking)—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)—
i
for “an EU” substitute “
a
”
; and
ii
omit “(EU declaration of conformity)”;
c
in paragraph (1)(b) for “CE” substitute “
UK
”
in both places in which it occurs;
d
in paragraph (2), omit “EU”;
e
for paragraph (3) substitute—
3
Where apparatus is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment concerned by its title.
Amendment to regulation 11I2378
In regulation 11 (retention of technical documentation and EU declaration of conformity), and in the heading to that regulation, omit “EU”.
Amendment to regulation 12I2389
In regulation 12 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 13I23910
In regulation 13 (information identifying manufacturer), in paragraph (4) for “in a language which can be easily understood by end-users and the competent national authority in the member State in which it is to be made available to such end-users” substitute “
clear, legible and in easily understandable English
”
.
Amendment to regulation 14I24011
For regulation 14 (instructions and information) substitute—
Instructions and information14
When placing apparatus on the market, a manufacturer must ensure that the apparatus is accompanied by instructions and the information referred to in regulation 36 (information concerning the use of apparatus) which are clear, legible and in clearly understandable English.
Amendment to regulation 15I24112
In regulation 15 (manufacturer's duty to take action in respect of apparatus placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other member State in which the manufacturer has made the apparatus available on the market,”.
Amendment to regulation 18I24213
In regulation 18 (requirements that must be satisfied before an importer places apparatus on the market), at paragraph (1)(c)(i) for “CE” substitute “
UK
”
.
Amendment to regulation 20I24314
Regulation 20 (information identifying importer) is amended as follows—
a
in paragraph (1) omit the words from “or, where” to “accompanying the apparatus”;
b
after paragraph (1) insert—
1A
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) F174on the packaging of the apparatus or on the apparatus, or
b
before placing the apparatus on the market, the importer sets out the information referred to in paragraph (1) in a document accompanying the apparatus.
c
in paragraph (2) for “competent national authority in the member State in which it is to be made available” substitute “
enforcing authority
”
.
Amendment to regulation 21I24415
In regulation 21 (instructions and information)—
a
in paragraph (1) for “is in a language which can be easily understood by consumers and other end users in the member State in which the apparatus is to be made available” substitute “
are clear, legible and in easily understandable English
”
; and
b
omit paragraph (2).
Amendment to regulation 23I24516
In regulation 23 (importer's duty to take action in respect of apparatus placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent authorities of any member State in which the importer has made the apparatus available on the market”.
Amendment to regulation 24]I24617
In regulation 24 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation, omit “EU”.
Amendment to regulation 27I24718
In paragraph (1) of regulation 27 (making available on the market)—
a
in sub-paragraph (a)(i) for “CE” substitute “
UK
”
; and
b
in sub-paragraph (a)(iii) for “is in a language which can be easily understood by consumers and other end-users in the member State in which the apparatus is to be made available on the market” substitute “
are clear, legible and in easily understandable English
”
.
Amendment to regulation 30I24819
In regulation 30 (duty to take action in respect of apparatus placed on the market or made available on the market which is considered not to be in conformity) in paragraph (2) omit “and the competent authorities of any other member State in which the distributor has made the apparatus available on the market”.
Amendment to regulation 31I24920
In regulation 31 (provision of information and co-operation) in paragraph (3)(b) for “in a language that can be easily understood by the enforcing authority” substitute “
clear, legible and in easily understandable English
”
.
Amendment to regulation 34I25021
Omit regulation 34 (translation of EU declaration of conformity).
Amendment to regulation 35I25122
In regulation 35 (prohibition on improper use of CE marking), and in the heading to that regulation, for “CE” in each place in which it occurs, substitute “
UK
”
.
Amendment to regulation 38I25223
In regulation 38 (appointment of an authorised representative)—
a
in paragraph (1) after “a person” insert “
established in the United Kingdom
”
;
b
in paragraph (2)(a) omit “EU”.
Insertion of regulation 38AI25324
After regulation 38 insert—
Obligations which are met by complying with obligations in the Directive38A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 3(25);
c
“harmonised standard” has the meaning given to it in Article 3(17).
2
Paragraph (3) applies where, before placing apparatus on the market, the manufacturer—
a
ensures that the apparatus has been designed and manufactured in accordance with the essential requirements set out in Annex I;
b
draws up the technical documentation relating to such apparatus referred to in Annex III;
c
ensures that the relevant conformity assessment procedure relating to such apparatus referred to in Article 14 has been carried out;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedure are prepared in or translated into English;
e
affixes a CE marking, in accordance with Articles 16 and 17(1) to (2);
f
draws up an EU declaration of conformity, in accordance with Article 15; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 8, 9, 10(1)(a) and (b) and (3) and 42(1) are to be treated as being satisfied;
b
regulations 2(2)(a), 10(2), 11, 12, 38(2) and 35 apply subject to the modifications in paragraph (8);
c
Part 4 does not apply; and
d
regulation 59 does not apply.
4
Paragraph (5) applies where, before placing a category apparatus on the market, the importer ensures that—
a
the relevant conformity assessment procedure referred to in Article 14 has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III; and
c
the apparatus bears the CE marking.
5
Where this paragraph applies—
a
the requirements of regulation 18(a) to (c) are to be treated as being satisfied; and
b
regulations 2(2)(a), 17, 19(1), 22 and 24 apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making apparatus available on the market, a distributor ensures that the apparatus bears the CE marking.
7
Where this paragraph applies—
a
regulation 27(1)(a) is to be treated as being satisfied; and
b
regulations 2(2)(a), 28(1) and 29 apply subject to the modifications in paragraph (10).
8
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential requirements” is to be read as a reference to the essential safety requirements referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 14;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III.
F177Expiry of regulation 38A38B
1
Subject to paragraph (2), regulation 38A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 38A—
a
any apparatus which was placed on the market pursuant to regulation 38A may continue to be made available on the market on or after the expiry of regulation 38A;
b
any obligation to which a person was subject under regulation 38A in respect of apparatus placed on the market pursuant to regulation 38A continues to have effect after the expiry of regulation 38A, in respect of that apparatus.
Qualifying Northern Ireland Goods38C
1
Where paragraph (2) applies, apparatus is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
apparatus—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the apparatus on the market, the importer—
a
complies with regulation 20;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the apparatus;
ii
the manufacturer has drawn up the technical documentation; and
iii
the apparatus bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“technical documentation” means the documentation a manufacturer must draw up, in accordance with regulation 9(b), as it applies in Northern Ireland.
Amendment to regulation 39I25425
In regulation 39 (presumption of conformity), paragraph (1) is amended as follows—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal”.
Amendment to regulation 41I25526
Regulation 41 (EU declaration of conformity) is amended as follows—
a
in the heading to that regulation, for “EU declaration”, substitute “
Declaration
”
; and
b
in that regulation, omit “EU”.
Amendment to regulation 42I256F17827
In regulation 42—
a
in the heading and in paragraph (2) in both places in which it occurs for “CE” substitute “
UK
”
;
b
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly—
a
to the apparatus;
b
to its data plate; or
c
where paragraph (1A) applies, to—
i
a label affixed to the apparatus or its data plate; or
ii
to a document accompanying the apparatus.
c
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the apparatus or its data plate; or
b
a document accompanying the apparatus.
d
in paragraph (2) after “Where” insert “
paragraph (1A) does not apply and
”
.
Amendment to Part 4I25728
For Part 4, substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies43
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 44 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 47 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F179IP completion day notified to the European Commission and the member State of the European Union, in accordance with Article 20 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 43(1)(b), as it had effect immediately before F179IP completion day;
“approved body requirements” means the requirements set out in Schedule 5.
Approval of conformity assessment bodies44
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of–
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of apparatus in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies45
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring46
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 44(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval47
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 46(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 43 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 46(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 43.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies48
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 44; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 6.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body to issue a Type-examination certificate referred to in Schedule 3.
Subsidiaries and contractors49
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M57.
Register of approved bodies50
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body notification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body51
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 46; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 50.
Amendment to regulation 55I25829
In regulation 55 (exercise of enforcement powers) omit paragraph (c).
Amendment to regulation 57I25930
Regulation 57 (enforcement action in respect of apparatus that is not in conformity and which present a risk) is amended as follows—
a
in paragraph (2) for “notified” substitute “
approved
”
;
b
omit paragraphs (4) and (7);
c
in paragraph (8) for “notices in paragraphs (6) and (7)”, substitute “
notice in paragraph (6)
”
; and
d
in paragraph (8)(f)(ii) for “harmonised” substitute “
designated
”
.
Amendment to regulation 58I26031
Omit regulation 58 (EU safeguard procedure).
Amendment to regulation 59I26132
Regulation 59 (enforcement action in respect of formal non-compliance) is amended as follows—
a
in paragraph (1)(a) for “CE” substitute “
UK
”
in each place in which it occurs;
b
in paragraph (1)(b)—
i
omit “EU” in each place in which it occurs;
ii
for “CE” substitute “
UK
”
.
Transitional provision in relation to EU ExitI23133
After regulation 74 (transitional provision) insert—
Transitional provision in relation to EU Exit74
1
In this regulation—
“pre-exit period” means the period beginning with 8th December 2016 and ending immediately before F181IP completion day;
“product” means electromagnetic equipment to which these Regulations apply.
2
Subject to paragraph (3) where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 20 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M58, any obligation to which a person was subject under these Regulations as they had effect immediately before F181IP completion day, continues to have effect as it did immediately before F181IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation to any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 40 as it had effect immediately before F181IP completion day;
that action has effect as if it had been done under regulation 40 as it has effect on and after F181IP completion day.
Amendment to regulation 75I26234
1
Regulation 75 (revocation and savings) is amended as follows—
2
In paragraph (2) before “as if” insert “
subject to the modifications in paragraph (2A),
”
3
After paragraph (2), insert—
2A
The modifications referred to in paragraph (2) are that in the Electromagnetic Compatibility Regulations 2006—
a
any reference to “the Community” is to be read as including the United Kingdom;
b
any reference to a “member State” is to be read as including the United Kingdom.
Amendment to Schedule 2I26335
Schedule 2 (Module A internal production control) is amended as follows—
a
in paragraph 5(d) —
i
for “harmonised” substitute “
designated
”
in each place in which it occurs;
ii
omit “the references of which have been published in the Official Journal”;
b
in the heading to paragraph 7—
i
for “CE” substitute “
UK
”
;
ii
omit “EU”;
c
in paragraph 7, for “CE” substitute “
UK
”
; and
d
in paragraph 8, omit “EU” in both places in which it occurs.
Amendment to Schedule 3I26436
Part 1 of Schedule 3 (applicable conformity assessment procedures Module B: EU-type Examination) is amended as follows—
a
in the heading, for “EU-Type” substitute “
Type
”
;
b
in paragraphs 1, 2, 3, 8, 9, 10, 12, 13, 14, 15 and 16 for “EU-type” substitute “
Type
”
in each place in which it occurs;
c
in paragraphs 1 and 14 for “a notified” substitute “
an approved
”
;
d
in paragraphs 3, 5, 6, 7, 10, 11, 12,13, 14 and 15 for “notified” substitute “
approved
”
in each place in which it occurs;
e
in paragraph 4(d)—
i
for “harmonised” substitute “
designated
”
in each place in which it occurs;
ii
omit “the references of which have been published in the Official Journal”;
f
in paragraph 7 for “an EU-type”, substitute “
a Type
”
;
g
in paragraph 15 for “The Commission, the Member States” substitute “
The Secretary of State
”
;
h
in the heading to paragraph 20—
i
for “CE” substitute “
UK
”
;
ii
omit “EU”;
i
in paragraph 20(1) for “CE” substitute “
UK
”
; and
j
in paragraphs 20(2) and (3) omit “EU” in each place in which it occurs.
I26537
Part 2 of Schedule 3 (applicable conformity assessment procedures) Module C (conformity to type based on internal production control) is amended as follows—
a
in paragraphs 18 and 19 for “EU-type” substitute “
Type
”
;
b
in the heading to paragraph 20—
i
for “CE” substitute “
UK
”
;
ii
omit “EU”;
c
in paragraph 20(1)—
i
for “CE” substitute “
UK
”
;
ii
for “EU-type” substitute “
Type
”
;
d
in paragraphs 20(2) and (3) omit “EU” in each place in which it occurs.
Amendment to Schedule 4I26638
Schedule 4 (EU declaration of conformity) is amended as follows—
a
in the heading, for “EU declaration” substitute “
Declaration
”
;
b
in the sub-heading, for “EU declaration” substitute “
Declaration
”
;
c
in paragraph 5, for “EU harmonisation legislation” substitute “
statutory requirements
”
;
d
in paragraph 6, for “harmonised” substitute “
designated
”
; and
e
in paragraph 7, for “notified” substitute “
approved
”
.
Amendment to Schedule 5I26739
Schedule 5 (requirements for notified bodies) is amended as follows—
a
in the heading, and in paragraphs 6, 9, 12 and 18, for “notified” substitute “
approved
”
;
b
in paragraph 10(c) for “a notified” substitute “
an approved
”
;
c
in paragraph 12(c)—
i
for “harmonised” substitute “
designated
”
;
ii
omit “of the Directive and”;
d
in paragraph 18 for “under the Directive” substitute “
by the Secretary of State
”
.
Amendment to Schedule 6I26840
Schedule 6 (operational obligations of notified bodies) is amended as follows—
a
in the heading, and in paragraphs 7 and 9 for “notified” substitute “
approved
”
;
b
in paragraphs 1, 2, 5, 6, 8, 10, 11, 12 and 13 for “a notified” substitute “
an approved
”
;
c
in paragraph 5, for “harmonised” substitute “
designated
”
;
d
in paragraph 12, for “notified under the Directive” substitute “
approved under these Regulations
”
;
e
in paragraph 13—
i
for “under the Directive” substitute “
by the Secretary of State
”
; and
ii
for “any notified” substitute “
any approved
”
.
SCHEDULE 21Amendment of the Simple Pressure Vessels (Safety) Regulations 2016
IntroductionI9021
The Simple Pressure Vessels (Safety) Regulations 2016 are amended in accordance with paragraphs 2 to 42.
Amendment to regulation 2I2692
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (4)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “the 1987 Act” insert—
“approved body” has the meaning given to it in regulation 45 (approved bodies);
F182d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
after the definition of “conformity assessment procedure” insert—
“designated standard” has the meaning given to it in regulation 2A;
h
in the definition of “the Directive” at the end insert “
(as it has effect immediately before F183 IP completion day)
”
;
i
omit the definition of “harmonised standard”;
j
for the definition of “importer” substitute—
F184“importer” means a person who—
a
is established in the United Kingdom and places a vessel from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a vessel on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F185k
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
l
omit the definition of “national accreditation body”;
m
omit the definition of “notified body requirements”;
n
omit the definition of “Official Journal”;
F186o
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
p
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
Omit paragraphs (6) and (7).
Insertion of regulation 2AI2713
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a vessel to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 4I2724
In regulation 4, in paragraph (2) (design and manufacture in accordance with essential safety requirements and sound engineering practice), for “the sound engineering practice of a Member State” substitute “
sound engineering practice
”
.
Amendment to regulation 6I2735
1
Regulation 6 (EU declaration of conformity, CE marking and inscriptions for category A vessels) is amended as follows.
2
In the heading to that regulation—
a
for “EU declaration” substitute “
Declaration
”
; and
b
for “CE” substitute “
UK
”
.
3
In paragraph (1)(a)—
a
for “an EU” substitute “
a
”
; and
b
omit “(EU declaration of conformity)”.
F1884
For paragraph (1)(b) substitute—
b
affix the information set out in paragraph (1B) to—
i
the vessel;
ii
its data plate; or
iii
where paragraph (1A) applies—
aa
to a label affixed to the vessel; or
bb
in a document accompanying the vessel;
F1874A
After paragraph (1) insert—
1A
This paragraph applies to a vessel that is placed on the market within a period of 24 months beginning with IP completion day.
1B
The information referred to in paragraph (1)(b) is—
a
the UK marking;
b
the last two digits of the year in which the UK marking is affixed;
c
the inscriptions.
5
In paragraph (4), omit “EU”.
6
For paragraph (5) substitute—
5
Where a category A vessel is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 8I2746
In regulation 8 (retention by manufacturers of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 9I2757
In regulation 9 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 11I2768
In regulation 11 (labelling of vessels) omit paragraph (3).
Amendment to regulation 12I2779
For regulation 12 (provision of instructions and safety information), substitute—
Provision of instructions and safety information12
When placing a vessel on the market, a manufacturer must ensure that a vessel is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 13I27810
In regulation 13 (duty of manufacturers to take action), in paragraph (2) omit “and the competent national authorities of any other Member State in which the manufacturer made the vessel available on the market,”.
Amendment to regulation 15I27911
In regulation 15 (appointment of authorised representative) after “a person” insert “
established in the United Kingdom
”
.
Amendment to regulation 16I28012
In regulation 16 (obligations of authorised representative), in paragraph (2)(a) omit “EU”.
Amendment to regulation 18I281F18913
In regulation 18 for paragraph (c) substitute—
c
the UK marking and the inscriptions have been affixed in accordance with regulation 6(1)(b) to (d);
Amendment to regulation 20I28214
In regulation 20 (requirements which must be satisfied before an importer places a category B vessel on the market), in paragraph (a) for “the sound engineering practice in a Member State” substitute “
sound engineering practice
”
.
Amendment to regulation 21I28315
In regulation 21 (information identifying importer)—
a
in paragraph (1) omit the words from “or, where” to “vessel”;
b
after paragraph (1) insert—
1A
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the vessel, or
b
before placing the vessel on the market, the importer sets out the information referred to in paragraph (1) in a document accompanying the vessel.
c
in paragraph (2) for “competent national authority in the Member State in which it is to be made available to such end-users” substitute “
enforcing authority
”
.
Amendment to regulation 22I28416
For regulation 22 (instructions and safety information) substitute—
Instructions and safety information22
When placing a vessel on the market, an importer must ensure that the vessel is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 25I28517
In regulation 25 (duty of importer to take action in respect of vessels placed on the market which are considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other Member State in which the importer made the vessel available on the market,”.
Amendment to regulation 26I28618
In regulation 26 (retention by importer of technical documentation and EU declaration of conformity), in the heading to that regulation and in paragraph (a), omit “EU”.
Amendment to regulation 29I28719
Regulation 29 (requirements which must be satisfied before a distributor makes available on the market a category A vessel), paragraph (a) is amended as follows—
a
in subparagraph (i) for “CE” substitute “
UK
”
; and
b
for subparagraph (ii) substitute—
ii
is accompanied by instructions and safety information that are clear, legible and in easily understandable English;
Amendment to regulation 31I28820
In regulation 31 (requirements which must be satisfied before a distributor makes a category B vessel available on the market)—
a
for paragraph (1)(a)(ii) substitute—
ii
is accompanied by instructions and safety information that are clear, legible and in easily understandable English;
b
omit paragraph (2).
Amendment to regulation 33I28921
In regulation 33 (duty for distributor to take action), in paragraph (2) omit “and the competent national authorities of any other Member States in which the distributor made the vessel available on the market”.
Omission of regulation 36I29022
Omit regulation 36 (translation of EU declaration of conformity).
Amendment to regulation 38I29123
In regulation 38 (improper use of CE marking) in the heading and in each place in which it occurs, for “CE” substitute “
UK
”
.
Insertion of regulations 38A and 38BI29224
After regulation 38 insert—
Obligations which are met by complying with obligations in the Directive38A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(17);
c
“harmonised standard” has the meaning given to it in Article 2(9).
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing a category A vessel on the market, the manufacturer—
a
ensures that the vessel has been designed and manufactured in accordance with the essential safety requirements set out in Annex I;
b
ensures that the relevant conformity assessment procedures that apply to that vessel in accordance with Article 13(1) and (2) have been carried out;
c
draws up the technical documentation referred to in Annex II;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the inscriptions, in accordance with Articles 15 and 16(1) to (4);
f
draws up an EU declaration of conformity, in accordance with Article 14; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 4(1), 5, 6(1) to (3) and (5) are to be treated as being satisfied;
b
regulations 2(5)(a), 6(4), 8, 9(2), 16(2) and 38 apply subject to the modifications in paragraph (10);
c
Part 3 does not apply; and
d
regulation 62 does not apply.
4
Subject to paragraphs (6) and (7), paragraph (5) applies where, before placing a category A vessel on the market, the importer ensures that—
a
the relevant conformity assessment procedures that apply to that vessel in accordance with Article 13(1) and (2) have been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex II; and
c
the vessel bears the CE marking and inscriptions referred to in point 1 of Annex III.
5
Where this paragraph applies—
a
the requirements of regulation 18(a) to (c) are to be treated as being satisfied; and
b
regulations 2(5)(a), 19(1), 23 and 26 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 12.
7
Where paragraph (6) applies, paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to have carried out—
a
the conformity assessment procedure set out in Article 13(1)(b); and
b
the relevant conformity assessment procedure that applies to that product in accordance with Article 13(2).
8
Paragraph (9) applies where, before making a category A vessel available on the market, a distributor ensures that the vessel bears the CE marking and inscriptions referred to in point 1 of Annex III.
9
Where this paragraph applies—
a
regulation 29(a)(i) is to be treated as being satisfied; and
b
regulations 2(5)(a), 30(1) and 32 apply subject to the modifications in paragraph (10).
10
The modifications referred to in paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the conformity assessment procedures that apply to the vessel in accordance with Article 13(1) and (2);
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex II.
Conformity assessment procedure obligation which is met by complying with the Directive38B
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“harmonised standard” has the meaning given to it in Article 2(9).
2
Subject to paragraphs (4) and (5), paragraph (3) applies where, prior to the manufacture of a category A vessel, the manufacturer ensures that the conformity assessment procedure that applies to that vessel in accordance with Article 13(1) has been carried out.
3
Where this paragraph applies—
a
regulation 40 is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 5(b), 6(1), 18(a), 38(b) and 43(c) is to be read as including the conformity assessment procedure referred to in Article 13(1); and
c
any reference to “technical documentation” in regulations 5(a), 8, 18(b) and 26(b) is to be read as including the technical documentation relating to the design of the vessel referred to in Annex II.
4
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 12.
5
Where paragraph (4) applies, paragraph (2) is to be treated as requiring the manufacturer to have carried out the conformity assessment procedure set out in Article 13(1)(b).
F193Expiry of regulations 38A and 38B38C
1
Subject to paragraph (2), regulation 38A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 38A—
a
any vessel which was placed on the market pursuant to regulation 38A may continue to be made available on the market after the expiry of regulation 38A;
b
any obligation to which a person was subject under regulation 38A in respect of a vessel placed on the market pursuant to regulation 38A continues to have effect after the expiry of regulation 38A, in respect of that vessel.
3
Subject to paragraph (4), regulation 38B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 38B in relation to a vessel prior to the expiry of regulation 38B, regulation 38B continues to apply in respect of that vessel where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after an EU-Type examination has been carried out in accordance with a conformity assessment procedure set out in point 1 of Annex II of the Directive.
Qualifying Northern Ireland Goods38D
1
Where paragraph (2) applies, a vessel is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
a vessel—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the vessel on the market, the importer—
a
complies with regulation 21;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the vessel, in accordance with Part 3, as that Part applies in Northern Ireland;
ii
the manufacturer has drawn up the technical documentation; and
iii
the vessel bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” means the documentation referred to in paragraph 2(2)(c) of Part 1 of Schedule 2 to these Regulations, as that Schedule applies in Northern Ireland.
Amendment to regulation 39I29325
In regulation 39 (presumption of conformity of category A vessels), in paragraph (1)—
a
for “harmonised” substitute “
designated
”
;
b
omit “the reference to which has been published in the Official Journal”.
Amendment to regulation 40I29426
In regulation 40 (conformity assessment procedures prior to manufacturer)—
a
in paragraphs (1) and (4) for “an EU-type”, substitute “
a Type
”
;
b
in paragraphs (2) and (3) for “harmonised” substitute “
designated
”
.
Amendment to regulation 42I29527
In regulation 42 (records and correspondence language requirements)—
a
for “an official language of the Member State in which the notified body is established” substitute “
English
”
;
b
for “that body” substitute “
the approved body
”
.
Amendment to regulation 43I29628
In regulation 43 (EU declaration of conformity)—
a
in the heading for “EU declaration” substitute “
Declaration
”
;
b
omit “EU”.
Amendment to regulation 44I29729
In regulation 44 (identification number)—
F194a
for paragraph (1) substitute—
1
The UK marking must be followed by the identification number of the approved body involved in the relevant conformity assessment procedure pursuant to regulation 41.
b
in paragraph (2) for “notified” in each place it in which it occurs, substitute “
approved
”
.
Amendment to Part 4I29830
For Part 4, substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies45
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 46 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 49 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F195IP completion day notified to the European Commission and the member States of the European Union in accordance with Article 17 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 45(1)(b), as it had effect immediately beforeF195IP completion day;
“approved body requirements” means the requirements set out in Part 1 of Schedule 4.
Approval of conformity assessment bodies46
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of vessels in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies47
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring48
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 46(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval49
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 48(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 45 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 48(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 45.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies50
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 46; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Part 2 of Schedule 4.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Schedule 2; or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 44 (identification number).
Subsidiaries and contractors51
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M59.
Register of approved bodies52
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body53
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 48; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 52.
Amendment to regulation 57I29931
In regulation 57 omit paragraph (c).
Amendment to regulation 59I30032
Regulation 59 (enforcement action in respect of vessels which are not in conformity) is amended as follows—
a
in paragraph (2) for “notified” substitute “
approved
”
;
b
omit paragraphs (4) and (7);
c
in paragraph (8) for “notices in paragraphs (6) and (7), substitute “
notice in paragraph (6)
”
;
d
in paragraph (8)(f)(ii) for “harmonised” substitute “
designated
”
; and
e
in paragraph (10) omit “throughout the EU”.
Omission of regulation 60I30133
Omit regulation 60 (EU safeguard procedure).
Amendment to regulation 61I30234
In regulation 61 (enforcement action in respect of vessels which are in conformity, but which present a risk)—
a
omit paragraph (3); and
b
in paragraph (4) for “notices referred to in paragraphs (2) and (3)” substitute “
notice referred to in paragraph (2)
”
.
Amendment to regulation 62I30335
Regulation 62 (enforcement action in respect of formal non-compliance) is amended as follows—
a
in paragraphs (1)(a)(i), (1)(a)(ii), (1)(a)(iv) and (1)(b)(i) for “CE” substitute “
UK
”
in each place in which it occurs;
b
in paragraphs (1)(a)(ii), (1)(a)(iv), (1)(b)(i) omit “EU” in each place in which it occurs;
c
in paragraph (1)(a)(iii) for “a notified” substitute “
an approved
”
;
d
in paragraphs (1)(a)(iii)(aa) and (bb) for “notified” substitute “
approved
”
;
e
in paragraph (4) omit “or 60(3) (the EU safeguard procedure)”.
Amendment to regulation 76I30436
In regulation 76 (transitional provisions) in paragraph (1) for “another” substitute “
a
”
.
Transitional provision in relation to EU ExitI27037
After regulation 76 insert—
Transitional provision in relation to EU Exit76A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F197IP completion day;
“product” means a vessel to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 21 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M60, any obligation to which a person was subject under these Regulations as they had effect immediately before F197IP completion day, continues to have effect as it did immediately before F197IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulations 40 or 41 as they had effect immediately before F197IP completion day in relation to that product
that action has effect as if it had been done under regulations 40 or 41 as they have effect on and after F197IP completion day.
Amendment to regulation 77I30538
1
Regulation 77 (revocations and savings) is amended as follows.
2
In paragraph (2) before “as if” insert “
subject to the modifications in paragraph (2A).
”
.
3
After paragraph (2) insert—
2A
The modifications referred to in paragraph (2) are as follows—
a
in the 1991 Regulations—
i
any reference to “the Community” is to be read as including the United Kingdom;
ii
any reference to “member State” is to be read as including the United Kingdom;
iii
in regulation 11 (EC type-examination certificate)—
aa
in paragraph (6)(a), for “the Commission, any other approved body or any other member State” substitute
“ or any other United Kingdom approved body ”;bb
in paragraph (8), omit “, with a view to this information being passed by him to the Commission and the other member States”;
iv
in regulation 17 (Functions of approved bodies in course of EC Surveillance)—
aa
paragraph (2) is to be read as if “or elsewhere” were omitted;
bb
paragraph (3) is to be read as if “, the Commission, any other approved body or any other member State” were omitted;
v
paragraph 6 of Schedule 5 (enforcement), is to be read as if “, with a view to this information being passed by him to the Commission” were omitted;
b
in the Simple Pressure Vessels (Safety) (Amendment) Regulations 1994, in regulation 5 (transitional provisions in respect of marking arrangements)—
i
any reference to “the Community” is to be read as including the United Kingdom; and
ii
paragraph (3) is to be read as if the following paragraph were substituted—
3
In the event of such election, to demonstrate compliance with the marking arrangements in force on 31 December 1994, the manufacturer or his authorised representative established in the Community, shall apply the principal Regulations (as amended by these Regulations)—
a
as if the amendments made by regulation 4(f) and (k) had not come into force; and
b
read subject to the modifications made by regulation 77(2A)(a) of the Simple Pressure Vessels (Safety) Regulations 2016 M61.
Amendment to Schedule 1I30639
In Schedule 1, in paragraph 21(2) for “a notified” in each place in which it occurs substitute “
an approved
”
.
Amendment to Schedule 2I30740
Schedule 2 (conformity assessment procedures) is amended as follows—
a
in the heading, omit “EU-”;
b
in paragraphs 1, 7(1), 8(3) to (5), 11(2) and 15(2) for “a notified” substitute “
an approved
”
;
c
in all places in which it occurs (other than those referred to in sub-paragraph (b)) for “notified” substitute “
approved
”
;
d
in paragraph 6(1) and (3) for “an EU-Type” substitute “
a Type
”
;
e
in all places in which it occurs (other than the paragraphs referred to in sub-paragraph (d)) for “EU-Type” substitute “
Type
”
;
f
in paragraphs 2(2)(e)(i), 3(1)(c)(iv) and (v) and 16(2) for “harmonised” substitute “
designated
”
;
g
in paragraph 3(c)(iv) omit “, the references to which have been published in the Official Journal”;
h
in paragraph 8(3) for “Commission, the member States” substitute “
Secretary of State
”
;
i
in paragraph 8(4), for “Commission and the member States” substitute “
Secretary of State
”
;
j
in paragraph 12(3) for “Member State in which the test is performed” substitute “
Secretary of State
”
;
k
in paragraphs 12(9) and 16(6) omit “other Member States and the Commission,”;
l
for the headings to paragraphs 13, 17 and 20 substitute— “
UK marking and declaration of conformity
”
;
m
in paragraphs 13, 17 and 20—
i
for “CE” substitute “
UK
”
; and
ii
for “EU declaration” substitute “
declaration
”
in each place in which it occurs; and
n
in paragraph 18 for “the Directive” substitute “
these Regulations
”
.
Amendment to Schedule 3I30841
Schedule 3 (EU Declaration of conformity) is amended as follows—
a
omit “EU” in each place in which it occurs;
b
for “declaration” in the first place in which it occurs substitute “
Declaration
”
;
c
in paragraph 5, for “Union harmonisation legislation” substitute “
statutory requirements
”
;
d
in paragraph 6, for “harmonised” substitute “
designated
”
; and
e
in paragraph 7 for “notified” substitute “
approved
”
.
Amendments to Schedule 4I30942
Schedule 4 (notified bodies) is amended as follows—
a
in paragraphs 8(b), 17, 18, 19, 20, 21, 24, 26, 27, 28 and 29 for “a notified” substitute “
an approved
”
;
b
in all places in which it occurs (other than the paragraphs referred to in subparagraph (a)) for “notified” substitute “
approved
”
;
c
in paragraph 10(c)–—
i
for “harmonised” substitute “
designated
”
; and
ii
omit “the relevant Directives and of”;
d
in paragraphs 16 and 29 for “under the Directive” substitute “
by the Secretary of State
”
;
e
in paragraph 21 for “harmonised” substitute “
designated
”
; and
f
in paragraph 26(b) omit “(notification)”.
SCHEDULE 22Amendment of the Lifts Regulations 2016
IntroductionI9031
The Lifts Regulations 2016 are amended in accordance with paragraphs 2 to 44.
Amendment to regulation 2I3102
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of the “1997 Regulations” insert—
“approved body” has the meaning given to it in regulation 51 (approved bodies);
F198d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
after the definition of “conformity assessment body” insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with—
a
in relation to lifts, regulation 8(1)(a) (declaration of conformity and UK marking: installer); and
b
in relation to safety components for lifts, regulation 17(1)(a) (declaration of conformity and UK marking: manufacturer);
h
after the definition of the “Department” insert—
“designated standard” has the meaning given to it in regulation 2A;
i
in the definition of “the Directive” at the end insert “
(as it has effect immediately before F199IP completion day
”
);
j
omit the definition of “European Commission”;
k
omit the definition of “EU declaration of conformity”;
l
omit the definition of “harmonised standard”;
m
for definition of “importer” substitute—
F200“importer” means a person who—
a
is established in the United Kingdom and places a safety component for lifts from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a safety component for lifts on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F201n
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
o
omit the definition of “national accreditation body”;
p
omit the definition of “notified body requirements”;
q
omit the definition of “Official Journal”;
F202r
in the definition of “place on the market” for “EU market” substitute “market of Great Britain” in both places in which it occurs;
s
in the definition of “safety component for lifts” omit the words after “Schedule 3”;
t
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
After paragraph (1) insert—
1A
Schedules 11 to 19 reproduce the provisions of Annexes IV to XII to the Directive (respectively) with amendments to correct deficiencies in retained EU law.
1B
A reference to any provision of Schedules 11 to 19 is a reference to the equivalent provision of the relevant Annex to the Directive as set out in the relevant Schedule.
4
Omit paragraphs (3) and (5).
Insertion of regulation 2AI3123
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a lift or a safety component for lifts to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 5I3134
In regulation 5 (lifts where risks are wholly or partly covered by other EU law) and in the heading to that regulation for “EU law” substitute “
enactments
”
.
Amendment to regulation 7I3145
In regulation 7 (technical documentation and conformity assessment)—
a
in paragraph (b)(i) for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
;
b
in paragraph (b)(ii) for “Annex XI to the Directive (as amended from time to time)” substitute “
Schedule 18
”
;
c
in paragraph (b)(iii) for “Annex VIII to the Directive (as amended from time to time)” substitute “
Schedule 15
”
.
Amendment to regulation 8I3156
1
Regulation 8 (EU declaration of conformity and CE marking) is amended as follows.
2
In the heading to that regulation—
a
for “EU declaration” substitute “
Declaration
”
; and
b
for “CE” substitute “
UK
”
.
3
In paragraph (1)—
a
in sub-paragraph (a) omit “EU”; and
b
in sub-paragraph (c) for “CE”, in both places it occurs, substitute “
UK
”
.
4
In paragraph (2) omit “EU”.
5
For paragraph (3) substitute—
3
Where a lift is subject to more than one enactment requiring the drawing up of a declaration of conformity, the installer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 9I3167
In regulation 9 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 10I3178
In regulation 10 (labelling and instructions)—
a
for paragraph (2) substitute—
2
the information referred to in paragraph (1) must be clear, legible and in easily understandable English.
b
omit paragraph (3).
Amendment to regulation 12I3189
In regulation 12 (duty to take action in respect of lifts placed on the market which are considered not to be in conformity) in paragraph (2), omit the words from “, and” to “market,”.
Amendment to regulation 16I31910
In regulation 16 (technical documentation and conformity assessment) in paragraph (b)—
a
in sub-paragraph (i) for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
; and
b
in sub-paragraph (ii) for “Annex VII to the Directive (as amended from time to time)” substitute “
Schedule 14
”
.
Amendment to regulation 17I32011
1
Regulation 17 (EU declaration of conformity and CE marking) is amended as follows.
2
In the heading to that regulation—
a
for “EU declaration” substitute “
Declaration
”
; and
b
for “CE” substitute “
UK
”
.
3
In paragraph (1)—
a
in sub-paragraph (a) omit “EU”; and
b
in sub-paragraph (c) for “CE”, in both places it occurs, substitute “
UK
”
.
4
In paragraph (2) omit “EU”.
5
For paragraph (3) substitute—
3
Where a safety component for lifts is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 18I32112
In regulation 18 (retention of technical documents and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 19I32213
In regulation 19 (labelling and instructions)—
a
for paragraph (2) substitute—
2
The information referred to in paragraph (1) must be clear, legible and in easily understandable English.
b
omit paragraph (4).
Amendment to regulation 20I32314
In regulation 20 (compliance procedures for series production) in paragraph 2(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 22I32415
In regulation 22 (duty to take action in respect of safety components for lifts placed on the market which are considered not to be in conformity) in paragraph (2) omit the words from “, and” to “market,”.
Amendment to regulation 24I32516
In regulation 24 (appointment of authorised representatives)—
a
in paragraph (1) after “a person” insert “
established in the United Kingdom
”
;
b
omit “EU” in each place it occurs; and
c
for “CE” substitute “
UK
”
in both places it occurs.
Amendment to regulation 26I32617
In regulation 26 (requirements which must be satisfied before an importer places a safety component for lifts on the market) in paragraph (1)(c)—
a
in paragraph (i) for “CE” substitute “
UK
”
; and
b
in paragraph (ii), omit “EU”.
Amendment to regulation 28I32718
In regulation 28 (information identifying importer)—
a
in paragraph (2) for “competent national authority” to the end substitute “
market surveillance authority
”
;
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information specified in paragraph (1) on the safety component for lifts; or
b
before placing the safety component for lifts on the market, the importer sets out the information specified in paragraph (1)—
i
on the packaging; or
ii
in a document accompanying the safety component for lifts.
Amendment to regulation 29I32819
In regulation 29 (instructions)—
a
for paragraph (1) substitute—
1
When placing a safety component for lifts on the market, an importer must ensure that it is accompanied by the instructions referred to in paragraph 7 of Schedule 1 and that they are clear, legible and in easily understandable English.
b
omit paragraph (2).
Amendment to regulation 33I32920
In regulation 33 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 37I33021
In regulation 37 (requirements which must be satisfied before a distributor makes a safety component for lifts available on the market)—
a
in paragraph (1)(a)—
i
in paragraph (i) for “CE” substitute “
UK
”
;
ii
in paragraph (ii) omit “EU”; and
iii
for paragraph (iii) substitute—
iii
is accompanied by the instructions referred to in paragraph 7 of Schedule 1 and that they are clear, legible and in easily understandable English;
b
omit paragraph (2).
Amendment to regulation 40I33122
In regulation 40 (duty to take action in respect of safety components for lifts made available on the market which are not in conformity with Part 2) in paragraph (2) omit the words from “, and” to “market,”.
Amendment to regulation 43I33223
Omit regulation 43 (translation of declaration of conformity).
Amendment to regulation 45I33324
In regulation 45 (prohibition on improper use of CE marking), in each place it occurs and in the heading to that regulation, for “CE” substitute “
UK
”
.
Insertion of regulations 45A and 45BI33425
After regulation 45 insert—
Obligations which are met by complying with obligations in the Directive45A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article of or an Annex to the Directive;
b
“CE marking” has the meaning given to it in Article 2(21);
c
“harmonised standard” has the meaning given to it in Article 2(13).
2
Paragraph (3) applies where, before placing a lift on the market, the installer—
a
ensures that the lift has been designed, manufactured, installed and tested in accordance with the essential health and safety requirements set out in Annex I;
b
ensures that the conformity assessment procedure that applies to that lift in accordance with Article 16 has been carried out;
c
draws up the technical documentation referred to in Article 7(2);
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and other markings, in accordance with Articles 18 and 19(1) to (5);
f
draws up an EU declaration of conformity, in accordance with Article 17; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 6, 7, 8(1) and 8(3) are to be treated as being satisfied;
b
regulations 2(2)(a), 8(2), 9, 24(2), 24(3) and 45 apply subject to the modifications in paragraph (10);
c
Part 3 does not apply; and
d
regulation 68 does not apply.
4
Paragraph (5) applies where, before placing a safety component for lifts on the market, the manufacturer—
a
ensures that the safety component has been designed and manufactured in accordance with Article 5(2);
b
ensures that the conformity assessment procedure that applies to that safety component in accordance with Article 15 has been carried out;
c
ensures that the relevant technical documentation referred to in Article 8(2) is drawn up;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and other markings, in accordance with Articles 18 and 19(1) to (5);
f
draws up an EU declaration of conformity, in accordance with Article 17; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
5
Where this paragraph applies—
a
the requirements of regulations 15, 16, 17(1) and (17)(3) are to be treated as being satisfied;
b
regulations 2(2)(a), 17(2), 18, 20(2), 24(2), 24(3) and 45 apply subject to the modifications in paragraph (10);
c
Part 3 does not apply; and
d
regulation 68 does not apply.
6
Paragraph (7) applies where, before placing a safety component for lifts on the market, the importer ensures that—
a
the conformity assessment procedure that applies to that lift in accordance with Article 15 has been carried out;
b
the manufacturer has drawn up the relevant technical documentation referred to in Article 8(2); and
c
the safety component for lifts—
i
bears the CE marking; and
ii
is accompanied by the EU declaration of conformity drawn up in accordance with Article 17.
7
Where this paragraph applies—
a
the requirements of regulation 26(1)(a) to (c)(i) are to be treated as being satisfied;
b
any requirement of regulation 26(1)(c)(ii), insofar as it relates to the declaration of conformity, is to be treated as being satisfied; and
c
regulations 2(2)(a), 27(1), 30 and 33 apply subject to the modifications in paragraph (10).
8
Paragraph (9) applies where, before making a safety component for lifts available on the market, a distributor ensures that the safety component for lifts—
a
bears the CE marking; and
b
is accompanied by an EU declaration of conformity drawn up in accordance with Article 17.
9
Where this paragraph applies—
a
the requirements of regulations 37(1)(a)(i) are to be treated as being satisfied;
b
any requirement of regulation 37(1)(a)(ii), insofar as it relates to the declaration of conformity, is to be treated as being satisfied; and
c
regulations 2(2)(a), 38(1) and 39 apply subject to the modifications in paragraph (10).
10
The modifications referred to in paragraphs (3)(b), (5)(b), (7)(c) and 9(c) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential health and safety requirements” is to be read as a reference to the essential health and safety requirements referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the conformity assessment procedure that applies to the lift or the safety component for the lift in accordance with Article 15 or Article 16, as the case may be;
f
any reference to “technical documentation” is a reference to the relevant technical documentation set out in Annexes IV to XII.
Conformity assessment procedure obligation which is met by complying with the Directive45B
1
In this regulation, any reference to an Article or an Annex is a reference to an Article of or an Annex to the Directive.
2
Paragraph (3) applies where, prior to the manufacture of a safety component, the manufacturer ensures that the conformity assessment procedure set out in Annex IV, Part A and referred to in Article 15(a) and (b) as EU-type examination, has been carried out in relation to a model of the safety component in accordance with Article 15(a) or (b).
3
Where this paragraph applies—
a
the requirement in regulation 48(a) or (b) to submit the model of the safety component for the conformity assessment procedure referred to in that regulation as Type examination is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 16(a), 17(1), 26(1)(a), 45(1)(b) and 49(b) is to be read as including the conformity assessment procedure referred to in Article 15(a) or (b) as EU-type examination; and;
c
any reference to “technical documentation” in regulations 16(b), 18, 26(1)(b) and 33(b) is to be read as including the technical documentation relating to the design of the safety component referred to in Annex IV, Part A.
4
Paragraph (5) applies where, a lift is designed and manufactured in accordance with a model lift that has undergone the conformity assessment procedure set out in Annex IV, Part B, referred to in Article 16(1)(a) as EU-type examination.
5
Where this paragraph applies—
a
the condition in regulation 47(1)(a) that the lift is designed and manufactured in accordance with a model lift which has undergone a Type examination set out in Part B of Schedule 11, is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 7(a), 8(1), 45(1)(b) and 49(b) is to be read as including the conformity assessment procedure set out in Annex IV, Part B and referred to in Article 16(1)(a) as EU-type examination; and
c
any reference to “technical documentation” in regulations 7(b) and 9 is to be read as including the technical documentation relating to the design of the lift referred to in Annex IV, Part B.
F206Expiry of regulations 45A and 45B45C
1
Subject to paragraph (2), regulation 45A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 38A—
a
any safety component for lifts which was placed on the market pursuant to regulation 45A may continue to be made available on the market on or after the expiry of regulation 45A;
b
any obligation to which a person was subject under regulation 45A in respect of a lift or safety component for lifts placed on the market pursuant to regulation 45A continues to have effect after the expiry of regulation 45A, in respect of that lift or safety component for lifts.
3
Subject to paragraph (4), regulation 45B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 45B in relation to a lift or a safety component for lifts prior to the expiry of regulation 45B, regulation 45B continues to apply in respect of that lift or safety component for lifts where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after—
a
in relation to a safety component for lifts, the conformity assessment procedure set out in Annex IV, Part A of the Directive and referred to in Article 15(a) and (b) of the Directive as EU-type examination, has been carried out in relation to a model of the safety component for lifts in accordance with Article 15(a) or (b) of the Directive; or
b
in relation to a lift that is designed and manufactured in accordance with a model, the conformity assessment procedure set out in Annex IV, Part B of the Directive, referred to in Article 16(1)(a) of the Directive as an EU-type examination has been carried out in relation to a model.
Qualifying Northern Ireland Goods45D
1
Where paragraph (2) applies a safety component for lifts is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
a safety component for lifts—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the safety component for lifts on the market, the importer—
a
complies with regulation 28;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in accordance with Part 3, as that Part applies in Northern Ireland;
ii
the manufacturer has drawn up the technical documentation; and
iii
the safety component bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” means the documentation a manufacturer must draw up in accordance with regulation 16(b), as it applies in Northern Ireland.
Amendment of regulation 46I33526
In paragraph (1) of regulation 46 (presumption of conformity)—
a
for “harmonised” substitute “
designated
”
; and
b
omit the words from “the reference” to “Journal”.
Amendment to regulation 47I33627
1
Regulation 47 (conformity assessment procedures for lifts) is amended as follows.
2
In paragraph (1)(a)—
a
for “EU-type” substitute “
Type
”
;
b
for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
;
c
in paragraph (i) for “Annex V to the Directive (as amended from time to time)” substitute “
Schedule 12
”
;
d
in paragraph (ii) for “Annex X to the Directive (as amended from time to time)” substitute “
Schedule 17
”
; and
e
in paragraph (iii) for “Annex XII to the Directive (as amended from time to time)” substitute “
Schedule 19
”
.
3
In paragraph (1)(b)—
a
for “Annex XI to the Directive (as amended from time to time)” substitute “
Schedule 18
”
;
b
in paragraph (i) for “Annex V to the Directive (as amended from time to time)” substitute “
Schedule 12
”
;
c
in paragraph (ii) for “Annex X to the Directive (as amended from time to time)” substitute “
Schedule 17
”
; and
d
in paragraph (iii) for “Annex XII to the Directive (as amended from time to time)” substitute “
Schedule 19
”
.
4
In paragraph (1)(c) for “Annex VIII to the Directive (as amended from time to time)” substitute “
Schedule 15
”
.
5
In paragraph (1)(d) for “Annex XI to the Directive (as amended from time to time)” substitute “
Schedule 18
”
.
Amendment to regulation 48I33728
Regulation 48 (conformity assessment procedures for safety components for lifts) is amended as follows—
a
for “EU type” substitute “
Type
”
in both places it occurs;
b
in paragraph (a)—
i
for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
; and
ii
for “Annex IX to the Directive (as amended from time to time)” substitute “
Schedule 16
”
;
c
in paragraph (b)—
i
for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
; and
ii
for “Annex VI to the Directive (as amended from time to time)” substitute “
Schedule 13
”
;
d
in paragraph (c) for “Annex VII to the Directive (as amended from time to time)” substitute “
Schedule 14
”
.
Amendment to regulation 49I33829
In regulation 49 (EU declaration of conformity)—
a
in the heading for “EU declaration” substitute “
Declaration
”
;
b
omit “EU” in both places it occurs; and
c
in paragraph (b) for “Annexes V to XII to the Directive (as amended from time to time)” substitute “
Schedules 12 to 19
”
.
Amendment to regulation 50I33930
1
Regulation 50 (CE marking) is amended as follows.
2
In the heading to that regulation for “CE” substitute “
UK
”
.
F2083
For paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly, and indelibly—
a
to the lift carrier;
b
to the safety component for lifts; or
c
where paragraph (1A) applies, to—
i
a label affixed to the lift carrier or the safety component; or
ii
to a document accompanying the lift or the safety component.
F2073A
After paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the lift carrier or the safety component; or
b
to a document accompanying the lift or the safety component.
3B
In the following paragraphs, for “CE” substitute “
UK
”
a
paragraph (2) (twice);
b
paragraph (3); and
c
paragraph (4).
3C
In paragraph (2) after “Where” insert “
paragraph (1A) does not apply and
”
;
3D
In paragraph (3) for “on a” substitute “
in respect of a
”
.
4
For “notified”, in each place it occurs, substitute “
approved
”
.
5
In paragraph (3)—
a
in sub-paragraph (a) for “Annex V to the Directive (as amended from time to time)” substitute “
Schedule 12
”
;
b
in sub-paragraph (b) for “Annex VIII to the Directive (as amended from time to time)” substitute “
Schedule 15
”
; and
c
in sub-paragraph (c) for “Annexes X, XI or XII to the Directive (as amended from time to time)” substitute “
Schedules 17, 18 or 19
”
.
6
In paragraph (4)—
F209ia
for “on a safety” substitute “
in respect of a safety
”
;
a
in sub-paragraph (a) for “Annex VI to the Directive (as amended from time to time)” substitute “
Schedule 13
”
;
b
in sub-paragraph (b) for “Annex VII to the Directive (as amended from time to time)” substitute “
Schedule 14
”
; and
c
in sub-paragraph (c) for “Annex IX to the Directive (as amended from time to time)” substitute “
Schedule 16
”
.
Amendment to Part 4I34031
For Part 4, substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies51
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 52 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 55 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F210IP completion day notified to the European Commission and the member State of the European Union, in accordance with Article 20 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 51(1)(b), as it had effect immediately before F210IP completion day; and
“approved body requirements” means the requirements set out in Schedule 4.
Approval of conformity assessment bodies52
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the relevant conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the product in respect of which the conformity assessment body claims to be competent, where “product” has the meaning given to it in Regulation 2A(8); and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies53
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring54
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 52(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval55
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 54(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 51 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 54(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 51.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies56
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 51; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 6.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type-examination certificate referred to in Schedule 11; or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 50.
Subsidiaries and contractors57
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M62.
Register of approved bodies58
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body notification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body59
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 54; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 58.
Amendment to regulation 63I34132
In regulation 63 (exercise of enforcement powers) omit paragraph (c).
Amendment to regulation 65I34233
In regulation 65 (enforcement action in respect of lifts and safety components for lifts which are not in conformity and which present a risk)—
a
in paragraph (2) for “notified” substitute “
approved
”
;
b
omit paragraphs (4) and (7);
c
in paragraph (8) for “paragraphs (6) and (7)” substitute “
paragraph (6)
”
.
Amendment to regulation 66I34334
Omit regulation 66 (EU safeguard procedure).
Amendment to regulation 67I34435
In regulation 67 (enforcement action in respect of lifts and safety components for lifts which are in conformity but present a risk)—
a
omit paragraph (3);
b
in paragraph (4) for “notices referred to paragraphs (2) and (3)” substitute “
notice referred to in paragraph (2)
”
.
Amendment to regulation 68I34536
Regulation 68 (enforcement action in respect of formal non-compliance) is amended as follows—
a
for “CE”, in each place it occurs, substitute “
UK
”
;
b
in paragraph (1)(b)—
i
for “a notified” substitute “
an approved
”
; and
ii
for “the notified” substitute “
the approved
”
;
c
in paragraph (1)(c) omit “EU” in each place it occurs.
Amendment to regulation 82I31137
In regulation 82 (transitional provisions) at the end insert—
3
In paragraphs (4), (5) and (6)—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F212IP completion day;
“product” means a lift or a safety component to lifts to which these Regulations apply.
4
Subject to paragraph (5), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 22 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M63, any obligation to which a person was subject under these Regulations as they had effect immediately before F213IP completion day, continues to have effect as it did immediately before F213IP completion day, in relation to that product.
5
Paragraph (4) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
6
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulations 47 or 48 as they had effect immediately before F214IP completion day in relation to that product,
that action has effect as if it had been done under regulations 47 or 48 as they have effect on and after F214IP completion day.
Amendment to regulation 83I34638
In regulation 83 (consequential revocations, savings and amendments)—
a
in paragraph (2) for “The” substitute “
Subject to the modifications made in paragraph (3A), the
”
;
b
in paragraph (3) after “effect” insert “
, subject to the modifications made in paragraph (3A),
”
;
c
after paragraph 3 insert—
3A
The modifications referred to in paragraphs (2) and (3) are as follows—
a
any reference to the “Community” is to be read as including the United Kingdom;
b
any reference to a “member State” is to be read as including the United Kingdom;
c
in regulation 11(3) omit paragraph (a);
d
in Schedule 5 in Part A and in Part B—
i
in paragraph (5) omit the words from “The Commission” to “carried out.”; and
ii
in paragraph (7) omit the words after “issued”;
e
in Schedules 7, 8, 11,12 and 13—
i
in paragraph (5), for “national” substitute
“ enforcement ”; andiv
in paragraph (6) omit “and withdrawn”;
f
in paragraph (6) of Schedule 15, omit “with a view to this information being passed by him to the Commission”.
Amendment to Schedule 1I34739
1
Schedule 1 (essential health and safety requirements) is amended as follows.
2
In paragraph 1(2), for “the Directive” substitute “
this Schedule
”
.
3
In the italic heading before paragraph 2, for “Directive 2006/42/EC” substitute “
the Supply of Machinery (Safety) Regulations 2008/1597
”
.
4
In paragraph 2—
a
for “Annex 1 to Directive 2006/42/EC of the European Parliament and of the Council” substitute “Schedule 2 to the Supply of Machinery (Safety) Regulations 2008/1597; and
b
for “1.1.2 of Annex I to Directive 2006/43/EC” substitute “
paragraph 1.1.2 of Schedule 2 to the Supply of Machinery (Safety) Regulations 2008/1597
”
.
F2154A
In paragraph 3(4) for “member States” substitute “
the Secretary of State
”
.
5
In paragraph (6)(1) for “1.7.3 of Annex I to Directive 2006/42/EC” substitute “
paragraph 1.7.3 of Schedule 2 to the Supply of Machinery (Safety) Regulations 2008/1597
”
.
Amendment to Schedule 3I34840
In Schedule 3, in the heading omit the words “referred to in Article 1(1) of the Directive”.
Amendment to Schedule 4I34941
In Schedule 4—
a
in the heading for “Notified” substitute “
Approved
”
;
b
in paragraph 5 for “notified” substitute “
approved
”
;
c
in paragraph 8 for “notified” substitute “
approved
”
;
d
in paragraph 9(b) for “a notified” substitute “
an approved
”
;
e
in paragraph 11(a) for “notified” substitute “
approved
”
; and
f
in paragraph 17 for “the Coordination Group of Notified Bodies for Lifts established under the Directive” substitute “
any coordination group of approved bodies for lifts established by the Secretary of State
”
.
Amendment to Schedule 5I35042
1
Schedule 5 (EU declaration of conformity) is amended as follows.
2
In the heading omit “EU”.
3
For “notified”, in each place it occurs, substitute “
approved
”
.
4
In the heading of Part 1, omit “EU”.
5
In paragraph (1)—
a
omit “EU”;
b
in sub-paragraph (f) for “the relevant Union harmonisation legislation” substitute “
relevant enactments
”
;
c
in sub-paragraph (h)—
i
for “EU-type”, in both places it occurs, substitute “
Type
”
; and
ii
for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 11
”
;
d
in sub-paragraph (i) for “Annex VIII to the Directive (as amended from time to time)” substitute “
Schedule 15
”
;
e
in sub-paragraph (j) for “Annex V to the Directive (as amended from time to time)” substitute “
Schedule 12
”
; and
f
in sub-paragraph (k) for “Annex X, XI or XII to the Directive (as amended from time to time)” substitute “
Schedules 17, 18 or 19
”
.
6
In the heading of Part 2, omit “EU”.
7
In paragraph (2)—
a
omit “EU”;
b
in sub-paragraph (i)—
i
for “EU-type”, in both place it occurs, substitute “
Type
”
; and
ii
for “Annex IV and Annex VI to the Directive (as amended from time to time)” substitute “
Schedule 11 and Schedule 13
”
;
c
in sub-paragraph (j) for “Annex IX to the Directive (as amended from time to time)” substitute “
Schedule 16
”
; and
d
in sub-paragraph (k) for “Annex VI or VII to the Directive (as amended from time to time)” substitute “
Schedule 13 or 14
”
.
Amendment to Schedule 6I35143
Schedule 6 (operational obligations of notified bodies) is amended as follows—
a
in the heading for “notified” substitute “
approved
”
;
b
for “A notified”, in each place it occurs, substitute “
An approved
”
;
c
in paragraphs 7 and 9, in each place it occurs, for “the notified” substitute “
the approved
”
;
d
in paragraph 12 for “notified under the Directive” substitute “
approved by the Secretary of State
”
; and
e
in paragraph (13) for the words “the Coordination Group of Notified Approved Bodies for Lifts established under the Directive” substitute “
any coordination group of approved bodies for lifts established by the Secretary of State
”
.
New Schedules 11 to 19I35244
After Schedule 10 (Compliance, withdrawal and recall notices) insert—
SCHEDULE 11TYPE EXAMINATION FOR LIFTS AND SAFETY COMPONENTS FOR LIFTS (Annex IV to the Directive)
MODULE B
A. Type examination of safety components for lifts1
Type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of a safety component for lifts and verifies and attests that the technical design of the safety component for lifts satisfies the applicable essential health and safety requirements of Schedule 1 and will enable a lift in which it is correctly incorporated to satisfy those requirements.
2
The application for Type examination shall be lodged by the manufacturer, or his authorised representative, with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well and the place of manufacture of the safety components for lifts;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation;
d
a representative specimen of the safety component for lifts or details of the place where it can be examined. The approved body may request further specimens if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution. This supporting evidence shall mention any documents, including other relevant technical specifications, that have been used, in particular where the relevant designated standards have not been applied in full. The supporting evidence shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer, or by another testing laboratory on his behalf and under his responsibility.
3
The technical documentation shall make it possible to assess whether the safety component for lifts meets the conditions referred to in point 1 and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the safety component for lifts.
The technical documentation shall contain, where applicable, the following:
a
a description of the safety component for lifts, including its area of use (in particular possible limits on speed, load and power) and conditions (in particular explosive environments and exposure to the elements);
b
design and manufacturing drawings and diagrams;
c
explanations necessary for the understanding of those drawings and diagrams and the operation of the safety component for lifts;
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to enable the safety component for lifts to meet the conditions referred to in point 1, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
e
results of design calculations performed by or for the manufacturer;
f
test reports;
g
a copy of the instructions for the safety components for lifts;
h
steps taken at the manufacturing stage to ensure that series-produced safety components for lifts conform to the safety component for lifts examined.
4
The approved body shall:
a
examine the technical documentation and the supporting evidence to assess the adequacy of the technical design of the safety component for lifts;
b
agree with the applicant on a location where the examinations and tests will be carried out;
c
verify that the representative specimen(s) has(have) been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
d
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the specifications of the relevant designated standards, these have been applied correctly;
e
carry out appropriate examinations and tests, or have them carried out, to check whether, where the specifications of the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications enable the safety component for lifts to meet the conditions referred to in point 1.
The approved body shall draw up an evaluation report that records the examinations, verifications and tests carried out and their outcome. Without prejudice to its obligations vis-à-vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
5
Where the type of the safety component for lifts meets the conditions referred to in point 1, the body shall issue a type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer the conclusions of the Type examination, any conditions of validity of the certificate and the particulars necessary to identify the approved type.
The Type examination certificate may have one or more annexes attached.
The Type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured safety components for lifts with the examined type to be evaluated and to allow for in-service control.
Where the type of the safety component for lifts does not satisfy the conditions referred to in point 1, the approved body shall refuse to issue a type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
The approved body shall keep a copy of the Type examination certificate, its annexes and additions, as well as the technical documentation and the evaluation report, for 15 years from the date of issue of that certificate.
6
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer meet the conditions referred to in point 1 and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
7
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination certificate of any modification to the approved type that may affect the conformity of the safety component for lifts with the conditions referred to in point 1 or the conditions of validity of the Type examination certificate.
The approved body shall examine the modification and inform the applicant whether the Type examination certificate remains valid or whether further examinations, verifications or tests are needed. As appropriate, the approved body shall issue an addition to the original Type examination certificate or ask for a new application for a type examination to be submitted.
8
Each approved body shall inform the Secretary of State concerning the Type examination certificates and any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the Type examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and/or additions thereto which it has issued.
9
The other approved bodies may, on request, obtain a copy of the Type examination certificates and additions thereto.
10
The manufacturer shall keep with the technical documentation a copy of the Type examination certificates, its annexes and additions at the disposal of the enforcing authorities for 10 years after the safety component for lifts has been placed on the market.
11
Authorised representative
The manufacturer's authorised representative may lodge the application referred to in point 2 and fulfil the obligations set out in points 7 and 10, provided that they are specified in the mandate.
B. Type examination of lifts1
Type examination of lifts is the part of a conformity assessment procedure in which an approved body examines the technical design of a model lift, or a lift for which there is no provision for an extension or variant, and verifies and attests that the technical design of the model lift or the lift meets the applicable essential health and safety requirements set out in Schedule 1.
Type examination of a lift includes an examination of a representative specimen of a complete lift.
2
The application for Type examination shall be lodged by the installer or his authorised representative with a single approved body of his choice.
The application shall include:
a
the name and address of the installer; and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation;
d
details of the place where the specimen lift can be examined. The specimen lift submitted for examination shall include the terminal parts and be capable of serving at least three levels (top, middle and bottom);
e
the supporting evidence for the adequacy of the technical design solution. This supporting evidence shall mention any documents, including other relevant technical specifications that have been used, in particular where the relevant designated standards have not been applied in full. The supporting evidence shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the installer, or by another testing laboratory on his behalf and under his responsibility.
3
The technical documentation shall make it possible to assess the conformity of the lift with the applicable essential health and safety requirements set out in Schedule 1.
The technical documentation shall contain, where applicable, the following:
a
a description of the model lift indicating clearly all the permitted variations of the model lift;
b
design and manufacturing drawings and diagrams;
c
explanations necessary for the understanding of those drawings and diagrams and of the operation of the lift;
d
a list of the essential health and safety requirements taken into consideration;
e
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
f
a copy of the declarations of conformity of the safety components for lifts incorporated in the lift;
g
results of design calculations performed by or for the installer;
h
test reports;
i
a copy of the instructions referred to in point 7.2 of Schedule 1;
j
steps taken at the installation stage to ensure that the series-produced lift conforms to the essential health and safety requirements set out in Schedule 1.
4
The approved body shall:
a
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the model lift or of the lift for which there is no provision for an extension or variant;
b
agree with the installer on a location where the examinations and tests will be carried out;
c
examine the specimen lift to check that it has been manufactured in accordance with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
d
carry out appropriate examinations and tests, or have them carried out, to check whether, where the installer has chosen to apply the specifications of the relevant designated standards, these have been applied correctly;
e
carry out appropriate examinations and tests, or have them carried out, to check whether, where the specifications of the relevant designated standards have not been applied, the solutions adopted by the installer applying other relevant technical specifications meet the corresponding essential health and safety requirements of these Regulations.
5
The approved body shall draw up an evaluation report that records the examinations, verifications and tests carried out and their outcome. Without prejudice to its obligations vis-à-vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the installer.
6
Where the type meets the essential health and safety requirements set out in Schedule 1 applicable to the lift concerned, the approved body shall issue a Type examination certificate to the installer. That certificate shall contain the name and address of the installer, the conclusions of the Type examination, any conditions of validity of the certificate and the particulars necessary to identify the approved type.
The Type examination certificate may have one or more annexes attached.
The Type examination certificate and its annexes shall contain all the information necessary to enable the conformity of lifts with the approved type to be assessed during the final inspection.
Where the type does not comply with the essential health and safety requirements set out in Schedule 1, the approved body shall refuse to issue a Type examination certificate and shall inform the installer accordingly, giving detailed reasons for its refusal.
The approved body shall keep a copy of the Type examination certificate, its annexes and additions, as well as the technical documentation and the evaluation report for 15 years from the date of issue of that certificate.
7
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the essential health and safety requirements set out in Schedule 1, and shall determine whether such changes require further investigation. If so, the approved body shall inform the installer accordingly.
8
The installer shall inform the approved body of any modifications to the approved type, including variations not specified in the original technical documentation, that may affect the conformity of the lift with the essential health and safety requirements set out in Schedule 1 or the conditions of validity of the Type examination certificate.
The approved body shall examine the modification and inform the installer whether the Type examination certificate remains valid or whether further examinations, verifications or tests are needed. As appropriate the approved body shall issue an addition to the original Type examination certificate or ask for a new application for a Type examination to be submitted.
9
Each approved body shall inform the Secretary of State concerning the Type examination certificates and any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the Type examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and additions thereto which it has issued.
10
The other approved bodies may, on request, obtain a copy of the Type examination certificates and additions thereto.
11
The installer shall keep with the technical documentation a copy of the Type examination certificate, including its annexes and additions, at the disposal of the enforcing authorities for 10 years after the lift has been placed on the market.
12
Authorised representative
The installer's authorised representative may lodge the application referred to in point 2 and fulfil the obligations set out in points 8 and 11, provided that they are specified in the mandate.
SCHEDULE 12FINAL INSPECTION FOR LIFTS (Annex V to the Directive)
1
Final inspection is the part of a conformity assessment procedure whereby an approved body ascertains and certifies that a lift subject to a Type examination certificate or designed and manufactured according to an approved quality system satisfies the essential health and safety requirements set out in Schedule 1.
Obligations of the installer2
The installer shall take all measures necessary to ensure that the lift being installed complies with the applicable essential health and safety requirements set out in Schedule 1 and with one of the following:
a
an approved type described in a Type examination certificate;
b
a lift designed and manufactured in accordance with a quality system pursuant to Schedule 18 and the design examination certificate if the design is not wholly in accordance with the designated standards.
Final inspection3
An approved body chosen by the installer shall carry out the final inspection of the lift about to be placed on the market in order to check the conformity of the lift with the applicable essential health and safety requirements set out in Schedule 1.
3
The installer shall lodge an application for final inspection with a single approved body of his choice and shall provide to the approved body the following documents:
a
the plan of the complete lift;
b
the plans and diagrams necessary for final inspection, in particular control circuit diagrams;
c
copy of the instructions referred to in Schedule 1, point 7.2;
d
a written declaration that the same application has not been lodged with any other approved body.
The approved body may not require detailed plans or precise information not necessary for verifying the conformity of the lift.
The appropriate examinations and tests set out in the relevant designated standard(s) or equivalent tests shall be carried out in order to check the conformity of the lift with the applicable essential health and safety requirements set out in Schedule 1.
3
The examinations shall include at least one of the following:
a
examination of the documents referred to in point 3.1 to check that the lift conforms with the approved type described in the Type examination certificate pursuant to Schedule 11, Part B;
b
examination of the documents referred to in point 3.1 to check that the lift conforms with the lift designed and manufactured in accordance with an approved quality system pursuant to Schedule 18 and if the design is not wholly in accordance with the designated standards, with the design examination certificate.
3
The tests of the lift shall include at least the following:
a
operation of the lift both empty and at maximum load to ensure correct installation and operation of the safety devices (end stops, locking devices, etc.);
b
operation of the lift at both maximum load and empty to ensure the correct functioning of the safety devices in the event of loss of power;
c
static test with a load equal to 1.25 times the rated load.
The rated load shall be that referred to in Schedule 1, paragraph 6.
After these tests, the approved body shall check that no distortion or deterioration which could impair the use of the lift has occurred.
4
If the lift satisfies the essential health and safety requirements set out in Schedule 1, the approved body shall affix or have affixed its identification number adjacent to the UK marking in accordance with regulation 8 (declaration of conformity and UK marking) and regulation 50 (UK marking) and shall issue a final inspection certificate which mentions the examinations and tests carried out.
The approved body shall fill in the corresponding pages in the logbook referred to in Schedule 1, paragraph 7(2).
If the approved body refuses to issue the final inspection certificate, it shall state the detailed reasons for refusal and indicate the necessary corrective measures to be taken. Where the installer again applies for final inspection, he shall apply to the same approved body.
UK marking and declaration of conformity5
5
The installer shall affix the UK marking in the car of each lift which satisfies the essential health and safety requirements of these Regulations, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number adjacent to the UK marking in the car of each lift.
5
The installer shall draw up a written declaration of conformity for each lift and keep a copy of the Declaration of conformity and the final inspection certificate at the disposal of the enforcing authorities for 10 years after the placing on the market of the lift. A copy of the Declaration of conformity shall be made available to the relevant authorities upon request.
Authorised representative6
The installer's obligations set out in points 3.1 and 5 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 13CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE FOR SAFETY COMPONENTS FOR LIFTS (Annex VI to the Directive)
MODULE E
1
Conformity to type based on product quality assurance for safety components for lifts is the part of the conformity assessment procedure whereby an approved body assesses the quality system of a manufacturer in order to ensure that the safety components for lifts are manufactured and monitored in conformity with the type described in the Type examination certificate, satisfy the applicable requirements of Schedule 1 and will enable a lift to which they are correctly incorporated to satisfy those requirements.
Obligations of the manufacturer2
The manufacturer shall operate an approved quality system for final inspection and testing of the safety components for lifts as specified in point 3, and shall be subject to surveillance as specified in point 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system for the safety components for lifts concerned with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the address of the premises where final inspection and testing of the safety components for lifts are carried out;
d
all relevant information on the safety components for lifts to be manufactured;
e
the documentation concerning the quality system;
f
the technical documentation of the approved safety components for lifts and a copy of the Type examination certificate.
3
Under the quality system, each safety component for lifts shall be inspected and appropriate tests as set out in the relevant designated standards or equivalent tests shall be carried out in order to ensure that it meets the conditions referred to in point 1. All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall contain in particular an adequate description of:
a
the quality objectives;
b
the organizational structure, responsibilities and powers of the management with regard to product quality;
c
the examinations and tests that will be carried out after manufacture;
d
the means of monitoring the effective operation of the quality system; and
e
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned, etc.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2. It shall presume conformity with those requirements in respect of the elements of the quality systems that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of assessment in the lift technology concerned and knowledge of the essential health and safety requirements set out in Schedule 1.
The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in point 3.1(f), in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the safety components for lifts with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising from the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer or his authorised representative shall keep the approved body which has approved the quality system informed of any intended changes of the quality system.
The approved body shall assess the modifications proposed and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall for assessment purposes allow the approved body access to the premises where final inspection, testing and storage are carried out and provide it with all necessary information, in particular:
a
the quality system documentation;
b
the technical documentation;
c
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned.
4
The approved body shall periodically carry out audits to ensure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
Additionally, the approved body may pay unexpected visits to the manufacturer's premises where final inspection and testing of safety components for lifts are carried out.
At the time of such visits, the approved body may, where necessary, carry out tests or have them carried out in order to check the proper functioning of the quality system. It shall provide the manufacturer, with a visit report and, if a test has been carried out, with a test report.
UK marking and Declaration of conformity5
5
The manufacturer shall affix the UK marking, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number to each individual safety component for lifts that meets the conditions referred to in point 1.
5
The manufacturer shall draw up a written declaration of conformity for each safety component for lifts and keep a copy of it at the disposal of the enforcing authorities for 10 years after the safety component for lifts has been placed on the market. The Declaration of conformity shall identify the safety component for lifts for which it has been drawn up.
6
The manufacturer shall for a period ending 10 years after the safety component for lifts has been placed on the market, keep at the disposal of the enforcing authorities:
a
the technical documentation referred to in point 3.1(f);
b
the documentation referred to in point 3.1(e);
c
the information relating to the change referred to in point 3.5;
d
the decisions and reports from the approved body which are referred to in the third paragraph of point 3.5 and in points 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approval decision(s) issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of approval decisions refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approval decision(s) which it has refused, suspended or withdrawn and, upon request, of approval decision(s) which it has issued.
Authorised representative8
The manufacturer's obligations set out in points 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 14CONFORMITY BASED ON FULL QUALITY ASSURANCE FOR SAFETY COMPONENTS FOR LIFTS (Annex VII to the Directive)
MODULE H
1
Conformity based on full quality assurance for safety components for lifts is the conformity assessment procedure whereby an approved body assesses the quality system of a manufacturer to ensure that the safety components for lifts are designed, manufactured, inspected and tested in order to satisfy the applicable requirements of Schedule 1 and to enable a lift to which they are correctly incorporated to satisfy those requirements.
Obligations of the manufacturer2
The manufacturer shall operate an approved quality system for the design, manufacture, final inspection and testing of safety components for lifts as specified in point 3 and shall be subject to surveillance as specified in point 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with a single approved body of his choice. The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
the address of the premises where the safety components for lifts are designed, manufactured, inspected and tested;
c
all relevant information on safety components for lifts to be manufactured;
d
the technical documentation described in point 3 of Schedule 11, Part A for one model of each category of safety component for lifts to be manufactured;
e
the documentation on the quality system;
f
a written declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the safety components for lifts with the conditions referred to in point 1. All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall contain in particular an adequate description of:
a
the quality objectives and the organizational structure, responsibilities and powers of the management with regard to the design and product quality;
b
the technical design specifications, including standards that will be applied and, where the relevant designated standards will not be applied or not applied in full, the means, including other relevant technical specifications, that will be used to ensure that the conditions referred to in point 1 will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the safety components for lifts;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned;
g
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2. It shall presume conformity with those requirements in respect of the elements of the quality systems that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of assessment in the lift technology concerned and knowledge of the essential health and safety requirements set out in Schedule 1. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in point 3.1(d) to verify the manufacturer's ability to identify the applicable essential health and safety requirements set out in Schedule 1 and to carry out the necessary examinations with a view to ensuring compliance of the safety components for lifts with those requirements.
The decision shall be notified to the manufacturer and, where appropriate, to his authorised representative. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising from the quality system as approved and maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body which has approved the quality system informed of any intended change to the quality system.
The approved body shall assess the modifications proposed and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the assessment and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall for assessment purposes allow the approved body access to the design, manufacture, inspection and testing, and storage locations, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records provided for in the design part of the quality system such as results of analyses, calculations, tests;
c
the technical documentation for the safety components for lifts manufactured;
d
the quality records provided for in the manufacturing part of the full quality system, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
Additionally, the approved body may pay unexpected visits to the manufacturer. At the time of such visits, the approved body may, where necessary, carry out tests or have them carried out in order to check the proper functioning of the quality system. It shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report
UK marking and Declaration of conformity5
5
The manufacturer shall affix the UK marking, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number to each individual safety component for lifts that meets the conditions referred to in point 1.
5
The manufacturer shall draw up a written declaration of conformity for each safety component for lifts and keep a copy of it at the disposal of the enforcing authorities for 10 years after the safety component for lifts has been placed on the market. The declaration of conformity shall identify the safety component for lifts for which it has been drawn up.
6
The manufacturer shall, for a period ending 10 years after the safety component for lifts has been placed on the market, keep at the disposal of the enforcing authorities:
a
the documentation referred to in point 3.1(e);
b
the technical documentation referred to in point 3.1(d);
c
the information relating to the change referred to in the first paragraph of point 3.5;
d
the decisions and reports from the approved body referred to in the third paragraph of point 3.5. and in points 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approval decision(s) issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of approval decisions refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approval decisions which it has refused, suspended or withdrawn and, upon request, of approval decisions which it has issued.
The approved body shall keep a copy of the approval decision issued, its annexes and additions, as well as the technical documentation for 15 years from the date of their issue.
Authorised representative8
The manufacturer's obligations set out in points 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 15CONFORMITY BASED ON UNIT VERIFICATION FOR LIFTS (Annex VIII to the Directive)
MODULE G
1
Conformity based on unit verification is the conformity assessment procedure whereby an approved body assesses whether a lift complies with the applicable essential health and safety requirements set out in Schedule 1.
Obligations of the installer2
2
The installer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the lift with the applicable essential health and safety requirements set out in Schedule 1.
2
The installer shall apply to a single approved body of his choice for unit verification.
The application shall contain:
a
the name and address of the installer, and if the application is lodged by the authorised representative, his name and address as well;
b
the location where the lift is installed;
c
a written declaration to the effect that a similar application has not been lodged with another approved body;
d
the technical documentation.
3
The technical documentation shall allow an assessment of the conformity of the lift with the applicable essential health and safety requirements set out in Schedule 1.
The technical documentation shall contain at least the following elements:
a
a description of the lift;
b
design and manufacturing drawings and diagrams;
c
explanations necessary for the understanding of those drawings and diagrams and of the operation of the lift;
d
a list of the essential health and safety requirements taken into consideration;
e
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
f
a copy of the Type examination certificates of the safety components for lifts incorporated in the lift;
g
results of design calculations performed by or for the installer;
h
test reports;
i
a copy of the instructions referred to in point 7.2 of Schedule 1.
Verification4
The approved body chosen by the installer shall examine the technical documentation and the lift and carry out the appropriate tests as set out in the relevant designated standard(s), or equivalent tests, to check its conformity with the applicable essential health and safety requirements set out in Schedule 1. The tests shall include at least the tests referred to in point 3.3 of Schedule 12.
If the lift meets the essential health and safety requirements set out in Schedule 1 the approved body shall issue a certificate of conformity relating to the tests carried out.
The approved body shall fill in the corresponding pages of the logbook referred to in point 7.2 of Schedule 1.
If the approved body refuses to issue the certificate of conformity, it shall state in detail its reasons for refusal and indicate the necessary corrective measures to be taken. When the installer reapplies for unit verification he shall apply to the same approved body.
On request, the approved body shall provide the Secretary of State with a copy of the certificate of conformity.
UK marking and Declaration of conformity5
5
The installer shall affix the UK marking in the car of each lift which satisfies the essential health and safety requirements of these Regulations, and, under the responsibility of the approved body referred to in point 2.2, the latter's identification number adjacent to the UK marking in the car of each lift.
5
The installer shall draw up a written declaration of conformity for each lift and keep a copy of the Declaration of conformity at the disposal of the enforcing authorities for 10 years after the placing on the market of the lift. A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
6
The installer shall keep with the technical documentation a copy of the certificate of conformity at the disposal of the enforcing authorities for 10 years from the date on which the lift is placed on the market.
Authorised representative7
The installer's obligations set out in points 2.2 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 16CONFORMITY TO TYPE WITH RANDOM CHECKING FOR SAFETY COMPONENTS FOR LIFTS (Annex IX to the Directive)
MODULE C2
1
Conformity to type with random checking is the part of the conformity assessment procedure whereby an approved body carries out checks on safety components for lifts to ensure that they are in conformity with the approved type as described in the Type examination certificate and satisfy the applicable requirements of Schedule 1 and will enable a lift in which they are correctly incorporated to satisfy those requirements.
Manufacturing2
The manufacturer shall take all measures necessary to ensure that the manufacturing process and its monitoring ensure that the manufactured safety components for lifts meet the conditions referred to in point 1.
3
The manufacturer shall lodge an application for random checking with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information on the safety components for lifts manufactured;
d
the address of the premises where the sample of the safety components for lifts can be taken.
4
The approved body shall carry out or have carried out checks on safety components for lifts at random intervals. An adequate sample of the final safety components for lifts, taken on site by the approved body, shall be examined and appropriate tests set out in the relevant designated standards, and/or equivalent tests set out in other relevant technical specifications, shall be carried out to check whether the safety components for lifts meets the conditions referred to in point 1. In cases where one or more of the safety components for lifts checked do not conform, the approved body shall take appropriate measures.
The points to be taken into account when checking the safety components for lifts will be defined by joint agreement between all the approved bodies responsible for this procedure, taking into consideration the essential characteristics of the safety components for lifts.
The approved body shall issue a certificate of conformity to type with respect to the examinations and tests carried out.
On request the approved body shall provide the Secretary of State with a copy of the certificate of conformity to type.
UK marking and Declaration of conformity5
5
The manufacturer shall affix the UK marking, and, under the responsibility of the approved body referred to in point 3, the latter's identification number to each individual safety component for lifts that meets the conditions referred to in point 1.
5
The manufacturer shall draw up a written declaration of conformity for each safety component for lifts and keep a copy of it at the disposal of the enforcing authorities for 10 years after the safety component for lifts has been placed on the market. The Declaration of conformity shall identify the safety component for lifts for which it has been drawn up.
Authorised representative6
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative shall not fulfil the manufacturer's obligations set out in point 2.
SCHEDULE 17CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE FOR LIFTS (Annex X to the Directive)
MODULE E
1
Conformity to type based on product quality assurance is the part of the conformity assessment procedure whereby an approved body assesses the product quality system of an installer to ensure that the lifts are in conformity with the approved type as described in the Type examination certificate or with a lift designed and manufactured under a full quality system approved in accordance with Schedule 18, and satisfy the applicable essential health and safety requirements set out in Schedule 1.
Obligations of the installer2
The installer shall operate an approved quality system for final inspection and testing of the lift as specified in point 3, and shall be subject to surveillance as specified in point 4.
Quality system3
3
The installer shall lodge an application for assessment of his quality system for the lifts concerned with a single approved body of his choice.
The application shall include:
a
the name and address of the installer, and if the application is lodged by the authorised representative, his name and address as well;
b
all relevant information on the lifts to be installed;
c
the documentation on the quality system;
d
the technical documentation of the lifts to be installed;
e
a written declaration that the same application has not been lodged with any other approved body.
3
Under the quality system, each lift shall be examined and appropriate tests as set out in the relevant designated standards or equivalent tests shall be carried out in order to ensure its conformity with the applicable essential health and safety requirements set out in Schedule 1.
All the elements, requirements and provisions adopted by the installer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and quality records.
It shall contain in particular an adequate description of:
a
the quality objectives;
b
the organisational structure, responsibilities and powers of the management with regard to product quality;
c
the examinations and tests that will be carried out before placing on the market, including at least the tests laid down in point 3.3 of Schedule 12;
d
the means of monitoring the effective operation of the quality system;
e
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2. It shall presume conformity with those requirements in respect of the elements of the quality systems that comply with the corresponding specifications of the relevant designated standard.
The auditing team shall have at least one member with experience of assessment in the lift technology concerned and knowledge of the essential health and safety requirements set out in Schedule 1. The audit shall include an assessment visit to the premises of the installer and a visit to the installation site.
The decision shall be notified to the installer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The installer shall undertake to fulfil the obligations arising from the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The installer shall keep the approved body which has approved the quality system informed of any intended change to the system.
3
The approved body shall assess the modifications proposed and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify its decision to the installer or, where appropriate, to his authorised representative. The notification shall contain the conclusions of the assessment and the reasoned assessment decision.
The approved body shall affix, or cause to be affixed, its identification number adjacent to the UK marking in accordance with regulation 50.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the installer duly fulfils the obligations arising out of the approved quality system.
4
The installer shall, for assessment purposes, allow the approved body access to the installation, inspection and testing locations, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the technical documentation;
c
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned, etc.
4
The approved body shall periodically carry out audits to ensure that the installer maintains and applies the quality system and shall provide the installer with an audit report.
4
Additionally, the approved body may pay unexpected visits to the lift installation sites.
At the time of such visits, the approved body may, where necessary, carry out tests or have them carried out in order to check the proper functioning of the quality system and of the lift. It shall provide the installer with a visit report and, if tests have been carried out, with a test report.
5
The installer shall, for 10 years after the last lift has been placed on the market, keep at the disposal of the enforcing authorities:
a
the documentation referred to in point 3.1(c);
b
the technical documentation referred to in point 3.1(d);
c
the information relating to the changes referred to in point 3.4.1;
d
the decisions and reports from the approved body which are referred to in the second paragraph of point 3.4.2 and in points 4.3 and 4.4.
6
Each approved body shall inform the Secretary of State of quality system approval decision(s) issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of approval decisions, refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approval decision(s) which it has refused, suspended or withdrawn and, upon request, of approval decision(s) which it has issued.
On request, the approved body shall provide the Secretary of State with a copy of the quality system approval decision(s) issued.
UK marking and declaration of conformity7
7
The installer shall affix the UK marking in the car of each lift which satisfies the essential health and safety requirements of these Regulations, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number adjacent to the UK marking in the car of each lift.
7
The installer shall draw up a written Declaration of conformity for each lift and keep a copy of the Declaration of conformity at the disposal of the enforcing authorities for 10 years after the placing on the market of the lift. A copy of the Declaration of conformity shall be made available to the relevant authorities upon request.
Authorised representative8
The installer's obligations set out in points 3.1, 3.4.1, 5 and 7 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 18CONFORMITY BASED ON FULL QUALITY ASSURANCE PLUS DESIGN EXAMINATION FOR LIFTS (Annex XI to the Directive)
MODULE H1
1
Conformity based on full quality assurance plus design examination for lifts is the conformity assessment procedure whereby an approved body assesses the quality system of an installer and, where appropriate, the design of the lifts, to ensure that the lifts satisfy the applicable essential health and safety requirements set out in Schedule 1.
Obligations of the installer2
The installer shall operate an approved quality system for the design, manufacture, assembly, installation, final inspection and testing of the lifts as specified in point 3, and shall be subject to surveillance as specified in point 4. The adequacy of the technical design of the lifts shall have been examined in accordance with point 3.3.
Quality system3
3
The installer shall lodge an application for assessment of his quality system with a single approved body of his choice.
The application shall include:
a
the name and address of the installer, and, if the application is lodged by the authorised representative, his name and address as well;
b
all relevant information on the lifts to be installed, in particular information which makes for an understanding of the relationship between the design and operation of the lift;
c
the documentation on the quality system;
d
the technical documentation described in point 3 of Schedule 11, Part B;
e
a written Declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the lifts with the applicable essential health and safety requirements set out in Schedule 1. All the elements, requirements and provisions adopted by the installer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and quality records.
It shall contain in particular an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards that will be applied and, where the relevant designated standards will not be applied in full, the means, including other relevant technical specifications that will be used to ensure that the applicable essential health and safety requirements set out in Schedule 1 will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the lifts;
d
the examinations and tests that will be carried out on acceptance of the supplies of materials, components and sub-assemblies;
e
the corresponding assembly, installation, quality control and quality assurance techniques, processes and systematic actions that will be used;
f
the examinations and tests that will be carried out before (inspection of installation conditions: shaft, housing of machinery, etc.), during and after installation (including at least the tests laid down in point 3.3 of Schedule 12);
g
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned;
h
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
3
Design examination
3
When the design is not entirely in accordance with designated standards, the approved body shall ascertain whether the design conforms to the essential health and safety requirements set out in Schedule 1 and, if it does, issue a design examination certificate to the installer, stating the limits of the certificate's validity and giving the details required for identification of the approved design.
3
Where the design does not satisfy the applicable essential health and safety requirements set out in Schedule 1, the approved body shall refuse to issue a design examination certificate and shall inform the installer accordingly, giving detailed reasons for its refusal.
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved design may no longer comply with the essential health and safety requirements set out in Schedule 1, and shall determine whether such changes require further investigation. If so, the approved body shall inform the installer accordingly.
3
The installer shall keep the approved body that has issued the design examination certificate informed of any modification to the approved design that may affect the conformity with the essential health and safety requirements set out in Schedule 1 or the conditions for validity of the certificate. Such modifications shall require additional approval — from the approved body that issued the design examination certificate — in the form of an addition to the original design examination certificate.
3
Each approved body shall inform the Secretary of State of the design examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of design examination certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of the design examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, of the certificates and/or additions thereto which it has issued.
3
The installer shall keep a copy of the design examination certificate, its annexes and additions together with the technical documentation at the disposal of the enforcing authorities for 10 years after the lift has been placed on the market.
3
Assessment of the quality system
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2. It shall presume conformity with those requirements in respect of the elements of the quality systems that comply with the corresponding specifications of the relevant designated standard.
The auditing team shall have at least one member with experience of assessment in the lift technology concerned and knowledge of the essential health and safety requirements set out in Schedule 1. The audit shall include an assessment visit to the installer's premises and a visit to an installation site.
The auditing team shall review the technical documentation referred to in point 3.1(d), to verify the installer's ability to identify the applicable essential health and safety requirements set out in Schedule 1 and to carry out the necessary examinations with a view to ensuring compliance of the lift with those requirements.
The decision shall be notified to the installer or, where appropriate, to his authorised representative. The notification shall contain the conclusions of the assessment and the reasoned assessment decision.
3
The installer shall undertake to fulfil the obligations arising from the quality system as approved and to maintain it so that it remains adequate and efficient.
The installer shall keep the approved body that has approved the quality system informed of any intended change to the system.
The approved body shall assess the modifications proposed and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify its decision to the installer or, where appropriate, to his authorised representative. The notification shall contain the conclusions of the assessment and the reasoned assessment decision.
The approved body shall affix, or cause to be affixed, its identification number adjacent to the UK marking in accordance with regulation 50.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the installer duly fulfils the obligations arising out of the approved quality system.
4
The installer shall, for assessment purposes, allow the approved body access to the design, manufacture, assembly, installation, inspection, testing and storage locations, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records provided for in the design part of the quality system, such as results of analyses, calculations, tests;
c
the quality records provided for in the part of the quality system concerning acceptance of supplies and installation, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the installer maintains and applies the quality system and shall provide the installer with an audit report.
4
Additionally, the approved body may pay unexpected visits to the premises of the installer or to the installation site of a lift. At the time of such visits, the approved body may, where necessary, carry out tests or have them carried out in order to check the proper functioning of the quality system. It shall provide the installer with a visit report and, if tests have been carried out, with a test report.
5
The installer shall, keep at the disposal of the enforcing authorities for a period ending 10 years after the lift has been placed on the market:
a
the documentation referred to in point 3.1(c);
b
the technical documentation referred to in point 3.1(d);
c
the information relating to the changes referred to in the second paragraph of point 3.5;
d
the decisions and reports from the approved body which are referred to in the fourth paragraph of point 3.5 and in points 4.3 and 4.4.
6
Each approved body shall inform the Secretary of State of full quality system approval decision(s) issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of approval decisions refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approval decision(s) which it has refused, suspended or withdrawn, and, upon request, of approval decisions which it has issued.
The approved body shall keep a copy of the approval decision issued, its annexes and additions, as well as the technical documentation for 15 years from the date of their issue.
On request, the approved body shall provide the Secretary of State with a copy of the quality system approval decision(s) issued.
UK marking and Declaration of conformity7
7
The installer shall affix the UK marking in the car of each lift which satisfies the essential health and safety requirements of these Regulations, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number adjacent to the UK marking in the car of each lift.
7
The installer shall draw up a written declaration of conformity for each lift and keep a copy of the Declaration of conformity at the disposal of the enforcing authorities for 10 years after the placing on the market of the lift. A copy of the Declaration of conformity shall be made available to the relevant authorities upon request.
Authorised representative8
The installer's obligations set out in points 3.1, 3.3.3, 3.3.5, 5 and 7 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 19CONFORMITY TO TYPE BASED ON PRODUCTION QUALITY ASSURANCE FOR LIFTS (Annex XII to the Directive)
MODULE D
1
Conformity to type based on production quality assurance for lifts is the part of the conformity assessment procedure whereby an approved body assesses the production quality system of an installer to ensure that the lifts installed are in conformity with the approved type as described in the Type examination certificate or with a lift designed and manufactured under a quality system approved in accordance with Schedule 18 , and satisfy the applicable essential health and safety requirements set out in Schedule 1.
Obligations of the installer2
The installer shall operate an approved quality system for manufacture, assembly, installation, final inspection and testing of the lifts as specified in point 3, and shall be subject to surveillance as specified in point 4.
Quality system3
3
The installer shall lodge an application for assessment of his quality system with a single approved body of his choice.
The application shall include:
a
the name and address of the installer, and, if the application is lodged by the authorised representative, his name and address as well;
b
all relevant information for the lifts to be installed;
c
the documentation on the quality system;
d
the technical documentation of the lifts to be installed;
e
a written declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the lifts with the applicable essential health and safety requirements set out in Schedule 1.
All the elements, requirements and provisions adopted by the installer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall contain in particular an adequate description of:
a
the quality objectives and the organizational structure, responsibilities and powers of the management with regard to the product quality;
b
the manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after installation;
d
the quality records, such as inspection reports and test data, calibration data, reports on the qualification of the personnel concerned;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.2. It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
The auditing team shall have at least one member with experience of assessment in the lift technology concerned and knowledge of the essential health and safety requirements set out in Schedule 1.
The audit shall include an assessment visit to the installer's premises and a visit to an installation site.
The decision shall be notified to the installer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The installer shall undertake to fulfil the obligations arising from the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The installer shall keep the approved body that has approved the quality system informed of any intended change to the system.
3
The approved body shall assess the modifications proposed and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.2 or whether a reassessment is necessary.
It shall notify its decision to the installer or, where appropriate, to his authorised representative. The notification shall contain the conclusions of the assessment and the reasoned assessment decision.
The approved body shall affix, or cause to be affixed, its identification number adjacent to the UK marking in accordance with regulation 50.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the installer duly fulfils the obligations arising out of the approved quality system.
4
The installer shall, for assessment purposes, allow the approved body access to the manufacture, assembly, installation, inspection, testing and storage locations, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the technical documentation;
c
the quality records, such as inspection reports and test data, calibration data, reports on the qualifications of the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the installer maintains and applies the quality system and shall provide the installer with an audit report.
4
Additionally, the approved body may pay unexpected visits to the installer. During such visits the approved body may, where necessary carry out tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the installer with a visit report and, if tests have been carried out, with a test report.
5
The installer shall, keep at the disposal of the enforcing authorities for a period ending 10 years after the lift has been placed on the market:
a
the documentation referred to in point 3.1(c);
b
the technical documentation referred to in point 3.1(d);
c
the information relating to the changes referred to in point 3.4.1;
d
the decisions and reports from the approved body which are referred to in the second paragraph of point 3.4.2, and in points 4.3 and 4.4.
6
Each approved body shall inform the Secretary of State of quality system approval decision(s) issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of approval decisions refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approval decision(s) which it has refused, suspended or withdrawn, and, upon request, of approval decision(s) which it has issued.
On request, the approved body shall provide the Secretary of State with a copy of the quality system approval decision(s) issued.
UK marking and Declaration of conformity7
7
The installer shall affix the UK marking in the car of each lift which satisfies the essential health and safety requirements of these Regulations, and, under the responsibility of the approved body referred to in point 3.1, the latter's identification number adjacent to the UK marking in the car of each lift.
7
The installer shall draw up a written Declaration of conformity for each lift and keep a copy of the Declaration of conformity at the disposal of the enforcing authorities for 10 years after the placing on the market of the lift. A copy of the Declaration of conformity shall be made available to the relevant authorities upon request.
Authorised representative8
The installer's obligations set out in points 3.1, 3.4.1, 5 and 7 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 23Amendment of the Electrical Equipment (Safety) Regulations 2016
IntroductionI9041
The Electrical Equipment (Safety) Regulations 2016 are amended in accordance with paragraphs 2 to 32.
Amendment to regulation 2I3532
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
F216a
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
b
omit the definition of “CE marking”;
c
after the definition of “authorised representative”, insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 6 (declaration of conformity);
“designated standard” has the meaning given to it in regulation 2A;
d
in the definition of “the Directive”, at the end, insert “
(as it has effect immediately before F217IP completion day)
”
;
e
omit the definition of “EU declaration of conformity”;
f
omit the definition of “harmonised standard”;
g
for the definition of “importer” substitute—
F218“importer” means a person who—
a
is established in the United Kingdom and places electrical equipment from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places electrical equipment on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
h
omit the definition of “international safety provision”;
F219i
in the definition of “making available on the market” for “EU market” substitute “market of Great Britain”;
j
omit the definition of “Official Journal”;
F220k
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
l
after the definition of “relevant economic operator” insert ----
“relevant international safety provision” means a safety provision of a standard set out by the International Commission on the Rules for the Approval of Electrical Equipment or the International Electrotechnical Commission, which has been published by the Secretary of State in a manner the Secretary of State considers appropriate;
m
after the definition of “technical specification”, insert—
UK marking” means the marking in the form set out in Annex 2 of RAMS;
3
Omit paragraph (5).
Insertion of regulation 2AI3553
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
the levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
the production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to electrical equipment to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 6I3564
Regulation 6 (EU declaration of conformity and CE marking) is amended as follows—
a
in the heading—
i
for “EU declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraphs (1)(a) and (2), omit “EU”;
c
in paragraph (1)(b), for “CE” substitute “
UK
”
, in both places in which it occurs; and
d
for paragraph (3) substitute—
3
Where electrical equipment is subject to more than one enactment requiring a declaration of conformity to be drawn up, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 7I3575
In regulation 7 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 9I3586
For regulation 9 (instructions and safety information), substitute—
Instructions and safety information9
When placing electrical equipment on the market, a manufacturer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 10I3597
In regulation 10 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 12I3608
In regulation 12 (duty to take action in respect of electrical equipment placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other member State in which the manufacturer made the electrical equipment available on the market,”.
Amendment to regulation 14I3619
In regulation 14 (manufacturer's authorised representatives)—
a
in paragraph (1) after “a person” insert “
established in the United Kingdom
”
;
b
in paragraph (2)(a) omit “EU”.
Amendment to regulation 16I36210
In regulation 16 (requirements which must be satisfied before an importer places electrical equipment on the market), in paragraph (c) for “CE” substitute “
UK
”
.
Amendment to regulation 18I36311
Regulation 18 (information identifying importer) is amended as follows –
a
in paragraph (2) omit “in the member State in which it is to be made available to such end-users”; and
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the electrical equipment; or
b
before placing the electrical equipment on the market, the importer sets out the information referred to in paragraph (1)—
i
on the packaging; or
ii
in a document accompanying the F222 electrical equipment.
Amendment to regulation 19I36412
For regulation 19 (instructions and safety information), substitute—
Instructions and safety information19
When placing electrical equipment on the market, an importer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 21I36513
In regulation 21 (retention of technical documentation and EU declaration of conformity), in the heading to that regulation and in paragraph (a), omit “EU”.
Amendment to regulation 23I36614
In regulation 23 (duty to take action in respect of electrical equipment placed on the market which is considered not to be in conformity), in paragraph (2), omit “and the competent national authorities of any other member State in which the manufacturer made the electrical equipment available on the market”.
Amendment to regulation 26I36715
Regulation 26 (requirements which must be satisfied before a distributor makes electrical equipment available on the market), is amended as follows—
a
in paragraph (1)(a)(i), for “CE” substitute “
UK
”
;
b
for paragraph (1)(a)(iii) substitute—
iii
is accompanied by instructions and safety information that are clear, legible and easily understandable English;
c
omit paragraph (3).
Amendment to regulation 29I36816
In regulation 29 (duty to take action in respect of electrical equipment made available on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of the other member States in which the distributor has made the electrical equipment available on the market,”.
Omission of regulation 32I36917
Omit regulation 32 (translation of declaration of conformity).
Amendment to regulation 34I37018
In regulation 34 (prohibition on improper use of CE marking), in the heading and all places where it occurs, for “CE” substitute “
UK
”
.
Insertion of regulation 34AI37119
After regulation 34 insert—
Obligations which are met by complying with obligations in the Directive34A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(14); and
c
“harmonised standard” has the meaning given to it in Article 2(9).
2
Paragraph (3) applies where, before placing electrical equipment on the market, the manufacturer—
a
ensures that the electrical equipment has been designed and manufactured in accordance with the principal elements of the safety objectives set out in Annex I;
b
ensures that the conformity assessment procedure that applies to that equipment in accordance with Annex III has been carried out;
c
draws up the technical documentation referred to in Annex III;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared or translated into English;
e
affixes a CE marking, in accordance with Articles 16 and 17(1) and (2);
f
draws up an EU declaration of conformity, in accordance with Article 15; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 4, 5, 6(1) and (3) are to be treated as being satisfied;
b
regulations 2(2)(a), 6(2), 7, 10(2), 14(2), and 34 apply subject to the modifications in paragraph (8);
c
Part 3 does not apply; and
d
regulation 48(1) does not apply.
4
Paragraph (5) applies where, before placing electrical equipment on the market, the importer ensures that—
a
the conformity assessment procedure that applies to that equipment in accordance with Annex III has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III; and
c
the equipment bears the CE marking.
5
Where this paragraph applies—
a
the requirements of regulation 16(a) to (c) are to be treated as being satisfied; and
b
regulations 2(2)(a), 17(1), 20 and 21 apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making electrical equipment available on the market, a distributor ensures that the equipment bears the CE marking.
7
Where this paragraph applies—
a
regulation 26(1)(a)(i) is to be treated as being satisfied; and
b
regulations 27(1) and 28 apply subject to the modifications in paragraph (10).
8
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (7)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “principal elements of the safety objectives” is to be read as a reference to the principal elements of the safety objectives referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard within the meaning of Article 2(9);
e
any reference to “conformity assessment procedure” is to be read as a reference to the conformity assessment procedure that applies to the equipment in accordance with Annex III; and
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III.
F225Expiry of regulation 34A34B
1
Subject to paragraph (2), regulation 34A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 34A—
a
any electrical equipment which was placed on the market pursuant to regulation 34A may continue to be made available on the market on or after the expiry of regulation 34A;
b
any obligation to which a person was subject under regulation 34A in respect of electrical equipment placed on the market pursuant to regulation 34A continues to have effect after the expiry of regulation 34A, in respect of that electrical equipment.
Qualifying Northern Ireland Goods34C
1
Where paragraph (2) applies electrical equipment is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
electrical equipment—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the electrical equipment on the market, the importer—
a
complies with regulation 18;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the electrical equipment, in accordance with Part 3, as that Part applies in Northern Ireland;
ii
the manufacturer has drawn up the technical documentation; and
iii
the electrical equipment bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 36I37220
Regulation 36 (presumption of conformity on the basis of harmonised standards), is amended as follows—
a
for “harmonised” substitute “
designated
”
in the heading and in paragraph (1); and
b
in paragraph (1), omit “the reference to which has been published in the Official Journal”.
Amendment to regulation 37I37321
Regulation 37 (conformity with other standards and requirements) is amended as follows—
a
in paragraph (1)—
i
for “harmonised” substitute “
designated
”
; and
ii
for “which satisfies safety provisions of international standards notified by the Commission” substitute “
which complies with relevant international safety provisions
”
;
b
in paragraph (2), for “harmonised” substitute “
designated
”
; and
c
omit paragraph (3).
Amendment to regulation 38I37422
Regulation 38 (EU declaration of conformity), is amended as follows-
a
in the heading, for “EU declaration” substitute “
Declaration
”
;
b
in the first line of the regulation, omit “EU”.
Amendment to regulation 39I375F22623
1
In regulation 39—
a
in the heading for “CE” substitute “
UK
”
;
b
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly to—
a
the electrical equipment;
b
its data plate; or
c
where paragraph (1A) applies, to—
i
a label affixed to the electrical equipment; or
ii
to a document accompanying the electrical equipment.
c
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the electrical equipment; or
b
to a document accompanying the electrical equipment;
d
in paragraph (2)—
i
after “Where” insert “
paragraph (1A) does not apply and
”
;
ii
for “CE” substitute “
UK
”
(twice).
Amendment to regulation 43I37624
In regulation 43 (exercise of enforcement powers), omit paragraph (c).
Amendment to regulation 45I37725
Regulation 45 (enforcement action in respect of electrical equipment which is not in conformity and which presents a risk) is amended as follows—
a
omit paragraphs (3) and (6);
b
in paragraph (7), for “notices in paragraphs (5) and (6)”, substitute “
notice in paragraph (5)
”
; and
c
in paragraph (7)(f)(ii), for “harmonised” substitute “
designated
”
.
Omission of regulation 46I37826
Omit regulation 46 (EU safeguard procedure).
Amendment to regulation 47I37927
Regulation 47 (enforcement action in respect of electrical equipment which is in conformity, but presents a risk) is amended as follows—
a
omit paragraph (3); and
b
in paragraph (4) for “notices referred to in paragraphs (2) and (3)” substitute “
notice referred to in paragraph (2)
”
.
Amendment to regulation 48I38028
Regulation 48 (enforcement action in cases of formal non-compliance) is amended as follows—
a
in paragraphs (1)(a) and (b) in all places where it occurs, for “CE” substitute “
UK
”
; and
b
in paragraph (1)(b) in all places where it occurs, omit “EU”.
Amendment to regulation 62I38129
In regulation 62, at the end of paragraph (1), insert “
subject to the modification that references to the Community are to be read as including the United Kingdom
”
.
Transitional provision in relation to EU ExitI35430
After Regulation 62 insert—
Transitional provision in relation to EU Exit62A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F227IP completion day;
“product” means electrical equipment to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 23 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M64, any obligation to which a person was subject under these Regulations as they had effect immediately before F227IP completion day, continues to have effect as it did immediately before F227IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the Member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
Amendment to Schedule 2I38231
Schedule 2 (conformity assessment procedures) is amended as follows—
a
in paragraph 2(4)(d)—
i
for “harmonised” substitute “
designated
”
in each place in which it occurs,
ii
omit “the references to which have been published in the Official Journal”;
b
in paragraph 4—
i
omit “EU” in the heading and each place in which it occurs,
ii
for “CE” substitute “
UK
”
in the heading and in sub-paragraph (1).
Amendment to Schedule 8I38332
Schedule 8 (EU declaration of conformity) is amended as follows—
a
in the title of the Schedule, omit “EU”;
b
in the heading before paragraph 1, for “EU declaration” substitute “
Declaration
”
;
c
in paragraph 5, for “Union harmonisation legislation” substitute “
statutory requirements
”
; and
d
in paragraph 6, for “harmonised” substitute “
designated
”
.
SCHEDULE 24Amendment of the Pressure Equipment (Safety) Regulations 2016
IntroductionI9051
The Pressure Equipment (Safety) Regulations 2016 are amended in accordance with paragraphs 2 to 50.
Amendment to regulation 2I3842
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
F228c
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
d
omit the definition of “CE marking”;
e
omit the definition of “Commission”;
f
for the definition of “conformity assessment procedure” substitute—
“conformity assessment procedure” means a procedure for conformity assessment set out in Schedule 1A;
g
after the definition of “conformity assessment procedure” insert—
“declaration of conformity” means a declaration of conformity drawn up in accordance with regulation 48 (declaration of conformity);
“designated standard” has the meaning given to it in regulation 2A;
h
in the definition of “the Directive”, at the end insert “
(as it has effect immediately before F232IP completion day)
”
;
i
omit the definition of “EU declaration of conformity”;
j
omit the definition of “European approval for materials”;
k
omit the definition of “harmonised standard”;
l
for the definition of “importer” substitute—
F229“importer” means a person who—
a
is established in the United Kingdom and places pressure equipment or an assembly from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places pressure equipment or an assembly on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge from an EEA state;
F230m
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
n
omit the definition of “national accreditation body”;
o
omit the definition of “Official Journal”;
F231p
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
q
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
3
Omit paragraphs (5) and (7).
Insertion of paragraph 2AI3863
After regulation 2 (interpretation) insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions; and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; or
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the publication is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other standardisation organisations.
6
The Secretary of State may remove from publication the reference to a standard that has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a product to which these Regulations apply.
9
The Secretary of State may be regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph (9) are to be made by statutory instrument.
11
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 8I3874
In regulation 8 (requirement for pressure equipment and assemblies to comply with sound engineering practice)—
a
in paragraph (2)(a) for “the sound engineering practice of a Member State” substitute “
sound engineering practice
”
; and
b
for paragraph (3) substitute—
3
Pressure equipment and assemblies to which this regulation applies must not bear the UK marking referred to in regulation 49 unless required to do so by other applicable UK legislation.
Insertion of Regulation 8AI3885
After regulation 8 insert—
Power to reclassify pressure equipment and assemblies8A
1
Where the condition in paragraph (2) is met, the Secretary of State may by regulations make provision that pressure equipment or assemblies referred to in regulation 8 are to satisfy the essential requirements in Schedule 2.
2
The condition referred to in paragraph (1) is that the Secretary of State considers that the provision is required to mitigate the effects of very serious safety concerns.
3
Regulations made under paragraph (1)—
a
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament; and
b
include power—
i
to make different provision for different cases; and
ii
to make such supplemental, consequential and transitional provision as the Secretary of State considers appropriate.
Amendment to regulation 10I3896
In regulation 10 (technical documentation and conformity assessment)—
a
in paragraph (2) for “Annex III to the Directive (as amended from time to time)”, substitute “
Schedule 1A to these Regulations
”
; and
b
for “EU-type” in each place where it occurs, substitute “
Type
”
.
Amendment to regulation 11I3907
In regulation 11 (EU declaration of conformity and CE marking)—
a
in the heading—
i
for “EU declaration”, substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a), omit “EU”;
c
in paragraph (1)(b), for “CE” substitute “
UK
”
;
F233ca
in paragraph (1)(c), for “notified” substitute “
approved
”
;
d
in paragraph (3), omit “EU”;
e
for paragraph (4), substitute—
4
Where pressure equipment or an assembly is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 12I3918
In regulation 12 (duty to keep technical documentation and EU declaration of conformity) and in the heading omit “EU”.
Amendment to regulation 13I3929
In regulation 13 (labelling of pressure equipment and assemblies), for paragraph (3) substitute—
3
The details set out in paragraph (1)(b) must be clear, legible and in easily understandable English.
Amendment to regulation 14I39310
In regulation 14 (instructions and safety information)—
a
in paragraphs (1) and (3), for “in a language which can be easily understood by consumers and other users” substitute “
that are clear, legible and in easily understandable English
”
;
b
omit paragraphs (4) and (5).
Amendment to regulation 15I39411
In regulation 15 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “EU”.
Amendment to regulation 17I39512
In regulation 17 (duty to take action in respect of pressure equipment or assemblies placed on the market which are considered not to be in conformity), in paragraph (2), omit “,and the competent national authorities of any other member State in which the manufacturer made the pressure equipment or assembly available on the market,”.
Amendment to regulation 19I39613
In regulation 19 (manufacturer's authorised representatives), in paragraph (2)(a), omit “EU”.
Amendment to regulation 21I39714
In regulation 21 (requirements which must be satisfied before an importer places pressure equipment or assemblies on the market), in paragraph (1)(c)(i), for “CE” substitute “
UK
”
.
Amendment to regulation 23I39815
In regulation 23 (information identifying importer)—
a
in paragraph (2), omit from “in the member State” to the end of the paragraph; and
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on F235the pressure equipment or assembly, or
b
Amendment to regulation 24I39916
In regulation 24 (instructions and safety information)—
a
in paragraphs (1) and (3), for “in a language which can be easily understood by consumers and other users”, substitute “
that are clear, legible and in easily understandable English
”
; and
b
omit paragraph (4).
Amendment to regulation 27I40017
In regulation 27 (duty to take action in respect of pressure equipment or assemblies placed on the market considered not to be in conformity), in paragraph (2), omit “of any other member State in which the importer made the pressure equipment or assembly available on the market”.
Amendment to regulation 28I40118
In regulation 28 (retention of technical documentation and EU declaration of conformity), in the heading and in paragraph (a), omit “EU”.
Amendment to regulation 29I40219
In regulation 29 (provision of information and cooperation), in paragraph (3)(b), for “must be in a language which can be easily understood by the enforcing authority”, substitute “
must be clear, legible and in easily understandable English
”
.
Amendment to regulation 31I40320
In regulation 31 (requirements which must be satisfied before a distributor makes pressure equipment or assemblies available on the market)—
a
in paragraphs (1)(a)(i) and (2)(d) for “CE”, substitute “
UK
”
;
b
for paragraph (1)(a)(iii), substitute “
is accompanied by instructions and safety information that are clear, legible and in easily understandable English
”
;
c
omit paragraph (3).
Amendment to regulation 34I40421
In regulation 34 (duty to take action in respect of pressure equipment made available on the market which are not in conformity), in paragraph (2), omit “of the member States in which the distributor has made the pressure equipment or assembly available on the market”.
Amendment to regulation 35I40522
In regulation 35 (provision of information and cooperation), in paragraph (2)(b), for “must be in a language which can clearly be understood by the enforcing authority”, substitute “
must be clear, legible and in easily understandable English
”
.
Amendment to regulation 37I40623
In regulation 37 (translation of EU declaration of conformity)—
a
in the heading to that regulation, and in paragraph (1), omit “EU”;
b
in paragraph (1), for “prepared in, or translated into, the language required by the member State in which it is to be made available on the market” substitute “
in English
”
; and
c
omit paragraph (2).
Amendment to regulation 39I40724
In regulation 39 (prohibition on improper use of CE marking), and in its heading, for “CE” substitute “
UK
”
in every place in which it occurs.
Insertion of regulations 39A and 39BI40825
After regulation 39 (prohibition on improper use of CE marking), insert—
Obligations which are met by complying with the obligations in the Directive39A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(31); and
c
“pressure equipment and assemblies” means the pressure equipment and assemblies referred to in Article 4(1) and (2).
2
Paragraph (3) applies where, before placing pressure equipment or an assembly on the market, the manufacturer—
a
ensures that the pressure equipment or assembly has been manufactured in accordance with the essential safety requirements set out in Annex I;
b
ensures that the relevant conformity assessment procedures referred to in Article 14 have been carried out;
c
draws up the technical documentation referred to in Annex III;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the identification number of the notified body (where that body is involved in the product control phase) in accordance with Articles 18 and 19(1) to (4);
f
draws up an EU declaration of conformity, in accordance with Article 17; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 9(1), 10 and 11(1) are to be treated as being satisfied;
b
regulations 2(2)(a), 11(3), 12, 15(2), 19(2) and 39 apply subject to the modifications in paragraph (8); and
c
Part 3 does not apply;
d
regulation 74 does not apply.
4
Paragraph (5) applies where, before placing pressure equipment or an assembly on the market, the importer ensures that—
a
the relevant conformity assessment procedure referred to in Article 14 has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III; and
c
the pressure equipment or assembly bears the CE marking and any notified body identification number.
5
Where this paragraph applies—
a
the requirements of regulation 21(1)(a) to (c) are to be treated as being satisfied; and
b
regulations 2(2)(a), 22(1), 25 and 28 apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making pressure equipment or an assembly available on the market, a distributor ensures that the pressure equipment or assembly bears the CE marking.
7
Where this paragraph applies—
a
regulation 31(1)(a)(i) is to be treated as being satisfied; and
b
regulations 2(2)(a) and 33(1) apply subject to the modifications in paragraph (8).
8
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (7)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex I;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard within the meaning of Article 2(24); and
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 14.
Conformity assessment procedure obligation which is met by complying with the Directive.F24139B
1
In this regulation any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive.
2
Paragraph (3) applies where, prior to the manufacture of pressure equipment or an assembly, the manufacturer ensures that the conformity assessment procedure that applies to that pressure equipment or assembly in accordance with Article 14(2), referred to as Module B and set out in Annex III, has been carried out.
3
Where this paragraph applies—
a
the requirement in regulation 42 to follow the conformity assessment procedure referred to in that regulation as Module B is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 10(1)(c), 11(1), 21(1)(a), 39(1)(b) and 48(b) is to be read as including the conformity assessment procedure referred to in Article 14(2), referred to as Module B and set out in Annex III; and
c
any reference to “technical documentation” in regulations 10(1)(d), 21(1)(b) and 28(b) is to be read as including the technical documentation relating to the design of the pressure equipment or assembly referred to as Module B as set out in Annex III.
F242Expiry of regulations 39A and 39B39C
1
Subject to paragraph (2), regulation 39A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 39A—
a
any pressure equipment or assembly which was placed on the market pursuant to regulation 39A may continue to be made available on the market on or after the expiry of regulation 39A;
b
any obligation to which a person was subject under regulation 39A in respect of any pressure equipment or assembly placed on the market pursuant to regulation 39A continues to have effect after the expiry of regulation 39A, in respect of that equipment or assembly.
3
Subject to paragraph (4), regulation 39B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 39B in relation to a pressure equipment or an assembly prior to the expiry of regulation 39B, regulation 39B continues to apply in respect of that pressure equipment or assembly where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after the conformity assessment referred to in the Directive as Module B and set out in Annex III of the Directive, has been carried out.
Qualifying Northern Ireland Goods39D
1
Where paragraph (2) applies any pressure equipment or assembly is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
any pressure equipment or assembly—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the pressure equipment or assembly on the market, the importer—
a
complies with regulation 23;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in accordance with Part 3, as that Part applies in Northern Ireland;
ii
the manufacturer has drawn up the technical documentation; and
iii
the pressure equipment or assembly bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 40I40926
In regulation 40 (presumption of conformity)—
a
in paragraph (1)—
i
for “harmonised”, substitute “
designated
”
; and
ii
omit “the reference to which has been published in the Official Journal”;
b
omit paragraph (2);
c
for paragraph (3) substitute—
3
The presumption in paragraph (1) is rebuttable.
Amendment to regulation 42I41027
In regulation 42 (conformity assessment procedures), in paragraph (1) for “Annex III to the Directive (as amended from time to time)”, substitute “
Schedule 1A to these Regulations
”
.
Insertion of regulation 42AI41128
After regulation 42 insert—
Power to amend applicable module42A
1
Where in order to mitigate the effects of very serious safety concerns the Secretary of State considers that an item or family of pressure equipment are to be subject to different categories of modules, the Secretary of State may by regulations make such provision.
2
Regulations made under paragraph (1)—
a
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament; and
b
include power—
i
to make different provision for different cases; and
ii
to make such supplemental, consequential and transitional provision as the Secretary of State considers appropriate.
Amendment to regulation 43I41229
In regulation 43, in paragraph (4), for “Annex III to the Directive (as amended from time to time)”, substitute “
Schedule 1A to these Regulations
”
.
Amendment to regulation 45I41330
In regulation 45 (conformity assessment procedures), in paragraph (a), for “CE”, substitute “
UK
”
.
Amendment to regulation 47I41431
For regulation 47 (conformity assessment procedures), substitute—
47
The records and correspondence relating to conformity assessment must be clear, legible and in easily understandable English.
Amendment to regulation 48I41532
In regulation 48 (EU declaration of conformity)—
a
in the heading and in the opening words of the regulation, omit “EU”; and
b
in paragraph (b), for “Annex III to the Directive (as amended from time to time)”, substitute “
Schedule 1A to these Regulations
”
.
Amendment to regulation 49I41633
In regulation 49 (CE marking)—
a
in the heading and in each place in which it occurs, for “CE” substitute “
UK
”
;
b
in each place in which it occurs for “notified” substitute “
approved
”
.
F243c
in paragraph (1)(b) for “dataplate.” substitute “
data plate; or
”
;
d
after paragraph (1)(b) insert—
c
where paragraph (1A) applies—
i
a label affixed to the pressure equipment or assembly; or
ii
to a document accompanying the pressure equipment or assembly.
e
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the pressure equipment or assembly; or
b
to a document accompanying the pressure equipment or assembly.
f
in paragraph (3) after “Where” insert “
paragraph (1A) does not apply and
”
.
Omission of regulation 50I41734
Omit regulation 50 (European approval for materials).
Substitution of Part 4I41835
For Part 4 substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies51
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 54 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 60 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F244IP completion day notified to the European Commission and the member States of the European Union as a notified body, in accordance with Article 20 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 51(1)(b), as it had effect immediately before F244IP completion day;
“approved body requirements” means the requirements set out in Schedule 4;
“product” means pressure equipment or assemblies;
“accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Recognised third party organisations52
1
A recognised third party organisation is a conformity assessment body which—
a
has been approved by the Secretary of State to be a recognised third party organisation, under regulation 54 (approval of conformity assessment bodies); or
b
immediately before F245IP completion day—
i
was a conformity assessment body which the Secretary of State had before F245IP completion day notified to the European Commission and the member States of the European Union as a recognised third party organisation, in accordance with Article 20 of the Directive;
ii
in respect of which no objections had been raised, as referred to in regulation 52(1)(b), as it had effect immediately before F245IP completion day; and
iii
in respect of which the Secretary of State had taken no action under regulation 62(1) or (2), as they had effect immediately before F245IP completion day to suspend or withdraw the body's status as a recognised third party organisation.
2
Paragraph (1) has effect subject to regulation 60 (restriction, suspension or withdrawal of approval).
User inspectorates53
1
A user inspectorate is a conformity assessment body which—
a
has been approved as a user inspectorate by the Secretary of State under regulation 54 (approval of conformity assessment bodies); or
b
immediately before F246IP completion day—
i
was a conformity assessment body which the Secretary of State had before F246IP completion day notified to the European Commission and the member States of the European Union as a user inspectorate, in accordance with Article 20 of the Directive;
ii
in respect of which no objections had been raised, as referred to in regulation 53(1)(b), as it had effect immediately before F246IP completion day; and
iii
in respect of which the Secretary of State had taken no action under regulation 62(1) or (2), as they had effect immediately before F246IP completion day, to suspend or withdraw the body's status as a recognised third party organisation.
2
Paragraph (1) has effect subject to regulation 61 (restriction, suspension or withdrawal of approval (user inspectorates)).
Approval of conformity assessment bodies54
1
The Secretary of State may approve only those conformity assessment bodies which—
a
qualify for approval as an approved body in accordance with regulation 55;
b
qualify for approval as a recognised third party organisation in accordance with regulation 56; or
c
qualify for approval as a user inspectorate in accordance with regulation 57.
2
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
Approval of approved bodies55
1
A conformity assessment body qualifies for approval as an approved body if the first and second conditions below are met.
2
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of products in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate, or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
3
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
4
For the purposes of paragraph (3), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (2)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
Approval of recognised third party organisations56
1
A conformity assessment body qualifies for approval as a recognised third party organisation if the conditions in paragraphs (2), (3) and (4) are met.
2
The first condition is that the conformity assessment body has applied to the Secretary of State to become a recognised third party organisation and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of products in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate, or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
3
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
4
The third condition is that the conformity assessment body must carry out approvals of only those activities referred to in paragraphs 21 and 22 of Schedule 2 (permanent joining and non-destructive tests).
5
For the purposes of paragraph (3), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (2)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
Approval of user inspectorates57
1
A conformity assessment body qualifies for approval as a user inspectorate if the conditions in paragraphs (2) to (7) are met.
2
The conformity assessment body must apply to the Secretary of State to become a user inspectorate and that application must be accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of products in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate, or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the user inspectorate requirements.
3
The Secretary of State must be satisfied that the conformity assessment body meets the user inspectorate requirements.
4
The conformity assessment procedures which a user inspectorate may carry out are modules A2, C2, F and G, set out in Part 2, Part 4, Part 9 and Part 10 of Schedule 1A respectively.
5
The group of which the user inspectorate is part must apply a common safety policy as regards the technical specifications for the design, manufacture, inspection, maintenance and use of products.
6
The user inspectorate must act exclusively for the group of which it is part.
7
Where the conformity of a product has been assessed by a user inspectorate, that product may only be used in establishments operated by the group of which the user inspectorate is part.
8
For the purposes of paragraph (3), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (2)(b), as sufficient evidence that the conformity assessment body meets the user inspectorate requirements.
Presumption of conformity of conformity assessment bodies58
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements or the user inspectorate requirements (as the case may be) covered by that standard (or part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring59
The Secretary of State must monitor each approved body, recognised third party organisation and user inspectorate with a view to verifying that the body—
a
continues to meet the approved body requirements or user inspectorate requirements, as applicable;
b
meets any conditions set—
i
in accordance with regulation 54(2)(b), or
ii
in the case of—
aa
an approved body which was a notified body immediately before F247IP completion day;
bb
a recognised third party organisation falling within regulation 52(1)(b); or
cc
a user inspectorate falling within regulations 53(1)(b);
in accordance with regulation 55(2)(b) as it applied immediately before F247IP completion day; and
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval (approved bodies and recognised third party organisations)60
1
Where the Secretary of State determines that an approved body or a recognised third party organisation—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 59(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body or a recognised third party organisation under regulation 51 or 52 (as the case may be).
2
Where the Secretary of State determines that an approved body or a recognised third party organisation no longer meets a condition referred to in regulation 59(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body or a recognised third party organisation under regulation 51 or 52 (as the case may be).
3
In deciding what action is required under paragraph (1) or (2) the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2) the Secretary of State must—
a
give notice in writing to the approved body or recognised third party organisation of the proposed action and the reasons for it;
b
give the approved body or recognised third party organisation an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body or recognised third party organisation.
5
Where the Secretary of State has taken action in respect of an approved body or recognised third party organisation under paragraph (1) or (2), or where an approved body or recognised third party organisation has ceased its activity, the approved body or recognised third party organisation must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body or recognised third party organisation to another approved body or recognised third party organisation or to the Secretary of State, or
b
keep its files relating to the activities it has undertaken as an approved body or recognised third party organisation available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken by an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Restriction, suspension or withdrawal of approval (user inspectorates)61
1
Where the Secretary of State determines that a user inspectorate—
a
no longer meets a user inspectorate requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 59(b),
the Secretary of State must restrict, suspend or withdraw the body's status as a user inspectorate under regulation 53.
2
Where the Secretary of State determines that a user inspectorate no longer meets a condition referred to in regulation 59(b), the Secretary of State may restrict, suspend or withdraw the body's status as a user inspectorate under regulation 53.
3
In deciding what action is required under paragraph (1) or (2) the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2) the Secretary of State must—
a
give notice in writing to the user inspectorate of the proposed action and the reasons for it;
b
give the user inspectorate an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the user inspectorate.
5
Where the Secretary of State has taken action in respect of a user inspectorate under paragraph (1) or (2), or where a user inspectorate has ceased its activity, the user inspectorate must at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as a user inspectorate to an approved body, a recognised third party organisation or to the Secretary of State, or
b
keep its files relating to the activities it has undertaken as a user inspectorate available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
Operational matters in relation to approved bodies, recognised third party organisations and user inspectorates62
1
Subject to the terms of its appointment, an approved body, recognised third party organisation or user inspectorate must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 55, 56 or 57 (as the case may be); or
b
in respect of which the body's notification to the European Commission was made as a notified body, a recognised third party organisation or a user inspectorate (as the case may be).
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 6.
3
An approved conformity assessment body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Schedule 1A, or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 49 (UK marking), where applicable.
Subsidiaries and contractors63
1
An approved body, recognised third party organisation or user inspectorate may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body, organisation or inspectorate is satisfied that the subcontractor or subsidiary meets the approved body requirements or user inspectorate requirements, as applicable;
b
the body, organisation or inspectorate has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meet those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body, recognised third party organisation or user inspectorate which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body, recognised third party organisation or user inspectorate subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the body, organisation or inspectorate must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documents concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M65.
Register of approved bodies64
1
The Secretary of State must—
a
assign—
i
an approved body identification number to each approved body;
ii
a recognised third party organisation identification number to each third party organisation;
iii
a user inspectorate identification number to each user inspectorate; and
b
compile and maintain a register of—
i
approved bodies, recognised third party organisations and user inspectorates;
ii
their identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
United Kingdom Accreditation Service65
The Secretary of State may authorise the United Kingdom Accreditation Service to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements or user inspectorate requirements (as applicable);
b
monitoring approved bodies, recognised third party organisations and user inspectorates in accordance with regulation 59; and
c
compiling and maintaining the register of approved bodies, recognised third party organisations and user inspectorates, in accordance with regulation 64.
Amendment to regulation 69I41936
In regulation 69 (exercise of enforcement powers) omit paragraph (c).
Amendment to regulation 71I42037
In regulation 71 (enforcement action in respect of pressure equipment or assemblies which are not in conformity and which present risk)—
a
in paragraph (2), for “notified”, substitute “
approved
”
;
b
omit paragraphs (3), (4) and (7);
c
in paragraph (8), for “notices in paragraphs (6) and (7)”, substitute “
notice in paragraph (6)
”
; and
d
in paragraph (8)(f)(ii) for “harmonised” substitute “
designated
”
.
Omission of regulation 72I42138
Omit regulation 72 (EU safeguard procedure).
Amendment to regulation 73I42239
In regulation 73 (pressure equipment or assemblies which are in conformity, but present a risk)—
a
omit paragraph (3); and
b
in paragraph (4), for “notices referred to in paragraphs (2) and (3)”, substitute “
notice referred to in paragraph (2)
”
.
Amendment to regulation 74I42340
In regulation 74 (enforcement action in cases of formal non-compliance)—
a
in paragraphs (1)(a) and (1)(c)(ii) for “CE” substitute “
UK
”
in each place in which it occurs;
F248aa
in paragraph (1)(b)—
i
for “a notified” substitute “
an approved
”
;
ii
for “the notified” substitute “
the approved
”
;
b
in paragraph (1)(c) omit “EU” in each place in which it occurs; and
c
in paragraph (4) omit “, 72 (EU safeguard procedure)”.
Insertion of regulation 88AI38541
After regulation 88 (transitional provisions) insert—
Transitional provision in relation to EU Exit88A
1
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 24 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M66, any obligation to which a person was subject under these Regulations as they had effect immediately before F249IP completion day, continues to have effect as it did immediately before F249IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 42 as it had effect immediately before F249IP completion day in relation to that product
that action has effect as if it had been done under regulation 42 as it had effect on and after F249IP completion day.
Amendment to regulation 90I42442
In regulation 90 (revocations, amendments and savings)—
a
in paragraph (1) after “paragraph (2)” insert “
and (2A)
”
;
b
for paragraph (2), substitute—
2
Subject to the modifications made in paragraph (2A), the Regulations referred to in paragraph (1) continue to apply, as if they had not been revoked, to pressure equipment or assemblies placed on the market before the commencement date.
2A
The modifications referred to in paragraph (2) are that in the 1999 Regulations—
a
references to “the Community” shall be read as including the United Kingdom;
b
references to a “member State” shall be read as including the United Kingdom; and
c
in Schedule 8 (enforcement), in paragraph 6, omit “with a view to this information being passed by him to the Commission”.
Amendment to Schedule 1I42543
In Schedule 1 (excluded pressure equipment and assemblies), paragraph 1—
a
b
for paragraph (f) substitute—
f
for equipment classified as no higher than category I in accordance with Schedule 1B to these Regulations and, to which, one of the following applies—
i
the Supply of Machinery (Safety) Regulations 2008 M69;
ii
the Lift Regulations 2016 M70;
iii
the Electrical Equipment (Safety) Regulations 2016 M71;
iv
the Medical Devices Regulations 2002 M72;
v
Regulation 2016/426 of the European Parliament and of the Council of 9 March on appliances burning gaseous fuels;
vi
the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016 M73.
c
for paragraph (g) substitute—
g
products connected with the production of trade in arms, munitions and war material;
Insertion of Schedule 1A and 1BI42644
After Schedule 1 insert—
SCHEDULE 1AConformity Assessment Procedures for Pressure Equipment and Assemblies
PART 1Module A: Internal Production Control
General1
Internal production control is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 4 and ensures and declares on their sole responsibility that the pressure equipment concerned satisfy the requirements of these Regulations.
Technical documentation2
1
The manufacturer shall establish the technical documentation.
2
The technical documentation shall—
a
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
b
include an adequate analysis and assessment of the risk;
c
specify the applicable requirements and contain, where applicable—
i
a general description;
ii
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits, and all other relevant parts, to component level;
iii
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
iv
a list of the designated standards;
v
results of design calculations made, examinations carried out and the results of any other relevant calculation or examination;
vi
test reports;
vii
manufacture; and
viii
operation,
of the pressure equipment or assembly.
3
The technical documentation must be kept at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured pressure equipment with the technical documentation referred to in paragraph 2 and the requirements of these Regulations.
UK marking and declaration of conformity4
The manufacturer shall—
a
affix the UK marking to each individual piece of pressure equipment, or assembly, that satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request; and
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Authorised representative5
The manufacturer's obligations set out in paragraph 4 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 2Module A2: Internal production control plus supervised pressure equipment checks at random
General6
Internal production control plus supervised pressure equipment checks at random intervals is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 ,4 and 5, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned satisfies the requirements of these Regulations.
Technical documentation7
1
The manufacturer shall establish the technical documentation.
2
The technical documentation shall—
a
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
b
include an adequate analysis and assessment of the risk;
c
specify the applicable requirements and contain, where applicable—
i
a general description;
ii
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits, and all other relevant parts, to component level;
iii
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
iv
a list of the designated standards;
v
results of design calculations made, examinations carried out, and the results of any other relevant calculation or examination;
vi
test reports;
vii
manufacture; and
viii
operation
of the pressure equipment or assembly.
4
The technical documentation must be kept at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Manufacturing8
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured pressure equipment with the technical documentation referred to in paragraph 7 and the requirements of these Regulations.
Final assessment and pressure equipment, assembly, checks9
1
The manufacturer shall perform a final assessment of the pressure equipment, or assembly, monitored by means of unexpected visits by an approved body chosen by the manufacturer.
2
The approved body shall carry out, or have carried out for them, product checks which shall—
a
be carried out at random intervals determined by the approved body;
b
verify the quality of the internal checks of the pressure equipment, or assembly (taking into account the technological complexity of the equipment, or assembly, and the quantity of production);
c
establish that the manufacturer performs final assessment in accordance with paragraphs 25 to 28 of Schedule 2 to these Regulations;
d
take samples of pressure equipment and assemblies at the manufacturing or storage premises in order to conduct checks (the approved body assesses the number of items of equipment to sample and whether it is necessary to perform, or have performed, all or part of the final assessment of the samples).
3
The acceptance sampling procedure to be applied is intended to determine whether the manufacturing process of the pressure equipment, or assembly, performs within acceptable limits, with a view to ensuring conformity of the pressure equipment, or assembly.
4
The approved body shall take appropriate measures where an item of pressure equipment or assembly does not conform.
5
The manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking and declaration of conformity10
The manufacturer shall—
a
affix the UK marking to each individual pressure equipment, or assembly, that satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request; and
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Authorised representative11
The manufacturer's obligations set out in paragraph 10 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 3Module B: Type examination
Type examination–production type
12
Type examination–production type is the part of a conformity assessment procedure in which an approved body examines the technical design of the pressure equipment, or assembly, and verifies and attests that the technical design of the pressure equipment meets the requirements of these Regulations.
13
Type examination–production type shall consist of an assessment of the adequacy of the technical design of the pressure equipment, or assembly, through examination of the technical documentation and supporting evidence referred to in paragraph 14, plus examination of a specimen, representative of the production envisaged, of the complete pressure equipment or assembly.
14
The manufacturer shall lodge an application with a single approved body of their choice. The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application had not been lodged with any other approved body;
c
the technical documentation which shall—
i
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
ii
include an adequate analysis and assessment of the risk;
iii
specify the applicable requirements and contain, where applicable—
aa
a general description;
bb
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits, and all other relevant parts, to component level;
cc
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
dd
a list of the designated standards;
ee
results of design calculations made, examinations carried out, and the results of any other relevant calculation or examination;
ff
test reports;
gg
information concerning the tests provided for in manufacture;
hh
information concerning the qualifications and approvals required under paragraphs 21 and 22 of Schedule 2 (essential safety requirements);
ii
manufacture; and
jj
operation;
d
specimens representative of the product envisaged which—
i
may cover several versions of the pressure equipment or assembly (provided that the differences between the versions do not affect the level of safety);
ii
the approved body may request further of, if needed for carrying out the test programme;
e
supporting evidence for the adequacy of the technical design solution which shall—
i
mention any documents that have been used, in particular where the relevant designated standards have not been applied in full; and
ii
include, where necessary, the results of tests carried out—
aa
by the appropriate laboratory of the manufacturer applying other relevant technical specifications; or
bb
by any other testing laboratory on their behalf and under their responsibility.
15
The approved body shall—
a
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the pressure equipment, or assembly, and the manufacturing procedures;
b
where the materials are not in conformity with the relevant designated standards, assess the materials and check the certificate issued by the material manufacturer in accordance with subparagraphs 31(5) to (8) of Schedule 2 to these Regulations;
c
approve the procedures for the permanent joining of pressure equipment, or assembly, parts, or check that they have been previously approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
d
verify that the personal undertaking in the permanent joining of pressure equipment, or assembly, parts and the non-destructive tests are qualified or approved in accordance with paragraphs 21 or 22 of Schedule 2 to these Regulations;
e
verify that the specimens have been manufactured in conformity with the technical documents and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards as well as the elements which have been designed using other relevant technical specifications without applying the relevant provisions of those standards;
f
carry out appropriate examinations and necessary tests to check whether—
i
when the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
ii
where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential safety requirements of these Regulations;
g
agree, with the manufacturer, on a location where the examinations and tests will be carried out;
h
draw up an evaluation report—
i
recording the activities undertaken, in accordance with this paragraph, and their outcomes; and
ii
only release the content, in full or in part, with the agreement of the manufacturer.
16
Where the type meets the requirements of these Regulations, the approved body shall issue a Type examination–production type certificate to the manufacturer.
17
The Type examination-production type certificate shall—
a
include—
i
the name and address of the manufacturer;
ii
the conclusions of the examination;
iii
any conditions for the certificate's validity; and
iv
necessary data for identification of the approved type;
b
include an annex listing the relevant parts of the technical documentation, a copy of which shall be kept by the approved body;
c
contain all relevant information to allow the conformity of manufactured equipment pressure equipment, or assemblies, with the examined type to be evaluated and to allow for in-service control;
d
be valid for 10 years, without prejudice to paragraphs 20 and 21, and be renewable.
18
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a Type examination-production type certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
19
Provision shall be made for an appeals procedure.
20
The approved body shall keep itself appraised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
21
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination-production type certificate of all modifications to the approved type that may affect the conformity of the pressure equipment, or assembly, with the essential safety requirements of these Regulations or the conditions for validity of the certificate. Such modifications shall require additional approval in the form of an addition to the original Type examination-production type certificate.
22
Each approved body shall inform the Secretary of State concerning Type examination-production type certificates and any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the enforcing authorities the list of certificates and any additions thereto refused, suspended or otherwise restricted.
23
Each approved body shall inform the other approved bodies concerning the Type examination-production type certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted and, upon request, concerning the certificates and additions thereto that it has issued.
24
Other approved bodies may, on request, obtain a copy of the Type examination-production type certificate and additions thereto.
25
The approved body shall keep a copy of the Type examination-production type certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of the certificate.
26
The manufacturer shall keep a copy of the Type examination-production type certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
27
The manufacturer's authorised representative may lodge the application referred to in paragraph 14 and fulfil the obligations set out in paragraphs 21 and 26, provided that they are specified in the mandate.
Type examination–design type
28
Type examination-design type is the part of a conformity assessment procedure in which an approved body examines the technical design of the pressure equipment, or assembly, and verifies and attests that the technical design of the pressure equipment, or assembly, meets the requirements of these Regulations.
29
Type examination-design type shall consist of an assessment of the adequacy of the technical design of the pressure equipment through examination of the technical documentation and supporting evidence referred to in paragraph 31, without examination of a specimen.
30
The experimental design method provided for at paragraph 6 of Schedule 2 to these Regulations shall not be used in the context of this module.
31
The manufacturer shall lodge an application with a single approved body of their choice. The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application had not been lodged with any other approved body;
c
the technical documentation which shall—
i
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
ii
include an adequate analysis and assessment of the risk;
iii
specify the applicable requirements and contain, where applicable—
aa
a general description;
bb
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits and all other relevant parts, to component level;
cc
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
dd
a list of the designated standards;
ee
information concerning the qualifications and approvals required under paragraphs 21 and 22 of Schedule 2;
d
supporting evidence for the adequacy of the technical design solution which shall—
i
mention any documents that have been used, in particular where the relevant designated standards have not been applied in full; and
ii
include, where necessary, the results of tests carried out—
aa
by the appropriate laboratory of the manufacturer applying other relevant technical specifications; or
bb
by any other testing laboratory on their behalf and under their responsibility.
32
The application may cover several versions of the pressure equipment, or assembly, provided that the differences between the versions do not affect the level of safety.
33
The approved body shall—
a
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the product;
b
assess the materials where they are not in conformity with the relevant designated standards;
c
approve the procedures for the permanent joining of pressure equipment, or assembly, parts or check that they have been previously approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
d
carry out appropriate examinations and necessary tests to check whether—
i
when the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
ii
where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential safety requirements of these Regulations;
e
draw up an evaluation report—
i
recording the activities undertaken, in accordance with this paragraph, and their outcomes;
ii
only release the content, in full or in part, with the agreement of the manufacturer.
34
Where the design meets the requirements of these Regulations, the approved body shall issue a Type examination–design type certificate to the manufacturer.
35
The Type examination-design certificate type shall—
a
include—
i
the name and address of the manufacturer;
ii
the conclusions of the examination;
iii
any conditions for the certificate's validity; and
iv
necessary data for identification of the approved type;
b
include an annex listing the relevant parts of the technical documentation, a copy of which shall be kept by the approved body;
c
contain all relevant information to allow the conformity of manufactured pressure equipment, or assemblies, with the examined design to be evaluated and to allow for in-service control;
d
be valid for 10 years, without prejudice to paragraphs 36 and 37, and be renewable.
36
Where the design does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a Type examination-design type certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
37
The approved body shall keep itself appraised of any changes in the generally acknowledged state of the art which indicate that the approved design may no longer comply with the applicable requirements of these Regulations and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
38
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination-design type certificate of all modifications to the approved type that may affect the conformity of the pressure equipment, or assembly, with the essential safety requirements of these Regulations or the conditions for validity of the certificate. Such modifications shall require additional approval in the form of an addition to the original Type examination-design type certificate.
39
Each approved body shall inform its approved authority concerning Type examination-design type certificates and any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to its approved authorities the list of certificates and any additions thereto refused, suspended or otherwise restricted.
40
Each approved body shall inform the other approved bodies concerning the Type examination-design type certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted and, upon request, concerning the certificates and additions thereto that it has issued.
41
Other approved bodies may, on request, obtain a copy of the Type examination-design type certificate and additions thereto.
42
The approved body shall keep a copy of the Type examination-design type certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of the certificate.
43
The manufacturer shall keep a copy of the Type examination-design type certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
44
The manufacturer's authorised representative may lodge the application referred to in paragraph 31 and fulfil the obligations set out in paragraphs 37 and 42, provided that they are specified in the mandate.
PART 4Module C2: Conformity to type based on internal production control plus supervised pressure equipment checks at random intervals
General45
Conformity to type based on internal production control plus supervised pressure equipment checks at random intervals is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 46, 47 and 48, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned is in conformity with the type described in the Type examination certificate and satisfies the requirements of these Regulations that apply to it.
Manufacturing46
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured pressure equipment, or assembly, with the type described in the Type examination certificate and with the requirements of these Regulations.
Final assessment and pressure equipment check47
1
The manufacturer shall choose an approved body to carry out checks, or have them carried out, at random intervals determined by that body.
2
Checks carried out by the approved body shall—
a
verify the quality of the final assessment;
b
verify the quality of the internal checks,
taking into account the technological complexity of the pressure equipment, or assembly, and the quantity of production.
3
The approved body shall establish that the manufacturer actually performs the final assessment in accordance with paragraphs 25 to 28 of Schedule 2 to these Regulations.
4
An adequate sample of the final pressure equipment, or assembly, taken on-site by the approved body before placing on the market, shall be examined and appropriate tests as identified by the relevant parts of the designated standards, or equivalent test applying other technical specifications, shall be carried out to check the conformity of the pressure equipment, or assembly, with the relevant requirements of these Regulations.
5
The approved body shall assess the number of items of equipment to sample and whether it is necessary to perform, or have performed, all or part of the final assessment on pressure equipment, or assembly, samples.
6
Where a sample does not conform to the acceptable quality level, the approving body shall take appropriate measures.
7
The acceptance sampling procedure to be applied is intended to determine whether the manufacturing process of the pressure equipment performs within acceptable limits, with a view to ensuring conformity of the pressure equipment or assembly.
8
Where the tests are carried out by an approved body, the manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking and declaration of conformity48
The manufacturer shall—
a
affix the UK marking to each individual pressure equipment, or assembly, that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request; and
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Authorised representative49
The manufacturer's obligations set out in paragraph 47 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 5Module D: Conformity to type based on quality assurance in the production process
General50
Conformity to type based on quality assurance in the production process is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 51 and 54 and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned is in conformity with the type described in the Type examination certificate and satisfies the requirements of these Regulations that apply to it.
Manufacturing51
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the pressure equipment, or assembly, concerned as specified in paragraph 52 and shall be subject to surveillance as specified in paragraph 53.
Quality system52
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information on the pressure equipment, or assembly, type envisaged;
d
the documentation concerning the quality; and
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure that the pressure equipment is in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations.
4
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to the quality of the pressure equipment or assembly;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used, particularly the procedures used for the permanent joining of parts as approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
e
the means of monitoring the achievement of the required quality and the effective operation of the quality system.
5
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems;
d
ensure that the audit—
i
is carried out by a team containing at least one member with experience in the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises;
iii
reviews the technical documentation referred to in paragraph 52, to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
6
The decision shall—
a
be notified to the manufacturer;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
7
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
8
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system informed of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body53
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the quality records, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned;
d
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, will be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
UK marking and declaration of conformity54
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 51(1), the latter's identification number, to each individual piece of pressure equipment, or assembly, that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
for a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation referred to in paragraph 52(1);
ii
any change referred to in paragraph 52(8)(a), as approved; and
iii
the decisions and reports of the approved body referred to in paragraphs 52(6) and (8) and 53(2) to (4),
2
Each approved body shall inform the Secretary of State of the quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Each approved body shall inform the other approved bodies of the quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative55
The manufacturer's obligations set out in paragraph 52(1) and (8) and paragraph 54 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 6Module D1: Quality assurance of the production process
General56
Quality assurance of the production process is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 57, 58 and 61, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned satisfies the requirements of these Regulations.
Technical documentation57
1
The manufacturer shall establish the technical documentation.
2
The technical documentation shall—
a
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
b
include an adequate analysis and assessment of the risk;
c
specify the applicable requirements and contain, where applicable—
i
a general description of the individual piece of equipment or the assembly;
ii
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits and all other relevant parts, to component level;
iii
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
iv
a list of the designated standards;
v
results of design calculations made, examinations carried out the results of any other relevant calculation or examination; and
vi
test reports.
3
The technical documentation must be kept at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Manufacturing58
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the pressure equipment, or assembly, concerned as specified in paragraph 59 and shall be subject to surveillance as specified in paragraph 60.
Quality system59
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information on the pressure equipment, or assembly, type envisaged;
d
the documentation concerning the quality system; and
e
the technical documentation referred to in paragraph 57.
3
The quality system shall ensure compliance of the pressure equipment, or assembly, with the requirements of these Regulations that apply to it.
4
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to the quality of the pressure equipment or assembly;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used, particularly the procedures used for the permanent joining of parts as approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
e
the means of monitoring the achievement of the required quality and the effective operation of the quality system.
5
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems; and
d
ensure that the audit—
i
is carried out by a team containing at least one member with experience in the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises; and
iii
reviews the technical documentation referred to in paragraph 56, to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
6
The decision shall—
a
be notified to the manufacturer;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
7
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
8
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system informed of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body60
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the technical documentation referred to in paragraph 57;
d
provide the quality records, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, etc.;
e
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, should be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits, the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
UK marking and declaration of conformity61
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 58(1), the latter's identification number, to each individual piece of pressure equipment, or assembly, that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
For a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation referred to in paragraphs 59(1) and (2);
ii
the change referred to in paragraph 59(8); and
iii
the decisions and reports of the approved body referred to in paragraphs 58(8) and 60(2) to (4),
2
Each approved body shall inform the Secretary of State of the quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Each approved body shall inform the other approved bodies of the quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative62
The manufacturer's obligations set out in paragraphs 59(1), (2) and (8) and paragraph 61 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 7Module E: Conformity to type based on pressure equipment quality assurance
General63
Conformity to type based on pressure equipment quality assurance is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 64 and 67, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned is in conformity with the type described in the Type examination certificate and satisfies the requirements of these Regulations.
Manufacturing64
The manufacturer shall operate an approved quality system for the final product inspection and testing of the pressure equipment, or assembly, concerned as specified in paragraph 65 and shall be subject to surveillance as specified in paragraph 66.
Quality system65
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information on the pressure equipment, or assembly, type envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure compliance of the products with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
4
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to the quality of the pressure equipment or assembly;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
d
the means of monitoring the effective operation of the quality system.
5
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems;
d
ensure that the audit—
i
is carried out by a team containing at least one member with experience in the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises;
iii
reviews the technical documentation referred to in paragraph 65(2)(e), to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
6
The decision shall—
a
be notified to the manufacturer;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
7
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
8
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system informed of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body66
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the technical documentation;
d
provide the quality records, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, etc.;
e
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, should be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits, the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
UK marking and declaration of conformity67
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 65(1), the latter's identification number, to each individual piece of pressure equipment, or assembly, that is conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
for a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation referred to in paragraphs 65(1) and (2);
ii
the change referred to in paragraph 65(8); and
iii
the decisions and reports of the approved body referred to in paragraphs 65(5) and (8) and 66(2) and (3).
2
Each approved body shall inform the Secretary of State of the quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Each approved body shall inform the other approved bodies of the quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative68
The manufacturer's obligations set out in paragraphs 65(1), (2) and (8) and paragraph 67 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate agreed between the manufacturer and representative.
PART 8Module E1: Quality assurance of final pressure equipment inspection and testing
General69
Quality assurance of final pressure equipment inspection and testing is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 70 (technical documentation), 71 (manufacturing) and 74 (UK marking and declaration of conformity), and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned satisfies the requirements of these Regulations that apply to it.
Technical documentation70
1
The manufacturer shall establish the technical documentation. The technical documentation shall—
a
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
b
include an adequate analysis and assessment of any risk;
c
specify the applicable requirements and contain, where applicable—
i
a general description;
ii
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits, and all other relevant parts, to component level;
iii
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
iv
a list of the designated standards;
v
results of design calculations made, examinations carried out and the results of any other relevant calculation or examination; and
vi
test reports.
2
The technical documentation must be kept at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Manufacturing71
The manufacturer shall operate an approved quality system for the final product inspection and testing of the pressure equipment, or assembly, concerned as specified in paragraph 72 and shall be subject to surveillance as specified in paragraph 73.
Quality system72
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information on the pressure equipment, or assembly, type envisaged;
d
the documentation concerning the quality system;
e
the technical documentation referred to in paragraph 70.
3
The quality system shall ensure compliance of the pressure equipment, or assembly, with the requirements of these Regulations that apply to it.
4
Under the quality system, each item of pressure equipment, or assembly, shall be examined and appropriate tests as set out in the designated standards and particularly final assessments as set out in paragraphs 25 to 28 of Schedule 2 shall be carried out in order to ensure its conformity with the requirements of these Regulations.
5
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to the quality of the pressure equipment or assembly;
b
the procedures used for the permanent joining of parts as approved with paragraph 21 of Schedule 2 to these Regulations;
c
the examinations and tests that will be carried out after manufacture;
d
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
e
the means of monitoring the achievement of the required quality and the effective operation of the quality system.
6
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems;
d
ensure that the audit—
i
is carried out by a team containing at least one member with experience in the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises;
iii
reviews the technical documentation referred to in paragraph 70, to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
7
The decision shall—
a
be notified to the manufacturer;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
8
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
9
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system informed of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body73
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the technical documentation referred to in paragraph 70;
d
provide the quality records, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, etc.;
e
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, should be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
UK marking and declaration of conformity74
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 72(1), the latter's identification number, to each individual piece of pressure equipment, or assembly, that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
for a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation referred to in paragraphs 70(1) and (2);
ii
the change referred to in paragraph 72(9); and
iii
the decisions and reports of the approved body referred to in paragraphs 72(9) and 73(2) to (4).
2
Each approved body shall inform the Secretary of State of the quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Each approved body shall inform the other approved bodies of the quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative75
The manufacturer's obligations set out in paragraphs 72(1), (2) and (9) and paragraphs 70 and 74 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 9Module F: Conformity to type based on pressure equipment verification
General76
Conformity to type based on pressure equipment verification is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 77 and 80, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned, which has been subject to the provisions of paragraph 78, is in conformity with the type described in the Type examination certificate and satisfies the requirements of these Regulations which apply to it.
Manufacturing77
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the approved type described in the Type examination certificate and with the requirements of these Regulations which apply to them.
Verification78
1
An approved body chosen by the manufacturer shall carry out the appropriate examinations and tests in order to check the conformity of the pressure equipment, or assembly, with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
2
The examinations and tests to check the conformity of the pressure equipment with the appropriate requirements shall be carried out by examination and testing of every product as specified in paragraph 79.
Verification of conformity by examination and testing of every item of pressure equipment or assembly79
1
All pressure equipment, or assemblies, shall be individually examined and appropriate tests set out in the relevant designated standards or equivalent tests shall be carried out in order to verify conformity with the approved type and described in the Type examination certificate and with the appropriate requirements of these Regulations.
2
In the absence of such a designated standard, the approved body concerned shall decide on the appropriate tests to be carried out.
3
The approved body shall—
a
verify that the personnel undertaking the permanent joining of parts and the non-destructive tests are qualified or approved in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations;
b
verify the certificate issued by the materials manufacturer in accordance with paragraphs 31(6) to (8) of Schedule 2 to these Regulations; and
c
carry out or have carried out the final inspection and proof test referred to in paragraphs 25 to 28 to Schedule 2 of these Regulations.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number or have it affixed under its responsibility to each approved item of pressure equipment or assembly.
5
The manufacturer shall keep certificates of conformity available for inspection by the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
UK marking and declaration of conformity80
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 78(1), the latter's identification number, to each individual item of pressure equipment, or assembly, that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for each pressure equipment model, or assembly, which identifies the pressure equipment model, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market; and
e
if the approved body referred to in paragraph 78(1) agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the pressure equipment, or assembly, during the manufacturing process.
Authorised representative81
The manufacturer's obligations may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligation set out in paragraph 77.
PART 10Module G: Conformity based on unit verification
General82
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 83, 84 and 85, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned, which has been subject to the provisions of paragraph 85, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation83
1
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in paragraph 85.
2
The technical documentation shall—
a
make it possible to assess the conformity of the pressure equipment, or the assembly, to the relevant requirements;
b
include an adequate analysis and assessment of the risk;
c
specify the applicable requirements and contain, where applicable—
i
a general description;
ii
the conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits and all other relevant parts, to component level;
iii
descriptions and explanations necessary for an understanding of those drawings and diagrams and the operation of the pressure equipment;
iv
a list of the designated standards;
v
results of design calculations made, examinations carried out the results of any other relevant calculation or examination;
vi
test reports; and
vii
appropriate details relating to the approval of the manufacturing and test procedures and of the qualifications or approvals of the personnel concerned in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations.
3
The technical documentation must be kept at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Manufacturing84
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured pressure equipment, or assembly, with the applicable requirements of these Regulations.
Verification85
1
An approved body chosen by the manufacturer shall carry out the appropriate examinations and tests, set out in the relevant designated standards or equivalent tests, to check the conformity of the pressure equipment, or assembly, with the appropriate requirements of these Regulations, or have them carried out.
2
In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out applying other technical specifications.
3
The approved body shall—
a
examine the technical documentation with respect to the design and manufacturing process;
b
assess the materials used where these are not in conformity with the relevant designated standards and check the certificate issued by the materials manufacturer in accordance with paragraphs 31(6) to (8) of Schedule 2 to these Regulations;
c
approve the procedures for the permanent joining of parts and check that they have been previously approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
d
verify the qualifications and approvals required under paragraphs 21 and 22 of Schedule 2 of these Regulations;
e
carry out the final inspection referred to in paragraphs 25 to 28 of Schedule 2 of these Regulations and perform or have performed the proof test, referred to in the same paragraphs, and examine safety devices, if applicable.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number or have it affixed under its responsibility to each approved item of pressure equipment or assembly.
5
The manufacturer shall keep certificates of conformity available for inspection by the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
UK marking and declaration of conformity86
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 85, the latter's identification number, to each individual item of pressure equipment, or assembly, that satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity which identifies the pressure equipment model, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request; and
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Authorised representative87
The manufacturer's obligations set out in paragraphs 83 and 85 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that the responsibilities are specified in the mandate set out between the manufacturer and representative.
PART 11Module H: Conformity based on full quality assurance
General88
Conformity based on full quality assurance is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 89 and 92, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned satisfies the requirements of these Regulations that apply to them.
Manufacturing89
The manufacturer shall operate an approved quality system for; the final design, manufacture, final product inspection and testing of the pressure equipment, or assembly concerned; as specified in paragraph 90 and shall be subject to surveillance as specified in paragraph 91.
Quality system90
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
the technical documentation for one model of each type of pressure equipment, or assembly, intended to be manufactured which shall, wherever applicable, contain—
i
a general description of the pressure equipment or assembly;
ii
conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits and all other relevant parts, to component level;
iii
descriptions and explanations necessary for the understanding of those drawings and diagrams and the operation of the pressure equipment or assembly;
iv
a list of the designated standards, applied in part or in full, and descriptions of the solutions adopted to meet the essential safety requirements of these Regulations where those designated standards have not been applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
v
results of design calculations made, examinations carried out and the results of any other relevant calculation or examination;
vi
test reports;
c
a written declaration that the same application has not been lodged with any other approved body; and
d
the documentation concerning the quality system.
3
The quality system shall ensure compliance of the pressure equipment, or assembly, with the requirements of these Regulations that apply to it.
4
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to the quality of the pressure equipment or assembly;
b
the technical design specifications, including standards, that will be applied and, where the relevant designated standards will not be applied in full, the means that will be used to ensure that the essential requirements of these Regulations that apply to the pressure equipment or assembly will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the pressure equipment, or assembly, pertaining to the product type covered, particularly with regard to materials in accordance with Part 4 of Schedule 2 to these Regulations;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used, particularly the procedures used for the permanent joining of parts as approved in accordance with paragraph 21 of Schedule 2 to these Regulations;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
g
the means of monitoring the achievement of the required design and pressure equipment quality and the effective operation of the quality system.
5
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems;
d
ensure that the audit—
i
is carried out by a team containing at least one member with knowledge of the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises;
iii
reviews the technical documentation referred to in paragraph 90(2)(b), to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
6
The decision shall—
a
be notified to the manufacturer, or his authorised representative;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
7
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
8
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system informed of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body91
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the quality records provided for by the design part of the quality system, such as results of analyses, calculations, tests and any other relevant quality records;
d
provide the quality records provided for by the manufacturing part of the quality system, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, and any other relevant quality records; and
e
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, should be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
UK marking and declaration of conformity92
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 90(1), the latter's identification number, to each individual item of pressure equipment, or assembly, that satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment model, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
for a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation referred to in paragraph 90(2);
ii
the documentation concerning the quality system referred to in paragraph 90(4);
iii
the change referred to in paragraph 90(8); and
iv
the decisions and reports of the approved body referred to in paragraphs 90(8) and 91(2) to (4);
2
Each approved body shall inform the Secretary of State of the quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Each approved body shall inform the other approved bodies of the quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative93
The manufacturer's obligations set out in paragraphs 90(1), (2) and (8) and paragraph 92 may be fulfilled by their authorised representative, on their behalf and under their responsibility, provided that they are specified in the mandate.
PART 12Module H1: Conformity based on full quality assurance plus design examination
General94
Conformity based on full quality assurance plus design examination and special surveillance of the final assessment is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 95 and 99, and ensures and declares on their sole responsibility that the pressure equipment, or assembly, concerned satisfies the requirements of these Regulations that apply to it.
Manufacturing95
The manufacturer shall operate an approved quality system for design, manufacture, final product inspection and testing of the products concerned as specified in paragraph 96 and shall be subject to surveillance as specified in paragraph 98. The adequacy of the technical design of the pressure equipment, or assembly, shall have been examined in accordance with paragraph 97.
Quality system96
1
The manufacturer shall lodge an application for assessment of their quality system with the approved body of their choice for the pressure equipment, or assembly, concerned.
2
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, their name and address as well;
b
the technical documentation for one model of each type of pressure equipment, or assembly, intended to be manufactured which shall, wherever applicable, contain—
i
a general description of the pressure equipment or assembly;
ii
conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits and any other relevant parts, to component level;
iii
descriptions and explanations necessary for the understanding of those drawings and diagrams and the operation of the pressure equipment or assembly;
iv
a list of the designated standards, applied in part or in full, and descriptions of the solutions adopted to meet the essential safety requirements of these Regulations where those designated standards have not been applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
v
results of design calculations made, examinations carried out and the results of any other relevant calculation or examination;
vi
test reports;
c
a written declaration that the same application has not been lodged with any other approved body; and
d
the documentation concerning the quality system.
3
The quality system shall ensure compliance of the pressure equipment, or assembly, with the requirements of these Regulations that apply to it.
4
All elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records, including—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards, that will be applied and, where the relevant designated standards will not be applied in full, the means that will be used to ensure that the essential requirements of these Regulations that apply to the pressure equipment or assembly will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the pressure equipment, or assembly, pertaining to the product type covered, particularly with regard to materials in accordance with Part 4 of Schedule 2 to these Regulations;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used, particularly the procedures used for the permanent joining of parts as approved with paragraph 21 of Schedule 2 to these Regulations;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, reports concerning the qualifications and approvals of the personnel concerned, particularly of those of the personnel undertaking the permanent joining of parts and the non-destructive test in accordance with paragraphs 21 and 22 of Schedule 2 to these Regulations; and
g
the means of monitoring the achievement of the required design and pressure equipment quality and the effective operation of the quality system.
5
The approved body shall—
a
assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraphs (3) and (4);
b
presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard;
c
provide a team with experience in quality management systems;
d
ensure that the audit—
i
is carried out by a team containing at least one member with knowledge of the relevant pressure equipment, or assembly, field and pressure equipment and assembly technology concerned, and knowledge of the applicable requirements of these Regulations;
ii
includes an inspection visit to the manufacturer's premises;
iii
reviews the technical documentation referred to in sub-paragraph (2)(b), to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
6
The decision shall—
a
be notified to the manufacturer, or his authorised representative;
b
contain the conclusions of the audit; and
c
contain a reasoned assessment decision.
7
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
8
Where the manufacturer intends to change the quality system—
a
the manufacturer shall inform the approved body that has approved the quality system of the intended change to the quality system;
b
the approved body shall evaluate the proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in subparagraph (5) or whether a reassessment is necessary;
c
the approved body shall notify the manufacturer of its decision; and
d
the notification shall contain the conclusions of the examination and the reasoned assessment decision.
Design examination97
1
The manufacturer shall lodge an application for the examination of the design of each item of pressure equipment, or assembly, not covered by a previous design examination with the approved body referred to in paragraph 96(1).
2
The application shall make it possible to understand the design, manufacture and operation of the pressure equipment, or assembly, and to assess the conformity with the requirements of these Regulations that apply to it.
3
The application shall include—
a
the name and address of the manufacturer;
b
the technical documentation for one model of each type of pressure equipment, or assembly, intended to be manufactured which shall, wherever applicable, contain—
i
a general description of the pressure equipment or assembly;
ii
conceptual design and manufacturing drawings and diagrams of components, sub-assemblies, circuits all other relevant parts, to component level;
iii
descriptions and explanations necessary for the understanding of those drawings and diagrams and the operation of the pressure equipment or assembly;
iv
a list of the designated standards, applied in part or in full, and descriptions of the solutions adopted to meet the essential safety requirements of these Regulations where those designated standards have not been applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
v
results of design calculations made, examinations carried out and the results of any other relevant calculation or examination;
vi
test reports;
c
a written declaration that the same application has not been lodged with any other approved body; and
d
the supporting evidence for the adequacy of the technical design. The supporting evidence shall mention any documents that have been used, in particular where the relevant designated standards have not been applied in full, and shall include, where necessary, the results of tests carried out by the appropriate laboratory of the manufacturer or by another testing laboratory on their behalf and under their responsibility.
4
Where the design meets the requirements of these Regulations the approved body shall issue a design examination certificate.
5
The design examination certificate—
a
must include—
i
the name and address of the manufacturer;
ii
the conclusions of the examination;
iii
the conditions (if any) for the validity of the certificate; and
iv
data necessary for identification of the approved design;
b
may have one or more annexes attached;
c
shall contain all relevant information to allow the conformity of manufactured products with the examined design to be evaluated, and to allow for in-service control, where applicable.
6
Where the design does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a design examination certificate and shall inform the applicant accordingly, giving details for its refusal.
7
The approved body shall keep itself appraised of any changes in the generally acknowledged state of the art which indicates that the approved design may no longer comply with the applicable requirements of these Regulations, and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
8
The manufacturer shall inform the approved body that issued the design examination certificate of all modifications to the approved design that may affect the conformity of the pressure equipment, or assembly, with the essential safety requirements of these Regulations or the conditions for validity of the certificate. Such modifications shall require additional approval, from the approved body that issued the design examination certificate, in the form of an addition to the original design examination certificate.
9
Each approved body shall inform the Secretary of State of the design examination certificates and any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of certificates and any additions thereto refused, suspended or otherwise restricted.
10
Each approved body shall inform the other approved bodies concerning the design examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted and, upon request, concerning the certificates and additions thereto that it has issued.
11
Other approved bodies may, on request, obtain a copy of the design examination certificate and additions thereto.
12
The approved body shall keep a copy of the design examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of the certificate.
13
The manufacturer shall keep a copy of the design examination certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market.
Surveillance under the responsibility of the approved body98
1
The manufacturer shall—
a
allow the approved body access to the manufacture, inspection, testing and storage sites for assessment;
b
provide the quality system documentation;
c
provide the quality records provided for by the design part of the quality system, such as results of analyses, calculations, tests and any other relevant quality records;
d
provide the quality records provided for by the manufacturing part of the quality system, such as inspection reports and test data, calibration data, qualification reports of the personnel concerned, and any other relevant quality records; and
e
provide any other information deemed necessary by the approved body.
2
The approved body shall—
a
carry our periodic audits to make sure that the manufacturer maintains and applies the quality system;
b
provide the manufacturer with an audit report;
c
ensure that the frequency of the periodic audits is such that a full reassessment is carried out every three years.
3
The approved body may pay unexpected visits to the manufacturer. The need for such additional visits, and their frequency, will be determined on the basis of a visit control system operated by the approved body. The following factors shall be considered in the visit control system—
a
the category of the pressure equipment or assembly;
b
the results of previous surveillance visits;
c
the need to follow up corrective actions;
d
special conditions linked to the approval of the system, where applicable; and
e
significant changes in manufacturing organisation, policies or techniques.
4
During unexpected visits, the approved body—
a
may carry out product tests or have them carried out, where necessary, in order to verify that the quality system is functioning correctly;
b
shall provide the manufacturer with a visit report; and
c
shall, where tests have been carried out, provide the manufacturer with a test report.
5
Final assessment as referred to in paragraphs 25 to 28 of Schedule 2 to these Regulations is subject to increased surveillance in the form of unexpected visits by the approved body. In the course of such visits, the approved body shall conduct examinations on the pressure equipment, or assembly, and provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity99
1
The manufacturer shall—
a
affix the UK marking and, under the responsibility of the approved body referred to in paragraph 96(1), the latter's identification number, to each individual items of pressure equipment, or assembly, that satisfies the applicable requirements of these Regulations;
b
draw up a written declaration of conformity for the pressure equipment model, or assembly, which identifies the pressure equipment, or assembly, for which it has been drawn up;
c
make a copy of the declaration of conformity available, to the relevant authorities, on request;
d
keep a copy of the declaration of conformity at the disposal of the national authorities for 10 years after the pressure equipment, or assembly, has been placed on the market;
e
for a period ending 10 years after the pressure equipment, or assembly, has been placed on the market, keep at the disposal of the national authorities—
i
the documentation concerning the quality system referred to in paragraph 96(2);
ii
the change referred to in paragraph 96(8); and
iii
the decisions and reports of the approved body referred to in paragraphs 96(8) and 98(2) and (3),
Authorised representative100
The manufacturer's authorised representative may lodge the application referred to in paragraphs 96(1) and (2) and fulfil the obligations set out in paragraphs 95(1), (2) and (8), 96(8) and (13) and paragraph 98, on the manufacturer's behalf and under his responsibility, provided that they are specified in the mandate set out between the manufacturer and his representative.
SCHEDULE 1BConformity Assessment Tables
1
The references in the tables to categories of modules are the following:
I
=
Module A
II
=
Modules A2, D1, E1
III
=
Modules B (design type) + D, B (design type) + F, B (production type) + E, B (production type) + C2, H
IV
=
Modules B (production type) + D, B (production type) + F, G, H1
1A
1
Where in order to mitigate the effects of very serious safety concerns the Secretary of State considers that an item or family of pressure equipment are to be subject to different categories of modules, the Secretary of State may by regulations make such provision.
2
Regulations made under paragraph (1)—
a
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament; and
b
include power—
i
to make different provision for different cases; and
ii
to make such supplemental, consequential and transitional provision as the Secretary of State considers appropriate.
2
The safety accessories defined in paragraph 5, are classified in category IV. However, by way of exception, safety accessories manufactured for specific equipment may be classified in the same category as the equipment they protect.
3
1
The pressure accessories defined in paragraph 6, are classified on the basis of:
a
their maximum allowable pressure PS;
b
their volume V or their nominal size DN, as appropriate;
c
the group of fluids for which they are intended.
2
The appropriate table for vessels or piping is to be used to determine the conformity assessment category.
3
Where both the volume and the nominal size are considered appropriate in subparagraph (1)(b), the pressure accessory shall be classified in the highest category.
4
1
The demarcation lines in the following conformity assessment tables indicate the upper limit for each category.
a
i
Table 1: Vessels for gases, liquefied gases, gases dissolved under pressure, vapours and also those liquids whose vapour pressure is greater than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 1 with a volume greater than 1L and a product PS and V greater than 25 bar.L, or with a pressure PS greater than 200 bar
ii
Exceptionally, vessels intended to contain an unstable gas and falling within categories I or II on the basis of table 1 shall be classified in category III.
b
i
Table 2: Vessels for gases, liquefied gases, gases dissolved under pressure, vapours and also those liquids whose vapour pressure is greater than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 2, with a volume greater than 1L and a product of PS and V is greater than 50 bar.L, or a pressure PS greater than 1000bar
ii
Exceptionally, portable extinguishers and bottles for breathing equipment shall be classified at least in category III.
c
Table 3: Vessels for liquids having a vapour pressure at the maximum allowable temperature of not more than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 1 with a volume greater than 1 L and a product of PS and V greater than 200 bar.L, or with a pressure PS greater than 500 bar
d
i
Table 4: Vessels for liquids having a vapour pressure at the maximum allowable temperature of not more than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 2 with a pressure PS greater than 10 bar and a product of PS and V greater than 10000 bar.L, or with a pressure PS greater than 1000 bar
ii
Exceptionally, assemblies intended for generating warm water at temperatures not greater than 110°C which are manually fed with solid fuels and have a PS.V, shall be subject either to a Type examination (Module B — design type) with respect to their conformity with the essential requirements referred to in paragraphs 14, 15, 16, 17 and 30 and subparagraphs 33(2)(a) and (d) of Schedule 2 to these Regulations, or to full quality assurance (Module H).
e
i
Table 5: Vessels fired or otherwise heated pressure equipment with the risk of overheating intended for generation of steam or superheated water at temperatures higher than 110°C having a volume greater than 2 L
ii
Exceptionally, the design of pressure-cookers shall be subject to a conformity assessment procedure equivalent to at least one of the category III modules.
f
i
Table 6: Piping intended for gases, liquefied gases, gases dissolved under pressure, vapours and those liquids whose vapour pressure at the maximum allowable temperature is greater than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 1 with a DN greater than 25
ii
Exceptionally, piping intended for unstable gases and falling within categories I or II on the basis of Table 6 shall be classified in category III.
g
i
Table 7: Piping intended for gases, liquefied gases, gases dissolved under pressure, vapours and those liquids whose vapour pressure at the maximum allowable temperature is greater than 0.5 bar above normal atmospheric pressure (1013 mbar), and for fluids in Group 2 with a DN greater than 32 and a product of PS and DN greater than 1000 bar
ii
Exceptionally, all piping containing fluids at a temperature greater than 350 °C and falling within category II on the basis of Table 7 shall be classified in category III.
h
Table 8: Piping intended for liquids having a vapour pressure at the maximum allowable temperature of not more than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 1 with a DN greater than 25 and a product of PS and DN greater than 2000 bar
i
Table 9: Piping intended for liquids having a vapour pressure at the maximum allowable temperature of not more than 0.5 bar above normal atmospheric pressure (1013 mbar) and, for fluids in Group 2 with a PS greater than 10 bar, a DN greater than 200 and a product of PS and DN greater than 5000 bar
5
In this Schedule “safety accessories” are defined as follows—
a
devices designed to protect pressure equipment against the allowable limits being exceeded, including devices for direct pressure limitation, such as safety valves, bursting disc safety devices, buckling rods, controlled safety pressure relief systems (CSPRS), and limiting devices, which either activate the means for correction or provide for shutdown or a shutdown and lockout, such as pressure switched or temperature switches or fluid level switches and safety related measurement control and regulation (SRMCR) devices; and
b
devices intended for equipment covered in the tables in paragraph 6 including where such equipment is incorporated into an assembly.
6
In this Schedule “pressure accessories” are defined as follows—
a
devices with an operational function and having pressure-bearing housings; and
b
devices intended for equipment covered in the tables in paragraph 6 including where such equipment is incorporated into an assembly.
Amendment to Schedule 2I42745
Schedule 2 (essential safety requirements) is amended as follows—
a
in paragraph 21(4), 31(4)(b)(i) and 35 (1) for “harmonised” substitute “
designated
”
;
b
in paragraph 29 (1) for “CE” substitute “
UK
”
; F251...
c
omit paragraph 31(4)(b)(ii) F252....
F250d
in paragraph 31(8) for “within the Union” substitute “
in the United Kingdom
”
Amendment to Schedule 3I42846
Schedule 3 (classification of pressure equipment) is amended as follows—
a
beneath the heading to “Schedule 3” insert the Part heading “
Part 1
”
;
b
for the heading “classification of pressure equipment” substitute “
classification of pressure equipment before F253IP completion day”
;
c
after paragraph 4 insert—
PART 2Classification of pressure equipment immediately on or after F254IP completion day
5
Pressure equipment referred to in regulation 6 (pressure equipment and assemblies subject to essential safety requirements) must be classified by category in accordance with Schedule 1B (conformity assessment tables) to these Regulations according to an ascending level of hazard.
6
1
In order to determine the appropriate category for classification of pressure equipment coming within regulations 6(a) to (c), the manufacturer must refer to the following tables within Schedule 1B to these Regulations—
a
for pressure equipment coming within—
i
regulation 6(a)(i)(aa), table 1;
ii
regulation 6(a)(i) (bb), table 2;
iii
regulation 6(a)(ii)(aa), table 3;
iv
regulation 6(a)(ii)(bb), table 4;
v
regulation 6(b), table 5;
vi
regulation 6(c)(i)(aa), table 6;
vii
regulation 6(c)(i)(bb), table 7;
viii
regulation 6(c)(ii)(aa), table 8;
ix
regulation 6(c)(ii)(bb), table 9;
b
for pressure equipment coming within regulation 6(d), the category must be determined in accordance with paragraphs 2 and 3 of Schedule 1B to these Regulations.
2
Where a vessel is composed of a number of chambers, it must be classified in the highest category applicable to the individual chambers and, where a chamber contains several fluids, classification must be on the basis of the fluid which requires the highest category.
7
For the purposes of the classification referred to in paragraph (5), fluids shall be divided up into the following groups—
a
group 1 consisting of substances and mixtures, as defined in points 7 and 8 of Article 2 of Regulation (EC) 1272/2008 of the European Parliament and of the Council of 16th December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EEC, and amending Regulation (EC) No 1907/2006, that are classified as hazardous in accordance with the following physical or health hazard classes laid down in Parts 2 and 3 of Annex 1 to that Regulation—
i
unstable explosives or explosives of Divisions 1.1. 1.2. 1.3, 1.4 and 1.5;
ii
flammable gases, category 1 and 2;
iii
oxidising gases, category 1;
iv
flammable liquids, categories 1 and 2;
v
flammable liquids, category 3 where the maximum allowable temperature is above the flashpoint;
vi
flammable solids, category 1 and 2;
vii
self-reactive substances and mixtures, type A to F;
viii
pyrophoric liquids, category 1;
ix
pyrophoric solids, category 1;
x
oxidising liquids, category 1, 2 and 3;
xi
substances and mixtures which in contact with water emit flammable gases, category 1, 2 and 3;
xii
oxidising liquids, category 1, 2 and 3;
xiii
oxidising solids, category 1, 2 and 3;
xiv
organic peroxides types A to F;
xv
acute oral toxicity, category 1 and 2;
xvi
acute dermal toxicity, category 1, 2 and 3;
xvii
acute inhalation toxicity, category 1, 2 and 3;
xviii
specific target organ toxicity – single exposure, category 1;
Group 1 also comprises substances and mixtures contained in pressure equipment with a maximum allowable temperature TS which exceeds the flashpoint of the fluid;
b
group 2 consisting of substances and mixtures not referred to in point (a).
8
Where a vessel is composed of a number of chambers, it shall be classified in the highest category applicable to the individual changes. Where a chamber contains several fluids, classification shall be on the basis of the fluid which requires the highest category.
Amendment to Schedule 4I42947
Schedule 4 (notified body requirements) is amended as follows—
a
in the heading and in every place in which it occurs, for “notified”, substitute “
approved
”
;
b
for “a notified body” substitute “
an approved body
”
in every place in which it occurs;
c
in paragraph 12(c) for “harmonised standards and of the Directive and” substitute “
designated standards
”
;
d
in paragraph 18 omit “established under the Directive”.
Amendment to Schedule 5I43048
Schedule 5 (user inspectorate requirements) is amended as follows—
a
in paragraph 10(c)—
i
for “harmonised” substitute “
designated
”
;
ii
omit “of the Directive and”;
b
in paragraph 16—
i
for “notified”, substitute “
approved
”
; and
ii
omit “established under the Directive”.
Amendment to Schedule 6I43149
Schedule 6 (operational obligations of notified bodies, recognised third party organisations and user inspectorates) is amended as follows—
a
in the heading and in every place in which it occurs, for “notified”, substitute “
approved
”
;
b
in every place in which it occurs, for “a notified body”, substitute “
an approved body
”
;
c
in paragraph 5, for “harmonised”, substitute “
designated
”
;
d
in paragraph 12, for “bodies notified under the Directive”, substitute “
other approved bodies
”
; and
e
in paragraph 13, for “established under the Directive”, substitute “
established by the Secretary of State
”
.
Amendment to Schedule 11I43250
Schedule 11 (EU Declaration of Conformity) is amended as follows—
a
omit “EU” in each place in which it occurs;
b
in paragraph 5, for “Union harmonisation legislation”, substitute “
statutory requirements
”
;
c
in paragraph 6, for “harmonised”, substitute “
designated
”
; and
d
in paragraph 7, for “notified”, substitute “
approved
”
.
SCHEDULE 25Amendment of the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016
IntroductionI9061
The Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016 are amended in accordance with paragraphs 2 to 41.
Amendment to regulation 2I4332
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
in the definition of the “1994 Directive” at the end insert “
(as it has effect immediately before F255IP completion day )
”
;
b
after the definition of the “1996 Regulations” insert—
“approved body” has the meaning given to it in regulation 42;
c
omit the definition of “accreditation certificate”;
d
in the definition of “attestation of conformity”—
i
omit “EU”; and
ii
for “CE” substitute “
UK
”
;
F256e
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
f
omit the definition of “CE Marking”;
g
omit the definition of “competent national authority”;
h
after the definition of “conformity assessment” insert—
“conformity assessment activities” means any activities connected with conformity assessment including calibration, testing, certification and inspection;
i
after the definition of “conformity assessment body” insert—
“conformity assessment procedure” means a procedure referred to in regulation 39 (conformity assessment procedures);
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 7(1)(a) (declaration of conformity and UK marking);
“designated standard” has the meaning given to it in regulation 2A;
j
for the definition of “equipment category” substitute—
“equipment category” means the classification of equipment, within each equipment group, specified in Schedule 1A to these Regulations;
k
in the definition of “equipment-group I” for “as set out in Annex I of the ATEX Directive (as amended from time to time)” substitute “
as set out in Schedule 1A to these Regulations
”
;
l
in the definition of “equipment-group II” for “as set out in Annex I of the ATEX Directive (as amended from time to time)” substitute “
as set out in Schedule 1A to these Regulations
”
;
m
omit the definition of “EU declaration of conformity”;
n
omit the definition of “European Commission”;
o
omit the definition of “harmonised standard”;
p
for the definition of “importer” substitute—
F257“importer” means a person who—
a
is established in the United Kingdom and places a product from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a product on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F258q
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
r
omit the definition of “national accreditation body”;
s
omit the definition of “notified body requirements”;
t
omit the definition of “Official Journal”;
F259u
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
v
in the definition of “putting into service” omit “within the EU market”;
w
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS.
3
After paragraph (1) insert—
1A
Schedule 1A reproduces the provisions of Annex I to the ATEX Directive with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedule 1A is a reference to the equivalent provisions of Annex I to the ATEX Directive as set out in Schedule 1A.
1C
Schedule 3A reproduces the provisions of Annexes III to IX to the ATEX Directive with amendments to correct deficiencies in retained EU law.
1D
A reference to any provision of Schedule 3A is a reference to the equivalent provisions of Annex III to IX of the ATEX Directive.
4
Omit paragraph (3).
5
Omit paragraph (6).
Insertion of regulation 2AI4353
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, service or system, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product, service or system as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product, service or system.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
9
Regulations made under paragraph (8) are to be made by statutory instrument.
10
A statutory instrument containing regulations made under paragraph (8) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 3I4364
In regulation 3 for paragraph (g) substitute—
g
products connected with the production of trade in arms, munitions and war material;
Amendment to regulation 6I4375
In regulation 6 (technical documentation and conformity assessment) for paragraph (b) substitute—
b
draw up the technical documentation referred to—
i
for a product in respect of which the conformity assessment procedure in regulation 39(1)(a) is being carried out, in paragraph 3(c) of Part 1 of Schedule 3A to these Regulations;
ii
for a product in respect of which the conformity assessment procedure in regulation 39(1)(b) is being carried out, in paragraph 3(c) of Part 1 of Schedule 3A to these Regulations;
iii
for a product in respect of which the conformity assessment procedure in regulation 39(1)(c) is being carried out, in paragraph 2 of Part 6 of Schedule 3A to these Regulations;
iv
for a product in respect of which the conformity assessment procedure in regulation 39(1)(d) is being carried out, in paragraph 2 of Part 7 of Schedule 3A to these Regulations.
Amendment to regulation 7I4386
Regulation 7 (EU declaration of conformity and CE marking) is amended as follows—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a) omit “EU”;
c
in paragraph (1)(b) for “CE” substitute “
UK
”
in each place in which it occurs;
d
in paragraphs (2), (4) and (5) omit “EU”;
e
for paragraph (6) substitute—
6
Where a product is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 8I4397
In regulation 8 (retention of technical documentation and EU declaration of conformity) and in the heading of that regulation omit “EU”.
Amendment to regulation 9I4408
In regulation 9 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Amendment to regulation 13I4419
In regulation 13 (information identifying manufacturer), for paragraph 3 substitute—
3
The information specified in paragraph (1) must be in a language which can be easily understood by end users and the market surveillance authority.
Amendment to regulation 14I44210
For regulation 14 (instructions and safety information) substitute—
Provision of instructions and safety information14
When placing a product on the market, a manufacturer must ensure that a product is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 15I44311
In regulation 15 (duty to take action in respect of a product placed on the market which is considered not to be in conformity), in paragraph (2) omit “, and the competent national authorities of any other Member State in which the manufacturer made the product available on the market,”.
Amendment to regulation 17I44412
In regulation 17 (authorised representatives)—
a
in paragraph (1) for “EU” substitute “
United Kingdom
”
;
b
in paragraph (4)(a) omit “EU”.
Amendment to regulation 19I44513
In regulation 19 (requirements which must be satisfied before an importer places a product on the market)—
a
in paragraph (1)(c)(i) for “CE” substitute “
UK
”
;
b
in paragraph (1)(c)(ii) omit “EU”;
c
in paragraph (2)(c) for “14(1) (instructions and safety information)” substitute “
14 (provision of instructions and safety information)
”
.
Amendment to regulation 21I44614
Regulation 21 (information identifying importer) is amended as follows—
a
in paragraph (2) for “by the competent national authority in the Member State in which it is to be made available to end-users” substitute “
the market surveillance authority
”
;
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the product, or
b
before placing the product on the market, the importer sets out the information referred to in paragraph (1)—
i
on the packaging; or
ii
in a document accompanying the product.
Amendment to regulation 22I44715
For regulation 22 (instructions and safety information) substitute—
Provision of Instructions and safety information22
When placing a product on the market, an importer must ensure that the product is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 25I44816
In regulation 25 (duty to take action in respect of a product placed on the market which is considered not to be in conformity), in paragraph (2) omit “, and the competent national authorities of any other Member State in which the importer made the product available on the market,”.
Amendment to regulation 27I44917
In regulation 27 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 29I45018
In regulation 29 (requirements which must be satisfied before a distributor makes a product available on the market)—
a
in paragraph (1)(a)(i) for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)(ii) omit “EU”;
c
for paragraph (1)(a)(iv) substitute—
iv
is accompanied by instructions and safety information that are clear, legible and in easily understandable English;
Amendment to regulation 32I45119
In regulation 32 (duty to take action in respect of products made available on the market which are not in conformity), in paragraph (2) omit “, and the competent national authorities of the other Member States in which the distributor has made the product available on the market,”.
Amendment to regulation 36I45220
In regulation 36 (prohibition on improper use of CE marking) in each place in which it occurs, and in the heading, for “CE” substitute “
UK
”
.
Insertion of regulations 36A and 36BI45321
After regulation 36 insert—
Obligations which are met by complying with obligations in the ATEX Directive36A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the ATEX Directive;
b
“CE marking” has the meaning given to it in Article 2(26); and
c
“harmonised standard” has the meaning given to in in Article 2(18).
2
Subject to paragraphs (6) and (7) paragraph (3) applies where, before placing the product on the market, the manufacturer—
a
ensures that the product has been designed and manufactured in accordance with the essential safety requirements set out in Annex II;
b
ensures that the relevant conformity assessment procedures that apply to that product in accordance with Article 13(1) and (2) have been carried out;
c
draws up the technical documentation referred to in Annexes III to IX;
d
ensures that the records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the inscriptions in accordance with Articles 15 and 16(1) to (4);
f
draws up an EU declaration of conformity, in accordance with Article 14; and
g
ensures that the declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 5, 6, 7(1), (3) and 7(6) are to be treated as being satisfied;
b
regulations 2(a), 7(6), 8, 9(2), 17(4), 36 and 59 apply subject to the modifications in paragraph (10);
c
Part 3 does not apply; and
d
regulation 57 does not apply.
4
Subject to paragraphs (6) and (7) paragraph (5) applies where, before placing a product on the market, the importer ensures that—
a
the relevant conformity assessment procedure referred to in Article 13 has been carried out;
b
the manufacturer has drawn up the technical documents relevant to the conformity assessment procedure followed; and
c
the product bears the CE marking and inscriptions referred to in point 1.0.5 of Annex II.
5
Where this paragraph applies—
a
the requirements of regulation 19(1)(a) to (c) are to be treated as being satisfied; and
b
regulations 2(a),18, 23 and 27 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 12.
7
Where paragraph (6) applies, paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to carry out—
a
the conformity assessment procedure set out in Article 13(1)(b); and
b
the relevant conformity assessment procedure that applies to that product in accordance with Article 13(2).
8
Paragraph (9) applies where, before making a product available on the market, a distributor ensures that the product bears the CE marking and inscriptions referred to in point 1.0.5 of Annex II.
9
Where this paragraph applies—
a
regulation 29(1)(a)(i) is to be treated as being satisfied; and
b
regulations 2(a), 30 and 31(1) apply subject to the modifications in paragraph (10).
10
The modifications referred to in subparagraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex II;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 13;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annexes III to IX.
Conformity assessment procedure obligation which is met by complying with the ATEX Directive36B
1
In this regulation any reference to an Article or Annex is a reference to an Article or an Annex of the ATEX Directive;
2
Paragraph (3) applies where, prior to the manufacture of a product, the manufacturer ensures that the conformity assessment procedure that applies to that product in accordance with Annex III as referred to in Article 13(1)(a) and (b) has been carried out.
3
Where this paragraph applies—
a
any requirement to follow the Type-examination set out in Part 1 of Schedule 3A in regulation 39 is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 6(a), 7(1), 19(a), 36(1)(b), 40(c) and 41(3) is to be read as including the conformity assessment procedure set out in Annex III as referred to in Article 13(1)(a) and (b); and
c
any reference to “technical documentation” in regulations 6(b), 8, 19(b) and 27(b) is to be read as including the technical documentation relating to the design of the product referred to in Annex III.
F263Expiry of regulations 36A and 36B36C
1
Subject to paragraph (2), regulation 36A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 36A—
a
any product which was placed on the market pursuant to regulation 36A may continue to be made available on the market on or after the expiry of regulation 36A;
b
any obligation to which a person was subject under regulation 36A in respect of any product placed on the market pursuant to regulation 36A continues to have effect after the expiry of regulation 36A, in respect of that product.
3
Subject to paragraph (4), regulation 36B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 36B in relation to a product prior to the expiry of regulation 36B, regulation 36B continues to apply in respect of that pressure equipment or assembly where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after the conformity assessment referred to in regulation 36B(2) has been carried out.
Qualifying Northern Ireland Goods36D
1
In this regulation—
“the 2017 Regulations” means the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations (Northern Ireland) 2017;
“CE marking” has the meaning given to it in regulation 2(1) of the 2017 Regulations;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1) of the 2017 Regulations;
“technical documentation” has the meaning given to it in regulation 2(1) of the 2017 Regulations.
2
Where paragraph (3) applies, a product is to be treated as being in conformity with Part 2.
3
This paragraph applies where—
a
a product—
i
is in conformity with Part 2, within the meaning of regulation 2(2) of the 2017 Regulations; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (4).
4
The obligations referred to in paragraph (3)(b) are that, before placing the product on the market, the importer—
a
complies with regulation 21;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the product;
ii
the manufacturer has drawn up the technical documentation; and
iii
the product bears the CE marking.
Omission of regulation 37I45422
Omit regulation 37 (translation of declaration of conformity).
Amendment to regulation 38I45523
In regulation 38 (presumption of conformity), in paragraph (1)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal”.
Amendment to regulation 39I45624
In regulation 39 (conformity assessment procedures)—
a
for paragraph (1)(a) substitute—
a
for equipment-groups I and II, equipment-categories M1 and 1, the manufacturer must follow either—
i
the Type-examination set out in Part 1 of Schedule 3A, in conjunction with either the procedure set out in—
aa
Part 2 of Schedule 3A, or
bb
Part 3 of Schedule 3A; or
ii
the conformity based on unit verification referred to in Part 7 of Schedule 3A;
b
for paragraph (1)(b) substitute—
b
for equipment-groups I and II, equipment-categories M2 and 2, the manufacturer must follow—
i
for internal combustion engines and electrical equipment in these groups and categories the Type examination set out in Part 1 of Schedule 3A, in conjunction with either the procedure set out in either Part 4 or Part 5 of Schedule 3A;
ii
for other equipment in these groups and categories the procedures set out in Part 6 of Schedule 3A;
c
for paragraph (1)(c) substitute—
c
for equipment group II, equipment-category 3, the procedure relating to internal production control referred to in Part 6 of Schedule 3A;
d
for paragraph (1)(d) substitute—
d
for equipment-groups I and II, instead of the procedures referred to in paragraphs (1)(a), (b) and (c), the manufacturer may follow conformity based on unit verification referred to in Part 7 of Schedule 3A.
e
in paragraph (3)(a)(i) for “CE” substitute “
UK
”
;
f
in paragraph (3)(a)(ii) omit “EU”;
g
in paragraph (4) for “Annex VIII to the ATEX Directive (as amended from time to time)” substitute “
Part 6 of Schedule 3A
”
;
h
in paragraph (5), omit “in the Member State concerned”;
i
in paragraph (6) for the words beginning with “the language” and ending with “market” substitute “
English
”
.
Amendment to regulation 40I45725
Regulation 40 (EU declaration of conformity) is amended as follows—
a
in the heading and in the body of the regulation, omit “EU”;
b
in paragraph (c) for “Annexes III to IX of the ATEX Directive (as amended from time to time)” substitute “
Schedule 3A to these Regulations
”
.
Amendment to regulation 41I45826
In regulation 41 (CE marking)—
F265a
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly—
a
to the product;
b
to its data plate; or
c
where paragraph (1A) applies, to—
i
a label affixed to the product; or
ii
a document accompanying the product.
F264aa
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the product; or
b
a document accompanying the product.
ab
in paragraph (2)—
i
after “Where” insert “
paragraph (1A) does not apply and
”
;
ii
for “paragraph (1)” substitute “
paragraph (1)(a) or (b)
”
;
ac
in the heading and in paragraphs (2) to (5) for “CE” substitute “
UK
”
in each place in which it occurs;
b
for “notified body” substitute “
approved body
”
in each place in which it occurs.
Amendment to Part 4I45927
For Part 4, substitute—
PART 4Approval of Conformity Assessment Bodies
Approved bodies42
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 43 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 46 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F266IP completion day notified to the European Commission and the member States of the European Union, in accordance with Article 17 of the ATEX Directive; and
- b
in respect of which no objections had been raised as referred to in regulation 42(1)(b) as it had effect immediately before F266IP completion day.
“approved body requirements” means the requirements set out in Schedule 2.
Approval of conformity assessment bodies43
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of products in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate, or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that applies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies44
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring45
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 43(6)(b), or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval46
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 45(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 42 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 45(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 42.
3
In deciding what action is required under paragraph (1) or (2) the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2) the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State, or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken by an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies47
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 43, or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 3.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Part 1 of Schedule 3B;
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 41 (UK marking).
Subsidiaries and contractors48
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meet the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meet those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documents concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M77;
Register of approved bodies49
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body notification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body50
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 45; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 49.
Amendment to regulation 54I46028
In regulation 54 (exercise of enforcement powers) omit paragraph (c).
Amendment to regulation 56I46129
Regulation 56 (enforcement action in respect of products which are not in conformity and which present a risk) is amended as follows—
a
in paragraph (2) for “notified” substitute “
approved
”
;
b
in paragraph (4) for “European Commission, Northern Ireland and the other Member states” substitute “
Health and Safety Executive for Northern Ireland
”
;
c
in paragraph (7) for “European Commission, Northern Ireland and the other Member States” substitute “
Health and Safety Executive for Northern Ireland
”
;
d
in subparagraph (8)(f)(ii) for “harmonised” substitute “
designated
”
.
Amendment to regulation 57I46230
Omit regulation 57 (EU safeguard procedure).
Amendment to regulation 58I46331
In regulation 58 (enforcement action in respect of products which are in conformity, but present a risk in paragraph (3) for “the European Commission and the other Member States” substitute “
the Health and Safety Executive for Northern Ireland
”
;
Amendment to regulation 59I46432
Regulation 59 (enforcement action in respect of formal non-compliance) is amended as follows—
a
in paragraphs (1)(a) and (1)(c)(ii) for “CE” substitute “
UK
”
in each place in which it occurs;
b
in paragraph (1)(b) for “a notified” substitute “
an approved
”
; and
c
in paragraph (1)(c) omit “EU” in each place in which it occurs.
Amendment to regulation 72I46533
In regulation 72 (transitional provisions) omit paragraph (2).
I43434
After regulation 72 insert—
Transitional provision in relation to EU Exit72A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F268IP completion day;
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 25 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M78, any obligation to which a person was subject under these Regulations as they had effect immediately before F268IP completion day, continues to have effect as it did immediately before F268IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 38 as it had effect immediately before F268IP completion day in relation to that product,
that action has effect as if it had been done under regulation 38 as it had effect on and after F268IP completion day.
Amendment to regulation 73I46635
Regulation 73 (revocations and savings) is amended as follows—
a
in paragraph (1) after “paragraph (3)” insert “
and (3A)
”
;
b
for paragraph (3) substitute—
3
Subject to the modifications made in paragraph (3A), the Regulations referred to in paragraph (1) continue to apply, as if they had not been revoked, to a product placed on the market before the commencement date.
c
After paragraph (3) (as substituted), insert—
3A
The modifications in the 1996 Regulations referred to in paragraph (3) are as follows—
i
any reference to “the Community” shall be read as including the United Kingdom;
ii
any reference to “member State” shall be read as including the United Kingdom;
iii
any reference to “notified body” shall be read as “approved body” as defined in the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations 2016 M79.
Amendment to Schedule 1I46736
Schedule 1 (essential health and safety requirements) is amended as follows—
a
in paragraph 5(1)(b) for “CE marking (see Annex II RAMS)” substitute “
UK marking;
”
;
b
at paragraph 13(2)—
i
for “other European Union legislation” substitute “
any other enactment
”
;
ii
for “European Union legislation” substitute “
enactment
”
.
Insertion of Schedule 1AI46837
After Schedule 1 insert—
SCHEDULE 1ACriteria determining the classification of equipment-groups into categories (Annex I to the ATEX Directive)
1
Equipment group I
a
Equipment category M 1 comprises equipment designed and, where necessary, equipped with additional special means of protection to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a very high level of protection.
Equipment in this category is intended for use in underground parts of mines as well as those parts of surface installations of such mines endangered by firedamp and/or combustible dust.
Equipment in this category is required to remain functional, even in the event of rare incidents relating to equipment, with an explosive atmosphere present, and is characterised by means of protection such that:
— either, in the event of failure of one means of protection, at least an independent second means provides the requisite level of protection,
— or the requisite level of protection is assured in the event of two faults occurring independently of each other.
Equipment in this category must comply with the supplementary requirements referred to in paragraph 30 of Schedule 1.
b
Equipment category M 2 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a high level of protection.
Equipment in this category is intended for use in underground parts of mines as well as those parts of surface installations of such mines likely to be endangered by firedamp and/or combustible dust.
This equipment is intended to be de-energised in the event of an explosive atmosphere.
The means of protection relating to equipment in this category assure the requisite level of protection during normal operation and also in the case of more severe operating conditions, in particular those arising from rough handling and changing environmental conditions.
Equipment in this category must comply with the supplementary requirements referred to in paragraph 31 of Schedule 1.
2
Equipment-group II
a
Equipment category 1 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a very high level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by mixtures of air and gases, vapours or mists or by air/dust mixtures are present continuously, for long periods or frequently.
Equipment in this category must ensure the requisite level of protection, even in the event of rare incidents relating to equipment, and is characterised by means of protection such that:
— either, in the event of failure of one means of protection, at least an independent second means provides the requisite level of protection,
— or the requisite level of protection is assured in the event of two faults occurring independently of each other.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 32 and 33 of Schedule 1.
b
Equipment category 2 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and of ensuring a high level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by gases, vapours, mists or air/dust mixtures are likely to occur occasionally.
The means of protection relating to equipment in this category ensure the requisite level of protection, even in the event of frequently occurring disturbances or equipment faults which normally have to be taken into account.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 34 and 35 of Schedule 1.
c
Equipment category 3 comprises equipment designed to be capable of functioning in conformity with the operating parameters established by the manufacturer and ensuring a normal level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by gases, vapours, mists, or air/dust mixtures are unlikely to occur or, if they do occur, are likely to do so only infrequently and for a short period only.
Equipment in this category ensures the requisite level of protection during normal operation.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 36 and 37 of Schedule 1.
Amendment to Schedule 2I46938
Schedule 2 (notified body requirements) is amended as follows—
a
in the heading and in paragraphs 6, 9, 12(a) and 18 for “notified” substitute “
approved
”
;
b
in paragraph 3 for “regulation 44 (notification)” substitute “
regulation 43 (approval of conformity assessment bodies)
”
;
c
in paragraph 10(b) for “a notified” substitute “
an approved
”
;
d
in paragraph 12(c) for “harmonised standards and of the ATEX Directive” substitute “
designated standards
”
; and
e
in paragraph 18 for “under the ATEX Directive” substitute “
by the Secretary of State
”
.
Amendment to Schedule 3I47039
In Schedule 3 (operational obligations of notified bodies) is amended as follows—
a
in the shoulder reference for “Regulation 49” substitute “
Regulation 47
”
;
b
in the heading and in paragraphs 7 and 9 for “notified” substitute “
approved
”
;
c
in all places in which it occurs (other than where stated in paragraph (b)) for “a notified” substitute “an approved";
d
in paragraph 10(b) for “regulation 44 (notification)” substitute “
regulation 43 (approval of conformity assessment bodies)
”
;
e
in paragraph 10(d) for “notification under regulation 44” substitute “
approval under regulation 43
”
;
f
in paragraph 12 for “bodies notified under the ATEX Directive” substitute “
bodies approved under these Regulations
”
;
g
in paragraph 13 for “notified body coordination group established under the ATEX Directive” substitute “
approved body coordination group established by the Secretary of State
”
.
Insertion of Schedule 3AI47140
After Schedule 3 insert—
SCHEDULE 3AConformity Assessment Procedures (Annexes III to IX of the ATEX Directive)
PART 1
TYPE EXAMINATION1
Type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of a product and verifies and attests that the technical design of the product meets the requirements of these Regulations that apply to it.
2
Type examination shall be carried out with the examination of a specimen, representative of the production envisaged, of the complete product (production type).
3
The manufacturer shall lodge an application for Type examination with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
a written declaration that the same application has not been lodged with any other approved body,
c
the technical documentation. The technical documentation shall make it possible to assess the product's conformity with the applicable requirements of these Regulations and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
i
a general description of the product,
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.,
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
iv
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
v
results of design calculations made, examinations carried out, etc., and
vi
test reports,
d
the specimens representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme.
4
The approved body shall:
4
examine the technical documentation, verify that the specimen(s) have been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential health and safety requirements of these Regulations;
4
agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body shall draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes. Without prejudice to its obligations vis-à-vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
6
Where the type meets the requirements of these Regulations that apply to the product concerned, the approved body shall issue a Type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The Type examination certificate may have one or more annexes attached.
The Type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured products with the examined type to be evaluated and to allow for in-service control.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a Type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
7
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the product with the essential health and safety requirements of these Regulations or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original Type examination certificate.
8
Each approved body shall inform the Secretary of State concerning the Type examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the Type examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and/or additions thereto which it has issued.
The Health and Safety Executive for Northern Ireland may, on request, obtain a copy of the Type examination certificates and/or additions thereto. On request, The Health and Safety Executive for Northern Ireland may obtain a copy of the technical documentation and the results of the examinations carried out by the approved body. The approved body shall keep a copy of the Type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
9
The manufacturer shall keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the product has been placed on the market.
10
The manufacturer's authorised representative may lodge the application referred to in paragraph 3 and fulfil the obligations set out in paragraphs 7 and 9, provided that they are specified in the mandate.
PART 2CONFORMITY TO TYPE BASED ON QUALITY ASSURANCE OF THE PRODUCTION PROCESS
1
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5, and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the products concerned as specified in paragraph 3 and shall be subject to surveillance as specified in paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the products concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
a written declaration that the same application has not been lodged with any other approved body,
c
all relevant information for the product category envisaged,
d
the documentation concerning the quality system,
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure that the products are in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality,
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used,
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out,
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc., and
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in paragraph 3.1(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation,
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model, other than a component and keep it at the disposal of the national authorities for 10 years after the product other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
6
The manufacturer shall, for a period ending 10 years after the product has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved,
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 3CONFORMITY TO TYPE BASED ON PRODUCT VERIFICATION
1
Conformity to type based on product verification is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5 and ensures and declares on his sole responsibility that the products concerned, which have been subject to the provisions of paragraph 3, are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the approved type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Verification3
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests in order to check the conformity of the products with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
The examinations and tests to check the conformity of the products with the appropriate requirements shall be carried out by examination and testing of every product as specified in paragraph 4.
Verification of conformity by examination and testing of every product4
4
All products shall be individually examined, and appropriate tests set out in the relevant designated standard(s) and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify conformity with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
In the absence of such a designated standard, the approved body concerned shall decide on the appropriate tests to be carried out.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to each approved product or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity available for inspection by the national authorities for 10 years after the product has been placed on the market.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3, the latter's identification number to each individual product other than a component that is in conformity with the approved type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model other than a component and keep it at the disposal of the national authorities, for 10 years after the product, other than a component, has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
If the approved body referred to in paragraph 3 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the products other than components.
6
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the products during the manufacturing process.
Authorised representative7
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in paragraph 2.
PART 4CONFORMITY TO TYPE BASED ON INTERNAL PRODUCTION CONTROL PLUS SUPERVISED PRODUCT TESTING
1
Conformity to type based on internal production control plus supervised product testing is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 4, and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Product checks3
For each individual product manufactured one or more tests on one or more specific aspects of the product shall be carried out by the manufacturer or on his behalf, in order to verify conformity with the type described in the Type examination certificate and with the corresponding requirements of these Regulations. The tests shall be carried out under the responsibility of an approved body, chosen by the manufacturer.
The manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking, declaration of conformity and attestation of conformity4
4
The manufacturer shall affix the UK marking to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for a product model other than a component and keep it at the disposal of the national authorities for 10 years after the product, other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
4
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative5
The manufacturer's obligations set out in paragraph 4 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 5CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE
1
Conformity to type based on product quality assurance is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5 and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for final product inspection and testing of the products concerned as specified in paragraph 3 and shall be subject to surveillance as specified in paragraph 4.
Quality System3
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the products concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well
b
a written declaration that the same application has not been lodged with any other approved body,
c
all relevant information for the product category envisaged,
d
the documentation concerning the quality system, and
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure compliance of the products with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality,
b
the examinations and tests that will be carried out after manufacture,
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.,
d
the means of monitoring the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in paragraph 3.1(e) in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation,
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model, other than a component and keep it at the disposal of the national authorities for 10 years after the product other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
6
The manufacturer shall, for a period ending 10 years after the product has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved,
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 6INTERNAL PRODUCTION CONTROL
1
Internal production control is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 4, and ensures and declares on his sole responsibility that the products concerned satisfy the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation. The documentation shall make it possible to assess the product's conformity to the relevant requirements and shall include an adequate analysis and assessment of the risk(s).
The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
a
a general description of the product,
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
e
results of design calculations made, examinations carried out, etc., and
f
test reports.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured products with the technical documentation referred to in paragraph 2 and with the requirements of these Regulations that apply to them.
UK marking, declaration of conformity and attestation of conformity4
4
The manufacturer shall affix the UK marking to each individual product other than a component that satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for a product model other than a component and keep it together with the technical documentation at the disposal of the national authorities for 10 years after the product, other than a component, has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
4
The manufacturer shall draw up a written attestation of conformity for each component model and keep it together with the technical documentation at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative5
The manufacturer's obligations set out in paragraph 4 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 7CONFORMITY BASED ON UNIT VERIFICATION
1
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 5, and ensures and declares on his sole responsibility that the product concerned, which has been subject to the provisions of paragraph 4, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation2
2
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in paragraph 4. The documentation shall make it possible to assess the product's conformity with the relevant requirements and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
a
a general description of the product,
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.,
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
e
results of design calculations made, examinations carried out, etc., and
f
test reports.
2
The manufacturer shall keep the technical documentation at the disposal of the relevant national authorities for 10 years after the product has been placed on the market.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with the applicable requirements of these Regulations.
Verification4
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests, set out in the relevant designated standards and/or equivalent tests set out in other relevant technical specifications, to check the conformity of the product with the applicable requirements of these Regulations, or have them carried out. In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to the approved product, or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the national authorities for 10 years after the product has been placed on the market.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 4, the latter's identification number to each product other than a component that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity and keep it at the disposal of the national authorities for 10 years after the product, other than a component has been placed on the market. The declaration of conformity shall identify such product for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
5
The manufacturer shall draw up a written attestation of conformity and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative6
The manufacturer's obligations set out in paragraphs 2.2 and 5 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
Amendment to Schedule 6I47241
Schedule 6 (EU Declaration of Conformity) is amended as follows—
a
omit “EU” from the heading;
b
in paragraph 5, for “Union harmonisation legislation” substitute “
statutory requirements
”
;
c
in paragraph 6, for “harmonised” substitute “
designated
”
;
d
in paragraph 7, for “notified” substitute “
approved
”
.
SCHEDULE 26Amendment of the Non-automatic Weighing Instruments Regulations 2016
IntroductionI9071
The Non-automatic Weighing Instruments Regulations 2016 are amended in accordance with paragraphs 2 to 45.
Amendment to regulation 2I4732
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
before the definition of “authorised representative” insert—
“approved body” has the meaning given to it in regulation 47 (approved bodies);
F270d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “Commission”;
g
after the definition of “conformity assessment body” insert—
“conformity assessment procedure” means a procedure referred to in regulation 36;”;
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with Chapter 2 of Part 3;”;
“designated standard” has the meaning given to it in regulation 2A;
h
F269ha
in the definition of “essential requirements” for “Annex I to the Directive” substitute “
Schedule 6
”
;
i
omit the definition of “EU declaration of conformity”;
j
omit the definition of “EU-type examination certificate”
k
omit the definition of “harmonised standard”;
l
for the definition of “importer” substitute—
F272“importer” means a person who—
a
is established in the United Kingdom and places a non-automatic weighing instrument from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a non-automatic weighing instrument on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
m
in the definition of “M marking” for “CE” substitute “
UK
”
;
F273n
in the definition of “make available on the market” for “European Economic Area market” substitute “market of Great Britain”;
o
omit the definition of “national accreditation body”;
p
omit the definition of “notified body requirements”;
q
omit the definition of “notifying authority”;
F274r
in the definition of “place on the market” for “in the European Economic Area” substitute “of Great Britain”;
s
in the definition of “technical documentation” for “Annex II to the Directive” substitute Schedule 7;
t
after the definition of “technical specification” insert—
“Type-examination certificate” means a type-examination certificate issued by an approved body in accordance with Module B of Schedule 7;
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
u
omit the definition of “Union harmonisation legislation”.
3
After paragraph (1) insert—
1A
Schedules 6 to 8 reproduce the provisions of Annexes I to III to the Directive (respectively) with amendments to correct deficiencies in retained EU law.
1B
A reference to any provision of Schedules 6 to 8 is a reference to the equivalent provision of the relevant Annex to the Directive as set out in the relevant Schedule.
4
Omit paragraph (2).
Insertion of regulation 2AI4753
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a non-automatic weighing instrument, process, service or system and which lays down one or more of the following—
a
the characteristics required of a non-automatic weighing instrument, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the non-automatic weighing instrument as regards the name under which the measuring instrument is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the non-automatic weighing instrument, where these have an effect on the characteristics of the non-automatic weighing instrument.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any persons who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
9
Regulations made under paragraph (8) are to be made by statutory instrument.
10
A statutory instrument containing regulations made under paragraph (8) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 4I4764
Regulation 4 (revocations and transitional and consequential provisions) is amended as follows—
a
in paragraph (2) after “in paragraph (1)” insert “
subject to the modifications made in paragraph (3A)
”
;
b
after paragraph (3) insert—
3A
The modifications referred to in paragraph (2) are as follows—
a
in the Non-automatic Weighing Instruments Regulations 2000 and the Non-automatic Weighing Instruments (Amendment) Regulations 2008—
i
any reference to “the Community” is to be read as including the United Kingdom;
ii
references to “member State” is to be read as including the United Kingdom;
b
in the Non-automatic Weighing Instruments Regulations 2000—
i
omit regulation 10(14);
ii
in regulations 25(6)(a)(i) and 25(7)(a) for “; and” substitute
“ . ”; andiii
omit regulations 25(6)(a)(ii), 25(6)(b) and 25(7)(b).
Transitional provision in relation to EU ExitI4745
After regulation 4 insert—
Transitional provision in relation to EU Exit4A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F276IP completion day.
2
Subject to paragraph (3), where a non-automatic weighing instrument was made available on the market during the pre-exit period, despite the amendments made by Schedule 26 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M80, any obligation to which a person was subject under these Regulations as they had effect immediately before F276IP completion day, continues to have effect as it did immediately before F276IP completion day, in relation to that non-automatic weighing instrument.
3
Paragraph (2) does not apply to—
a
any obligation of any competent authority to inform the European Commission or Member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that non-automatic weighing instrument.
4
Where during the pre-exit period—
a
a non-automatic weighing instrument has not been placed on the market; and
b
a manufacturer has taken any action under regulation 36 as it had effect immediately before F276IP completion day in relation to that non-automatic weighing instrument,
that action has effect as if it had been done under regulation 36 as it had effect on and after F276IP completion day.
Amendment to regulation 6I477F2776
In regulation 6 (manufacturer's responsibilities - design, conformity assessment and marking of regulated non-automatic weighing instruments)—
a
the existing provision is renumbered paragraph (1);
b
in paragraph (1)(d) (as so renumbered) for “an EU” substitute “
a
”
;
c
in paragraph (1)(e) (as so renumbered)—
i
after “instrument” insert “
or where paragraph (2) applies, in respect of the UK marking, to a label affixed to a product or to a document accompanying the product
”
;
ii
for “CE” substitute “
UK
”
;
d
after the renumbered paragraph (1) insert—
2
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the instrument; or
b
to a document accompanying the instrument.
Amendment to regulation 7I4787
In regulation 7 (manufacturers' obligations in respect of records) omit “EU”.
Amendment to regulation 8I4798
In regulation 8 (manufacturers' obligations to ensure continuing conformity with essential requirements) in paragraph (2)(b) for “harmonised” substitute “
designated
”
.
Amendment to regulation 10I4809
In regulation 10 (manufacturers to mark contact details on regulated non-automatic weighing instruments) in paragraph (3) for “in a language” to “be in”, substitute “
clear, legible and in easily understandable
”
.
Amendment to regulation 11I48110
In regulation 11 (documentation to accompany regulated non-automatic weighing instruments)—
a
omit paragraph (2);
b
in paragraph (3) for “clear, understandable and intelligible” substitute “
clear, legible and in easily understandable English
”
.
Amendment to regulation 12I48211
In regulation 12 (action to be taken where regulated non-automatic weighing instruments placed on the market are not in conformity with the essential requirements) in paragraph (3) for “national” to “market” substitute “
authority
”
.
Amendment to regulation 14I48312
In regulation 14 (use of authorised representatives by manufacturers) in paragraph (3)(a) omit “EU”.
Amendment to regulation 15I48413
In regulation 15 (introductory) for “European Economic Area” substitute “
United Kingdom
”
.
Amendment to regulation 16I48514
In regulation 16 (ensuring compliance of regulated non-automatic weighing instruments) in paragraph (2)(c) for “CE” substitute “
UK
”
.
Amendment to regulation 18I48615
In regulation 18 (requirements to mark importers' details on regulated non-automatic weighing instruments) —
a
for paragraph (2) substitute—
2
Paragraph (1) does not apply where—
a
either—
i
the importer would have to open the packaging in order to indicate the information on the instrument; or
b
before placing the instrument on the market, the importer sets out the information referred to in paragraph (1)—
i
where sub-paragraph (a)(i) applies, on the packaging and in a document accompanying the instrument;
ii
where sub-paragraph (a)(ii) applies, in a document accompanying the instrument.
b
in paragraph (3) for “in a language” to “be in” substitute “
clear, legible and in easily understandable
”
.
Amendment to regulation 19I48716
In regulation 19 (importers' duty to ensure that regulated non-automatic weighing instruments are accompanied by relevant documentation)—
a
in paragraph (1) for “in a language easily understood by end users” substitute “
which are clear, legible and in easily understandable English
”
; and
b
omit paragraph (2).
Amendment to regulation 23I48817
In regulation 23 (requirement for importer to keep copy of EU declaration of conformity) and in the heading to that regulation, omit “EU”.
Amendment to regulation 27I48918
Regulation 27 (distributors-verification obligations) is amended as follows—
a
in paragraph (1) for “CE” substitute “
UK
”
;
b
in paragraph (2) for “easily understood by end users” substitute “
which are clear, legible and in easily understandable English
”
; and
c
omit paragraph (3).
Insertion of regulation 32AI49019
After regulation 32 insert—
Obligations which are met by complying with obligations in the Directive32A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(19);
c
“Module B” means the conformity assessment procedure set out in point 1 of Annex II;
d
“EU-type examination certificate” means an EU-type examination certificate issued in accordance with Module B;
e
“harmonised standard” has the meaning given to it in Article 2(11).
2
Paragraph (3) applies where, before placing a non-automatic weighing instrument on the market, the manufacturer—
a
ensures that the non-automatic weighing instrument has been designed and manufactured in accordance with the essential requirements set out in Annex I;
b
ensures that the relevant conformity assessment procedures that apply to that non-automatic weighing instrument in accordance with Article 13 have been carried out;
c
draws up the technical documentation referred to in Annex II;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes the CE marking and the supplementary metrology marking, in accordance with Articles 16 and 17(1) to (5);
f
affixes the inscriptions provided for in points 1 or 2 of Annex III in accordance with Article 6(5);
g
affixes where required in accordance with Article 6(5) the restrictive use symbol as provided for in Article 18 and in point 3 of Annex III;
h
draws up an EU declaration of conformity, in accordance with Article 14; and
i
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 6, 9(3) and (4), 41 and 45(2) are to be treated as being satisfied;
b
regulations 7, 8(2), 44, 63(1)(a) to (e), 67, 68 and 71 apply subject to the modifications in paragraph (8); and
c
Regulations 34 to 36 do not apply.
4
Paragraph (5) applies where, before placing a regulated non-automatic weighing instrument on the market, the importer ensures that—
a
the relevant conformity assessment procedure referred to in Article 13 has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex II; and
c
the non-automatic weighing instrument bears the CE marking and supplementary metrology marking in accordance with Articles 16 and 17(1) to (5).
5
Where this paragraph applies—
a
the requirements of regulation 16(2)(a) to (c) are to be treated as being satisfied; and
b
regulations 23, 63(1)(a) to (e), 67 and 68 apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making a regulated non-automatic weighing instrument available on the market, a distributor ensures that the non-automatic weighing instrument bears the CE marking and the inscriptions referred to in point 1 of Annex III.
7
Where this paragraph applies—
a
regulation 27(1) is to be treated as being satisfied; and
b
regulations 28(1), 28(2), 29, 63(1)(a), 63(1)(b), 67, 68 and 71 apply subject to the modifications in paragraph (8).
8
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (7)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “designated standard” is to be read as a reference to a harmonised standard;
d
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 13;
e
any reference to “technical documentation” is to be read as a reference to the technical documentation referred to in Annex II;
f
any reference to “type examination certificate” is to be read as a reference to an EU-type examination certificate;
g
any reference to “M marking” is to be read as a reference to the supplementary metrology marking;
h
any reference to “approved body” is to be read as a reference to the body that undertook any conformity assessment procedure in accordance with Article 13;
i
any reference to “authorised mark” includes the CE marking and the supplementary metrology marking.
Conformity assessment procedure obligations that are met by complying with the Directive32B
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“EU-type examination certificate” means an EU-type examination certificate issued in accordance with the conformity assessment procedure set out in point 1 of Annex II (Module B);
c
any reference to “the first stage of the conformity assessment procedure” is a reference to one or both of the following—
i
all examinations and tests which are not gravity dependent and which are included in the conformity assessment procedures set out in points 2 to 5 of Annex II;
ii
the examinations and tests included in the conformity assessment procedures set out in points 2 to 5 of Annex II that may be carried out at the manufacturer's works or any other location where—
aa
the transport of the instrument to its place of use requires dismantling of the instrument; or
bb
the putting into service of the instrument in its place of use requires assembly of the instrument or other technical installation work that is likely to affect the instrument's performance.
2
Paragraph (3) applies where, prior to the manufacture of a non-automatic weighing instrument the manufacturer has ensured that the conformity assessment procedure as set out in point 1 of Annex II (Module B) has been carried out.
3
Where this paragraph applies—
a
the reference in regulation 36(a) to “Module B as set out in point 1 of Schedule 7” is to be read as a reference to the conformity assessment procedure as set out in point 1 of Annex II (Module B); and
b
regulations 6(b) and (c), 7, 16(2)(a) and (b), 63(1)(e), 67(2)(b), 68(4)(b) and paragraph 1 of Schedule 1 apply subject to the modifications in paragraph (6).
4
Paragraph (5) applies where—
a
in accordance with point 7.1 of Annex II, the procedures set out in points 2 to 5 of that Annex may be carried out in two stages; and
b
the first stage of the conformity assessment procedure is carried out in accordance with any of the following points of Annex II—
i
point 2 (Module D);
ii
point 3 (Module D1);
iii
point 4 (Module F); or
iv
point 5 (Module F1).
5
Where this paragraph applies—
a
the reference in regulation 36(1)(a)(i) to “Module D as set out in point of Schedule 7” is to be read as including the first stage of the conformity assessment procedure as set out in point 2 of Annex II (Module D);
b
the reference in regulation 36(1)(a)(ii) to “Module F as set out in point 4 of Schedule 7” is to be read as including the first stage of the conformity assessment procedure as set out in point 4 of Annex II (Module F);
c
the reference in regulation 36(3)(a) to “Module D1 as set out in point 3 of Schedule 7” is to be read as including the first stage of the conformity assessment procedure as set out in point 3 of Annex II (Module D1);
d
the reference in regulation 36(3)(b) to “Module F1 as set out in point 5 of Schedule 7” is to be read as including the first stage of the conformity assessment procedure as set out in point 5 of Annex II (Module F1);
e
regulations 6(b) and (c), 7, 16(2)(a) and (b), 45(6) and (7), 63(1)(c) and (e) and 67(1)(c) apply subject to the modifications in paragraph (6).
6
The modifications referred to in paragraphs (3)(b) and (5)(e) are that—
a
any reference to “relevant conformity assessment procedure” is to be read as including—
i
where paragraph (3) applies, the conformity assessment procedure set out in point 1 of Annex II;
ii
where paragraph (5) applies, the relevant first stage conformity assessment procedure;
b
any reference to “type examination” is to be read as a reference to the EU-Type examination certificate;
c
any reference to “technical documentation” is to be read as including the technical documentation required by points 1 to 5 of Annex II (as applicable);
d
any reference to “approved body” is to be read as including the body which undertook the first stage conformity assessment procedure.
F281Expiry of regulations 32A and 32B32C
1
Subject to paragraph (2), regulation 32A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 32A—
a
any non-automatic weighing instrument which was placed on the market pursuant to regulation 32A may continue to be made available on the market on or after the expiry of regulation 32A;
b
any obligation to which a person was subject under regulation 32A in respect of any non-automatic weighing instrument placed on the market pursuant to regulation 32A continues to have effect after the expiry of regulation 32A, in respect of that instrument.
3
Subject to paragraph (4), regulation 32B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 32B in relation to a non-automatic weighing instrument prior to the expiry of regulation 32B, regulation 32B continues to apply in respect of that instrument where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” has the meaning given to it in regulation 32B(1)(b).
Qualifying Northern Ireland Goods32D
1
Where paragraph (2) applies—
a
a non-automatic weighing instrument is to be treated as being in conformity with the essential requirements; and
b
each relevant economic operator is to be treated as having complied or as complying with the obligations imposed on them under Part 2.
2
This paragraph applies where—
a
a non-automatic weighing instrument is—
i
in conformity with the essential requirements, within the meaning of that term in regulation 2, as it applies in Northern Ireland; and
ii
qualifying Northern Ireland goods;
b
each relevant economic operator has complied or is complying with the obligations imposed on them under Part 2, as that Part applies in Northern Ireland; and
c
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(c) are that, before placing the non-automatic weighing instrument on the market, the importer—
a
complies with regulation 18;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in accordance with Part 3, as that Part applies in Northern Ireland;
ii
the manufacturer has drawn up the technical documentation; and
iii
the non-automatic weighing instrument bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 34I49120
In regulation 34 (methods of establishing conformity with the essential requirements)—
a
in sub-paragraph (a) for “harmonised” the first time it appears, substitute “
designated
”
; and
b
omit from “where” to “European Union”.
Amendment to regulation 35I49221
In regulation 35 (presumptions of conformity of regulated non-automatic weighing instruments) for “harmonised” substitute “
designated
”
.
Amendment to regulation 36I49322
In regulation 36 (conformity assessment procedures)—
a
in paragraphs (1)(a) and (6) for “Annex II to the Directive” substitute “
Schedule 7
”
;
b
in paragraphs (1)(a)(i), (1)(a)(ii), (1)(b), (3)(a) and (3)(b) for “Annex II” substitute “
Schedule 7
”
;
c
in paragraph (4), for “A notified” substitute “
An approved
”
.
Amendment to regulation 37I49423
Omit regulation 37 (subsidiaries and contractors).
Amendment to regulation 38I49524
In regulation 38 (fees)—
a
for “a United Kingdom notified”, substitute “
an approved
”
in both places in which it occurs;
b
In sub-paragraph (2)(a), for “United Kingdom notified” substitute “
approved
”
.
Amendment to regulation 39I49625
In regulation 39 (application of Chapter) and in the heading to Chapter 2 omit “EU” in both places in which it occurs.
Amendment to regulation 40I49726
In regulation 40 (form and contents of EU declaration of conformity etc.)—
a
in paragraph (1) and in the heading omit “EU”;
b
in paragraph (1)(b) for “Annex IV to the Directive” substitute Schedule 9;
c
in paragraph (1)(c) for “Annex II to the Directive” substitute Schedule 7; and
d
in paragraph (2) omit “EU”.
Amendment to regulation 41I49827
For regulation 41 (regulated instruments that require more than one declaration of conformity) substitute—
41
Where a non-automatic weighing instrument is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 42I49928
In regulation 42 (responsibility of manufacturer that draws up declaration of conformity) for “an EU” substitute “
a
”
.
Amendment to regulation 43I50029
In regulation 43 (conformity with Directive requirements to be indicated by the CE marking)—
a
in the heading omit “Directive”; and
b
in the regulation and in the heading for “CE” substitute “
UK
”
.
Amendment to regulation 44I50130
For regulation 44 (general principles relating to the M marking) substitute—
Prohibition on improper use of UK marking and the M marking44
1
An economic operator must not affix the UK marking or the M marking to a regulated non-automatic weighing instrument unless—
a
that economic operator is the manufacturer of the non-automatic weighing instrument; and
b
the conformity of the non-automatic weighing instrument with the essential requirements has been demonstrated by a conformity assessment procedure.
2
An economic operator must not affix a marking to a regulated non-automatic weighing instrument which is not the UK marking or the M marking but which purports to attest that the non-automatic weighing instrument satisfies the essential requirements.
3
An economic operator must not affix to a regulated non-automatic weighing instrument any other marking if the visibility, legibility and meaning of the UK marking or the M marking would be impaired as a result.
Amendment to regulation 45I50231
Regulation 45 (rules and conditions for affixing the CE marking and the M marking etc.) is amended as follows—
a
in paragraphs (1) and (4) and in the heading for “CE” substitute “
UK
”
;
F282ab
in paragraph (2) for “or its data plate” substitute “
, its data plate, or where regulation 6(2) applies in respect of the UK marking, to a label affixed to the regulated non-automatic weighing instrument, or to a document accompanying the regulated non-automatic weighing instrument;
”
b
in paragraph (5) for “Annex II to the Directive” substitute “
Schedule 7
”
; and
c
in paragraphs (5), (6) and (7) for “notified” substitute “
approved
”
in each place in which it occurs.
Amendment to Part 5I50332
For Part 5, substitute—
PART 5APPROVAL OF CONFORMITY ASSESSMENT BODIES
Approved bodies47
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 48 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 51 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F283IP completion day notified to the European Commission and the member State of the European Union, in accordance with Article 27 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 47(2)(b), as it had effect immediately before F283IP completion day;
“approved body requirements” means the requirements set out in Schedule 3.
Approval of conformity assessment bodies48
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the class of regulated non-automatic weighing instruments in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b)(i), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies49
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring50
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 48(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval51
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 50(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 48 (approval of conformity assessment bodies).
2
With the consent of an approved body, or where the Secretary of State determines that an approved body no longer meets a condition in accordance with regulation 50(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 48 (approval of conformity assessment bodies).
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the failure.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of that notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must—
a
on the request of the Secretary of State, transfer its files to another approved body or to the Secretary of State; or
b
in the absence of a request under sub-paragraph (a), ensure that its files relating to the activities it has undertaken as an approved body are kept available for the Secretary of State and competent authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
7
The Secretary of State may impose a monetary penalty on an approved body that fails to comply with any requirement imposed by or under paragraph (5).
8
Schedule 5 has effect in relation to monetary penalties imposed under paragraph (7).
Subsidiaries and contractors52
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M81.
Register of approved bodies53
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body54
1
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements; and
b
monitoring approved bodies in accordance with regulation 50.
2
Where the Secretary of State authorises the UK national accreditation body pursuant to paragraph (1), the Secretary of State remains fully responsible for anything done pursuant to that authorisation.
Amendment to regulation 58I50433
Regulation 58 (regulated non-automatic weighing instruments presenting a risk) is amended as follows—
a
in paragraph (5), for “notified” substitute “
approved
”
;
b
omit paragraph (6);
c
in paragraph (7) for “on the market throughout the European Economic Area” substitute “
in the United Kingdom
”
;
d
in paragraph (9) for “Commission and the other EEA States” substitute “
Secretary of State
”
; and
e
in paragraph (10)(f)(ii) for “harmonised” substitute “
designated
”
.
Amendment to regulation 59I50534
Omit regulation 59 (EU safeguard procedure).
Amendment to regulation 60I50635
In regulation 60 (compliant regulated non-automatic weighing instruments which present a risk) in paragraph (3), for “Commission and the other EEA states” substitute “
Secretary of State
”
.
Amendment to regulation 63I50736
In regulation 63 (compliance notice procedure) in paragraph (1)—
a
in sub-paragraphs (a) and (b), for “CE” substitute “
UK
”
in both places in which it occurs;
b
in sub-paragraph (a) for “Article 30 of the RAMS Regulation or the requirements of these Regulations” substitute “
regulation 44 or regulation 45
”
;
c
in sub-paragraph (c) for “notified” substitute “
approved
”
; and
d
in sub-paragraph (d) omit “EU”.
Amendment to regulation 64I50837
In regulation 64 (enforcement notice procedure)—
a
in paragraph (5)—
i
for “a United Kingdom notified” substitute “
an approved
”
;
ii
for “that notified” substitute “
that approved
”
; and
b
omit paragraph (6).
Amendment to regulation 67I50938
In regulation 67 (disqualification)—
a
in paragraph (1)—
i
in sub-paragraph (a) for “CE” substitute “
UK
”
;
ii
in sub-paragraph (c) for “notified” substitute “
approved
”
; and
b
in paragraph 2(b) omit “EU-”.
Amendment to regulation 68I51039
In regulation 68 (requalification)—
a
in paragraph (3)(c)—
i
omit “notified”; and
ii
for “Annex II to the Directive” substitute “
Schedule 7
”
;
b
in paragraph (3)(d)—
i
for “notified” substitute “
approved
”
; and
ii
for “Annex II to the Directive” substitute Schedule 7;
c
in paragraph (4)(b) omit “EU-”.
Amendment to regulation 71I51140
In regulation 71 (unauthorised application of authorised marks) in paragraph (5)—
a
in sub-paragraph (a) for “CE” substitute “
UK
”
; and
b
in sub-paragraph (c) for “notified” substitute “
approved
”
.
Amendment to Schedule 1I51241
In Schedule 1 (information to be marked on regulated non-automatic weighing instruments)—
a
in paragraph 1 omit “EU-”; and
b
in paragraph 9 for “the Annex III to the Directive” substitute “
Schedule 8
”
.
Amendment to Schedule 2I51342
In Schedule 2 (operational obligations of notified bodies)—
a
in paragraphs 3, 4 and 6 for “a notified” substitute “
an approved
”
;
b
in all places in which it occurs (other than the paragraphs referred to in sub-paragraph (a)), including in the heading, for “notified” substitute “
approved
”
;
c
in paragraph 7 for “notifying authority” substitute “
Secretary of State
”
;
d
in paragraph 7(b) for “notification” substitute “
approval
”
;
e
in paragraph 8 for “this Directive” substitute “
these Regulations
”
; and
f
omit paragraph 9.
Amendments to Schedule 3I51443
In Schedule 3 (requirements related to notified bodies)—
a
for the heading, substitute— “
SCHEDULE 3
Approved Body requirements
”
;
b
in paragraph 1 for “under the national law of an EEA state” substitute “
in the United Kingdom
”
;
c
in paragraph 8 for “Annex II to the Directive” substitute “
Schedule 7
”
;
d
in paragraph 9(c) for “a notified” substitute “
an approved
”
;
e
in all places in which it occurs (other than the paragraph referred to in sub-paragraph (d)), including in the heading, for “notified” substitute “
approved
”
;
f
in paragraph 11(c)—
i
for “Annex I to the Directive” substitute “
Schedule 6
”
;
ii
for “harmonised” substitute “
designated
”
; and
iii
for “European harmonisation legislation and of national legislation” substitute “
these Regulations
”
;
g
in paragraph 16 for “under the European Union harmonisation legislation” substitute “
by the Secretary of State
”
.
Amendments to Schedule 4I51544
In Schedule 4 (use for trade of regulated non-automatic weighing instruments in Great Britain) in paragraphs 1(3) and 1(5) for “Annex I to the Directive” substitute “
Schedule 6
”
.
Insertion of Schedule 6I51645
After Schedule 5 (monetary penalties) insert—
SCHEDULE 6(Annex I to the Directive)
ESSENTIAL REQUIREMENTS
The terminology used is that of the International Organisation of Legal Metrology
Preliminary observationWhere an instrument includes, or is connected to, more than one indicating or printing device used for the applications listed in sub-paragraphs (a) to (f) of regulation 3(2), those devices which repeat the results of the weighing operation and which cannot influence the correct functioning of the instrument shall not be subject to the essential requirements if the weighing results are printed or recorded correctly and indelibly by a part of the instrument which meets the essential requirements and the results are accessible to both parties concerned by the measurement. However, in the case of instruments used for direct sales to the public, display and printing devices for the vendor and the customer must fulfil the essential requirements.
Metrological requirements
1
Units of mass
The units of mass used shall be the legal units within the meaning of the Weights and Measures Act 1985 relating to units of measurement M82.
Subject to compliance with this condition, the following units are permitted—
a
SI units: kilogram, microgram, milligram, gram, tonne;
b
imperial unit: troy ounce, if weighing precious metals;
c
other non-SI unit: metric carat, if weighing precious stones.
For instruments that make use of the imperial unit of mass referred to above, the relevant essential requirements specified below shall be converted to that unit, using simple interpolation.
2
Accuracy classes
2
The following accuracy classes have been defined—
a
I special
b
II high
c
III medium
d
IIII ordinary
The specifications of these classes are given in Table 1.
Table 1
Accuracy classes
Class
Verification scale interval (e)
Minimum capacity (Min)
Number of verification scale intervals n = ((Max)/(e))
minimum value
minimum value
maximum value
I
0,001 g ≤ e
100 e
50 000
II
0,001 g ≤ e ≤ 0,05 g
20 e
100
100 000
0,1 g ≤ e
50 e
5 000
100 000
III
0,1 g ≤ e ≤ 2 g
20 e
100
10 000
5 g ≤ e
20 e
500
10 000
IIII
5 g ≤ e
10 e
100
1 000
The minimum capacity is reduced to 5 e for instruments in classes II and III for determining a conveying tariff.
2
Scale intervals
2
The actual scale interval (d) and the verification scale interval (e) shall be in the form—
1 x 10k, 2 x 10k, or 5 x 10k mass units,
k being any integer or zero.
2
For all instruments other than those with auxiliary indicating devices—
d = e.
2
For instruments with auxiliary indicating devices the following conditions apply—
e = 1 x 10kg;
d < e ≤ 10 d.
Those conditions do not apply for instruments of class I with d < 10–4 g, for which e = 10–3 g.
3
Classification
3
Instruments with one weighing range
Instruments equipped with an auxiliary indicating device shall belong to class I or class II. For these instruments the minimum capacity lower limits for these two classes are obtained from Table 1 by replacement in column 3 of the verification scale interval (e) by the actual scale interval (d).
If d < 10–4 g, the maximum capacity of class I may be less than 50 000 e.
3
Instruments with multiple weighing ranges
Multiple weighing ranges are permitted, provided they are clearly indicated on the instrument. Each individual weighing range is classified according to point 3.1. If the weighing ranges fall into different accuracy classes the instrument shall comply with the severest of the requirements that apply for the accuracy classes in which the weighing ranges fall.
3
Multi-interval instruments
3
Instruments with one weighing range may have several partial weighing ranges (multi-interval instruments).
Multi-interval instruments shall not be equipped with an auxiliary indicating device.
3
Each partial weighing range i of multi-interval instruments is defined by—
— its verification scale interval ei
with e(i + 1) > ei
— its maximum capacity Maxi
with Maxr = Max
— its minimum capacity Mini
with Mini = Max (i – 1)
and Min1 = Min
Where:
i = 1, 2, …r,
i = partial weighing range number,
r = the total number of partial weighing ranges
All capacities are capacities of net load, irrespective of the value of any tare used.
3
The partial weighing ranges are classified according to Table 2. All partial weighing ranges shall fall into the same accuracy class, that class being the instrument's accuracy class.
Table 2
Multi-level instruments
i = 1, 2, …r,
i = partial weighing range number,
r = the total number of partial weighing ranges
Class
Verification scale interval (e)
Minimum capacity (Min)
Number of verification scale intervals
Minimum value
Minimum value M83
n = ((Maxi) / (e(i+1)))
Maximum value
n = ((Maxi) / (ei))
I
0,001 g ≤ ei
100 e1
50 000
II
0,001 g ≤ ei ≤ 0,05 g
20 e1
5 000
100 000
0,1 g ≤ ei
50 e1
5 000
100 000
III
0,1 g ≤ ei
20 e1
500
10 000
IIII
5g ≤ ei
10 e1
50
1 000
4
Accuracy
4
On implementation of the procedures laid down in regulation 36, the error of indication shall not exceed the maximum permissible error of indication as shown in Table 3. In the case of digital indication the error of indication shall be corrected for the rounding error.
The maximum permissible errors apply to the net value and tare value for all possible loads, excluding preset tare values.
Table 3
Maximum permissible errors
Load
Maximum permissible error
Class I
Class II
Class III
Class IIII
0 ≤ m ≤ 50 000 e
0 ≤ m ≤ 5 000 e
0 ≤ m ≤ 500 e
0 ≤ m ≤ 50 e
± 0,5 e
50 000 e < m ≤ 200 000 e
5 000 e < m ≤ 20 000 e
500 e < m ≤
2 000 e
50 e < m ≤ 200 e
± 1,0 e
200 000 e < m
20 000 e < m ≤ 100 000 e
2 000 e < m ≤ 10 000 e
200 e < m ≤
1 000 e
± 1,5 e
4
The maximum permissible errors in service are twice the maximum permissible errors fixed in Section 4.1.
5
Weighing results of an instrument shall be repeatable, and shall be reproducible by the other indicating devices used and in accordance with other methods of balancing used.
The weighing results shall be sufficiently insensitive to changes in the position of the load on the load receptor.
6
The instrument shall react to small variations in the load.
7
Influence quantities and time
7
Instruments of classes II, III and IIII, liable to be used in a tilted position, shall be sufficiently insensitive to the degree of tilting that can occur in normal use.
7
The instruments shall meet the metrological requirements within the temperature range specified by the manufacturer. The value of this range shall be at least equal to—
a
5 °C for an instrument in class I;
b
15 °C for an instrument in class II;
c
30 °C for an instrument in class III or IIII.
In the absence of a manufacturer's specification, the temperature range of – 10 °C to + 40 °C applies.
7
Instruments operated from a mains power supply shall meet the metrological requirements under conditions of power supply within the limits of normal fluctuation.
Instruments operated from battery power shall indicate whenever the voltage drops below the minimum required value and shall under those circumstances either continue to function correctly or be automatically put out of service.
7
Electronic instruments, except those in class I and in class II if e is less than 1 g, shall meet the metrological requirements under conditions of high relative humidity at the upper limit of their temperature range.
7
Loading an instrument in class II, III or IIII for a prolonged period of time shall have a negligible influence on the indication at load or on the zero indication immediately after removal of the load.
7
Under other conditions the instruments shall either continue to function correctly or be automatically put out of service.
Design and construction
8
General requirements
8
Design and construction of the instruments shall be such that the instruments will preserve their metrological qualities when properly used and installed and when used in an environment for which they are intended. The value of the mass must be indicated.
8
When exposed to disturbances, electronic instruments shall not display the effects of significant faults, or shall automatically detect and indicate them.
Upon automatic detection of a significant fault, electronic instruments shall provide a visual or audible alarm that shall continue until the user takes corrective action or the fault disappears.
8
The requirements of points 8.1 and 8.2 shall be met on a lasting basis during a period of time that is normal in view of the intended use of such instruments.
Digital electronic devices shall always exercise adequate control of the correct operation of the measuring process, of the indicating device, and of all data storage and data transfer.
Upon automatic detection of a significant durability error, electronic instruments shall provide a visual or audible alarm that shall continue until the user takes corrective action or the error disappears.
8
When external equipment is connected to an electronic instrument through an appropriate interface the metrological qualities of the instrument shall not be adversely influenced.
8
The instruments shall have no characteristics likely to facilitate fraudulent use, whereas possibilities for unintentional misuse shall be minimal. Components that may not be dismantled or adjusted by the user shall be secured against such actions.
8
Instruments shall be designed to permit ready execution of the statutory controls laid down by these Regulations.
9
Indication of weighing results and other weight values
The indication of the weighing results and other weight values shall be accurate, unambiguous and non-misleading and the indicating device shall permit easy reading of the indication under normal conditions of use.
The names and symbols of the units referred to in point 1 of this Schedule shall comply with the provisions of the Weights and Measures Act 1985 with the addition of the symbol for the metric carat which shall be the symbol ‘ct’.
Indication shall be impossible above the maximum capacity (Max), increased by 9 e.
An auxiliary indicating device is permitted only to the right of the decimal mark. An extended indicating device may be used only temporarily, and printing shall be inhibited during its functioning.
Secondary indications may be shown, provided that they cannot be mistaken for primary indications.
10
Printing of weighing results and other weight values
Printed results shall be correct, suitably identified and unambiguous. The printing shall be clear, legible, non-erasable and durable.
11
Levelling
When appropriate, instruments shall be fitted with a levelling device and a level indicator, sufficiently sensitive to allow proper installation.
12
Zeroing
Instruments may be equipped with zeroing devices. The operation of these devices shall result in accurate zeroing and shall not cause incorrect measuring results.
13
Tare devices and preset tare devices
The instruments may have one or more tare devices and a preset tare device. The operation of the tare devices shall result in accurate zeroing and shall ensure correct net weighing. The operation of the preset tare device shall ensure correct determination of the calculated net value.
14
Instruments for direct sales to the public, with a maximum capacity not greater than 100 kg: additional requirements
Instruments for direct sale to the public shall show all essential information about the weighing operation and, in the case of price-indicating instruments, shall clearly show the customer the price calculation of the product to be purchased.
The price to pay, if indicated, shall be accurate.
Price-computing instruments shall display the essential indications long enough for the customer to read them properly.
Price-computing instruments may perform functions other than per-article weighing and price computation only if all indications related to all transactions are printed clearly and unambiguously and are conveniently arranged on a ticket or label for the customer.
Instruments shall bear no characteristics that can cause, directly or indirectly, indications the interpretation of which is not easy or straightforward.
Instruments shall safeguard customers against incorrect sales transactions due to their malfunctioning.
Auxiliary indicating devices and extended indicating devices are not permitted.
Supplementary devices are permitted only if they cannot lead to fraudulent use.
Instruments similar to those normally used for direct sales to the public which do not satisfy the requirements of this Section must carry near to the display the indelible marking ‘Not to be used for direct sale to the public’.
15
Price labelling instruments
Price labelling instruments shall meet the requirements of price indicating instruments for direct sale to the public, as far as applicable to the instrument in question. The printing of a price label shall be impossible below a minimum capacity.
SCHEDULE 7(Annex II to the Directive)
CONFORMITY ASSESSMENT PROCEDURES
1
Module B: type examination
1
type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of an instrument and verifies and attests that the technical design of the instrument meets the requirements of these Regulations that apply to it.
1
type examination may be carried out in any of the following manners—
— examination of a specimen, representative of the production envisaged, of the complete instrument (production type);
— assessment of the adequacy of the technical design of the instrument through examination of the technical documentation and supporting evidence referred to in point 1.3, plus examination of specimens, representative of the production envisaged, of one or more critical parts of the instrument (combination of production type and design type);
— assessment of the adequacy of the technical design of the instrument through examination of the technical documentation and supporting evidence referred to in point 1.3, without examination of a specimen (design type).
1
The manufacturer shall lodge an application for type examination with a single approved body of his choice.
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation. The technical documentation shall make it possible to assess the instrument's conformity with the applicable requirements of these Regulations and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument. The technical documentation shall contain, wherever applicable, at least the following elements—
i
a general description of the instrument;
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the instrument;
iv
a list of the designated standards applied in full or in part, and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
v
results of design calculations made, examinations carried out, etc.;
vi
test reports;
d
the specimens representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution. This supporting evidence shall mention any documents that have been used, in particular where the relevant designated standards have not been applied in full. The supporting evidence shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer or by another testing laboratory on his behalf and under his responsibility.
1
The approved body shall—
For the instrument—
1
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the instrument;
For the specimen(s)—
1
verify that the specimen(s) have been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
1
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
1
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential requirements of these Regulations;
1
agree with the manufacturer on a location where the examinations and tests will be carried out.
1
The approved body shall draw up an evaluation report that records the activities undertaken in accordance with point 1.4 and their outcomes. Without prejudice to its obligations vis-à-vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
1
Where the type meets the requirements of these Regulations, that apply to the instrument concerned, the approved body shall issue a type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The type examination certificate may have one or more annexes attached.
The type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured instruments with the examined type to be evaluated and to allow for in-service control.
The type examination certificate shall have a validity period of 10 years from the date of its issue, and may be renewed for subsequent periods of 10 years each. In the event of fundamental changes to the design of the instrument, e.g. as a result of the application of new techniques, the validity of type examination certificate may be limited to two years and extended by three years.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
1
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations, and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly. The manufacturer shall inform the approved body that holds the technical documentation relating to the type examination certificate of all modifications to the approved type that may affect the conformity of the instrument with the essential requirements of these Regulations or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original type examination certificate.
1
Each approved body shall inform the Secretary of State concerning the type examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the type examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and/or additions thereto which it has issued.
The other approved bodies and the Secretary of State may, on request, obtain a copy of the type examination certificates and/or additions thereto. On request, the Secretary of State may obtain a copy of the technical documentation and the results of the examinations carried out by the approved body. The approved body shall keep a copy of the type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
1
The manufacturer shall keep a copy of the type examination certificate, its annexes and additions together with the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
1
The manufacturer's authorised representative may lodge the application referred to in point 1.3 and fulfil the obligations set out in points 1.7 and 1.9, provided that they are specified in the mandate.
2
Module D: Conformity to type based on quality assurance of the production process
2
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 2.2 and 2.5, and ensures and declares on his sole responsibility that the instruments concerned are in conformity with the type described in the type examination certificate and satisfy the requirements of these Regulations that apply to them.
2
Manufacturing
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the instruments concerned as specified in point 2.3, and shall be subject to surveillance as specified in point 2.4.
2
Quality system
2
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the instruments concerned.
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system; and
e
the technical documentation of the approved type and a copy of the type examination certificate.
2
The quality system shall ensure that the instruments are in conformity with the type described in the type examination certificate and comply with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
2
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 2.3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in point 2.3.1(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
2
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
2
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in point 2.3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
2
Surveillance under the responsibility of the approved body
2
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
2
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
2
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
2
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
2
Conformity marking and declaration of conformity
2
The manufacturer shall affix the UK marking and the M metrology marking set out in these Regulations, and, under the responsibility of the approved body referred to in point 2.3.1, the latter's identification number to each individual instrument that is in conformity with the type described in the type examination certificate and satisfies the applicable requirements of these Regulations.
2
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
2
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities—
a
the documentation referred to in point 2.3.1;
b
the information relating to the change referred to in point 2.3.5, as approved;
c
the decisions and reports of the approved body referred to in points 2.3.5, 2.4.3 and 2.4.4.
2
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
2
Authorised representative
The manufacturer's obligations set out in points 2.3.1, 2.3.5, 2.5 and 2.6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
3
Module D1: Quality assurance of the production process
3
Quality assurance of the production process is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 3.2, 3.4 and 3.7, and ensures and declares on his sole responsibility that the instruments concerned satisfy the requirements of these Regulations that apply to them.
3
Technical documentation
The manufacturer shall establish the technical documentation. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument. The technical documentation shall, wherever applicable, contain at least the following elements—
a
a general description of the instrument;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the instrument;
d
a list of the designated standards applied in full or in part, and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
e
results of design calculations made, examinations carried out, etc.;
f
test reports.
3
The manufacturer shall keep the technical documentation at the disposal of the relevant national authorities for 10 years after the instrument has been placed on the market.
3
Manufacturing
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the instruments concerned as specified in point 3.5, and shall be subject to surveillance as specified in point 3.6.
3
Quality system
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the instruments concerned.
The application shall include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation referred to in point 3.2.
3
The quality system shall ensure compliance of the instruments with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in point 3.5.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in point 3.2 in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in point 3.5.2 or whether reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
3
Surveillance under the responsibility of the approved body
3
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
3
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular—
a
the quality system documentation;
b
the technical documentation referred to in point 3.2;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
3
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
3
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
3
Conformity marking and declaration of conformity
3
The manufacturer shall affix the UK marking and the M metrology marking, set out in these Regulations, and, under the responsibility of the approved body referred to in point 3.5.1, the latter's identification number to each individual instrument that satisfies the applicable requirements of these Regulations.
3
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
3
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities—
a
the documentation referred to in point 3.5.1;
b
the information relating to the change referred to in point 3.5.5, as approved;
c
the decisions and reports of the approved body referred to in points 3.5.5, 3.6.3 and 3.6.4.
3
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
3
Authorised representative
The manufacturer's obligations set out in points 3.3, 3.5.1, 3.5.5, 3.7 and 3.8 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
4
Module F: Conformity to type based on product verification
4
Conformity to type based on product verification is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 4.2 and 4.5 and ensures and declares on his sole responsibility that the instruments concerned, which have been subject to the provisions of point 4.3, are in conformity with the type described in the type examination certificate and satisfy the requirements of these Regulations that apply to them.
4
Manufacturing
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured instruments with the approved type described in the type examination certificate and with the requirements of these Regulations that apply to them.
4
Verification
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests in order to check the conformity of the instruments with the approved type described in the type examination certificate and with the appropriate requirements of these Regulations.
The examinations and tests to check the conformity of the instruments with the appropriate requirements shall be carried out by examination and testing of every instrument as specified in point 4.4.
4
Verification of conformity by examination and testing of every instrument
4
All instruments shall be individually examined and appropriate tests set out in the relevant designated standard(s), and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify conformity with the approved type described in the type examination certificate and with the appropriate requirements of these Regulations.
In the absence of such a designated standard, the approved body concerned shall decide on the appropriate tests to be carried out.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity available for inspection by the market surveillance authorities for 10 years after the instrument has been placed on the market.
4
Conformity marking and declaration of conformity
4
The manufacturer shall affix the UK marking and the M metrology marking, set out in these Regulations, and, under the responsibility of the approved body referred to in point 4.3, the latter's identification number to each individual instrument that is in conformity with the approved type described in the type examination certificate and satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities, for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
If the approved body referred to in point 4.3 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the instruments.
4
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the instruments during the manufacturing process.
4
Authorised representative
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in point 4.2.
5
Module F1: Conformity based on product verification
5
Conformity based on product verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 5.2, 5.3 and 5.6 and ensures and declares on his sole responsibility that the instruments concerned, which have been subject to the provisions of point 5.4, are in conformity with the requirements of these Regulations that apply to them.
5
Technical documentation
5
The manufacturer shall establish the technical documentation. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument. The technical documentation shall, wherever applicable, contain at least the following elements:
a
a general description of the instrument;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the instrument;
d
a list of the designated standards applied in full or in part, and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
e
results of design calculations made, examinations carried out, etc.;
f
test reports.
5
The manufacturer shall keep the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
5
Manufacturing
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured instruments with the applicable requirements of these Regulations.
5
Verification
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests to check the conformity of the instruments with the applicable requirements of these Regulations.
The examinations and tests to check the conformity with those requirements shall be carried out by examination and testing of every instrument as specified in point 5.5.
5
Verification of conformity by examination and testing of every instrument
5
All instruments shall be individually examined and appropriate tests, set out in the relevant designated standards and/or equivalent tests set out in other relevant technical specifications, shall be carried out to verify conformity with the requirements that apply to them. In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out.
5
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
5
Conformity marking and declaration of conformity
5
The manufacturer shall affix the UK marking and the M metrology marking, set out in these Regulations, and, under the responsibility of the approved body referred to in point 5.4, the latter's identification number to each individual instrument that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the relevant authorities upon request.
If the approved body referred to in point 5.5 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the instruments.
5
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the instruments during the manufacturing process.
5
Authorised representative
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in points 5.2.1 and 5.3.
6
Module G: Conformity based on unit verification
6
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in points 6.2, 6.3 and 6.5, and ensures and declares on his sole responsibility that the instrument concerned, which has been subject to the provisions of point 6.4, is in conformity with the requirements of these Regulations that apply to it.
6
Technical documentation
6
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in point 6.4. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument. The technical documentation shall, wherever applicable, contain at least the following elements—
a
a general description of the instrument;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the instrument;
d
a list of the designated standards applied in full or in part, and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied;
e
results of design calculations made, examinations carried out, etc.;
f
test reports.
6
The manufacturer shall keep the technical documentation at the disposal of the relevant market surveillance authorities for 10 years after the instrument has been placed on the market.
6
Manufacturing
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured instrument with the applicable requirements of these Regulations.
6
Verification
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests, set out in the relevant designated standards and/or equivalent tests set out in other relevant technical specifications, to check the conformity of the instrument with the applicable requirements of these Regulations, or have them carried out. In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to the approved instrument, or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
6
Conformity marking and declaration of conformity
6
The manufacturer shall affix the UK marking and the M metrology marking, set out in these Regulations, and, under the responsibility of the approved body referred to in point 6.4, the latter's identification number to each instrument that satisfies the applicable requirements of these Regulations.
6
The manufacturer shall draw up a written declaration of conformity and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
6
Authorised representative
The manufacturer's obligations set out in points 6.2.2 and 6.5 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
7
Common provisions
7
The conformity assessment according to Module D, D1, F, F1 or G may be carried out at the manufacturer's works or any other location if transport to the place of use does not require dismantling of the instrument, if the putting into service at the place of use does not require assembly of the instrument or other technical installation work likely to affect the instrument's performance, and if the gravity value at the place of putting into service is taken into consideration or if the instrument's performance is insensitive to gravity variations. In all other cases, it shall be carried out at the place of use of the instrument.
7
If the instrument's performance is sensitive to gravity variations the procedures referred to in point 7.1 may be carried out in two stages, with the second stage comprising all examinations and tests of which the outcome is gravity-dependent, and the first stage all other examinations and tests. The second stage shall be carried out at the place of use of the instrument.
7
Where a manufacturer has opted for execution in two stages of one of the procedures mentioned in point 7.1, and where these two stages will be carried out by different parties, an instrument which has undergone the first stage of the procedure shall bear the identification number of the approved body involved in that stage.
7
The party which has carried out the first stage of the procedure shall issue for each of the instruments a certificate containing the data necessary for identification of the instrument and specifying the examinations and tests that have been carried out.
The party which carries out the second stage of the procedure shall carry out those examinations and tests that have not yet been carried out.
The manufacturer or his authorised representative shall ensure that he is able to supply the approved body's certificates of conformity on request.
7
A manufacturer who has opted for Module D or D1 in the first stage may either use this same procedure in the second stage or decide to continue in the second stage with Module F or F1 as appropriate.
7
The UK marking and the M metrology marking shall be affixed to the instrument on completion of the second stage, along with the identification number of the approved body which took part in the second stage.
SCHEDULE 8(Annex III to the Directive)
INSCRIPTIONS
1
Instruments intended to be used for the applications listed in sub-paragraphs (a) to (f) of regulation 3(2).
1
Those instruments shall bear visibly, legibly and indelibly the following inscriptions—
i
the number of the type examination certificate, where appropriate;
ii
the manufacturer's name, registered trade name or registered trade mark;
iii
the accuracy class, enclosed in an oval or in two horizontal lines joined by two half circles;
iv
maximum capacity, in the form Max …;
v
minimum capacity, in the form Min …;
vi
verification scale interval, in the form e = …;
vii
type, batch or serial number and when applicable;
viii
for instruments consisting of separate but associated units: identification mark on each unit;
ix
scale interval if it is different from e, in the form d = …;
x
maximum additive tare effect, in the form T = + …;
xi
maximum subtractive tare effect if it is different from Max, in the form T = – …;
xii
tare interval if it is different from d, in the form dT = …;
xiii
maximum safe load if it is different from Max, in the form Lim …;
xiv
the special temperature limits, in the form … oC/… oC;
xv
ratio between load receptor and load.
1
Those instruments shall have adequate facilities for the affixing of the conformity marking and inscriptions. These shall be such that it shall be impossible to remove the conformity marking and inscriptions without damaging them, and that the conformity marking and inscriptions shall be visible when the instrument is in its regular operating position.
1
Where a data plate is used it shall be possible to seal the plate unless it cannot be removed without being destroyed. If the data plate is sealable it shall be possible to apply a control mark to it.
1
The inscriptions Max, Min, e, and d, shall also be shown near the display of the result if they are not already located there.
1
Each load measuring device which is connected or can be connected to one or more load receptors shall bear the relevant inscriptions relating to the said load receptors.
2
Instruments not intended to be used for the applications listed in points (a) to (f) of regulation 3(2) shall bear visibly, legibly and indelibly—
— the manufacturer's name, registered trade name or registered trade mark;
— maximum capacity, in the form Max ….
Those instruments shall not bear the conformity marking as set out in these Regulations.
3
Restrictive use symbol referred to in regulation 9(3).
The restrictive use symbol shall be constituted by a capital letter ‘M’ printed in black on a red background at least 25 mm × 25 mm square with two intersecting diagonals forming a cross.
SCHEDULE 9DECLARATION OF CONFORMITY (No XXXX) M84
1
Instrument model/Instrument (product, type, batch or serial number):
2
Name and address of the manufacturer and, where applicable, his authorised representative:
3
This declaration of conformity is issued under the sole responsibility of the manufacturer.
4
Object of the declaration (identification of instrument allowing traceability; it may, where necessary for the identification of the instrument, include an image):
5
The object of the declaration described above is in conformity with the relevant UK legislation:
6
References to the relevant designated standards used or references to the other technical specifications in relation to which conformity is declared:
7
The approved body … (name, number) performed … (description of intervention) and issued the certificate:
8
Additional information:
— Signed for and on behalf of:
— (place and date of issue):
— (name, function) (signature):
SCHEDULE 27Amendment of the Measuring Instruments Regulations 2016
IntroductionI9081
The Measuring Instruments Regulations 2016 are amended in accordance with paragraphs 2 to 53.
Amendment to regulation 2I5172
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “active electrical energy meter”, insert—
“approved body” has the meaning given to it in regulation 53 (approved bodies);
F286d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “Commission”;
g
in the definition of “conformity assessment” before “measuring” insert “
regulated
”
;
h
after the definition of “conformity assessment body”, insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with chapter 3 of Part 4;
“designated standard” has the meaning given to it in regulation 2A;
“design examination certificate” means a design certificate issued by an approved body in accordance with Module H1 in Schedule 1B
i
omit the definition of “dimensional measuring instrument”;
j
in the definition of “distributor” before “measuring” insert “
regulated
”
;
k
in the definition of “essential requirements”—
i
before “measuring” insert “
regulated
”
;
ii
for “1”, substitute “
1A and 1C to 1J
”
;
l
omit the definition of “EU declaration of conformity”;
m
omit the definition of “EU-design examination certificate”;
n
omit the definition of “EU-type examination certificate”;
o
in the definition of “exhaust gas analyser” before “measuring” insert “
regulated
”
;
p
omit the definition of “harmonised standard”;
q
for the definition of “importer” substitute—
F287“importer” means a person who—
a
is established in the United Kingdom and places a regulated measuring instrument from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a regulated measuring instrument on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
r
in the definition of “M marking”—
i
before “measuring” insert “
regulated
”
;
ii
for “CE”, substitute “
UK
”
;
s
omit the definition of “measuring instrument”;
t
in the definition of “make available on the market”—
i
before “measuring” insert “
regulated
”
;
F288ii
for “the European Economic Area market” substitute “market of Great Britain”;
u
in the definition of “manufacturer” insert “
regulated
”
before “measuring” in each place it occurs;
v
in the definition of “market surveillance authority”, omit from “and” to “EEA state”;
w
omit the definition of “national accreditation body”;
x
omit the definition of “non-prescribed measuring instrument”;
y
omit the definition of “notified body”;
z
omit the definition of “notified body requirements”;
aa
omit the definition of “notifying authority”;
bb
in the definition of “place on the market”—
i
before “measuring” insert “
regulated
”
;
F285ii
for “, in the European Economic Area” substitute “of Great Britain”;
cc
in the definition of “putting into use” insert “
regulated
”
before “measuring”;
dd
in the definition of “relevant conformity assessment procedure”—
i
before “measuring” insert “
regulated
”
;
ii
for “Schedule 1”, substitute “
Schedules 1C to 1J
”
;
ee
in the definition of “relevant economic operator” insert “
regulated
”
before “measuring” in both places it occurs;
ff
omit the definition of “sub-assembly”;
gg
in the definition of “taximeter” insert “
regulated
”
before “measuring”;
hh
in the definition of “technical specification” insert “
regulated
”
before “measuring”;
ii
after the definition of “technical specification” insert—
“type examination certificate” means a type examination certificate issued by an approved body in accordance with Module B in Schedule 1B;
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
jj
omit the definition of “thermal energy meter”;
kk
omit the definition of “Union harmonisation legislation”;
ll
omit the definition of “United Kingdom Accreditation Service”;
mm
omit the definition of “volume conversion device”;
nn
in the definition of “withdraw” insert “
regulated
”
before “measuring” in both places it occurs.
3
After paragraph (1) insert—
1A
Schedules 1A to 1J reproduce the provisions of Annexes I to V, VII to X and XII to the Directive (respectively) with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedules 1A to 1J is a reference to the equivalent provision of the relevant Annex to the Directive as set out in the relevant Schedule.
4
Omit paragraph (2).
Insertion of regulation 2AI5193
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a regulated measuring instrument, process, service or system and which lays down one or more of the following—
a
the characteristics required of a regulated measuring instrument, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the regulated measuring instrument as regards the name under which the regulated measuring instrument is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the regulated measuring instrument, where these have an effect on the characteristics of the regulated measuring instrument.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
9
Regulations made under paragraph (8) are to be made by statutory instrument.
10
A statutory instrument containing regulations made under paragraph (9) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 3I5204
1
In regulation 3 (meaning of “measuring instrument” and related expressions and application of these Regulations) in the heading—
a
before “measuring” insert “
regulated
”
;
b
omit “and related expressions”; and
2
omit paragraphs (1) and (3).
Amendment to regulation 7I521F2895
In regulation 7 (manufacturer's responsibilities - design, conformity assessment and marking of regulated measuring instruments)—
a
the existing paragraph is renumbered paragraph (1);
b
in paragraph (1)(d) (as so renumbered) for “an EU” substitute “
a
”
;
c
in paragraph (1)(e) (as so renumbered)—
i
after “instrument” insert “
or where paragraph (2) applies in respect of the UK marking, to a label affixed to a product or to a document accompanying the product
”
;
ii
for “CE” substitute “
UK
”
;
d
after the renumbered paragraph (1) insert—
2
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the instrument; or
b
to a document accompanying the instrument.
Amendment to regulation 8I5226
In regulation 8 (manufacturers - obligations in respect of records) omit “EU”.
Amendment to regulation 9I5237
In regulation 9 (manufacturers' obligations to ensure continuing conformity with essential requirements), in paragraph (2)(b), for “harmonised”, substitute “
designated
”
.
Amendment to regulation 11I1I5248
In regulation 11 (manufacturers to mark contact details on regulated measuring instruments where possible), for paragraph (4) substitute—
4
The contact details required by this regulation must be clear, legible and in easily understandable English.
Amendment to regulation 12I5259
In regulation 12 (documentation to accompany regulated measuring instruments)—
a
in paragraph (1)(a), omit “EU”;
b
omit paragraph (3); and
c
in paragraph (4), for “understandable and intelligible” substitute “
legible and in easily understandable English
”
.
Amendment to regulation 13I52610
In regulation 13 (action to be taken where regulated measuring instruments placed on the market are not in conformity with the essential requirements), in paragraph (3), for “national” to “market”, substitute “
authority
”
.
Amendment to regulation 15I52711
In regulation 15 (use of authorised representatives by manufacturers)—
a
in paragraph (1) for “an” substitute “
a person established in the United Kingdom as their
”
;
b
in paragraph (3)(a), omit “EU”.
Amendment to regulation 16I52812
In regulation 16 (introductory), omit “European Economic Area that is imported into the”.
Amendment to regulation 17I52913
In regulation 17 (ensuring compliance of regulated measuring instruments), in paragraph (2)—
a
in sub-paragraph (c), for “CE”, substitute “
UK
”
; and
b
in sub-paragraph (d), omit “EU”.
Amendment to regulation 19I53014
In regulation 19 (requirements to mark importers' details on regulated measuring instruments)—
a
for paragraph (2) substitute—
2
Paragraph (1) does not apply where—
a
either—
i
the regulated measuring instrument it too small or too sensitive a composition to allow it to bear the information required by paragraph (1); or
b
before placing the regulated measuring instrument on the market, the importer sets out the information referred to in paragraph (1)—
i
where sub-paragraph (a)(i) applies, on any packaging in which the instrument is supplied and in any accompanying documents;
ii
where sub-paragraph (a)(ii) applies, in a document accompanying the instrument.
b
for paragraph (3) substitute—
3
Any contact details required by this regulation must be clear, legible and in easily understandable English.
Amendment to regulation 20I53115
In regulation 20 (importers' duty to ensure that regulated measuring instruments are accompanied by relevant documentation)—
a
in paragraph (1), omit “in a language easily understood by end-users”; and
b
for paragraph (2), substitute—
2
The instructions and information referred to in paragraph (1) must be clear, legible and in easily understandable English.
Amendment to regulation 24I53216
In regulation 24 (requirement for importer to keep copy of EU declaration of conformity), and in the heading to that regulation, omit “EU”.
Amendment to regulation 28I53317
In regulation 28 (distributors - verification obligations)—
a
in paragraph (1), for “CE”, substitute “
UK
”
;
b
in paragraph (2)(a), omit “EU”; and
c
for paragraph (4), substitute—
4
Instructions and information supplied in accordance with this regulation must be clear, legible and in easily understandable English.
Insertion of regulation 33A F31733B and 33CI53418
After regulation 33 insert—
Obligations which are met by complying with obligations in the Directive33A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 4(22);
c
“Module B” means the conformity assessment procedure set out under the heading “MODULE B: EU-TYPE EXAMINATION” in Annex II;
d
“Module H1” means the conformity assessment procedure set out under the heading “MODULE H1: CONFORMITY BASED ON FULL QUALITY ASSURANCE PLUS DESIGN EXAMINATION” in Annex II;
e
“EU-design examination certificate” means an EU design certificate issued in accordance with Module H1;
f
“EU-type examination certificate” means an EU-type examination certificate issued in accordance with Module B;
g
“harmonised standard” has the meaning given to it in Article 4(14);
h
“instrument-specific Annexes” means Annexes III to XII.
2
Paragraph (3) applies where, before placing a regulated measuring instrument on the market, the manufacturer—
a
ensures that the regulated measuring instrument has been designed and manufactured in accordance with the essential requirements set out in Annex I and in the relevant instrument-specific Annex which applies to the regulated measuring instrument;
b
ensures that the one of the relevant conformity assessment procedures listed in the relevant instrument-specific Annex that apply to that regulated measuring instrument in accordance with Article 17 have been carried out;
c
draws up the technical documentation referred to in Article 18;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the supplementary metrology marking, in accordance with Articles 21 and 22(1) to (6);
f
draws up an EU declaration of conformity, in accordance with Article 19; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 7(a) to (e), 48 and 52(2) are to be treated as being satisfied;
b
regulations 8, 9(2), 51, 68(1)(a) to (e), 72, 73 and 75 apply subject to the modifications in paragraph (8); and
c
regulations 36 to 39 do not apply.
4
Paragraph (5) applies where, before placing a regulated measuring instrument on the market, the importer ensures that—
a
the relevant conformity assessment procedures that apply to that measuring instrument in accordance with Article 17 have been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Article 18; and
c
the measuring instrument bears the CE marking, and the supplementary metrology marking referred to in Article 21(2).
5
Where this paragraph applies—
a
the requirements of regulation 17(2)(a) to (c) are to be treated as being satisfied; and
b
regulations 18, 21, 23, 51, 68(1)(a) to (e), 72, 73 and 75 apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before making a regulated measuring instrument available on the market, a distributor ensures that the regulated measuring instrument bears the CE marking, and the supplementary metrology marking referred to in Article 21(2).
7
Where this paragraph applies—
a
regulation 28(1) is to be treated as being satisfied; and
b
regulations 29(1),30, 68(1)(a), 68(1)(b), 72 and 73 apply subject to the modifications in paragraph (8).
8
The modifications referred to in paragraphs (3)(b), (5)(b) and (7)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential requirements” is to be read as a reference to the essential requirements referred to in Annex I and in the relevant instrument-specific Annex which applies to the regulated measuring instrument;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures that apply to the regulated measuring instrument in accordance with Article 17;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Article 18;
g
any reference to “type examination certificate” is to be read as a reference to an EU-type examination certificate; and
h
any reference to “design examination certificate” is to be read as a reference to an EU-design examination certificate;
i
any reference to “M marking” is to be read as a reference to the supplementary metrology marking; and
j
any reference to “approved body” is to be read as a reference to the body that undertook any conformity assessment procedure in accordance with Article 13;
k
any reference to “authorised mark” includes the CE marking and the supplementary metrology marking.
Conformity assessment procedure obligation which is met by complying with the Directive33B
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“Module B” means the conformity assessment procedure set out under the heading “MODULE B: EU-TYPE EXAMINATION” in Annex II;
c
“EU-type examination certificate” means an EU-type examination certificate issued in accordance with Module B;
d
“harmonised standard” has the meaning given to it in Article 4(14);
e
“instrument-specific Annexes” means Annexes III to XII.
2
Paragraph (3) applies where—
a
in accordance with Article 17, one of the conformity assessment procedures listed in the instrument-specific Annex that applies to the regulated measuring instrument is Module B; and
b
before placing a regulated measuring instrument on the market, the manufacturer ensures that—
i
the regulated measuring instrument has been designed in accordance with the essential requirements set out in Annex I and in the relevant instrument-specific Annex which applies to the regulated measuring instrument; and
ii
Module B has been complied with in respect of that regulated measuring instrument.
3
Where this paragraph applies—
a
any reference in regulation 7(c) to “relevant conformity assessment procedure” includes Module B;
b
any reference to “type examination certificate” in regulations 45(1)(j), 72(3)(b) and 73(3)(b) is to be read as a reference to “EU-type examination certificate”; and
c
any reference to “designated standard” in regulation 45(1)(f) is to be read as a reference to “harmonised standard.
F318Expiry of regulations 33A and 33B33C
1
Subject to paragraph (2), regulation 33A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 33A—
a
any regulated measuring instrument which was placed on the market pursuant to regulation 33A may continue to be made available on the market on or after the expiry of regulation 33A;
b
any obligation to which a person was subject under regulation 33A in respect of any regulated measuring instrument placed on the market pursuant to regulation 33A continues to have effect after the expiry of regulation 33A, in respect of that instrument.
3
Subject to paragraph (4), regulation 33B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 33B in relation to a regulated measuring instrument prior to the expiry of regulation 33B, regulation 33B continues to apply in respect of that instrument where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” has the meaning given to it in regulation 33B(1)(c).
Qualifying Northern Ireland Goods33D
1
Where paragraph (2) applies—
a
a regulated measuring instrument is to be treated as being in conformity with the essential requirements; and
b
each relevant economic operator is to be treated as having complied or as complying with the obligations imposed on them under Part 2.
2
This paragraph applies where—
a
a regulated measuring instrument is—
i
in conformity with the essential requirements, within the meaning of that term in regulation 2, as it applies in Northern Ireland; and
ii
qualifying Northern Ireland goods; and
b
each relevant economic operator has complied or is complying with the obligations imposed on them under Part 2, as that Part applies in Northern Ireland; and
c
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(c) are that, before placing the non-automatic weighing instrument on the market, the importer—
a
complies with regulation 19;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out.
ii
the manufacturer has drawn up the technical documentation; and
iii
the regulated measuring instrument bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given it in in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to Part 3I53519
Omit Part 3 (non-prescribed measuring instruments).
Amendment to regulation 36I53620
For regulation 36 (introductory), substitute—
36
This chapter applies for the purposes of establishing whether a regulated measuring instrument complies with the essential requirements.
Amendment to regulation 37I53721
In regulation 37 (methods of establishing conformity with the essential requirements)—
a
in the opening words insert “
regulated
”
before “measuring”;
b
in paragraph (a)—
i
for “harmonised”, the first time it appears, substitute “
designated
”
; and
ii
omit from “where” to “Union”;
c
in paragraph (b), for “in the Official Journal of the European Union”, substitute “
by the Secretary of State
”
.
Amendment to regulation 38I53822
In regulation 38 (presumptions of conformity of measuring instruments)—
a
in paragraphs (1) and (2) and in the heading, before “measuring” insert “
regulated
”
;
b
in paragraphs (1) and (3), for “harmonised”, substitute “
designated
”
.
Amendment to regulation 39I53923
In regulation 39 (conformity assessment procedures)—
a
in paragraph (1)—
i
before “measuring” insert “
regulated
”
in both places it occurs;
ii
for “1” substitute “
1C to 1J
”
; and
b
in paragraph (2), for “A notified” substitute “
An approved
”
.
Insert of regulation 39AI54024
After regulation 39, insert—
Power to amend Schedules 1C to 1J39A
1
Where the one or more of the conditions in paragraph (2) are met, the Secretary of State may by regulations make provision to amend Schedules 1C to 1J in relation to any of the following matters—
a
maximum permissible errors (MPEs) and accuracy classes;
b
rated operating conditions;
c
critical change values; and
d
disturbances.
2
The conditions referred to in paragraph (1) are that the Secretary of State considers that the purpose of the provision is to—
a
take into account scientific or technical progress; or
b
provide adequate protection of consumers or other end users.
3
The power to make regulations under this regulation includes the power—
a
to make different provision for different cases; and
b
to make such supplemental, consequential and transitional provision as the Secretary of State considers appropriate
4
Regulations made under paragraph (1) are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 40I54125
In regulation 40 (capacity serving measures – accredited in house bodies)—
a
in paragraph (2) for “of Annex II to the Directive” substitute “
in Schedule 1B
”
;
b
in paragraphs (3) and (4)(c), before “measuring” insert “
regulated
”
; and
c
for paragraph (5) substitute—
5
An accredited in-house body need not be approved by the Secretary of State, but information concerning its accreditation must be given by the undertaking of which it forms part to the Secretary of State at the request of the Secretary of State.
Amendment to regulation 41I54226
For regulation 41 (subsidiaries and contractors) substitute—
Subsidiaries and contractors41
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M85.
Amendment to regulation 42I54327
In regulation 42 (fees)—
a
for “a United Kingdom notified” substitute “
an approved
”
in both places in which it occurs;
b
in paragraph (2)(a) for “United Kingdom notified” substitute “
approved
”
.
Amendment to regulation 44I54428
In regulation 44 (general requirements to be met by technical documentation)—
a
in paragraphs (1)(a), (2)(b) and (2)(c) before “measuring” insert “
regulated
”
;
b
in paragraph (1)(b) for “the Directive” substitute “
these Regulations
”
.
Amendment to regulation 45I54529
In regulation 45 (specific information to be included in technical documentation), in paragraph (1)—
a
before “measuring” insert “
regulated
”
in each place it occurs;
b
in sub-paragraph (f)—
i
for “harmonised”, substitute “
designated
”
; and
ii
omit “, the references of which have been published in the Official Journal of the European Union”;
c
in sub-paragraph (g) for “harmonised” substitute “
designated
”
;
d
in sub-paragraph (i)(i) for “the Directive” substitute “
these Regulations
”
;
e
in sub-paragraph (i)(ii ) for “, water and thermal-energy” substitute “
and water
”
;
f
in sub-paragraph (j)—
i
for “EU-type” substitute “
type
”
;
ii
omit “EU” in the second place it occurs.
Amendment to the heading of Chapter 3I54630
In the heading to Chapter 3 omit “EU”.
Amendment to regulation 46I54731
In regulation 46 (application of Chapter)—
a
omit “EU”; and
b
before “measuring” insert “
regulated
”
.
Amendment to regulation 47I54832
In regulation 47 (form and contents of EU declaration of conformity etc)—
a
in paragraphs (1) and (2) and in the heading, omit “EU”;
b
in paragraph (1)(b) for “Annex II to the Directive” substitute “
Schedule 1B
”
; and
c
in paragraph (1)(c) for “Annex XIII to the Directive” substitute “
Schedule 1K
”
.
Amendment to regulation 48I54933
For regulation 48 (measuring instruments that require more than one declaration of conformity) substitute—
Regulated measuring instruments that require more than one declaration of conformity48
Where a regulated measuring instrument is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 49I55034
In regulation 49 (responsibility of manufacturer that draws up declaration of conformity)—
a
for “an EU” substitute “
a
”
; and
b
before “measuring” insert “
regulated
”
in both places it occurs.
Amendment to regulation 50I55135
In regulation 50 (conformity with Directive requirements to be indicated by the CE marking)—
a
in the heading omit “Directive”; and
b
in the regulation and in the heading, for “CE”, substitute “
UK
”
.
Amendment to regulation 51I55236
For regulation 51 (general principles relating to the M marking), substitute—
Prohibition on improper use of the UK marking and the M marking51
1
An economic operator must not affix the UK marking or the M marking to a regulated measuring instrument unless—
a
that economic operator is the manufacturer of the regulated measuring instrument; and
b
the conformity of the regulated measuring instrument with the essential requirements has been demonstrated by a relevant conformity assessment procedure.
2
An economic operator must not affix a marking to a regulated measuring instrument which is not the UK marking or the M marking but which purports to attest that the regulated measuring instrument satisfies the essential requirements.
3
An economic operator must not affix to a regulated measuring instrument a marking, sign or inscription which is likely to mislead any other person as to the meaning or form of the marking.
4
An economic operator must not affix to a regulated measuring instrument any other marking if the visibility, legibility and meaning of the UK marking or the M marking would be impaired as a result.
Amendment to regulation 52I55337
In regulation 52 (rules and conditions for affixing the CE marking and the M marking)—
a
in paragraphs (1) to (6) before “measuring” insert “
regulated
”
in each place it occurs;
b
in paragraphs (1) and (7) and in the heading, for “CE”, substitute “
UK
”
;
F319bb
in paragraph (2) for “or its data plate” substitute “
, its data plate, or where regulation 7(2) applies, to a label affixed to the measuring instrument or to a document accompanying the measuring instrument;
”
c
in paragraph (4) omit “, not being sub-assemblies”;
d
in paragraph (8) for “Annex II to the Directive” substitute “
Schedule 1B
”
; and
e
in paragraphs (8), (9) and (10) for “notified” substitute “
approved
”
.
Amendment to Part 5I55438
For Part 5 substitute—
PART 5Approval of Conformity Assessment Bodies
Approved bodies53
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 54 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 57 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F320IP completion day notified to the European Commission and the member States of the European Union, in accordance with Article 23 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 53(2)(b), as it had effect immediately before F320IP completion day;
“approved body requirements” means the requirements set out in Schedule 5.
Approval of conformity assessment bodies54
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent; and
iii
the regulated measuring instrument for which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b)(i), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may–
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies55
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring56
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 54(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval57
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 54(6)(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 54 (approval of conformity assessment bodies).
2
With the consent of an approved body, or where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 56(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 54 (approval of conformity assessment bodies).
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must—
a
on the request of the Secretary of State, transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
in the absence of a request under sub-paragraph (a), ensure that its files relating to the activities it has undertaken as an approved body are kept available for the Secretary of State and competent authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
7
The Secretary of State may impose a monetary penalty on an approved body that fails to comply with any requirement imposed by or under paragraph (5).
8
Schedule 7 has effect in relation to a monetary penalty imposed under paragraph (7).
Register of approved bodies58
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body59
1
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements; and
b
monitoring approved bodies in accordance with regulation 56;
2
Where the Secretary of State authorises the UK national accreditation body pursuant to paragraph (1), the Secretary of State remains fully responsible for anything done pursuant to that authorisation.
Amendment to regulation 63I55539
In regulation 63 (regulated measuring instruments presenting a risk)—
a
in paragraph (5) for “notified”, substitute “
approved
”
;
b
omit paragraph (6);
c
in paragraph (7) for “on the market throughout the European Economic Area”, substitute “
in the United Kingdom
”
;
F322d
omit paragraph (9);
F323e
omit paragraph (10).
Amendment to regulation 64I55640
Omit regulation 64 (EU safeguard procedure).
Amendment to regulation 65I55741
In regulation 65 (compliant regulated measuring instruments which present a risk) omit paragraph (3).
Amendment to regulation 68I55842
In regulation 68 (compliance notice procedure), in paragraph (1)—
a
in sub-paragraphs (a) and (b) for “CE” substitute “
UK
”
in each place in which it occurs;
b
in sub-paragraph (a) for “Article 30 of the RAMS regulation or the requirements of these Regulations” substitute “
regulation 51 or regulation 52
”
;
c
in sub-paragraph (c) for “notified” substitute “
approved
”
in each place in which it occurs; and
d
in sub-paragraph (d) omit “EU”.
Amendment to regulation 69I55943
In regulation 69 (enforcement notices)—
a
in paragraph (5)—
i
for “a United Kingdom notified” substitute “
an approved
”
;
ii
for “that notified” substitute “
that approved
”
; and
b
omit paragraph (6).
Amendment to regulation 72I56044
In regulation 72 (disqualification)—
a
in paragraph (2)—
i
in sub-paragraph (a) for “CE”, substitute “
UK
”
;
ii
in sub-paragraph (c) for “notified”, substitute “
approved
”
; and
b
in paragraph 3(b) omit “EU-” in each place it occurs.
Amendment to regulation 73I56145
In regulation 73 (requalification) in paragraph 3(b), omit “EU-” in each place it occurs.
Amendment to regulation 75I56246
In regulation 75 (unauthorised application of authorised marks), paragraph (5)—
a
in sub-paragraph (a) for “CE” substitute “
UK
”
; and
b
in sub-paragraph (c) for “notified” substitute “
approved
”
.
Amendment to regulation 81I56347
In regulation 81 (review) omit paragraph (2).
Amendment to Schedule 1I56448
Omit Schedule 1 (essential requirements and applicable conformity assessment procedures).
Insertion of SchedulesI56549
Before Schedule 2 insert—
SCHEDULE 1AESSENTIAL REQUIREMENTS (Annex I to the Directive)
A regulated measuring instrument shall provide a high level of metrological protection in order that any party affected can have confidence in the result of measurement, and shall be designed and manufactured to a high level of quality in respect of the measurement technology and security of the measurement data.
The essential requirements that shall be met by regulated measuring instruments are set out below and are supplemented, where appropriate, by instrument-specific requirements in Schedules 1C to 1J that provide more detail on certain aspects of the general requirements.
The solutions adopted in the pursuit of the essential requirements shall take account of the intended use of the instrument and any foreseeable misuse thereof.
DEFINITIONS
Measurand
The measurand is the particular quantity subject to measurement
Influence quantity
An influence quantity is a quantity that is not the measurand but that affects the result of measurement.
Rated Operating Conditions
The rated operating conditions are the values for the measurand and influence quantities making up the normal working conditions of an instrument.
Disturbance
An influence quantity having a value within the limits specified in the appropriate requirement but outside the specified rated operating conditions of the regulated measuring instrument. An influence quantity is a disturbance if for that influence quantity the rated operating conditions are not specified.
Critical change value
The critical change value is the value at which the change in the measurement result is considered undesirable.
Material Measure
A material measure is a device intended to reproduce or supply in a permanent manner during its use one or more known values of a given quantity.
Direct sales
A trading transaction is direct sales if:
— the measurement result serves as the basis for the price to pay; and
— at least one of the parties involved in the transaction related to measurement is a consumer or any other party requiring a similar level of protection; and
— all the parties in the transaction accept the measurement result at that time and place.
Climatic environments
Climatic environments are the conditions in which regulated measuring instruments may be used. To cope with climatic differences, a range of temperature limits has been defined.
Utility
A utility is regarded as a supplier of electricity, gas or water.
ESSENTIAL REQUIREMENTS
Allowable Errors1
1
Under rated operating conditions and in the absence of a disturbance, the error of measurement shall not exceed the maximum permissible error (MPE) value as laid down in the appropriate instrument-specific requirements.
Unless stated otherwise in the instrument-specific Schedules, MPE is expressed as a bilateral value of the deviation from the true measurement value.
1
Under rated operating conditions and in the presence of a disturbance, the performance requirement shall be as laid down in the appropriate instrument-specific requirements.
Where the instrument is intended to be used in a specified permanent continuous electromagnetic field the permitted performance during the radiated electromagnetic field-amplitude modulated test shall be within MPE.
1
The manufacturer shall specify the climatic, mechanical and electromagnetic environments in which the instrument is intended to be used, power supply and other influence quantities likely to affect its accuracy, taking account of the requirements laid down in the appropriate instrument-specific Schedules.
1
Climatic environments
The manufacturer shall specify the upper temperature limit and the lower temperature limit from any of the values in Table 1 unless otherwise specified in Schedules 1C to 1J and indicate whether the instrument is designed for condensing or non-condensing humidity as well as the intended location for the instrument, i.e. open or closed.
Table 1
Temperature Limits
Upper temperature limit
30 oC
40 oC
55 oC
70 oC
Lower temperature limit
5 oC
-10 oC
-25 oC
-40 oC
1
(a) Mechanical environments are classified into classes M1 to M3 as described below.
M1
This class applies to instruments used in locations with vibration and shocks of low significance, e.g. for instruments fastened to light supporting structures subject to negligible vibrations and shocks transmitted from local blasting or pile-driving activities, slamming doors, etc.
M2
This class applies to instruments used in locations with significant or high levels of vibration and shock, e.g. transmitted from machines and passing vehicles in the vicinity or adjacent to heavy machines, conveyor belts, etc.
M3
This class applies to instruments used in locations where the level of vibration and shock is high and very high, e.g. for instruments mounted directly on machines, conveyor belts, etc.
b
The following influence quantities shall be considered in relation with mechanical environments:
— vibration
— mechanical shock
1
(a) Electromagnetic environments are classified into classes E1, E2 or E3 as described below, unless otherwise laid down in the appropriate instrument-specific Schedules.
E1
This class applies to instruments used in locations with electromagnetic disturbances corresponding to those likely to be found in residential, commercial and light industrial buildings.
E2
This class applies to instruments used in locations with electromagnetic disturbances corresponding to those likely to be found in other industrial buildings.
E3
This class applies to instruments supplied by the battery of a vehicle. Such instruments shall comply with the requirements of E2 and the following additional requirements:
— voltage reductions caused by energising the starter-motor circuits of internal combustion engines,
— load dump transients occurring in the event of a discharged battery being disconnected while the engine is running.
b
The following influence quantities shall be considered in relation with electromagnetic environments:
— voltage interruptions;
— short voltage reductions;
— voltage transients on supply lines and/or signal lines;
— electrostatic discharges;
— radio frequency electromagnetic fields;
— conducted radio frequency electromagnetic fields on supply lines and/or signal lines;
— surges on supply lines and/or signal lines.
1
Other influence quantities to be considered, where appropriate, are:
— voltage variation;
— mains frequency variation;
— power frequency magnetic fields;
— any other quantity likely to influence in a significant way the accuracy of the instrument.
1
When carrying out the tests as envisaged in these Regulations, the following points shall apply:
1
Basic rules for testing and the determination of errors
Essential requirements specified in paragraphs 1.1 and 1.2 shall be verified for each relevant influence quantity. Unless otherwise specified in the appropriate instrument-specific Schedule, these essential requirements apply when each influence quantity is applied and its effect evaluated separately, all other influence quantities being kept relatively constant at their reference value.
Metrological tests shall be carried out during or after the application of the influence quantity, whichever condition corresponds to the normal operational status of the instrument when that influence quantity is likely to occur.
1
Ambient humidity
a
According to the climatic operating environment in which the instrument is intended to be used either the damp heat-steady state (non-condensing) or damp heat cyclic (condensing) test may be appropriate.
b
The damp heat cyclic test is appropriate where condensation is important or when penetration of vapour will be accelerated by the effect of breathing. In conditions where non-condensing humidity is a factor the damp-heat steady state is appropriate.
Reproducibility2
The application of the same measurand in a different location or by a different user, all other conditions being the same, shall result in the close agreement of successive measurements. The difference between the measurement results shall be small when compared with the MPE.
Repeatability3
The application of the same measurand under the same conditions of measurement shall result in the close agreement of successive measurements. The difference between the measurement results shall be small when compared with the MPE.
Discrimination and Sensitivity4
A regulated measuring instrument shall be sufficiently sensitive and the discrimination threshold shall be sufficiently low for the intended measurement task.
Durability5
A regulated measuring instrument shall be designed to maintain an adequate stability of its metrological characteristics over a period of time estimated by the manufacturer, provided that it is properly installed, maintained and used according to the manufacturer's instruction when in the environmental conditions for which it is intended.
Reliability6
A regulated measuring instrument shall be designed to reduce as far as possible the effect of a defect that would lead to an inaccurate measurement result, unless the presence of such a defect is obvious.
Suitability7
7
A regulated measuring instrument shall have no feature likely to facilitate fraudulent use, whereas possibilities for unintentional misuse shall be minimal.
7
A regulated measuring instrument shall be suitable for its intended use taking account of the practical working conditions and shall not require unreasonable demands of the user in order to obtain a correct measurement result.
7
The errors of a utility measuring instrument at flows or currents outside the controlled range shall not be unduly biased.
7
Where a regulated measuring instrument is designed for the measurement of values of the measurand that are constant over time, the regulated measuring instrument shall be insensitive to small fluctuations of the value of the measurand, or shall take appropriate action.
7
A regulated measuring instrument shall be robust and its materials of construction shall be suitable for the conditions in which it is intended to be used.
7
A regulated measuring instrument shall be designed so as to allow the control of the measuring tasks after the instrument has been placed on the market and put into use. If necessary, special equipment or software for this control shall be part of the instrument. The test procedure shall be described in the operation manual.
When a regulated measuring instrument has associated software which provides other functions besides the measuring function, the software that is critical for the metrological characteristics shall be identifiable and shall not be inadmissibly influenced by the associated software.
Protection against corruption8
8
The metrological characteristics of a regulated measuring instrument shall not be influenced in any inadmissible way by the connection to it of another device, by any feature of the connected device itself or by any remote device that communicates with the regulated measuring instrument.
8
A hardware component that is critical for metrological characteristics shall be designed so that it can be secured. Security measures foreseen shall provide for evidence of an intervention.
8
Software that is critical for metrological characteristics shall be identified as such and shall be secured.
Software identification shall be easily provided by the regulated measuring instrument.
Evidence of an intervention shall be available for a reasonable period of time.
8
Measurement data, software that is critical for measurement characteristics and metrologically important parameters stored or transmitted shall be adequately protected against accidental or intentional corruption.
8
For utility measuring instruments the display of the total quantity supplied or the displays from which the total quantity supplied can be derived, whole or partial reference to which is the basis for payment, shall not be able to be reset during use.
Information to be borne by and to accompany the instrument9
9
A regulated measuring instrument shall bear the following inscriptions:
a
manufacturer's name, registered trade name or registered trade mark;
b
information in respect of its accuracy; and, where applicable:
c
information in respect of the conditions of use;
d
measuring capacity;
e
measuring range;
f
identity marking;
g
number of the type examination certificate or the design examination certificate;
h
information whether or not additional devices providing metrological results comply with the provisions of these Regulations on legal metrological control.
9
An instrument of dimensions too small or of too sensitive a composition to allow it to bear the relevant information shall have its packaging, if any, and the accompanying documents required by the provisions of these Regulations suitably marked.
9
The instrument shall be accompanied by information on its operation, unless the simplicity of the regulated measuring instrument makes this unnecessary. Information shall be easily understandable and shall include where relevant:
a
rated operating conditions;
b
mechanical and electromagnetic environment classes;
c
the upper and lower temperature limit, whether condensation is possible or not, open or closed location;
d
instructions for installation, maintenance, repairs, permissible adjustments;
e
instructions for correct operation and any special conditions of use;
f
conditions for compatibility with interfaces or regulated measuring instruments.
9
Groups of identical regulated measuring instruments used in the same location or used for utility measurements do not necessarily require individual instruction manuals.
9
Unless specified otherwise in an instrument-specific Schedule, the scale interval for a measured value shall be in the form 1 × 10n, 2 × 10n, or 5 × 10n, where n is any integer or zero. The unit of measurement or its symbol shall be shown close to the numerical value.
9
A material measure shall be marked with a nominal value or a scale, accompanied by the unit of measurement used.
9
The units of measurement used and their symbols shall be in accordance with the relevant enactments on units of measurement and their symbols.
9
All marks and inscriptions required under any requirement shall be clear, non-erasable, unambiguous and non-transferable.
Indication of result10
10
Indication of the result shall be by means of a display or hard copy.
10
The indication of any result shall be clear and unambiguous and accompanied by such marks and inscriptions necessary to inform the user of the significance of the result. Easy reading of the presented result shall be permitted under normal conditions of use. Additional indications may be shown provided they cannot be confused with the metrologically controlled indications.
10
In the case of hard copy the print or record shall also be easily legible and non-erasable.
10
A regulated measuring instrument for direct sales trading transactions shall be designed to present the measurement result to both parties in the transaction when installed as intended. When critical in case of direct sales, any ticket provided to the consumer by an ancillary device not complying with the appropriate requirements of these Regulations shall bear appropriate restrictive information.
10
Whether or not a regulated measuring instrument intended for utility measurement purposes can be remotely read it shall in any case be fitted with a metrologically controlled display accessible without tools to the consumer. The reading of this display is the measurement result that serves as the basis for the price to pay.
Further processing of data to conclude the trading transaction11
11
A regulated measuring instrument other than a utility measuring instrument shall record by a durable means the measurement result accompanied by information to identify the particular transaction, when:
a
the measurement is non-repeatable; and
b
the regulated measuring instrument is normally intended for use in the absence of one of the trading parties.
11
Additionally, a durable proof of the measurement result and the information to identify the transaction shall be available on request at the time the measurement is concluded.
Conformity evaluation12
A regulated measuring instrument shall be designed so as to allow ready evaluation of its conformity with the appropriate requirements of these Regulations.
SCHEDULE 1BCONFORMITY ASSESSMENT PROCEDURES (Annex II to the Directive)
MODULE A2:INTERNAL PRODUCTION CONTROL PLUS SUPERVISED INSTRUMENT CHECKS AT RANDOM INTERVALS
1
Internal production control plus supervised instrument checks at random intervals is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3, 4, and 5, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned satisfy the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation set out in regulations 44 and 45. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured instruments with the technical documentation referred to in paragraph 2 and with the requirements of these Regulations that apply to them.
Instrument checks4
At the choice of the manufacturer, either an accredited in-house body or an approved body, chosen by the manufacturer, shall carry out instrument checks or have them carried out at random intervals determined by the body, in order to verify the quality of the internal checks of the instrument, taking into account, inter alia, the technological complexity of the instruments and the quantity of production. An adequate sample of the final regulated measuring instruments, taken on site by the body before the placing on the market, shall be examined and appropriate tests as identified by the relevant parts of the designated standard, and/or normative document, and/or equivalent tests set out in other relevant technical specifications, shall be carried out to verify the conformity of the instruments with the relevant requirements of these Regulations. In the absence of a relevant designated standard or normative document, the accredited in-house body or approved body concerned shall decide on the appropriate tests to be carried out.
In those cases where a relevant number of instruments in the sample do not conform to an acceptable quality level, the accredited in-house body or approved body shall take appropriate measures.
Where the tests are carried out by an approved body, the manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
Conformity marking and declaration of conformity5
5
The manufacturer shall affix the UK marking and the M marking set out in these Regulations to each individual instrument that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for an instrument model and keep it together with the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument for which it was drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
Authorised representative6
The manufacturer's obligations set out in paragraph 5 may be fulfilled by his authorised representative, on his behalf and under his responsibility provided that they are specified in the mandate.
MODULE BTYPE EXAMINATION
1
‘Type examination’ is the part of a conformity assessment procedure in which an approved body examines the technical design of an instrument and verifies and attests that the technical design of the instrument meets the requirements of these Regulations that apply to it.
2
Type examination may be carried out in either of the following manners:
a
examination of a specimen, representative of the production envisaged, of the complete regulated measuring instrument (production type),
b
assessment of the adequacy of the technical design of the instrument through examination of the technical documentation and supporting evidence referred to in paragraph 3, plus examination of specimens, representative of the production envisaged, of one or more critical parts of the instrument (combination of production type and design type);
c
assessment of the adequacy of the technical design of the instrument through examination of the technical documentation and supporting evidence referred to in paragraph 3, without examination of a specimen (design type).
The approved body decides on the appropriate manner and the specimens required.
3
The manufacturer shall lodge an application for type examination with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation set out in regulations 44 and 45. The technical documentation shall make it possible to assess the instrument's conformity with the applicable requirements of these Regulations and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
The application shall in addition contain, wherever applicable:
d
the specimens, representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution. This supporting evidence shall mention any documents that have been used, in particular where the relevant designated standards, and/or normative documents have not been applied in full. The supporting evidence shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer, or by another testing laboratory on his behalf and under his responsibility.
4
The approved body shall:
For the instrument:
4
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the instrument;
For the specimen(s):
4
verify that the specimen(s) have been manufactured in conformity with the technical documentation and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards and/or normative documents, as well as the elements which have been designed in accordance with other relevant technical specifications;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards and normative documents, these have been applied correctly;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards, and/or normative documents have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential requirements of these Regulations;
4
agree with the manufacturer on the location where the examinations and tests will be carried out.
For the other parts of the regulated measuring instrument:
4
examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the other parts of the regulated measuring instrument.
5
The approved body shall draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes. Without prejudice to its obligations vis-à-vis the Secretary of State, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
6
Where the type meets the requirements of these Regulations, the approved body shall issue a type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The type examination certificate may have one or more annexes attached.
The type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured regulated measuring instruments with the examined type to be evaluated and to allow for in-service control. In particular, to allow the conformity of manufactured instruments to be evaluated with the examined type regarding the reproducibility of their metrological performances, when they are properly adjusted using appropriate means, content shall include:
— the metrological characteristics of the type of instrument;
— measures required for ensuring the integrity of the instruments (sealing, identification of software, etc.);
— information on other elements necessary for the identification of the instruments and to check their visual external conformity to type;
— if appropriate, any specific information necessary to verify the characteristics of manufactured instruments.
The type examination certificate shall have a validity of 10 years from the date of its issue, and may be renewed for subsequent periods of 10 years each.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
7
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations, and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
8
The manufacturer shall inform the approved body that holds the technical documentation relating to the type examination certificate of all modifications to the approved type that may affect the conformity of the instrument with the essential requirements of these Regulations or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original type examination certificate.
9
Each approved body shall inform the Secretary of State concerning the type examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and/or any additions thereto refused, suspended or otherwise restricted.
The other approved bodies and the Secretary of State may, on request, obtain a copy of the type examination certificates and/or additions thereto. On request, the Secretary of State may obtain a copy of the technical documentation and the results of the examinations carried out by the approved body.
The approved body shall keep a copy of the type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer until the expiry of the validity of that certificate.
10
The manufacturer shall keep a copy of the type examination certificate, its annexes and additions together with the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
11
The manufacturer's authorised representative may lodge the application referred to in paragraph 3 and fulfil the obligations set out in paragraphs 8 and 10, provided that they are specified in the mandate.
MODULE D:CONFORMITY TO TYPE BASED ON QUALITY ASSURANCE OF THE PRODUCTION PROCESS
1
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned are in conformity with the type described in the type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 3 and shall be subject to surveillance as specified in paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with an approved body of his choice, for the regulated measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
a written declaration that the same application has not been lodged with any other approved body,
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the type examination certificate.
3
The quality system shall ensure that the regulated measuring instruments are in conformity with the type described in the type examination certificate and comply with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during, and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in paragraph 3.1(e), to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change of the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a re-assessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity5
5
The manufacturer shall affix the UK marking and the M marking set out in these Regulations and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual regulated measuring instrument that is in conformity with the type described in the type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
6
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the documentation referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved;
c
the decisions and reports from the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE D1:QUALITY ASSURANCE OF THE PRODUCTION PROCESS
1
Quality assurance of the production process is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 4 and 7, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned satisfy the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation set out in regulations 44 and 45. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
3
The manufacturer shall keep the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Manufacturing4
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 5 and shall be subject to surveillance as specified in paragraph 6.
Quality system5
5
The manufacturer shall lodge an application for assessment of his quality system with an approved body of his choice, for the measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation referred to in paragraph 2.
5
The quality system shall ensure compliance of the regulated measuring instruments with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during, and after manufacture, and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
5
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 5.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in paragraph 2 in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
5
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
5
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change of the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 5.2 or whether a re-assessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body6
6
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
6
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the technical documentation referred to in paragraph 2;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned.
6
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
6
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity7
7
The manufacturer shall affix the UK marking, the M marking set out in these Regulations, and, under the responsibility of the approved body referred to in paragraph 5.1, the latter's identification number to each individual regulated measuring instrument that satisfies the applicable requirements of these Regulations.
7
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
8
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the documentation referred to in paragraph 5.1;
b
the information relating to the change referred to in paragraph 5.5, as approved;
c
the decisions and reports of the approved body referred to in paragraphs 5.5, 6.3 and 6.4.
9
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Authorised representative10
The manufacturer's obligations set out in paragraphs 3, 5.1, 5.5, 7 and 8 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE E:CONFORMITY TO TYPE BASED ON INSTRUMENT QUALITY ASSURANCE
1
Conformity to type based on instrument quality assurance is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned are in conformity with the type described in the type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 3 and shall be subject to surveillance, as specified in paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with an approved body of his choice, for the regulated measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the type examination certificate.
3
The quality system shall ensure compliance of the regulated measuring instruments with the type described in the type examination certificate and with the applicable requirements of these Regulations.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
d
the means of monitoring the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in paragraph 3.1(e), in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a re-assessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity5
5
The manufacturer shall affix the UK marking, the M marking set out in these Regulations, and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual instrument that is in conformity with the type described in the type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
6
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the documentation referred to in paragraph 3.1;
c
the information relating to the change referred to in paragraph 3.5, as approved;
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE E1:QUALITY ASSURANCE OF FINAL INSTRUMENT INSPECTION AND TESTING
1
Quality assurance of final instrument inspection and testing is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 4 and 7, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned satisfy the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation set out in regulations 44 and 45. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
3
The manufacturer shall keep the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Manufacturing4
The manufacturer shall operate an approved quality system for final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 5 and shall be subject to surveillance as specified in paragraph 6.
Quality system5
5
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the regulated measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the instrument category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation referred to in paragraph 2.
5
The quality system shall ensure compliance of the regulated measuring instruments with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
d
the means of monitoring the effective operation of the quality system.
5
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 5.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in paragraph 2 in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
5
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
5
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 5.2 or whether a re-assessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body6
6
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
6
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the technical documentation referred to in paragraph 2;
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned.
6
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
6
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity7
7
The manufacturer shall affix the UK marking, the M marking set out in these Regulations, and, under the responsibility of the approved body referred to in paragraph 5.1, the latter's identification number to each individual regulated measuring instrument that satisfies the applicable requirements of these Regulations.
7
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
8
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the documentation referred to in paragraph 5.1,
b
the information relating to the change referred to in paragraph 5.5, as approved;
c
the decisions and reports from the approved body referred to in paragraphs 5.5, 6.3 and 6.4.
9
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Authorised representative10
The manufacturer's obligations set out in paragraphs 3, 5.1, 5.5, 7 and 8 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE F:CONFORMITY TO TYPE BASED ON PRODUCT VERIFICATION
1
Conformity to type based on product verification is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 5.1 and 6, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned, which have been subject to the provisions of paragraph 3, are in conformity with the type described in the type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured regulated measuring instruments with the approved type described in the type examination certificate and with the requirements of these Regulations that apply to them.
Verification3
An approved body chosen by the manufacturer shall carry out the appropriate examinations and tests, or have them carried out, to verify the conformity of the instruments with the type as described in the type examination certificate and the appropriate requirements of these Regulations.
The examinations and tests to verify the conformity of the regulated measuring instruments with the appropriate requirements shall be carried out, at the choice of the manufacturer, either by examination and testing of every instrument as specified in paragraph 4, or by examination and testing of the regulated measuring instruments on a statistical basis as specified in paragraph 5.
4
Verification of conformity by examination and testing of every instrument
4
All regulated measuring instruments shall be individually examined and appropriate tests set out in the relevant designated standard(s) and/or normative documents, and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify their conformity with the approved type described in the type examination certificate and with the appropriate requirements of these Regulations.
In the absence of a designated standard or normative document, the approved body concerned shall decide on the appropriate tests to be carried out.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity available for inspection by the market surveillance authorities for 10 years after the instrument has been placed on the market.
Statistical verification of conformity5
5
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure the homogeneity of each lot produced, and shall present his regulated measuring instruments for verification in the form of homogeneous lots.
5
A random sample shall be taken from each lot according to the requirements of paragraph 5.3. All regulated measuring instruments in a sample shall be individually examined and appropriate tests set out in the relevant designated standard(s) and/or normative document(s), and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify their conformity with the type described in the type examination certificate and with the applicable requirements of these Regulations, and to determine whether the lot is accepted or rejected. In the absence of such designated standard or normative document, the approved body concerned shall decide on the appropriate tests to be carried out.
5
The statistical procedure shall meet the following requirements:
The statistical control will be based on attributes. The sampling system shall ensure:
a
a level of quality corresponding to a probability of acceptance of 95 %, with a non-conformity of less than 1 %;
b
a limit quality corresponding to a probability of acceptance of 5 %, with a non-conformity of less than 7 %.
5
If a lot is accepted, all regulated measuring instruments of the lot shall be considered approved, except for those regulated measuring instruments from the sample that have been found not to satisfy the tests.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
5
If a lot is rejected, the approved body shall take appropriate measures to prevent the placing on the market of that lot. In the event of frequent rejection of lots the approved body may suspend the statistical verification and take appropriate measures.
Conformity marking and declaration of conformity6
6
The manufacturer shall affix the UK marking and the M marking set out in these Regulations, and, under the responsibility of the approved body referred to in paragraph 3, the latter's identification number to each individual instrument that is in conformity with the approved type described in the type examination certificate and satisfies the applicable requirements of these Regulations.
6
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
If the approved body referred to in paragraph 3 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the regulated measuring instruments.
7
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the regulated measuring instruments during the manufacturing process.
Authorised representative8
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in paragraphs 2 and 5.1.
MODULE F1:CONFORMITY BASED ON PRODUCT VERIFICATION
1
Conformity based on product verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3, 6.1 and 7 and ensures and declares on his sole responsibility that the regulated measuring instruments concerned which have been subject to the provisions of paragraph 4, are in conformity with the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation set out in regulations 44 and 45. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
The manufacturer shall keep the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured regulated measuring instruments with the applicable requirements of these Regulations.
Verification4
An approved body chosen by the manufacturer shall carry out the appropriate examinations and tests, or have them carried out, to verify the conformity of the regulated measuring instruments with the applicable requirements of these Regulations.
The examinations and tests to verify the conformity with the requirements shall be carried out, at the choice of the manufacturer, either by examination and testing of every instrument as specified in paragraph 5, or by examination and testing of the regulated measuring instruments on a statistical basis as specified in paragraph 6.
Verification of conformity by examination and testing of every instrument5
5
All regulated measuring instruments shall be individually examined and appropriate tests, set out in the relevant designated standards and/or normative documents, and/or equivalent tests set out in other relevant technical specifications, shall be carried out to verify their conformity with the requirements that apply to them. In the absence of such a designated standard, or normative document, the approved body concerned shall decide on the appropriate tests to be carried out.
5
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Statistical verification of conformity6
6
The manufacturer shall take all measures necessary so that the manufacturing process ensures the homogeneity of each lot produced, and shall present his regulated measuring instruments for verification in the form of homogeneous lots.
6
A random sample shall be taken from each lot according to the requirements of paragraph 6.4.
6
All regulated measuring instruments in the sample shall be individually examined and appropriate tests set out in the relevant designated standards and/or normative documents, and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify their conformity with the applicable requirements of these Regulations and to determine whether the lot is accepted or rejected. In the absence of such designated standard, or normative document, the approved body concerned shall decide on the appropriate tests to be carried out.
6
The statistical procedure shall meet the following requirements:
The statistical control will be based on attributes. The sampling system shall ensure:
a
a level of quality corresponding to a probability of acceptance of 95 %, with a non-conformity of less than 1 %;
b
a limit quality corresponding to a probability of acceptance of 5 %, with a non-conformity of less than 7 %.
6
If a lot is accepted, all regulated measuring instruments of the lot shall be considered approved, except for those regulated measuring instruments from the sample that have been found not to satisfy the tests.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out, and shall affix its identification number to each approved instrument or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
If a lot is rejected, the approved body shall take appropriate measures to prevent that lot from being placed on the market. In the event of frequent rejection of lots the approved body may suspend the statistical verification and take appropriate measures.
Conformity marking and declaration of conformity7
7
The manufacturer shall affix the UK marking and the M marking set out in these Regulations, and under the responsibility of the approved body referred to in paragraph 4, the latter's identification number to each individual regulated measuring instrument that satisfies the applicable requirements of these Regulations.
7
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual regulated measuring instruments in those cases where a large number of instruments is delivered to a single user.
If the approved body referred to in paragraph 5 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the regulated measuring instruments.
8
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the regulated measuring instruments during the manufacturing process.
9
Authorised representative
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in the first paragraph of paragraph 2, paragraph 3 and paragraph 6.1.
MODULE GCONFORMITY BASED ON UNIT VERIFICATION
1
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 5 and ensures and declares on his sole responsibility that the instrument concerned, which has been subject to the provisions of paragraph 4, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation2
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in paragraph 4. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument.
The manufacturer shall keep the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured instrument with the applicable requirements of these Regulations.
Verification4
An approved body chosen by the manufacturer shall carry out the appropriate examinations and tests set out in the relevant designated standards, and/or normative documents, or equivalent tests set out in other relevant technical specifications, to verify the conformity of the instrument with the applicable requirements of these Regulations, or have them carried out. In the absence of such a designated standard, or normative document, the approved body concerned shall decide on the appropriate tests to be carried out.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and affix its identification number to the approved instrument, or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Conformity marking and declaration of conformity5
5
The manufacturer shall affix the UK marking and the M marking set out in these Regulations and, under the responsibility of the approved body referred to in paragraph 4, the latter's identification number to each instrument that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with the regulated measuring instrument.
Authorised representative6
The manufacturer's obligations set out in paragraphs 2 and 5 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE H:CONFORMITY BASED ON FULL QUALITY ASSURANCE
1
Conformity based on full quality assurance is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for design, manufacture and final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 3, and shall be subject to surveillance as specified in paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the regulated measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
the technical documentation for one model of each category of regulated measuring instruments intended to be manufactured. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the instrument,
c
the documentation concerning the quality system, and
d
a written declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the regulated measuring instruments with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards, that will be applied and, where the relevant designated standards, and/or normative documents will not be applied in full, the means that will be used to ensure that the essential requirements of these Regulations that apply to the regulated measuring instruments will be met applying other relevant technical specifications;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the regulated measuring instruments pertaining to the instrument category covered;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
g
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member experienced as an assessor in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The auditing team shall review the technical documentation referred to in paragraph 3.1(b) to verify the manufacturer's ability to identify the applicable requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the instrument with those requirements.
The manufacturer or his authorised representative shall be notified of the decision. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a re-assessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the design, manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records as provided for by the design part of the quality system, such as results of analyses, calculations, tests.;
c
the quality records as provided for by the manufacturing part of the quality system, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to check the proper functioning of the quality system. It shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity5
5
The manufacturer shall affix the UK marking, the M marking set out in these Regulations and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual instrument that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
6
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the technical documentation referred to in paragraph 3.1,
b
the documentation concerning the quality system referred to in paragraph 3.1,
c
the information relating to the change referred to in paragraph 3.5, as approved;
d
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
MODULE H1:CONFORMITY BASED ON FULL QUALITY ASSURANCE PLUS DESIGN EXAMINATION
1
Conformity based on full quality assurance plus design examination is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 6, and ensures and declares on his sole responsibility that the regulated measuring instruments concerned satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for design, manufacture and final product inspection and testing of the regulated measuring instruments concerned as specified in paragraph 3, and shall be subject to surveillance as specified in paragraph 5.
The adequacy of the technical design of the regulated measuring instruments shall have been examined in accordance with paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of the quality system with the approved body of his choice for the regulated measuring instruments concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well;
b
all relevant information for the instrument category envisaged;
c
the documentation concerning the quality system;
d
a written declaration that the same application has not been lodged with any other approved body.
3
The quality system shall ensure compliance of the regulated measuring instruments with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. This quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards, that will be applied and, where the relevant designated standards and/or normative documents will not be applied in full, the means that will be used to ensure that the essential requirements of these Regulations that apply to the regulated measuring instruments will be met, applying other relevant technical specifications;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the regulated measuring instruments pertaining to the instrument category covered;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned;
g
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2. It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member experienced as an assessor in the relevant instrument field and instrument technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises.
The manufacturer or his authorised representative shall be notified of the decision. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a re-assessment is necessary.
It shall notify the manufacturer or his authorised representative of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
3
Each approved body shall inform the Secretary of State of quality system approvals issued or withdrawn, and shall periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
Design examination4
4
The manufacturer shall lodge an application for examination of the design with the approved body referred to in paragraph 3.1.
4
The application shall make it possible to understand the design, manufacture and operation of the instrument, and to assess the conformity with the requirements of these Regulations that apply to it.
It shall include:
a
the name and address of the manufacturer;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation. The documentation shall make it possible to assess the instrument's conformity with the relevant requirements, and shall include an adequate analysis and assessment of the risk(s). It shall, as far as relevant for such assessment, cover the design and operation of the instrument;
d
the supporting evidence for the adequacy of the technical design. This supporting evidence shall mention any documents that have been used, in particular where the relevant designated standards and/or normative documents have not been applied in full, and shall include, where necessary, the results of tests carried out in accordance with other relevant technical specifications, by the appropriate laboratory of the manufacturer, or by another testing laboratory on his behalf and under his responsibility.
4
The approved body shall examine the application, and where the design meets the requirements of these Regulations that apply to the instrument it shall issue a design examination certificate to the manufacturer. That certificate shall give the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the data necessary for identification of the approved design. That certificate may have one or more annexes attached.
That certificate and its annexes shall contain all relevant information to allow the conformity of manufactured regulated measuring instruments with the examined design to be evaluated and to allow for in-service control. It shall allow the evaluation of conformity of the manufactured instruments with the examined design regarding the reproducibility of their metrological performances, when they are properly adjusted using appropriate means, including:
a
the metrological characteristics of the design of the instrument;
b
measures required for ensuring the integrity of the instruments (sealing, identification of software, etc.);
c
information on other elements necessary for the identification of the instrument and to check its visual external conformity to the design;
d
if appropriate, any specific information necessary to verify the characteristics of manufactured instruments;
e
in the case of a sub-assembly, all necessary information to ensure the compatibility with other sub-assemblies or regulated measuring instruments.
The approved body shall establish an evaluation report in this regard and keep it at the disposal of the Secretary of State. Without prejudice to paragraph 9 of Schedule 5, the approved body shall release the content of this report, in full or in part, only with the agreement of the manufacturer.
The certificate shall have a validity of 10 years from the date of its issue, and may be renewed for subsequent periods of 10 years each.
Where the design does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a design examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
4
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved design may no longer comply with the applicable requirements of these Regulations, and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
The manufacturer shall keep the approved body that has issued the design examination certificate informed of any modification to the approved design that may affect the conformity with the essential requirements of these Regulations or the conditions for validity of the certificate. Such modifications shall require additional approval – from the approved body that issued the design examination certificate – in the form of an addition to the original design examination certificate.
4
Each approved body shall inform the Secretary of State of the design examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of certificates and/or any additions thereto refused, suspended or otherwise restricted.
The other approved bodies and the Secretary of State may, on request, obtain a copy of the design examination certificates and/or additions thereto. On request, the Secretary of State may obtain a copy of the technical documentation and of the results of the examinations carried out by the approved body.
The approved body shall keep a copy of the design examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer until the expiry of the validity of the certificate.
4
The manufacturer shall keep a copy of the design examination certificate, its annexes and additions with the technical documentation at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market.
Surveillance under the responsibility of the approved body5
5
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
5
The manufacturer shall, for assessment purposes, allow the approved body access to the design, manufacture, inspection, testing and storage sites, and shall provide it with all necessary information, in particular:
a
the quality system documentation;
b
the quality records as provided for by the design part of the quality system, such as results of analyses, calculations, tests, etc.;
c
the quality records as provided for by the manufacturing part of the quality system, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
5
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
5
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out instrument tests, or have them carried out, in order to check the proper functioning of the quality system. It shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
Conformity marking and declaration of conformity6
6
The manufacturer shall affix the UK marking and the M marking set out in these Regulations, and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual instrument that satisfies the applicable requirements of these Regulations.
6
The manufacturer shall draw up a written declaration of conformity for each instrument model and keep it at the disposal of the market surveillance authorities for 10 years after the instrument has been placed on the market. The declaration of conformity shall identify the instrument model for which it has been drawn up and shall mention the number of the design examination certificate.
A copy of the declaration of conformity shall be made available to the market surveillance authorities upon request.
A copy of the declaration of conformity shall be supplied with each regulated measuring instrument that is placed on the market. However, this requirement may be interpreted as applying to a batch or consignment rather than individual instruments in those cases where a large number of instruments is delivered to a single user.
7
The manufacturer shall, for a period ending 10 years after the instrument has been placed on the market, keep at the disposal of the market surveillance authorities:
a
the documentation concerning the quality system referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved;
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 5.3 and 5.4.
Authorised representative8
The manufacturer's authorised representative may lodge the application referred to in paragraphs 4.1 and 4.2 and fulfil the obligations set out in paragraphs 3.1, 3.5, 4.4, 4.6, 6 and 7, on his behalf and under his responsibility, provided that they are specified in the mandate.
SCHEDULE 1CWATER METERS (MI-001) (Annex III to the Directive)
The relevant requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule, apply to water meters intended for the measurement of volumes of clean, cold or heated water in residential, commercial and light industrial use.
DEFINITIONS
Minimum Flowrate (Q1)
The lowest flowrate at which the water meter provides indications that satisfy the requirements concerning the maximum permissible errors (MPEs.)
Transitional Flowrate (Q2)
The transitional flowrate is the flowrate value occurring between the permanent and minimum flowrates, at which the flowrate range is divided into two zones, the ‘upper zone’ and the ‘lower zone’. Each zone has a characteristic MPE.
Permanent Flowrate (Q3)
The highest flowrate at which the water meter operates in a satisfactory manner under normal conditions of use, i.e. under steady or intermittent flow conditions.
Overload Flowrate (Q4)
The overload flowrate is the highest flowrate at which the meter operates in a satisfactory manner for a short period of time without deteriorating.
SPECIFIC REQUIREMENTS
Rated Operating ConditionsThe manufacturer shall specify the rated operating conditions for the instrument, in particular:
1
The flowrate range of the water.
The values for the flowrate range shall fulfil the following conditions:
Q3/Q1 ≥ 40
Q2/Q1 = 1.6
Q4/Q3 = 1.25
2
The temperature range of the water.
The values for the temperature range shall fulfil the following conditions:
0.1 °C to at least 30 °C
3
The relative pressure range of the water, the range being 0.3 bar to at least 10 bar at Q3.
4
For the power supply: the nominal value of the AC voltage supply and/or the limits of DC supply.
MPE
5
The MPE, positive or negative, on volumes delivered at flowrates between the transitional flowrate (Q2) (included) and the overload flowrate (Q4) is:
2 % for water having a temperature ≤ 30 °C,
The meter shall not exploit the MPE or systematically favour any party.
6
The MPE, positive or negative, on volumes delivered at flowrates between the minimum flowrate (Q1) and the transitional flowrate (Q2) (excluded) is 5 % for water having any temperature.
The meter shall not exploit the MPE or systematically favour any party.
Permissible Effect of Disturbances
Electromagnetic immunity7
7
The effect of an electromagnetic disturbance on a water meter shall be such that:
— the change in the measurement result is no greater than the critical change value as defined in paragraph 7.1.3, or
— the indication of the measurement result is such that it cannot be interpreted as a valid result, such as a momentary variation that cannot be interpreted, memorised or transmitted as a measuring result.
7
After undergoing an electromagnetic disturbance the water meter shall:
— recover to operate within MPE, and
— have all measurement functions safeguarded, and
— allow recovery of all measurement data present just before the disturbance.
7
The critical change value is the smaller of the two following values:
— the volume corresponding to half of the magnitude of the MPE in the upper zone on the measured volume;
— the volume corresponding to the MPE on the volume corresponding to one minute at flowrate Q3.
Durability7
After an appropriate test, taking into account the period of time estimated by the manufacturer, has been performed, the following criteria shall be satisfied:
7
The variation of the measurement result after the durability test, when compared with the initial measurement result, shall not exceed:
— 3 % of the metered volume between Q1 included and Q2 excluded;
— 1.5 % of the metered volume between Q2 included and Q4 included.
7
The error of indication for the volume metered after the durability test shall not exceed:
— ± 6 % of the metered volume between Q1 included and Q2 excluded;
— ± 2.5 % of the metered volume between Q2 included and Q4 included for water meters intended to meter water with a temperature between 0.1 °C and 30 °C,
Suitability8
The meter shall be able to be installed to operate in any position unless clearly marked otherwise.
8
The manufacturer shall specify whether the meter is designed to measure reverse flow. In such a case, the reverse flow volume shall either be subtracted from the cumulated volume or shall be separately recorded. The same MPE shall apply to both forward and reverse flow.
Water meters not designed to measure reverse flow shall either prevent reverse flow or shall withstand an accidental reverse flow without any deterioration or change in metrological properties.
Units of Measurement9
Metered volume shall be displayed in cubic metres.
Putting into Use10
The requirements under paragraphs 1, 2 and 3 are determined by the utility or the person legally designated for installing the meter, so that the meter is appropriate for the accurate measurement of consumption that is foreseen or foreseeable.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to water meters that the manufacturer can choose between are:
a
B and F;
b
B and D; or
c
H1.
SCHEDULE 1DGAS METERS (MI-002) (Annex IV to the Directive)
The relevant requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule, apply to gas meters.
DEFINITIONS
Minimum flowrate (Qmin)
The lowest flowrate at which the gas meter provides indications that satisfy the requirements regarding maximum permissible error (MPE).
Maximum flowrate (Qmax)
The highest flowrate at which the gas meter provides indications that satisfy the requirements regarding MPE.
Transitional flowrate (Qt)
The transitional flowrate is the flowrate occurring between the maximum and minimum flowrates at which the flowrate range is divided into two zones, the ‘upper zone’ and the ‘lower zone’. Each zone has a characteristic MPE.
Overload Flowrate (Qr)
The overload flowrate is the highest flowrate at which the meter operates for a short period of time without deteriorating.
Base conditions
The specified conditions to which the measured quantity of fluid is converted.
PART ISPECIFIC REQUIREMENTS
GAS METERS
1
Rated operating conditions
The manufacturer shall specify the rated operating conditions of the gas meter, taking into account:
1
The flowrate range of the gas shall fulfil at least the following conditions:
Class
Qmax/Qmin
Qmax/Qt
Qr/Qmax
1.5
≥ 150
≥ 10
1.2
1.0
≥ 20
≥ 5
1.2
1
The temperature range of the gas, with a minimum range of 40 °C.
The fuel/gas related conditions1
The gas meter shall be designed for the range of gases and supply pressures of the United Kingdom. In particular the manufacturer shall indicate:
— the gas family or group;
— the maximum operating pressure.
1
A minimum temperature range of 50 °C for the climatic environment.
1
The nominal value of the AC voltage supply and/or the limits of DC supply.
Maximum permissible error (MPEs)2
Gas meter indicating the volume at metering conditions or mass2
Table 1
Class
1.5
1.0
Qmin ≤ Q < Qt
3 %
2 %
Qt ≤ Q ≤ Qmax
1.5 %
1 %
The gas meter shall not exploit the MPEs or systematically favour any party.
2
For a gas meter with temperature conversion, which only indicates the converted volume, the MPE of the meter is increased by 0.5 % in a range of 30 °C extending symmetrically around the temperature specified by the manufacturer that lies between 15 °C and 25 °C. Outside this range, an additional increase of 0.5 % is permitted in each interval of 10 °C.
Permissible effect of disturbances3
Electromagnetic immunity3
3
The effect of an electromagnetic disturbance on a gas meter shall be such that:
— the change in the measurement result is no greater than the critical change value as defined in paragraph 3.1.3, or
— the indication of the measurement result is such that it cannot be interpreted as a valid result, such as a momentary variation that cannot be interpreted, memorised or transmitted as a measuring result.
3
After undergoing a disturbance, the gas meter shall:
— recover to operate within MPE, and
— have all measurement functions safeguarded, and
— allow recovery of all measurement data present just before the disturbance.
3
The critical change value is the smaller of the two following values:
— the quantity corresponding to half of the magnitude of the MPE in the upper zone on the measured volume;
— the quantity corresponding to the MPE on the quantity corresponding to one minute at maximum flowrate.
Effect of upstream-downstream flow disturbances3
Under installation conditions specified by the manufacturer, the effect of the flow disturbances shall not exceed one third of the MPE.
Durability4
After an appropriate test, taking into account the period of time estimated by the manufacturer, has been performed, the following criteria shall be satisfied:
Class 1.5 Gas Meters4
4
The variation of the measurement result after the durability test when compared with the initial measurement result for the flow rates in the range Qt to Qmax shall not exceed the measurement result by more than 2 %.
4
The error of indication after the durability test shall not exceed twice the MPE in paragraph 2.
Class 1.0 Gas Meters4
4
The variation of the measurement result after the durability test when compared with the initial measurement result shall not exceed one-third of the MPE in paragraph 2.
4
The error of indication after the durability test shall not exceed the MPE in paragraph 2.
Suitability5
5
A gas meter powered from the mains (AC or DC) shall be provided with an emergency power supply device or other means to ensure, during a failure of the principal power source, that all measuring functions are safeguarded.
5
A dedicated power source shall have a lifetime of at least five years. After 90 % of its lifetime an appropriate warning shall be shown.
5
An indicating device shall have a sufficient number of digits to ensure that the quantity passed during 8,000 hours at Qmax does not return the digits to their initial values.
5
The gas meter shall be able to be installed to operate in any position declared by the manufacturer in its installation instruction.
5
The gas meter shall have a test element, which shall enable tests to be carried out in a reasonable time.
5
The gas meter shall respect the MPE in any flow direction or only in one flow direction clearly marked.
Units6
Metered quantity shall be displayed in cubic metre, or in kilogram.
PART IIPUTTING INTO USE AND CONFORMITY ASSESSMENT
7
Putting into use
a
The measurement of residential use must be performed by means of any Class 1.5 gas meter, or by Class 1.0 gas meters which have a Qmax/Qmin ratio equal to or greater than 150.
b
Measurement of commercial and/or light industrial use must be performed by any Class 1.0 or Class 1.5 gas meter.
c
The person responsible for installing a gas meter must have regard to the requirements under paragraphs 1.2 and 1.3 of Part I of this Schedule and must ensure that the gas meter is appropriate for the accurate measurement of consumption that is foreseen or foreseeable.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to gas meters that the manufacturer can choose between are:
a
B and F;
b
B and D; or
c
H1.
SCHEDULE 1EACTIVE ELECTRICAL ENERGY METERS (MI-003) (Annex V to the Directive)
The relevant requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule, apply to active electrical energy meters.
Note:
Electrical energy meters may be used in combination with external instrument transformers, depending upon the measurement technique applied. However, this Schedule covers only electrical energy meters but not instrument transformers.
DEFINITIONS
An active electrical energy meter is a device which measures the active electrical energy consumed in a circuit.
I
=
the electrical current flowing through the meter;
In
=
the specified reference current for which the transformer operated meter has been designed;
Ist
=
the lowest declared value of I at which the meter registers active electrical energy at unity power factor (polyphase meters with balanced load);
Imin
=
the value of I above which the error lies within maximum permissible errors (MPEs) (polyphase meters with balanced load);
Itr
=
the value of I above which the error lies within the smallest MPE corresponding to the class index of the meter;
Imax
=
the maximum value of I for which the error lies within the MPEs;
U
=
the voltage of the electricity supplied to the meter;
Un
=
the specified reference voltage;
f
=
the frequency of the voltage supplied to the meter;
fn
=
the specified reference frequency;
PF
=
power factor = cosφ = the cosine of the phase difference φ between I and U.
SPECIFIC REQUIREMENTS
Accuracy1
The manufacturer shall specify the class index of the meter. The class indices are defined as: Class A, B and C.
Rated operating conditions2
The manufacturer shall specify the rated operating conditions of the meter; in particular:
The values of fn, Un, In, Ist, Imin, Itr and Imax that apply to the meter. For the current values specified, the meter shall satisfy the conditions given in Table 1;
Table 1
Class A
Class B
Class C
For direct-connected meters
Ist
≤ 0.05 ∙ Itr
≤ 0.04 ∙ Itr
≤ 0.04 ∙ Itr
Imin
≤ 0.5 ∙ Itr
≤ 0.5 ∙ Itr
≤ 0.3 ∙ Itr
Imax
≥ 50 ∙ Itr
≥ 50 ∙ Itr
≥ 50 ∙ Itr
For transformer-operated meters
Ist
≤ 0.06 ∙ Itr
≤ 0.04 ∙ Itr
≤ 0.02 ∙ Itr
Imin
≤ 0.4 ∙ Itr
≤ 0.2 ∙ Itr1
≤ 0.2 ∙ Itr
In
= 20 ∙ Itr
= 20 ∙ Itr
= 20 ∙ Itr
Imax
≥ 1.2 ∙ In
≥ 1.2 ∙ In
≥ 1.2 ∙ In
1 For Class B electromechanical meters Imin≤ 0.4 ∙ Itr shall apply.
The voltage, frequency and power factor ranges within which the meter shall satisfy the MPE requirements are specified in Table 2. These ranges shall recognise the typical characteristics of electricity supplied by public distribution systems.
The voltage and frequency ranges shall be at least:
0.9 ∙ Un≤ U ≤ 1.1 ∙ Un
0.98 ∙ fn≤ f ≤ 1.02 ∙ fn
power factor range at least from cosφ = 0.5 inductive to cosφ = 0.8 capacitive.
MPEs3
The effects of the various measurands and influence quantities (a, b, c,…) are evaluated separately, all other measurands and influence quantities being kept relatively constant at their reference values. The error of measurement, that shall not exceed the MPE stated in Table 2, is calculated as:
When the meter is operating under varying-load current, the percentage errors shall not exceed the limits given in Table 2.
Table 2
Operating temperatures
Operating temperatures
Operating temperatures
Operating temperatures
MPEs in percent at rated operating conditions and defined load current levels and operating temperature
Operating temperatures
Operating temperatures
Operating temperatures
Operating temperatures
+ 5 °C … + 30 °C
– 10 °C … + 5 °C
or
+ 30 °C … + 40 °C
– 25 °C … – 10 °C
or
+ 40 °C … + 55 °C
– 40 °C … – 25 °C
or
+ 55 °C … + 70 °C
Meter class
A
B
C
A
B
C
A
B
C
A
B
C
Single phase meter; polyphase meter if operating with balanced loads
Imin ≤ I < Itr
3.5
2
1
5
2.5
1.3
7
3.5
1.7
9
4
2
Itr ≤ I < Imax
3.5
2
0
4.5
2.5
1
7
3.5
1.3
9
4
1.5
Polyphase meter if operating with single phase load
Itr ≤ I < Imax
, see exception below
4
2.5
1
5
3
1.3
7
4
1.7
9
4.5
2
For electromechanical polyphase meters the current range for single-phase load is limited to 5Itr ≤ I ≤ Imax
When a meter operates in different temperature ranges the relevant MPE values shall apply.
The meter shall not exploit the MPEs or systematically favour any party.
4
Permissible effect of disturbances
4
General
As electrical energy meters are directly connected to the mains supply and as mains current is also one of the measurands, a special electromagnetic environment is used for electricity meters.
The meter shall comply with the electromagnetic environment E2 and the additional requirements in paragraphs 4.2 and 4.3.
The electromagnetic environment and permissible effects reflect the situation that there are disturbances of long duration which shall not affect the accuracy beyond the critical change values and transient disturbances, which may cause a temporary degradation or loss of function or performance but from which the meter shall recover and shall not affect the accuracy beyond the critical change values.
When there is a foreseeable high risk due to lightning or where overhead supply networks are predominant, the metrological characteristics of the meter shall be protected.
Effect of disturbances of long duration4
Table 3
Critical change values for disturbances of long duration
Disturbance
Critical change values in percent for meters of class
A
B
C
Reversed phase sequence
1.5
1.5
0.3
Voltage unbalance (only applicable to polyphase meters)
4
2
1
Harmonic contents in the current circuits
1
0.8
0.5
DC and harmonics in the current circuit
6
3
1.5
Fast transient bursts
6
4
2
Magnetic fields; HF (radiated RF) electromagnetic field; Conducted disturbances introduced by radio-frequency fields; and Oscillatory waves immunity
3
2
1
In the case of electromechanical electricity meters, no critical change values are defined for harmonic contents in the current circuits and for DC and harmonics in the current circuit.
Permissible effect of transient electromagnetic phenomena4
4
The effect of an electromagnetic disturbance on an electrical energy meter shall be such that during and immediately after a disturbance:
— any output intended for testing the accuracy of the meter does not produce pulses or signals corresponding to an energy of more than the critical change value,
and in reasonable time after the disturbance the meter shall:
— recover to operate within the MPE limits, and
— have all measurement functions safeguarded, and
— allow recovery of all measurement data present prior to the disturbance, and
— not indicate a change in the registered energy of more than the critical change value.
(m being the number of measuring elements of the meter, Un in Volts and Imax in Amps).
4
For overcurrent the critical change value is 1.5 %.
Suitability5
5
Below the rated operating voltage the positive error of the meter shall not exceed 10 %.
5
The display of the total energy shall have a sufficient number of digits to ensure that when the meter is operated for 4,000 hours at full load (I = Imax, U = Un and PF = 1) the indication does not return to its initial value and shall not be able to be reset during use.
5
In the event of loss of electricity in the circuit, the amounts of electrical energy measured shall remain available for reading during a period of at least 4 months.
Running with no load5
When the voltage is applied with no current flowing in the current circuit (current circuit shall be open circuit), the meter shall not register energy at any voltage between 0.8 ∙ Un and 1.1 Un.
Starting5
The meter shall start and continue to register at Un, PF = 1 (polyphase meter with balanced loads) and a current which is equal to Ist.
Units6
The electrical energy measured shall be displayed in kilowatt-hours or in megawatt-hours.
Putting into use7
a
Subject to sub-paragraph (b), measurement may be performed by means of any active electrical energy meter provided that the temperature range to which an active electrical energy meter is exposed is not wider than the range specified by the manufacturer in relation to that active electrical energy meter in accordance with paragraph 1.3.1 and Table 1 in Schedule 1A to these Regulations.
b
Class A active electrical energy meters may not be used when operating outside the temperature range of an upper temperature limit of 30°C to a lower temperature limit of 5°C.
c
The person responsible for installing the active electrical energy meter must determine the correct current range and assess the climatic environment.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to active electrical energy meters that the manufacturer can choose between are:
a
B and F;
b
B and D; or
c
H1.
SCHEDULE 1FMEASURING SYSTEMS FOR THE CONTINUOUS AND DYNAMIC MEASUREMENT OF QUANTITIES OF LIQUIDS OTHER THAN WATER (MI-005) (Annex VII to the Directive)
The relevant essential requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule, apply to measuring systems intended for the continuous and dynamic measurement of quantities (volumes or masses) of liquids other than water. If appropriate, the terms ‘volume, and L’ in this Schedule can be read as: ‘mass and kg’.
DEFINITIONS
Meter
An instrument designed to measure continuously, memorise and display the quantity at metering conditions of liquid flowing through the measurement transducer in a closed, fully charged conduit.
Calculator
A part of a meter that receives the output signals from the measurement transducer(s) and possibly, from associated regulated measuring instruments and displays the measurement results.
Associated Measuring Instrument
An instrument connected to the calculator for measuring certain quantities which are characteristic of the liquid, with a view to make a correction and/or conversion.
Conversion Device
A part of the calculator which by taking account of the characteristics of the liquid (temperature, density, etc.) measured using associated regulated measuring instruments, or stored in a memory, automatically converts:
— the volume of the liquid measured at metering conditions into a volume at base conditions and/or into mass, or
— the mass of the liquid measured at metering conditions into a volume at metering conditions and/or into a volume at base conditions
Note:
A conversion device includes the relevant associated measuring instruments.
Base conditions
The specified conditions to which the measured quantity of liquid at metering conditions is converted.
Measuring System
A system that comprises the meter itself and all devices required to ensure correct measurement or intended to facilitate the measuring operations.
Fuel dispenser
A measuring system intended for the refuelling of motor vehicles, small boats and small aircraft.
Self-service arrangement
An arrangement that allows the customer to use a measuring system for the purpose of obtaining liquid for his own use.
Self-service device
A specific device that is part of a self-service arrangement and which allows one of more measuring systems to perform in this self-service arrangement.
Minimum measured quantity (MMQ)
The smallest quantity of liquid for which the measurement is metrologically acceptable for the measuring system.
Direct indication
The indication, either volume or mass, corresponding to the measure and that the meter is physically capable of measuring.
Note:
The direct indication may be converted into another quantity using a conversion device.
Interruptible/non-interruptible
A measuring system is considered as interruptible/non-interruptible when the liquid flow can/cannot be stopped easily and rapidly.
Flowrate range
The range between the minimum flowrate (Qmin) and maximum flowrate (Qmax).
SPECIFIC REQUIREMENTS
Rated operating conditions1
The manufacturer shall specify the rated operating conditions for the instrument, in particular;
The flowrate range1
The flowrate ran ge is subject to the following conditions:
i
the flowrate range of a measuring system shall be within the flowrate range of each of its elements, in particular the meter.
ii
meter and measuring system:
Table 1
Specific measuring system
Characteristic of liquid
Minimum ratio of Qmax: Qmin
Fuel dispensers
Not Liquefied gases
10: 1
Liquefied gases
5: 1
Measuring system
Cryogenic liquids
5: 1
Measuring systems on pipeline and systems for loading ships
All liquids
Suitable for use
All other measuring systems
All liquids
4: 1
1
The properties of the liquid to be measured by the instrument by specifying the name or type of the liquid or its relevant characteristics, for example:
— Temperature range;
— Pressure range;
— Density range;
— Viscosity range.
1
The nominal value of the AC voltage supply and/or limits of the DC voltage supply.
1
The base conditions for converted values.
This is without prejudice to the Secretary of State's obligations to require use of a temperature of 15 °C in accordance with section 12(1) of the Finance Act 1993 M86.
Accuracy classification and maximum permissible errors (MPEs)2
2
For quantities equal to or greater than 2 litres the MPE on indications is:
Table 2
Accuracy Class
Accuracy Class
0.3
0.5
1.0
1.5
2.5
Measuring systems (A)
0.3 %
0.5 %
1.0 %
1.5 %
2.5 %
Meters (B)
0.2 %
0.3 %
0.6 %
1.0 %
1.5 %
2
For quantities less than two litres the MPE on indications is:
Table 3
Measured volume V
MPE
V < 0.1 L
4 × value in Table 2, applied to 0.1 L
0.1 L ≤ V < 0.2 L
4 × value in Table 2
0.2 L ≤ V < 0.4 L
2 × value in Table 2, applied to 0.4 L
0.4 L ≤ V < 1 L
2 × value in Table 2
1 L ≤ V < 2 L
Value in Table 2, applied to 2 L
2
However, no matter what the measured quantity may be, the magnitude of the MPE is given by the greater of the following two values:
— the absolute value of the MPE given in Table 2 or Table 3,
— the absolute value of the MPE for the minimum measured quantity (Emin).
2
For minimum measured quantities greater than or equal to 2 litres the following conditions apply:
Condition 1
Emin shall fulfil the condition: Emin ≥ 2 R, where R is the smallest scale interval of the indication device.
Condition 2
Emin is given by the formula: Emin = (2MMQ)x (A/100) where:
— MMQ is the minimum measured quantity,
— A is the numerical value specified in line A of Table 2.
2
For minimum measured quantities of less than two litres, the above mentioned condition 1 applies and Emin is twice the value specified in Table 3, and related to line A of Table 2.
Converted indication2
In the case of a converted indication the MPEs are as in line A of Table 2.
Conversion devices2
MPEs on converted indications due to a conversion device are equal to ± (A — B), A and B being the values specified in Table 2.
Parts of conversion devices that can be tested separately
a
Calculator
MPEs on quantities of liquid indications applicable to calculation, positive or negative, are equal to one-tenth of the MPEs as defined in line A of Table 2.
b
Associated regulated measuring instruments
Associated regulated measuring instruments shall have an accuracy at least as good as the values in Table 4:
Table 4
MPE on Measurements
Accuracy classes of the measuring system
0.3
0.5
1.0
1.5
2.5
Temperature
± 0.3 °C
± 0.5 °C
± 1.0 °C
Pressure
Less than 1 MPa: ± 50 kPa
From 1 to 4 MPa: ± 5 %
Over 4 MPa: ± 200 kPa
Density
± 1 kg/m3
± 2 kg/m3
± 5 kg/m3
These values apply to the indication of the characteristic quantities of the liquid displayed by the conversion device.
c
Accuracy for calculating function
The MPE for the calculation of each characteristic quantity of the liquid, positive or negative, is equal to two fifths of the value fixed in (b).
2
The requirement (a) in paragraph 2.6 applies to any calculation, not only conversion.
2
The measuring system shall not exploit the MPEs or systematically favour any party.
Maximum permissible effect of disturbances3
3
The effect of an electromagnetic disturbance on a measuring system shall be one of the following:
— the change in the measurement result is not greater than the critical change value as defined in paragraph 3.2, or
— the indication of the measurement result shows a momentary variation that cannot be interpreted, memorised or transmitted as a measuring result. Furthermore, in the case of an interruptible system, this can also mean the impossibility to perform any measurement, or
— the change in the measurement result is greater than the critical change value, in which case the measuring system shall permit the retrieval of the measuring result just before the critical change value occurred and cut off the flow.
3
The critical change value is the greater of MPE/5 for a particular measured quantity or Emin.
Durability4
After an appropriate test, taking into account the period of time estimated by the manufacturer, has been performed, the following criterion shall be satisfied:
The variation of the measurement result after the durability test, when compared with the initial measurement result, shall not exceed the value for meters specified in line B of table 2.
Suitability5
5
For any measured quantity relating to the same measurement, the indications provided by various devices shall not deviate one from another by more than one scale interval where devices have the same scale interval. In the case where the devices have different scale intervals, the deviation shall not be more than that of the greatest scale interval.
However, in the case of a self-service arrangement the scale intervals of the main indicating device on the measuring system and the scale intervals of the self-service device shall be the same and results of measurement shall not deviate one from another.
5
It shall not be possible to divert the measured quantity in normal conditions of use unless it is readily apparent.
5
Any percentage of air or gas not easily detectable in the liquid shall not lead to a variation of error greater than:
— 0.5 % for liquids other than potable liquids and for liquids of a viscosity not exceeding 1 mPa.s, or
— 1 % for potable liquids and for liquids of a viscosity exceeding 1 mPa.s.
However, the allowed variation shall never be smaller than 1 % of MMQ. This value applies in the case of air or gas pockets.
Instruments for direct sales5
5
A measuring system for direct sales shall be provided with means for resetting the display to zero.
It shall not be possible to divert the measured quantity.
5
The display of the quantity on which the transaction is based shall be permanent until all parties in the transaction have accepted the measurement result.
5
Measuring systems for direct sales shall be interruptible.
5
Any percentage of air or gas in the liquid shall not lead to a variation of error greater than the values specified in paragraph 5.3.
Fuel Dispensers5
5
Displays on fuel dispensers shall not be capable of being reset to zero during a measurement.
5
The start of a new measurement shall be inhibited until the display has been reset to zero.
5
Where a measuring system is fitted with a price display, the difference between the indicated price and the price calculated from the unit price and the indicated quantity shall not exceed the price corresponding to EminHowever this difference need not be less than the smallest monetary value.
Power supply failure6
A measuring system shall either be provided with an emergency power supply device that will safeguard all measuring functions during the failure of the main power supply device or be equipped with means to save and display the data present in order to permit the conclusion of the transaction in progress and with means to stop the flow at the moment of the failure of the main power supply device.
Putting into use7
Table 5
Accuracy class
Types of Measuring system
0.3
Measuring systems on pipeline
0.5
All measuring systems if not differently stated elsewhere in this Table, in particular:
fuel dispensers (not for liquefied gases),
measuring systems on road tankers for liquids of low viscosity (< 20 mPa.s)
1.0
Measuring systems for liquefied gases under pressure measured at a temperature equal to or above – 10 °C
Measuring systems normally in class 0.3 or 0.5 but used for liquids
whose temperature is less than – 10 °C or greater than 50 °C
whose dynamic viscosity is higher than 1,000 mPa.s
whose maximum volumetric flowrate is not higher than 20 L/h
1.5
Measuring systems for liquefied gases under pressure measured at a temperature below – 10 °C (other than cryogenic liquids)
2.5
Measuring systems for cryogenic liquids (temperature below – 153 °C)
Units of measurement8
The metered quantity shall be displayed in millilitres, cubic centimetres, litres, cubic metres, grams, kilograms or tonnes.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to measuring systems for the continuous and dynamic measurement of quantities of liquids other than water that the manufacturer can choose between are:
a
B and F;
b
B and D;
c
H1; or
d
G.
SCHEDULE 1GAUTOMATIC WEIGHING INSTRUMENTS (MI-006) (Annex VIII to the Directive)
The relevant essential requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in Chapter I of this Schedule, apply to automatic weighing instruments defined below, intended to determine the mass of a body by using the action of gravity on that body.
DEFINITIONS
Automatic weighing instrument
An instrument that determines the mass of a product without the intervention of an operator and follows a predetermined programme of automatic processes characteristic of the instrument.
Automatic catchweigher
An automatic weighing instrument that determines the mass of pre-assembled discrete loads (for example prepackages) or single loads of loose material.
Weight labeller
An automatic catchweigher that labels individual articles with the weight value.
Weight/price labeller
An automatic catchweigher that labels individual articles with the weight value, and price information.
Automatic gravimetric filling instrument
An automatic weighing instrument that fills containers with a predetermined and virtually constant mass of product from bulk.
Discontinuous totaliser (totalising hopper weigher)
An automatic weighing instrument that determines the mass of a bulk product by dividing it into discrete loads. The mass of each discrete load is determined in sequence and summed. Each discrete load is then delivered to bulk.
Continuous totaliser
An automatic weighing instrument that continuously determines the mass of a bulk product on a conveyor belt, without systematic subdivision of the product and without interrupting the movement of the conveyor belt.
Rail-weighbridge
An automatic weighing instrument having a load receptor inclusive of rails for conveying railway vehicles.
SPECIFIC REQUIREMENTS
CHAPTER IRequirements common to all types of automatic weighing instruments
Rated Operating Conditions1
The manufacturer shall specify the rated operating conditions for the instrument as follows:
1
For the measurand:
The measuring range in terms of its maximum and minimum capacity.
1
For the electrical supply influence quantities:
In case of AC voltage supply
:
the nominal AC voltage supply, or the AC voltage limits.
In case of DC voltage supply
:
the nominal and minimum DC voltage supply, or the DC voltage limits.
1
For the mechanical and climatic influence quantities:
The minimum temperature range is 30 °C unless specified otherwise in the following chapters of this Schedule.
The mechanical environment classes according to Schedule 1A, paragraph 1.3.2 are not applicable. For instruments which are used under special mechanical strain, e.g. instruments incorporated into vehicles, the manufacturer shall define the mechanical conditions of use.
1
For other influence quantities (if applicable):
The rate(s) of operation.
The characteristics of the product(s) to be weighed.
2
Permissible effect of disturbances — Electromagnetic environment
The required performance and the critical change value are given in the relevant Chapter of this Schedule for each type of instrument.
Suitability3
3
Means shall be provided to limit the effects of tilt, loading and rate of operation such that maximum permissible errors (MPEs) are not exceeded in normal operation.
3
Adequate material handling facilities shall be provided to enable the instrument to respect the MPEs during normal operation.
3
Any operator control interface shall be clear and effective.
3
The integrity of the display (where present) shall be verifiable by the operator.
3
Adequate zero setting capability shall be provided to enable the instrument to respect the MPEs during normal operation.
3
Any result outside the measurement range shall be identified as such, where a printout is possible.
Conformity assessment4
The conformity assessment procedures specified in the modules in Schedule 1B applicable to automatic weighing instruments that the manufacturer can choose between are:
a
For mechanical systems:
i
B and D;
ii
B and E;
iii
B and F;
iv
D1;
v
F1;
vi
G; or
vii
H1.
b
For electromechanical instruments:
i
B and D;
ii
B and E;
iii
B and F;
iv
G; or
v
H1.
c
For electronic systems or systems containing software:
i
B and D;
ii
B and F;
iii
G; or
iv
H1.
CHAPTER IIAutomatic Catchweighers
1
These categories are divided into four accuracy classes:
Y(I), Y(II), Y(a) & Y(b)
which shall be specified by the manufacturer.
MPE2
2
MPE Category Y instruments
Table 1
Net Load (m) in verification scale intervals (e)
Maximum permissible mean error
Maximum permissible error
Y(I)
Y(II)
Y(a)
Y(b)
Static
Automatic
0 < m ≤ 50,000
0 < m ≤ 5,000
0 < m ≤ 500
0 < m ≤ 50
± 0.5 e
± 1 e
50,000 < m ≤ 200,000
5,000 < m ≤ 20,000
500 < m ≤ 2,000
50 < m ≤ 200
± 1.0 e
± 1.5 e
200,000 < m
20,000 < m ≤ 100,000
2,000 < m ≤ 10,000
200 < m ≤ 1,000
± 1.5
± 2 e
Verification scale interval — single interval instruments2
Table 2
Accuracy classes
Verification scale interval
Number of verification scale intervalsn = Max/e
Minimum
Maximum
Minimum
Maximum
XI
Y(I)
0.001 g ≤ e
50,000
XII
Y(II)
0.001 g ≤ e ≤ 0.05 g
100
100,000
0.1 g ≤ e
5,000
100,000
XIII
Y(a)
0.1 g ≤ e ≤ 2 g
100
10,000
5 g ≤ e
500
10,000
XIIII
Y(b)
5 g ≤ e
100
1,000
Verification scale interval — multi-interval instruments2
Table 3
Verification scale interval
Number of verification scale intervalsn = Max/e
Minimum value1
n = Maxi /e(i+1)
For i = r the corresponding column of Table 2 applies with e replaced by er.
Maximum value
n = Maxi /ei
Y(I)
0.001 g ≤ ei
50,000
Y(II)
0.001 g ≤ ei≤ 0.05 g
5,000
100,000
0.1 g ≤ ei
5,000
100,000
Y(a)
0.1 g ≤ ei
500
10,000
Y(b)
5 g ≤ ei
50
1 000
1 For i = r the corresponding column of Table 2 applies with e replaced by er.
Where:
i = 1, 2, … r
i = partial weighing range
r = total number of partial ranges
Measurement Range3
In specifying the measurement range for class Y instruments the manufacturer shall take account that the minimum capacity shall not be less than:
class Y(I)
:
100 e
class Y(II)
:
20 e for 0.001 g ≤ e ≤ 0.05 g, and 50 e for 0.1 g ≤ e
class Y(a)
:
20 e
class Y(b)
:
10 e
Scales used for grading, e.g. postal scales and garbage weighers
:
5 e
Dynamic Setting4
4
The dynamic setting facility shall operate within a load range specified by the manufacturer.
4
When fitted, a dynamic setting facility that compensates for the dynamic effects of the load in motion shall be inhibited from operating outside the load range, and shall be capable of being secured.
Performance Under Influence Factors And Electromagnetic Disturbances5
5
The MPEs due to influence factors are:
5
For category Y instruments
— For each load in automatic operation; as specified in Table 1,
— For static weighing in non-automatic operation; as specified in Table 1.
5
The critical change value due to a disturbance is one verification scale interval.
5
Temperature range:
— For class Y(I) the minimum range is 5 °C,
— For class Y(II) the minimum range is 15 °C.
CHAPTER IIIAutomatic Gravimetric Filling Instruments
Accuracy classes1
1
The manufacturer shall specify both the reference accuracy class Ref(x) and the operational accuracy class(es) X(x).
1
An instrument type is designated a reference accuracy class, Ref(x), corresponding to the best possible accuracy for instruments of the type. After installation, individual instruments are designated for one or more operational accuracy classes, X(x), having taken account of the specific products to be weighed. The class designation factor (x) shall be ≤ 2, and in the form 1 × 10k, 2 × 10k or 5 × 10k where k is a negative whole number or zero.
1
The reference accuracy class, Ref(x) is applicable for static loads.
1
For the operational accuracy class X(x), X is a regime relating accuracy to load weight and (x) is a multiplier for the limits of error specified for class X(1) in paragraph 2.2.
MPE2
Static weighing error2
2
For static loads under rated operating conditions, the MPE for reference accuracy class Ref(x), shall be 0.312 of the maximum permissible deviation of each fill from the average; as specified in Table 5; multiplied by the class designation factor (x).
2
For instruments where the fill may be made up from more than one load (e.g. cumulative or selective combination weighers) the MPE for static loads shall be the accuracy required for the fill as specified in paragraph 2.2 (i.e. not the sum of the maximum permissible deviation for the individual loads).
Deviation from average fill2
Table 4
Value of the mass, m (g), of the fills
Maximum permissible deviation of each fill from the average for class X(1)
m ≤ 50
7.2 %
50 < m ≤ 100
3.6 g
100 < m ≤ 200
3.6 %
200 < m ≤ 300
7.2 g
300 < m ≤ 500
2.4 %
500 < m ≤ 1,000
12 g
1,000 < m ≤ 10,000
1.2 %
10,000 < m ≤ 15,000
120 g
15,000 < m
0.8 %
Note:
The calculated deviation of each fill from the average may be adjusted to take account for the effect of material particle size.
Error relative to pre-set value (setting error)2
For instruments where it is possible to pre-set a fill weight; the maximum difference between the pre-set value and the average mass of the fills shall not exceed 0.312 of the maximum permissible deviation of each fill from the average, as specified in Table 4.
Performance Under Influence Factor And Electromagnetic Disturbance3
3
The MPE due to influence factors shall be as specified in paragraph 2.1.
3
The critical change value due to a disturbance is a change of the static weight indication equal to the MPE as specified in paragraph 2.1 calculated for the rated minimum fill, or a change that would give equivalent effect on the fill in the case of instruments where the fill consists of multiple loads. The calculated critical change value shall be rounded to the next higher scale interval (d).
3
The manufacturer shall specify the value of the rated minimum fill.
CHAPTER IVDiscontinuous Totalisers
1
Accuracy Classes
Instruments are divided into four accuracy classes as follows: 0.2; 0.5; 1; 2.
2
MPEs
Table 5
Accuracy class
MPE of totalised load
0.2
± 0.10 %
0.5
± 0.25 %
1
± 0.50 %
2
± 1.00 %
Totalisation scale interval3
The totalisation scale interval (dt) shall be in the range: 0.01 % Max ≤ dt ≤ 0.2 % Max
Minimum Totalised Load (Σmin)4
The minimum totalised load (Σmin) shall be not less than the load at which the MPE is equal to the totalisation scale interval (dt) and not less than the minimum load as specified by the manufacturer.
Zero Setting5
Instruments that do not tare weigh after each discharge shall have a zero setting device. Automatic operation shall be inhibited if zero indication varies by:
— 1 dt on instruments with automatic zero setting device;
— 0.5 dt on instruments with a semi-automatic, or non-automatic, zero setting device
Operator Interface6
Operator adjustments and reset function shall be inhibited during automatic operation.
Printout7
On instruments equipped with a printing device, the reset of the total shall be inhibited until the total is printed. The printout of the total shall occur if automatic operation is interrupted.
Performance under influence factors and electromagnetic disturbances8
8
The MPEs due to influence factors shall be as specified in Table 6.
Table 6
Load (m) in totalisation scale intervals (dt)
MPE
0 < m ≤ 500
± 0.5 dt
500 < m ≤ 2,000
± 1.0 dt
2,000 < m ≤ 10,000
± 1.5 dt
8
The critical change value due to a disturbance is one totalisation scale interval for any weight indication and any stored total.
CHAPTER V
Accuracy classes1
Instruments are divided into three accuracy classes as follows: 0.5; 1; 2.
Measurement Range2
2
The manufacturer shall specify the measurement range, the ratio between the minimum net load on the weighing unit and the maximum capacity, and the minimum totalised load.
2
The minimum totalised load Σmin shall not be less than
800 d for class 0.5,
400 d for class 1,
200 d for class 2.
Where d is the totalisation scale interval of the general totalisation device.
MPE3
Table 7
Accuracy class
MPE for totalised load
0.5
± 0.25 %
1
± 0.5 %
2
± 1.0 %
Speed of the belt4
The speed of the belt shall be specified by the manufacturer. For single-speed beltweighers, and variable-speed beltweighers having a manual speed setting control, the speed shall not vary by more than 5 % of the nominal value. The product shall not have a different speed than the speed of the belt.
General Totalisation Device5
It shall not be possible to reset the general totalisation device to zero.
Performance under influence factors and electromagnetic disturbances6
6
The MPE due to influence factor, for a load not less than the Σmin, shall be 0.7 times the appropriate value specified in Table 7, rounded to the nearest totalisation scale interval (d).
6
The critical change value due to a disturbance shall be 0.7 times the appropriate value specified in Table 7, for a load equal to Σmin, for the designated class of the beltweigher; rounded up to the next higher totalisation scale interval (d).
CHAPTER VIAutomatic Rail Weighbridges
Accuracy classes1
Instruments are divided into four accuracy classes as follows: 0.2; 0.5; 1; 2.
MPE2
2
The MPEs for weighing-in-motion of a single wagon or a total train are shown in Table 8.
Table 8
Accuracy class
MPE
0.2
± 0.1 %
0.5
± 0.25 %
1
± 0.5 %
2
± 1.0 %
2
The MPEs for the weight of coupled or uncoupled wagons weighing-in-motion shall be one of the following values, whichever is the greatest:
— the value calculated according to Table 8, rounded to the nearest scale interval;
— the value calculated according to Table 8, rounded to the nearest scale interval for a weight equal to 35 % of the maximum wagon weight (as inscribed on the descriptive markings);
— one scale interval (d).
2
The MPEs for the weight of train weighing-in-motion shall be one of the following values, whichever is the greatest:
— the value calculated according to Table 9, rounded to the nearest scale interval;
— the value calculated according to Table 9, for the weight of a single wagon equal to 35 % of the maximum wagon weight (as inscribed on the descriptive markings) multiplied by the number of reference wagons (not exceeding 10) in the train, and rounded to the nearest scale interval;
— one scale interval (d) for each wagon in the train, but not exceeding 10 d.
2
When weighing coupled wagons; the errors of not more than 10 % of the weighing results taken from one or more passes of the train may exceed the appropriate MPE given in paragraph 2.2, but shall not exceed twice the MPE.
Scale interval (d)3
The relationship between the accuracy class and the scale interval shall be as specified in Table 9.
Table 9
Accuracy class
Scale interval (d)
0.2
d ≤ 50 kg
0.5
d ≤ 100 kg
1
d ≤ 200 kg
2
d ≤ 500 kg
Measurement range4
4
The minimum capacity shall not be less than 1 t, and not greater than the value of the result of the minimum wagon weight divided by the number of partial weighings.
4
The minimum wagon weight shall not be less than 50 d.
Performance under influence factor and electromagnetic disturbance5
5
The MPE due to an influence factor shall be as specified in Table 10.
Table 10
Load (m) in verification scale intervals (d)
MPE
0 < m ≤ 500
± 0.5 d
500 < m ≤ 2,000
± 1.0 d
2,000 < m ≤ 10,000
± 1.5 d
5
The critical change value due to a disturbance is one scale interval.
SCHEDULE 1HTAXIMETERS (MI-007) (Annex IX to the Directive)
The relevant requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule apply to taximeters.
DEFINITIONS
Appropriate Licensing Authority
Within this Schedule, “appropriate licensing authority” means –
a
in relation to the area to which the Metropolitan Public Carriage Act 1869 M87 applies, Transport for London;
b
in relation to any other area in England and Wales, the authority responsible for licensing taxis in that area;
c
in relation to Scotland, the district or islands council responsible for licensing taxis in that area;
d
and in relation to Northern Ireland, the Department of the Environment for Northern Ireland.
Taximeter
A device that works together with a signal generator M88 to make a regulated measuring instrument.
This device measures duration, calculates distance on the basis of a signal delivered by the distance signal generator. Additionally, it calculates and displays the fare to be paid for a trip on the basis of the calculated distance and/or the measured duration of the trip.
Fare
The total amount of money due for a trip based on a fixed initial hire fee and/or the length and/or the duration of the trip. The fare does not include a supplement charged for extra services.
Cross-over speed
The speed value found by division of a time tariff value by a distance tariff value.
Normal calculation mode S (single application of tariff)
Fare calculation based on application of the time tariff below the cross-over speed and application of the distance tariff above the cross-over speed.
Normal calculation mode D (double application of tariff)
Fare calculation based on simultaneous application of time tariff and distance tariff over the whole trip.
Operating position
The different modes in which a taximeter fulfils the different parts of its functioning. The operating positions are distinguished by the following indications:
‘For Hire’
:
The operating position in which the fare calculation is disabled
‘Hired’
:
The operating position in which the fare calculation takes place on the basis of a possible initial charge and a tariff for distance travelled and/or time of the trip
‘Stopped’
:
The operating position in which the fare due for the trip is indicated and at least the fare calculation based on time is disabled.
DESIGN REQUIREMENTS
1
The taximeter shall be designed to calculate the distance and to measure the duration of a trip.
2
The taximeter shall be designed to calculate and display the fare, incrementing in steps equal to the resolution fixed by the appropriate licensing authority in the operation position ‘Hired’. The taximeter shall also be designed to display the final value for the trip in the operating position ‘Stopped’.
3
A taximeter shall be able to apply the normal calculation modes S and D. It shall be possible to choose between these calculation modes by a secured setting.
4
A taximeter shall be able to supply the following data through an appropriate secured interface(s):
— operation position: ‘For Hire’, ‘Hired’ or ‘Stopped’;
— totaliser data according to paragraph 15.1;
— general information: constant of the distance signal generator, date of securing, taxi identifier, real time, identification of the tariff;
— fare information for a trip: total charged, fare, calculation of the fare, supplement charge, date, start time, finish time, distance travelled;
— tariff(s) information: parameters of tariff(s).
Where a device is required to be connected to the interface(s) of a taximeter, it shall be possible, by way of a secured setting, to inhibit automatically the operation of the taximeter for reasons of the non-presence or improper functioning of the required device.
5
If relevant, it shall be possible to adjust a taximeter for the constant of the distance signal generator to which it is to be connected and to secure the adjustment.
RATED OPERATING CONDITIONS
6
The mechanical environment class that applies is M3.
6
The manufacturer shall specify the rated operating conditions for the instrument, in particular:
— a minimum temperature range of 80 °C for the climatic environment;
— the limits of the DC power supply for which the instrument has been designed.
MAXIMUM PERMISSIBLE ERRORS (MPEs)
7
The MPE, excluding any errors due to application of the taximeter in a taxi, are:
— For the time elapsed: ± 0.1 %
minimum value of mpe: 0.2 s;
minimum value of mpe: 4 m;
minimum, including rounding: corresponding to the least significant digit of the fare indication.
PERMISSIBLE EFFECT OF DISTURBANCES
8
Electromagnetic immunity
8
The electromagnetic class that applies is E3.
8
The MPE laid down in paragraph 7 shall also be respected in the presence of an electromagnetic disturbance. POWER SUPPLY FAILURE
9
In case of a reduction of the voltage supply to a value below the lower operating limit as specified by the manufacturer, the taximeter shall:
— continue to work correctly or resume its correct functioning without loss of data available before the voltage drop if the voltage drop is temporary, i.e. due to restarting the engine;
— abort an existing measurement and return to the position ‘For Hire’ if the voltage drop is for a longer period.
OTHER REQUIREMENTS
10
The conditions for the compatibility between the taximeter and the distance signal generator shall be specified by the manufacturer of the taximeter.
11
If there is a supplement charge for an extra service, entered by the driver on manual command, this shall be excluded from the fare displayed. However, in that case a taximeter may display temporarily the value of the fare including the supplementary charge.
12
If the fare is calculated according to calculation mode D a taximeter may have an additional display mode in which only the total distance and duration of the trip are displayed in real time.
13
All values displayed for the passenger shall be suitably identified. These values as well as their identification shall be clearly readable under daylight and night conditions.
14
If the fare to be paid or the measures to be taken against fraudulent use can be affected by the choice of functionality from a pre-programmed setting or by free data setting, it shall be possible to secure the instrument settings and data entered.
14
The securing possibilities available in a taximeter shall be such that separate securing of the settings is possible.
14
The provisions in paragraph 8.3 of Schedule 1A apply also to the tariffs.
15
A taximeter shall be fitted with non-resettable totalisers for all of the following values:
— The total distance travelled by the taxi;
— The total distance travelled when hired;
— The total number of hirings;
— The total amount of money charged as supplements;
— The total amount of money charged as fare.
The totalised values shall include the values saved according to paragraph 9 under conditions of loss of power supply.
15
If disconnected from power, a taximeter shall allow the totalised values to be stored for one year for the purpose of reading out the values from the taximeter to another medium.
15
Adequate measures shall be taken to prevent the display of totalised values from being used to deceive passengers.
16
Automatic change of tariffs is allowed due to the:
— distance of the trip;
— duration of the trip;
— time of the day;
— date;
— day of the week.
17
If properties of the taxi are important for the correctness of the taximeter, the taximeter shall provide means to secure the connection of the taximeter to the taxi in which it is installed.
18
For the purpose of testing after installation, the taximeter shall be equipped with the possibility to test separately the accuracy of time and distance measurement and the accuracy of the calculation.
19
A taximeter and its installation instructions specified by the manufacturer shall be such that, if installed according to the manufacturer's instructions, fraudulent alterations of the measurement signal representing the distance travelled are sufficiently excluded.
20
The general essential requirement dealing with fraudulent use shall be fulfilled in such a way that the interests of the customer, the driver, the driver's employer and the fiscal authorities are protected.
21
A taximeter shall be designed so that it can respect the MPEs without adjustment during a period of one year of normal use.
22
The taximeter shall be equipped with a real-time clock by means of which the time of the day and the date are kept, one or both can be used for automatic change of tariffs. The requirements for the real-time clock are:
— the timekeeping shall have an accuracy of 0.02 %;
— the correction possibility of the clock shall be not more than 2 minutes per week. Correction for summer and wintertime shall be performed automatically;
— correction, automatic or manually, during a trip shall be prevented.
23
The values of distance travelled and time elapsed, when displayed or printed in accordance with these Regulations, shall use the following units:
Distance travelled:
— kilometres;
— miles.
Time elapsed:
— seconds, minutes or hours, as may be suitable; keeping in mind the necessary resolution and the need to prevent misunderstandings.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to taximeters that the manufacturer can choose between are:
a
B and F;
b
B and D; or
c
H1.
SCHEDULE 1IMATERIAL MEASURES (MI-008) (Annex X to the Directive)
CHAPTER 1Material measures of length
The relevant essential requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this chapter, apply to material measures of length defined below. However, the requirement for the supply of a copy of declarations of conformity may be interpreted as applying to a batch or consignment rather than each individual instrument.
SPECIFIC REQUIREMENTS
Reference Conditions1
For tapes of length equal to or greater than 5 metres, the maximum permissible errors (MPEs) are to be met when a tractive force of fifty newtons or other force values as specified by the manufacturer and marked on the tape accordingly, or in the case of rigid or semi-rigid measures no tractive force is needed, is applied.
1
The reference temperature is 20 °C unless otherwise specified by the manufacturer and marked on the measure accordingly.
MPEs2
The MPE, positive or negative in mm, between two non-consecutive scale marks is (a + bL), where:
— L is the value of the length rounded up to the next whole metre; and
— a and b are given in Table 1 below.
When a terminal interval is bounded by a surface, the MPE for any distance beginning at this point is increased by the value c given in Table 1.
Table 1
Accuracy Class
a(mm)
b
c(mm)
I
0.1
0.1
0.1
II
0.3
0.2
0.2
III
0.6
0.4
0.3
The MPE for the length between consecutive scale marks, and the maximum permissible difference between two consecutive intervals, are given in Table 2 below.
Table 2
Length i of the interval
MPE or difference in millimetres according to accuracy class
I
II
III
i ≤ 1 mm
0.1
0.2
0.3
1 mm < i ≤ 1 cm
0.2
0.4
0.6
Where a rule is of the folding type, the jointing shall be such as not to cause any errors, supplementary to those above, exceeding: 0.3 mm for Class II, and 0.5 mm for Class III.
Materials3
Materials used for material measures shall be such that length variations due to temperature excursions up to ± 8 °C about the reference temperature do not exceed the MPE.
3
Measures made from material whose dimensions may alter materially when subjected to a wide range of relative humidity, may only be included in Classes II or III.
Markings4
The nominal value shall be marked on the measure. Millimetre scales shall be numbered every centimetre and measures with a scale interval greater than 2 cm shall have all scale marks numbered.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to material measures of length that the manufacturer can choose between are:
a
F1;
b
D1;
c
B and D;
d
H; or
e
G.
CHAPTER IICapacity serving measures
The relevant essential requirements of Schedule 1A, and the specific requirements and the conformity assessment procedures listed in this chapter, apply to capacity serving measures defined below. However, the requirement for the supply of a copy of declarations of conformity may be interpreted as applying to a batch or consignment rather than each individual instrument. Also, the requirement for the instrument to bear information in respect of its accuracy shall not apply.
DEFINITIONS
Line measure
A capacity serving measure marked with a line to indicate nominal capacity.
Brim measure
A capacity serving measure for which the internal volume is equal to the nominal capacity.
Transfer measure
A capacity serving measure from which it is intended that the liquid is decanted prior to consumption.
Capacity
The capacity is the internal volume for brim measures or internal volume to a filling mark for line measures.
SPECIFIC REQUIREMENTS
Reference Conditions1
1
Temperature: the reference temperature for measurement of capacity is 20 °C.
1
Position for correct indication: free standing on a level surface.
MPEs2
Table 1
Line
Brim
Transfer measures
Transfer measures
< 100 ml
± 2 ml
– 0
+ 4 ml
≥ 100 ml
± 3 %
– 0
+ 6 %
Serving measures
< 200 ml
± 5 %
– 0
+ 10 %
≥ 200 ml
± (5 ml + 2.5 %)
– 0
+ 10 ml + 5 %
Materials3
Capacity serving measures shall be made of material which is sufficiently rigid and dimensionally stable to maintain capacity within the MPE.
Shape4
4
Transfer measures shall be designed so that a change of contents equal to the MPE causes a change in level of at least 2 mm at the brim or filling mark.
4
Transfer measures shall be designed so that the complete discharge of the liquid being measured will not be impeded.
Marking5
5
The nominal capacity declared shall be clearly and indelibly marked on the measure.
5
Capacity serving measures may also be marked with up to three clearly distinguishable capacities, none of which shall lead to confusion one to the other.
5
All filling marks shall be sufficiently clear and durable to ensure that MPEs are not exceeded in use.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to capacity serving measures that the manufacturer can choose between are:
a
A2;
b
F1;
c
D1;
d
E1;
e
B and E;
f
B and D; or
g
H.
SCHEDULE 1JEXHAUST GAS ANALYSERS (MI-010) (Annex XII to the Directive)
The relevant requirements of Schedule 1A, the specific requirements of this Schedule and the conformity assessment procedures listed in this Schedule, apply to exhaust gas analysers to the extent that they are also regulated measuring instruments.
The volume fractions of the exhaust gas components are expressed as a percentage (% vol) for carbon monoxide (CO), carbon dioxide (CO2) and oxygen (O2) and in parts per million (ppm vol) for hydrocarbons (HC).
The content of HC has to be expressed as concentration of n-hexane (C6H14), measured with near-infrared absorption techniques.
DEFINITIONS
Lambda
Lambda is a dimensionless value representative of the burning efficiency of an engine in terms of air/fuel ratio in the exhaust gases.
SPECIFIC REQUIREMENTS
Instrument Classes1
Two classes (0 and I) are being defined for exhaust gas analysers. The relevant minimum measuring ranges for these classes are shown in Table 1.
Table 1
Classes and measuring ranges
Parameter
Classes 0 and I
CO fraction
from 0 to 5 % vol
CO2 fraction
from 0 to 16 % vol
HC fraction
from 0 to 2,000 ppm vol
O2 fraction
from 0 to 21 % vol
λ
from 0.8 to 1.2
Rated operating conditions2
The values of the operating conditions shall be specified by the manufacturer as follows:
2
For the climatic and mechanical influence quantities:
— a minimum temperature range of 35 °C for the climatic environment;
— the mechanical environment class that applies is M1.
2
For the electrical power influence quantities:
— the voltage and frequency range for the AC voltage supply
— the limits of the DC voltage supply.
2
For the ambient pressure:
— the minimum and the maximum values of the ambient pressure are for both classes: pmin ≤ 860 hPa, pmax ≥ 1,060 hPa.
Maximum permissible errors (MPEs)3
The MPEs are defined as follows:
3
For each of the fractions measured, the maximum error value permitted under rated operating conditions according to paragraph 1.1 of Schedule 1A is the greater of the two values shown in Table 2. Absolute values are expressed in % vol or ppm vol, percentage values are percent of the true value.
Table 2
Parameter
Class 0
Class I
MPEs
CO fraction
± 0.03 % vol
± 5 %
± 0.06 % vol
± 5 %
CO2 fraction
± 0.5 % vol
± 0.5 % vol
±5 %
± 5 %
HC fraction
± 10 ppm vol
± 12 ppm vol
± 5 %
± 5 %
O2 fraction
± 0.1 % vol
± 0.1 % vol
± 5 %
± 5 %
3
The MPE on lambda calculation is 0.3 %. The conventional true value is calculated according to the formula set out in point 5.3.7.3 of Regulation No 83 of the Economic Commission for Europe of the United Nations (UN/ECE).
For this purpose, the values displayed by the instrument are used for calculation.
Permissible effect of disturbances4
For each of the volume fractions measured by the instrument, the critical change value is equal to the MPE for the parameter concerned.
5
The effect of an electromagnetic disturbance shall be such that:
— either the change in the measurement result is not greater than the critical change value laid down in paragraph 4; or
— the presentation of the measurement result is such that it cannot be taken for a valid result.
Other requirements6
The resolution shall be equal to or of one order of magnitude higher than the values shown in Table 3.
Table 3
Resolution
CO
CO2
O2
HC
Class O and class I
0.01 % vol
0.1 % vol
0.01 % vol for measurand values below or equal to 4 % vol, otherwise 0.1 % vol.
1 ppm vol
The lambda value shall be displayed with a resolution of 0.001.
The standard deviation of 20 measurements shall not be greater than one third of the modulus of the MPE for each applicable gas volume fraction.
8
For measuring CO, CO2 and HC, the instrument, including the specified gas handling system, must indicate 95 % of the final value as determined with calibration gases within 15 seconds after changing from a gas with zero content, e.g. fresh air. For measuring O2, the instrument under similar conditions must indicate a value differing less than 0.1 % vol from zero within 60 seconds after changing from fresh air to an oxygen-free gas.
9
The components in the exhaust gas, other than the components whose values are subject to the measurement, shall not affect the measurement results by more than the half of the modulus of the MPEs when those components are present in the following maximum volume fractions:
6 % vol CO,
16 % vol CO2,
10 % vol O2,
5 % vol H2,
0.3 % vol NO,
2,000 ppm vol HC (as n-hexane),
water vapour up to saturation.
10
An exhaust gas analyser shall have an adjustment facility that provides operations for zero-setting, gas calibration and internal adjustment. The adjustment facility for zero-setting and internal adjustment shall be automatic.
11
For automatic or semi-automatic adjustment facilities, the instrument shall be unable to make a measurement as long as the adjustments have not been made.
12
An exhaust gas analyser shall detect hydrocarbon residues in the gas handling system. It shall not be possible to carry out a measurement if the hydrocarbon residues, present before any measurement, exceed 20 ppm vol.
13
An exhaust gas analyser shall have a device for automatically recognising any malfunctioning of the sensor of the oxygen channel due to wear or a break in the connecting line.
14
If the exhaust gas analyser is capable to operate with different fuels (e.g. petrol or liquefied gas), there shall be the possibility to select the suitable coefficients for the Lambda calculation without ambiguity concerning the appropriate formula.
CONFORMITY ASSESSMENT The conformity assessment procedures specified in the modules in Schedule 1B applicable to exhaust gas analysers that the manufacturer can choose between are:
a
B and F;
b
B and D; o
c
H1.
SCHEDULE 1KDeclaration of Conformity
Declaration of Conformity (No. XXXX) M891
Instrument model/instrument (product, type, batch or serial number):
2
Name and address of the manufacturer and, where applicable, his authorised representative:
3
This declaration of conformity is issued under the sole responsibility of the manufacturer.
4
Object of the declaration (identification of the instrument allowing traceability; it may, where necessary for the identification of the instrument, include an image):
5
The object of the declaration described above is in conformity with the relevant statutory requirements:
6
References to the relevant designated standards or normative documents used or references to the other technical specifications in relation to which conformity is declared:
7
The approved body (name, number) performed … (description of intervention) and issued the certificate:
8
Additional information:
Signed for and on behalf of: (place and date of issue): (name, function) (signature):
Amendment to Schedule 3I51850
In Schedule 3 (revocations and transitional and consequential provisions)—
a
after paragraph 2, insert—
Transitional provisions relating to UK withdrawal from the EU2A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F324IP completion day;
2
Subject to paragraph (3), where a regulated measuring instrument was made available on the market during the pre-exit period, despite the amendments made by Schedule 27 of the Product Safety and Metrology (Amendment etc.) (EU Exit) Regulations 2019 M90, any obligation to which a person was subject under these Regulations as they had effect immediately before F324IP completion day, continues to have effect as it did immediately before F324IP completion day, in relation to that regulated measuring instrument.
3
Paragraph (2) does not apply to—
a
any obligation of any competent authority to inform the European Commission or Member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that regulated measuring instrument.
4
Where during the pre-exit period—
a
a regulated measuring instrument has not been placed on the market; and
b
a manufacturer has taken any action under regulation 39 as it had effect immediately before F324IP completion day in relation to that regulated measuring instrument,
that action has effect as if it had been done under regulation 39 as it had effect on and after F324IP completion day.
b
in paragraph 4—
i
in sub-paragraphs (4)(a), (4)(b), (6)(c)(i) and (6)(c)(ii), for “Annex IV to the Directive”, substitute “
Schedule 1D to the Measuring Instruments Regulations 2016 M91
”
in each place it occurs;
ii
in sub-paragraphs (4)(b), (6)(c)(i) and (6)(c)(ii), for “Annex IV”, substitute “
Schedule 1D to the Measuring Instruments Regulations 2016
”
;
c
in paragraph 5—
i
in sub-paragraphs (4)(a) and (4)(b) for “Annex IV to the Directive”, substitute “
Schedule 1D to the Measuring Instruments Regulations 2016
”
in each place it occurs;
ii
in sub-paragraphs (4)(a) and (4)(b), for “Annex IV”, substitute “
Schedule 1D to the Measuring Instruments Regulations 2016
”
in each place it occurs;
d
in paragraphs 6 and 7, for “Annex V to the Directive”, substitute “
Schedule 1E of the Measuring Instruments Regulations 2016
”
in each place it occurs.
Amendment to Schedule 4I56651
In Schedule 4 (operational obligations of notified bodies)—
a
in paragraphs 3, 4 and 6 for “a notified” substitute “
an approved
”
;
b
in all places in which it occurs (other than the paragraphs referred to in paragraph 51(a)) including in the heading, for “notified” substitute “
approved
”
;
c
in paragraph 7—
i
for “notifying authority” substitute “
Secretary of State
”
; and
ii
in subparagraphs (b) and (d) for “notification” substitute “
approval
”
in both places in which it occurs;
d
in paragraph 8—
i
after “bodies”, the second time it occurs, insert “
approved
”
;
ii
for “this Directive” substitute “
these Regulations
”
; and
e
in paragraph 9 omit from “convened” to “Directive”.
Amendment to Schedule 5I56752
In Schedule 5 (requirements related to notified bodies)—
a
in paragraph 1 for “under the national law of an EEA state” substitute “
in the United Kingdom
”
;
b
in paragraph 5(1) for “1” substitute “
1B
”
;
c
in paragraph 6(c)(ii) for “harmonised” substitute “
designated
”
;
d
in paragraph 6(c)(iii)—
i
omit “of Union harmonisation legislation and”;
ii
for “national” substitute “
applicable
”
;
e
in paragraph 9(1) omit from “except” to “carried out”;
f
in paragraph 10 for “under the relevant Union harmonisation legislation” substitute “
by the Secretary of State
”
; and
g
in paragraph 5(2)(c) for “a notified” substitute “
an approved
”
;
h
in all places in which it occurs, including in the heading, for “notified” substitute “
approved
”
.
Amendment to Schedule 6I56853
In Schedule 6 (in service requirements for certain regulated measuring instruments in Great Britain), Part 5, paragraph 14 for “the Directive” substitute “
Schedule 1G
”
.
SCHEDULE 28Amendment of the Recreational Craft Regulations 2017 and related amendment
PART 1Amendment to the Recreational Craft Regulations 2017
IntroductionI9091
The Recreational Craft Regulations 2017 are amended in accordance with paragraphs 2 to 54.
Amendment to regulation 2I5692
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “adaptor” insert—
“approved body” has the meaning given to it in regulation 55 (approved bodies);
F303d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
in the definition of “components” omit “EU”;
h
after the definition of “conformity assessment body” insert—
“declaration of conformity” means the declaration required to be drawn up in accordance with regulation 10;
“designated standard” has the meaning given to it in regulation 2A;
i
omit the definition of “Decision 768/2008”;
j
in the definition of the “Directive” at the end insert “
(as it had effect immediately before F304IP completion day)
”
;
k
omit the definition of “EU declaration of conformity”;
l
omit the definition of “harmonised standard”;
m
in the definition of “hull length” for “harmonised” substitute “
designated
”
;
n
for the definition of “importer” substitute—
F305“importer” means a person who—
a
is established in the United Kingdom and places a product from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places a product on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F306o
in the definition of “making available on the market” for “EU market” substitute “market of Great Britain”;
p
omit the definition of “national accreditation body”;
q
omit the definition of “notified body requirements”;
F307r
in the definition of “placing on the market” for “EU market” substitute “market of Great Britain”;
s
for the definition of “private importer” substitute—
“private importer” means a person who—
a
is established in the United Kingdom; and
b
imports in the course of a non-commercial activity a watercraft from a country outside of the United Kingdom into the United Kingdom with the intention of putting it into service for the person's own use;
F308t
in the definition of “putting into service” for “EU market” substitute “market of Great Britain”;
u
after the definition of “technical documentation” insert—
“technical specification” means a document that prescribes technical requirements to be fulfilled by a product;
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
Omit paragraphs (4) and (5).
Insertion of regulation 2AI5713
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations, a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation, a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
9
Regulations made under paragraph (8) are to be made by statutory instrument.
10
A statutory instrument containing regulations made under paragraph (8) is subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 4I572F3254
In regulation 4(1)(g) omit “EU”.
Amendment to regulation 7I5735
In regulation 7 (making available and putting into service)—
a
omit paragraph (1)(c)(ii);
b
in paragraph (2)—
i
in sub-paragraph (a) omit “either Directive 97/68/EC or”;
ii
in sub-paragraph (b) omit “either the Directive or”.
Substitution of regulation 10I5746
For regulation 10 (EU declaration of conformity and CE marking) substitute—
Declaration of conformity and UK marking10
1
Where the conformity of a product with the essential requirements has been demonstrated by a relevant conformity assessment procedure, the manufacturer must, before placing the product on the market—
a
draw up a declaration of conformity in accordance with regulation 53; and
b
affix the UK marking F326... in accordance with regulation 54.
2
The declaration of conformity must follow the format set out in Schedule 4.
3
But where a declaration of conformity relates to a partly-completed watercraft, the declaration must follow the format set out in Schedule 3.
4
The manufacturer must keep the declaration of conformity up to date.
5
Where a product is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 11I5757
In regulation 11 (duty of manufacturers to retain technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 12I5768
In regulation 12 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Amendment to regulation 15I5779
In regulation 15 (instructions and safety information)—
a
in paragraph (1) for “a language which can be easily understood by consumers and other end-users in the Member State in which the product is to be made available” substitute “
English
”
;
b
omit paragraph (2).
Amendment to regulation 16I57810
In regulation 16 (duty to take action in respect of product placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities in any Member State in which the manufacturer has made the product available on the market”.
Amendment to regulation 19I57911
In regulation 19 (requirements that must be satisfied before an importer places a product on the market)—
a
in paragraph (1)—
i
in sub-paragraph (a) after “assessment” insert “
procedure
”
;
ii
in sub-paragraph (c)(i) for “CE” substitute “
UK
”
;
b
in paragraph (2)(a) omit “EU”.
Amendment to regulation 21I58012
In regulation 21, for paragraph (2) substitute—
2
Paragraph (1) does not apply where—
a
either—
i
in the case of a component, it is not possible to indicate the information specified in paragraph (1) on the component, or
b
before placing the product on the market, the importer indicates the information specified in paragraph (1)—
i
in the case of component, in a document accompanying the product or on the packaging; or
ii
in all other cases, in a document accompanying the product.
Amendment to regulation 22I58113
In regulation 22 (instructions and safety information)—
a
in paragraph (1) for “a language which can be easily understood by consumers and other end-users in the Member State in which the product is to be made available” substitute “
English
”
;
b
omit paragraph (2).
Amendment to regulation 24I58214
In regulation 24 (duty to take action in respect of product placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other Member State in which the importer has made the product available on the market”.
Amendment to regulation 25I58315
In regulation 25 (duty of importers to retain technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 28I58416
In regulation 28 (making available on the market), in paragraph (1)(a)—
a
in paragraph (i) for “CE” substitute “
UK
”
;
b
in paragraph (iii) for “a language that can be easily understood by consumers and other end-users in the Member State in which the product is to be made available on the market” substitute “
English
”
.
Amendment to regulation 29I58517
In regulation 29 (duty not to make available a product on the market where a distributor suspects that it is not in conformity), in paragraph (2) omit “and the competent national authorities of other Member States in which the distributor has made the product available on the market”.
Amendment to regulation 31I58618
In regulation 31 (duty to take action in respect of watercraft placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other Member State in which the distributor has made the product available on the market”.
Omission of regulation 35I58719
Omit regulation 35 (translation of EU declaration of conformity).
Amendment to regulation 36I58820
In regulation 36 (private importers)—
a
in paragraph (1)(b)—
i
in paragraph (ii) for “(EU declaration of conformity and CE marking)” substitute “
(declaration of conformity and UK marking)
”
;
ii
in paragraph (iii) omit “EU”;
b
in paragraph (4) for “notified” substitute “
approved
”
.
Amendment to regulation 39I58921
In regulation 39 (authorised representatives)—
a
in paragraph (1) for “EU” substitute “
United Kingdom
”
;
b
in paragraph (3)(a)—
i
in paragraph (i) omit “EU”;
ii
omit “and competent national authorities”;
c
in paragraph (3)(c) for “competent national authorities” substitute “
enforcing authority
”
.
Amendment to regulation 40I59022
In regulation 40 (prohibition on improper use of CE marking) in each place in which it occurs, and in the heading to that regulation, for “CE” substitute “
UK
”
.
Insertion of regulations 40A and 40BI59123
After regulation 40 insert—
Obligations that are met by complying with obligations in the Directive40A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article of or Annex to the Directive;
b
“CE marking” has the meaning given in Article 3(28);
c
“harmonised standard” has the meaning given in Article 3(20).
2
For the purposes of this regulation, references to the requirements set out in Article 4(1) and Annex I are to be read as if they include a requirement that the owner's manuals referred to in point 2.5 of Part A of Annex I and point 4 of Part B of that Annex must be in English (instead of in a language or languages which can be easily understood by consumers and other end-users, as determined by the member State concerned).
3
Where a product meets the requirements set out in Article 4(1) and Annex I—
a
the requirements of regulation 6(a) and (b) are to be treated as being satisfied;
b
regulation 2(2)(a) applies subject to the modification set out in paragraph (15)(c).
4
Subject to paragraphs (8) and (9), paragraph (5) applies where, before placing a product on the market, the manufacturer—
a
ensures that the product has been designed and manufactured in accordance with the requirements set out in Article 4(1) and Annex I;
b
draws up the technical documentation in accordance with Article 25;
c
carries out the conformity assessment procedure applicable to the product in accordance with Articles 19 to 22 and 24 or has it carried out;
d
ensures that the technical documentation and any other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking to the product in accordance with Articles 16 to 18;
f
draws up an EU declaration of conformity in accordance with Article 15; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
5
Where this paragraph applies—
a
the requirements of regulations 8, 9 and 10(1), (2), (3) and (5) are to be treated as being satisfied;
b
regulations 10(4), 11, 12(2), 39(3) and 40 apply subject to the modifications set out in paragraph (15);
c
Part 3 (except for regulations 43(2) and (3) and 48) does not apply;
d
regulation 71 does not apply.
6
Subject to paragraphs (8) and (9), paragraph (7) applies where, before placing a product on the market, the importer ensures that—
a
the conformity assessment procedure applicable to the product in accordance with Articles 19 to 22 and 24 has been carried out;
b
the manufacturer has drawn up the technical documentation in accordance with Article 25; and
c
the product bears the CE marking in accordance with Articles 16 to 18.
7
Where this paragraph applies—
a
the requirements of regulation 19(1)(a), (b) and (c)(i) are to be treated as being satisfied;
b
regulations 18, 19(2), 20, 23 and 25 apply subject to the modifications set out in paragraph (15).
8
This paragraph applies where there is no designated standard or part of a designated standard that corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 14.
9
Where paragraph (8) applies, paragraphs (4)(c) and (6)(a) of this regulation are to be read as requiring—
a
in respect of products referred to in Article 20(1)(b)(i), one of the conformity assessment procedures (combination of procedures) referred to in the second indent of Article 20(1)(b)(i);
b
in respect of exhaust emissions for products referred to in points (d) and (e) of Article 2(1), one of the conformity assessment procedures (or combinations of procedures) referred to in Article 21(b);
c
in respect of noise emissions for products referred to in Article 22(1)—
i
the conformity assessment procedure referred to in Article 22(1)(b); or
ii
where applicable in accordance with Article 22(1)(c), one of the conformity assessment procedures referred to in Article 22(1)(c);
d
in respect of noise emissions for products referred to in Article 22(2), the conformity assessment procedure referred to in Article 22(2)(b).
10
Paragraph (11) applies where, before making a product available on the market, the distributor ensures that the product bears the CE marking in accordance with Articles 16 to 18.
11
Where this paragraph applies—
a
the requirement of regulation 28(1)(a)(i) is to be treated as being satisfied;
b
regulations 19(2) (which contains the definition of “required documents” for the purposes of regulation 28), 29 and 30 apply subject to the modifications set out in paragraph (15).
12
Paragraph (13) applies where the private importer—
a
ensures before putting a product into service that the product has been designed and manufactured in accordance with the requirements set out in Article 4(1) and Annex I; and
b
ensures that the name and postal address of the notified body that carried out the conformity assessment procedure applicable to the product in accordance with Articles 19 to 22 and 24 is marked on the product.
13
Where this paragraph applies, the requirements of regulation 36(1)(a) and 36(4) are to be treated as being satisfied.
14
Where, before placing a product on the market or putting a product into service, a person applies the procedure referred to in Article 23 to the product, the requirements of regulation 43 are to be treated as being satisfied.
15
The modifications referred to in paragraphs (3)(b), (5)(b), (7)(b) and (11)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “designated standard” is to be read as a reference to a harmonised standard;
c
any reference to “essential requirements” is to be read as a reference to the requirements set out in Article 4(1) and Annex I (as modified by paragraph (2));
d
any reference to “UK marking” is to be read as a reference to the CE marking;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the conformity assessment procedures that apply to the product in accordance with Articles 19 to 22 and 24;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Article 25.
Conformity assessment procedure obligation which is met by complying with the Directive40B
1
In this regulation any reference to an Article or an Annex is a reference to an Article of or Annex to the Directive.
2
Paragraph (3) applies where—
a
Articles 20 or 21 provide that the conformity assessment procedure referred to as Module B in those Articles may be carried out in relation to a product; and
b
prior to the manufacture of a product, the manufacturer ensures that—
i
the product has been designed in accordance with the essential requirements set out in Annex I to the Directive;
ii
the conformity assessment procedure referred to as Module B in Articles 20 and 21 has been carried out in relation to that product, in accordance with those Articles and with Article 24(1).
3
Where this paragraph applies—
a
the requirement in regulation 42 to apply the conformity assessment procedure referred to in regulations 44 and 45 as Module B is to be treated as being satisfied in relation to that product;
b
any reference to “relevant conformity assessment procedure” in regulations 9, 10(1), 19(1)(a), 36(4), 40(1)(b) and 53(b) is to be read as including the conformity assessment procedure referred to in Articles 20, 21 and 24 as Module B; and
c
any reference to “technical documentation” in regulations 9(b), 11, 19(1)(b), 25(b) and 36(3) is to be read as including the technical documentation relating to the design of the product referred to in Article 25 of the Directive;
d
the reference to “approved body” in regulation 36(4) is to be read as the body that undertook the conformity assessment procedure referred to as Module B in Articles 20 or 21.
F329Expiry of regulations 40A and 40B40C
1
Subject to paragraph (2), regulation 40A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 40A—
a
any product which was placed on the market pursuant to regulation 40A may continue to be made available on the market on or after the expiry of regulation 40A;
b
any obligation to which a person was subject under regulation 40A in respect of a product placed on the market pursuant to regulation 40A continues to have effect after the expiry of regulation 40A, in respect of that product.
3
Subject to paragraph (4), regulation 40B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 40B in relation to a product prior to the expiry of regulation 40B, regulation 40B continues to apply in respect of that product where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after the conformity assessment procedure referred to as Module B in Articles 20 and 21 of the Directive has been carried out in relation to that product, in accordance with Article 24(1) of the Directive.
Qualifying Northern Ireland Goods40D
1
Where paragraph (2) applies a product is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
a product—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the product on the market, the importer—
a
complies with regulation 21;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the product;
ii
the manufacturer has drawn up the technical documentation; and
iii
the product bears the CE marking.
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 41I59224
In regulation 41 (presumption of conformity), in paragraph (1)—
a
for “harmonised” substitute “
designated
”
;
b
omit “the reference to which has been published in the Official Journal of the European Union”.
Amendment to regulation 42I59325
In regulation 42 (applicable conformity assessment procedures), for “Annex II of Decision 768/2008” substitute “
Schedule 15
”
.
Amendment to regulation 44I59426
1
Regulation 44 (design and construction) is amended as follows.
2
In paragraph (1)—
a
for “Annex II to Decision 768/2008/EC” substitute “
Schedule 15
”
;
b
in sub-paragraph (a) for “(EU-type examination)” in both places in which it occurs substitute “
(type examination)
”
;
c
in sub-paragraph (b)—
i
for “EU type-examination” in each place in which it occurs substitute “
type examination
”
;
ii
for “harmonised” in both places in which it occurs substitute “
designated
”
;
d
in sub-paragraph (c)(iii) for “EU- type examination” substitute “
type examination
”
.
3
In paragraphs (2) and (3) for “Annex II to Decision 768/2008/EC” substitute “
Schedule 15
”
.
4
In paragraph (2)(c) for “EU type-examination” substitute “
type examination
”
.
5
In paragraph (3)(a) for “(EU type-examination)” substitute “
(type examination)
”
.
Amendment to regulation 45I59527
Regulation 45 (exhaust emissions) is amended as follows—
a
for “Annex II to Decision 768/2008/EC” substitute “
Schedule 15
”
;
b
in paragraphs (a) and (b) for “harmonised” substitute “
designated
”
;
c
in paragraph (a)(i) for “(EU-type examination)” substitute “
(type examination)
”
;
d
in paragraph (b)(i) for “(the EU-type examination)” substitute “
(type examination)
”
.
Amendment to regulation 46I59628
In regulation 46 (noise emissions: recreational craft)—
a
in paragraph (1) for “Annex II to Decision 768/2008/EC” substitute “
Schedule 15
”
;
b
in paragraphs (2) and (3) for “harmonised” substitute “
designated
”
.
Amendment to regulation 47I59729
In regulation 47 (noise emissions: personal watercraft)—
a
in paragraph (1) for “Annex II to Decision 768/2008/EC” substitute “
Schedule 15
”
;
b
in paragraphs (2) and (3) for “harmonised” substitute “
designated
”
.
Omission of regulation 49I59830
Omit regulation 49 (conformity assessments carried out under Module B (EU-type examination)).
Amendment to regulation 50I59931
In regulation 50 (conformity assessments carried out under Module A1 (internal production control plus supervised product testing))—
a
in paragraph (1) for “of Annex II to Decision 768/2008/EC” substitute “
as set out in Schedule 15
”
;
b
omit paragraph (2).
Amendment to regulation 51I60032
In regulation 51 (conformity assessments carried out under Module F (conformity to type based on product verification)), for “of Annex II to Decision 768/2008/EC” substitute “
as set out in Schedule 15
”
.
Amendment to regulation 52I60133
In regulation 52 (conformity assessments carried out under Module C (conformity to type based on internal production control))—
a
in paragraph (1)—
i
in sub-paragraph (a) for “of Annex II of Decision 768/2008/EC” substitute “
set out in Schedule 15
”
;
ii
in sub-paragraph (c) for “of Annex II to Decision 768/2008/EC” substitute “
set out in Schedule 15
”
;
b
in paragraph (2)—
i
for “A notified” substitute “
An approved
”
;
ii
for “the notified” substitute “
the approved
”
.
Amendment to regulation 53I60234
In regulation 53 (EU declaration of conformity)—
a
in the heading, for “EU declaration” substitute “
Declaration
”
;
b
omit “EU”;
c
in paragraph (b) after “assessment” insert “
procedure
”
.
Amendment to regulation 54I60335
In regulation 54 (CE marking)—
a
for the heading substitute “
UK marking
”
;
F332b
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly—
a
to the product; or
b
where paragraph (1A) applies, to—
i
a label affixed to the product; or
ii
a document accompanying the product.
F330ba
in paragraph (4) for “notified” substitute “
approved
”
;
F331ba
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the product; or
b
a document accompanying the product.
bb
in paragraph (2)—
i
after “Where” insert “
paragraph (1A) does not apply and
”
;
ii
for “CE” substitute “
UK
”
(twice);
iii
for “paragraph (1)” substitute “
paragraph (1)(a)
”
;
bc
in paragraph (3)—
i
at the beginning insert “
Except where paragraph (3A) applies
”
;
ii
for “CE” substitute “
UK
”
(twice);
bd
after paragraph (3) insert—
3A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the watercraft or propulsion engine; or
b
a document accompanying the watercraft or propulsion engine.
be
in paragraph (4) for “CE” substitute “
UK
”
;
c
in paragraph (4)(a) for “Annex II of Decision 768/2008” substitute “
Schedule 15
”
;
d
in paragraph (5) for “notified” in each place in which it occurs substitute “
approved
”
.
Substitution of Part 4I60436
For Part 4 substitute—
PART 4APPROVAL OF CONFORMITY ASSESSMENT BODIES
Approved bodies55
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 56 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 59 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F333IP completion day notified to the European Commission and the member States of the European Union in accordance with Article 26 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 55(b), as it had effect immediately before F333IP completion day;
“approved body requirements” means the requirements set out in Schedule 11.
Approval of conformity assessment bodies56
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and the application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the relevant conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the product in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
8
For the purposes of this regulation, “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies57
1
Where a conformity assessment body demonstrates its conformity with the criteria set out in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of the standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring of approved bodies58
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any condition set—
i
in accordance with regulation 56(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval59
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement; or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 58(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 55 (approved bodies).
2
With the consent of the approved body or where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 58(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 55.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activities, the approved body must—
a
at the request of the Secretary of State, transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
in the absence of a request under sub-paragraph (a), keep its files relating to the activities it has undertaken as an approved body available for inspection by the Secretary of State and the market surveillance authorities for a period of 10 years from the date they were created.
5
The activities undertaken as an approved body referred to in paragraph (4) include any activities that the body has undertaken as a notified body.
Notice of proposed restriction, suspension or withdrawal of approval60
1
Where the Secretary of State proposes to restrict, suspend or withdraw a body's status as an approved body in accordance with regulation 59 (restriction, suspension or withdrawal of approval), the Secretary of State must give notice in writing to the approved body that its approval will be restricted, suspended or withdrawn.
2
A notice provided in accordance with paragraph (1) must—
a
state the date on which the notice is issued;
b
state the reasons why the approval is being restricted, suspended or withdrawn;
c
state the date on which the restriction, suspension or withdrawal of the approval is to take effect;
d
where an approval is being restricted or suspended, state what the effect of that restriction or suspension is on the approved body;
e
inform the approved body of its right to make written representations to the Secretary of State against the proposal within 14 days of the date of the notice.
3
Where an approved body submits written representations to the Secretary of State within 14 days of the notice in accordance with paragraph (2)(e), the Secretary of State must respond to the representations within 21 days of the date on which the representations are received, stating whether, having considered the representations, the notice issued under paragraph (1) will be modified or withdrawn.
Operational requirements of approved bodies61
When an approved body carries out a relevant conformity assessment procedure, Schedule 12 (operational requirements of approved bodies) has effect.
Subsidiaries and contractors62
1
Where an approved body subcontracts specific tasks connected with conformity assessment, or has such tasks carried out by a subsidiary, the tasks are to be treated as having been carried out by an approved body for the purposes of regulations 44 to 47 only where the conditions in paragraphs (2) and (3) are satisfied.
2
The approved body must—
a
ensure that the subcontractor or subsidiary meets the approved body requirements; and
b
inform the Secretary of State accordingly.
3
The approved body must have obtained the agreement of the client economic operator to the use of a subcontractor or subsidiary.
4
Where an approved body subcontracts specific tasks connected with conformity assessment, or has such tasks carried out by a subsidiary, the approved body must, for a period of 10 years beginning on the day on which the tasks are carried out, keep at the disposal of the Secretary of State the documentation concerning—
a
the assessment of the qualifications of the subcontractor or subsidiary; and
b
the conformity assessment activities carried out by the subcontractor or subsidiary.
5
When monitoring an approved body in accordance with regulation 58 (monitoring of approved bodies), the Secretary of State must treat the approved body as responsible for the tasks performed by a subcontractor or subsidiary, wherever the subcontractor or subsidiary is established.
6
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M92.
Register of approved bodies63
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
Authorisation of UK national accreditation body64
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 58;
c
compiling and maintaining the register of approved bodies in accordance with regulation 63.
Amendment to regulation 69I60537
In regulation 69 (enforcement action in respect of products that are not in conformity and which present a risk)—
a
in paragraph (2) for “the notified” substitute “
any approved
”
;
b
omit paragraphs (4) and (7);
c
in paragraph (8)—
i
for “notices referred to in paragraphs (6) and (7)” substitute “
notice referred to in paragraph (6)
”
;
ii
in sub-paragraph (f)(ii) for “a harmonised standards referred to in regulation 41 (presumption of conformity) which confer” substitute “
a designated standard referred to in regulation 41 (presumption of conformity) which confers
”
.
Omission of regulation 70I60638
Omit regulation 70 (EU safeguard procedure).
Amendment to regulation 71I60739
In regulation 71 (enforcement action in respect of formal non-compliance), in paragraph (1)—
a
in sub-paragraph (a), for “CE” substitute “
UK
”
in each place in which it occurs;
b
in sub-paragraph (b) omit “EU”.
Insertion of Part 5AI60840
After regulation 83 insert—
PART 5APOWERS OF THE SECRETARY OF STATE
Power to amend Schedules83A
1
The Secretary of State may by regulations amend any of the provisions specified in paragraph (2) where the Secretary of State considers it necessary to do so in order to take into account technical progress and new scientific evidence.
2
The provisions referred to in paragraph (1) are—
a
in Schedule 1—
i
points 2.3, 2.4, 2.5 and Section 3 of Part B;
ii
Section 3 of Part C;
b
Schedule 7;
c
Schedule 9.
3
The Secretary of State may by regulations amend Schedule 5 where the Secretary of State considers it necessary to do so in order to take into account technical progress, the adequacy of ensuring equivalent conformity and new scientific evidence.
4
Regulations made under this regulation may—
a
make different provisions for different cases; and
b
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
5
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Power to make provision for application of conformity assessments and of Schedule 183B
1
Where one or both of the conditions in paragraph (2) is met, the Secretary of State may by regulations make provision about—
a
detailed procedures for the operation of regulations 50 to 52 and paragraph 2 of Module B (as set out in Schedule 15), taking into account the specific conformity assessment needs of the products covered by these Regulations;
b
the application of the watercraft design categories set out in point 1 of Part A of Schedule 1, including on the use of weather terminology and measurement scales used in those categories;
c
the information on the builder's plate set out in point 2.2 of Part A of Schedule 1;
d
the application of the Regulations on navigation lights set out in point 5.7 of Part A of Schedule 1;
e
arrangements for discharge prevention, in particular as regards operation of holding tanks, set out in point 5.8 of Part A of Schedule 1;
f
the installation and testing of gas appliances and permanently installed gas systems on watercraft, as referenced in point 5.5 of Part A of Schedule 1.
2
The conditions referred to in paragraph (1) are that the Secretary of State considers it necessary to make such provision in order to—
a
take into account the progress of technical knowledge; and
b
ensure that these Regulations are applied in a uniform manner.
3
Before making regulations under this regulation, the Secretary of State must consult such persons as the Secretary of State considers appropriate.
4
Regulations made under this regulation may—
a
make different provisions for different cases; and
b
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
5
Regulations made under this regulation are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Transitional provision in relation to EU ExitI57041
After regulation 89 insert—
Transitional provisions in relation to EU Exit89A
1
In this regulation, “pre-exit period” means the period beginning on the commencement date and ending immediately before F335IP completion day.
2
Subject to paragraph (3), where a product was made available on the market or put into service during the pre-exit period, despite the amendments made by Schedule 28 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M93, any obligation to which a person was subject under these Regulations as they had effect immediately before F335IP completion day, continues to have effect as it did immediately before F335IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or a member State of any matter; or
b
any obligation to take action outside of the market in respect of the product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
the manufacturer has taken any action under regulation 42 or a person has taken action under regulation 43(2) or (3), as those provisions had effect immediately before F335IP completion day in relation to that product,
that action has effect as if it had been done under regulation 42 or 43 as they have effect on and after F335IP completion day.
5
Where during the pre-exit period—
a
a product has not been placed on the market or put into service; and
b
the private importer or a person to whom regulation 43(2) applies, has taken any action under Schedule 5 as it had effect immediately before F335IP completion day in relation to that product,
that action has effect as if it had been done under Schedule 5 as it has effect on and after F335IP completion day.
Amendment to regulation 90I60942
1
Regulation 90 (revocations and savings) is amended as follows.
2
After paragraph (1) insert—
1A
For the purposes of paragraph (1), the Recreational Craft Regulations 1996 M94 have effect with the following modifications—
a
any reference to “the Community” is to be read as including the United Kingdom;
b
any reference to a “member State” is to be read as including the United Kingdom;
c
in Schedule 7 (EC type-examination (module B))—
i
in paragraph 7 omit “and withdrawn”;
ii
omit paragraph 8;
d
in Schedules 9 (production quality assurance (module D)) and 12 (full quality assurance (module H))—
i
in paragraph 5, for “national” substitute
“ enforcement ”; andii
in paragraph 6 omit “and withdrawn”;
e
in Schedule 15 (enforcement), in paragraph 2 omit “with a view to this information being passed by the Secretary of State to the Commission”.
3
After paragraph (2) insert—
3
“For the purposes of paragraph (2), the Recreational Craft Regulations 2004 M95 have effect with the following modifications—
a
any reference to “the Community” or “the European Union” is to be read as including the United Kingdom;
b
any reference to a “member State” is to be read as including the United Kingdom;
c
in Schedule 7 (EC type-examination)—
i
in paragraph 7 omit “and withdrawn”;
ii
omit paragraph 8;
d
in Schedules 9 (production quality assurance), 12 (full quality assurance) and 15 (product quality assurance (module E))—
i
in paragraph 5, for “national” substitute
“ enforcement ”; andii
in paragraph 6 omit “and withdrawn;
e
in Schedule 17 (enforcement), in paragraph 2 omit “with a view to this information being passed by the Secretary of State to the Commission”.
Amendment to Schedule 1I61043
1
Schedule 1 (essential requirements) is amended as follows.
2
In Part A (essential requirements for the design and construction of products referred to in Article 2(1))—
a
in the Explanatory Notes to Section 1 (Watercraft Design Categories), in the final paragraph, for “Annex” substitute “
Schedule
”
;
b
in paragraph 2.1—
i
in paragraph (2) for “the national authority of the Member State” substitute “
or on behalf of the Secretary of State
”
;
ii
for “harmonised” substitute “
designated
”
;
c
for paragraph 2.2—
i
for paragraph (b) substitute—
b
UK marking, as provided for in regulation 54;
ii
for “notified” substitute “
approved
”
;
d
in paragraph 2.5 for “in accordance with Article 7(7) and Article 9(4)” substitute “
including the instructions and safety information referred to in regulations 15 and 22
”
.
3
In Part B (essential requirements for exhaust emissions from propulsion engines)—
a
for paragraph 1.1(d) substitute—
d
UK marking, as provided for in regulation 54.
b
in paragraphs 2.3 and 2.5—
i
for “Notified” substitute “
Approved
”
;
ii
for “harmonised” substitute “
designated
”
;
c
in paragraph 4—
i
for “a language or languages which can be easily understood by consumers and other end-users, as determined by the Member State in which the engine is to be marketed” substitute “
English
”
;
ii
in sub-paragraph (b) for “harmonised” substitute “
designated
”
.
Amendment to Schedule 3I61144
Schedule 3 (declaration by the manufacturer or the importer of the partly completed watercraft (Article 6(2)) is amended as follows—
a
for “established in the Union referred to in Article 6(2)” substitute “
established in the United Kingdom referred to in regulation 7(1)(b)
”
;
b
in paragraph (b) for “established in the Union” substitute “
established in the United Kingdom
”
;
c
in paragraph (d)—
i
for “harmonised” substitute “
designated
”
;
ii
for “this Directive” substitute “
these Regulations
”
.
Amendment to Schedule 4I61245
Schedule 4 (EU declaration of conformity No xxxxx) is amended as follows—
a
in the heading omit “EU”;
b
in paragraph 3 for “Article 19(3) or (4) of Directive 2013/53/EU” substitute “
regulation 43(2) or (3) of the Recreational Craft Regulations 2017 (S.I. 2017/737, “the Regulations”)
”
;
c
in paragraph 5 for “Union harmonisation legislation” substitute “
statutory requirements
”
;
d
in paragraph 6 for “harmonised” substitute “
designated
”
;
e
in paragraph 7 for “notified” substitute “
approved
”
;
f
in paragraph 9—
i
omit “EU” in both places in which it occurs;
ii
for “points (b) and (c) of Article 6(4)” substitute “
regulation 7(1)(c)(iii)
”
;
iii
for “this Directive” substitute “
the Regulations
”
in both places in which it occurs;
iv
omit sub-paragraph (a)(ii);
v
for “Article 55(2)” substitute “
regulation 89(2)
”
.
Amendment to Schedule 5I61346
1
Schedule 5 (equivalent conformity based on post-construction assessment (module PCA)) is amended as follows.
2
For “this Directive” substitute “
these Regulations
”
in each place in which it occurs.
3
In paragraph 1, for “Article 19(2), (3) or (4) substitute “
regulation 43(1), (2) or (3)
”
.
4
In paragraph 2—
a
for “a notified” substitute “
an approved
”
;
b
for “the notified” in both places in which it occurs substitute “
the approved
”
;
c
for “relevant national authorities” substitute “
enforcing authority
”
.
5
In paragraph 3—
a
for “notified” in each place in which it occurs substitute “
approved
”
;
b
for “national authorities” substitute “
enforcing authority
”
;
c
for “CE” substitute “
UK
”
;
d
for “Annex I” substitute “
Schedule 1
”
;
e
for “the national authority of the Member State” substitute “
or on behalf of the Secretary of State
”
.
6
In paragraph 4—
a
for the heading substitute “
UK marking and declaration of conformity
”
;
b
in sub-paragraph 1—
i
for “CE” substitute “
UK
”
;
ii
for “notified” in both places in which it occurs substitute “
approved
”
;
c
in sub-paragraph 2—
i
for “an EU” substitute “
a
”
;
ii
for “national authorities” substitute “
enforcing authority
”
;
iii
for “the EU” substitute “
the
”
;
iv
for “relevant authorities” substitute “
enforcing authority
”
;
d
in sub-paragraph 3, for “Annex I” in both places in which it occurs substitute “
Schedule 1
”
.
7
In paragraph 5 for “notified” substitute “
approved
”
.
Amendment to Schedule 6I61447
Schedule 6 (supplementary requirements when internal production control plus supervised production tests set out in module A1 is used (Article 24(2))) is amended as follows—
a
for “Annex I” substitute “
Schedule 1
”
in each place in which it occurs;
b
for “a notified” in both places in which it occurs substitute “
an approved
”
;
c
for “Annex VII” substitute “
Schedule 7
”
.
Amendment to Schedule 7I61548
In Schedule 7 (conformity of production assessment for exhaust and noise emissions) in paragraph 1, for “notified” substitute “
approved
”
.
Amendment to Schedule 8I2I61649
Schedule 8 (supplementary procedure to be applied under conformity to type based on internal production control (module C)) is amended as follows—
a
for “Article 24(5)” substitute “
regulation 52
”
;
b
for “Annex I” in both places in which it occurs substitute “
Schedule 1
”
;
c
for “this Directive” substitute “
these Regulations
”
;
d
for “Annex VII” substitute “
Schedule 7
”
.
Amendment to Schedule 9I61750
Schedule 9 is amended as follows—
a
omit “referred to in Article 7(2) and Article 25”;
b
for “Article 14” in both places in which it occurs substitute “
regulation 41
”
;
c
for “Annex I” in each place in which it occurs substitute “
Schedule 1
”
.
Amendment to Schedule 10I61851
Schedule 10 (EU-type examination) is omitted.
Amendment to Schedule 11I61952
Schedule 11 (requirements of notified bodies) is amended as follows—
a
for the heading substitute “
Requirements of approved bodies
”
;
b
in paragraph 11(c) for “a notified” substitute “
an approved
”
;
c
in each place in which it occurs (other than that referred to in sub-paragraph (b)), for “notified” substitute “
approved
”
.
Amendment to Schedule 12I62053
Schedule 12 (operational requirements of notified bodies) is amended as follows—
a
for the heading substitute “
Operational requirements of approved bodies
”
;
b
in paragraph 1 for “Notified” substitute “
Approved
”
;
c
in paragraph 3 for “the Directive” substitute “
these Regulations
”
;
d
in paragraphs 4 and 5 for “a notified” substitute “
an approved
”
;
e
in paragraph 4 for “harmonised” substitute “
designated
”
;
f
in paragraphs 6 and 9 for “notified” substitute “
approved
”
;
g
in paragraphs 7(1), 7(2), 8, 9 and 10 for “A notified” substitute “
An approved
”
;
h
in paragraph 7—
i
for “notification” in both places in which it occurs substitute “
approval
”
;
ii
for “the notified” substitute “
the approved
”
;
i
in paragraph 10 for “any notified body coordination group established under the Directive” substitute “
any approved body coordination group established by the Secretary of State
”
.
Insertion of Schedule 15I62154
After Schedule 14 insert—
SCHEDULE 15Conformity assessment procedures
MODULE AInternal production control
Internal production control1
Internal production control is the conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 to 4; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned meets the essential requirements that apply to it.
Technical documentation2
1
The manufacturer must draw up the technical documentation.
2
The technical documentation must—
a
make it possible to assess the product's conformity with the essential requirements that apply to it;
b
include an adequate analysis and assessment of any risks;
c
specify the essential requirements that apply to the product; and
d
cover, as far as relevant for the assessment, the design, manufacture and operation of the product.
3
The technical documentation must contain, where applicable, at least the following—
a
a general description of the product;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product;
d
a list of the designated standards and other relevant technical specifications applied in full or in part (and where designated standards have been applied in part, the technical documentation must specify the parts which have been applied);
e
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements;
f
results of design calculations made and examinations carried out;
g
test reports.
Manufacturing3
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured product with—
a
the technical documentation referred to in paragraph 2; and
b
the essential requirements that apply to it.
UK marking and declaration of conformity4
1
The manufacturer must affix the UK marking to each individual product that meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it together with the technical documentation at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
MODULE A1Internal production control plus supervised product testing
Internal production control plus supervised product testing1
Internal production control plus supervised product testing is the conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 to 5; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned meets the essential requirements that apply to it M96.
Technical documentation2
1
The manufacturer must draw up the technical documentation.
2
The technical documentation must—
a
make it possible to assess the product's conformity with the essential requirements that apply to it;
b
include an adequate analysis and assessment of any risks;
c
specify the essential requirements that apply to the product; and
d
cover, as far as relevant for the assessment, the design, manufacture and operation of the product.
3
The technical documentation must contain, where applicable, at least the following—
a
a general description of the product;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product;
d
a list of the designated standards and other relevant technical specifications applied in full or in part (and where designated standards have been applied in part, the technical documentation must specify the parts which have been applied);
e
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements;
e
results of design calculations made and examinations carried out;
f
test reports.
Manufacturing3
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured product with—
a
the technical documentation referred to in paragraph 2; and
b
the essential requirements that apply to it.
Product checks4
1
For each individual product manufactured, one or more tests on one or more specific aspects of the product must be carried out on the manufacturer's behalf in order to verify the product's conformity with the essential requirements that apply to it.
2
The tests must be carried out under the responsibility of an approved body chosen by the manufacturer.
3
The manufacturer must, under the responsibility of the approved body, affix the approved body's identification number to the product during the manufacturing process.
UK marking and declaration of conformity5
1
The manufacturer must affix the UK marking to each individual product that meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it together with the technical documentation at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
MODULE BType examination
Type examination1
Type examination is the part of a conformity assessment procedure in which an approved body—
a
examines the technical design of a product; and
b
verifies and attests that the technical design of the product meets the essential requirements that apply to it.
How type examination must be carried out, etc.2
1
The conformity assessment procedure must include an assessment of the adequacy of the technical design of the product through examination of the technical documentation and supporting evidence referred to in paragraph 3, plus examination of specimens, representative of the production envisaged, of one or more critical parts of the product (combination of production type and design type).
2
The assessment referred to in sub-paragraph (1) may cover several versions of the product if—
a
the differences between the versions of the product do not affect the level of safety and the other requirements concerning the performance of the product; and
b
the different versions of the product are referred to in the corresponding type examination certificate, if necessary by means of amendments to the original certificate.
Application for type examination3
1
The manufacturer must lodge an application for type examination with a single approved body of the manufacturer's choice.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by an authorised representative, the name and address of the authorised representative;
b
a declaration that the same application has not been lodged with any other approved body;
c
the technical documentation;
d
the specimens representative of the production envisaged; and
e
the supporting evidence for the adequacy of the technical design solution.
3
The manufacturer must, if requested by the approved body, provide further specimens if needed for carrying out the test programme.
4
The technical documentation referred to in sub-paragraph (2)(c) must—
a
make it possible to assess the product's conformity with the essential requirements that apply to it;
b
include an adequate analysis and assessment of any risks;
c
specify the essential requirements that apply to the product; and
d
cover, as far as relevant for the assessment, the design, manufacture and operation of the product.
5
The technical documentation referred to in sub-paragraph (2)(c) must contain, where applicable, at least the following—
a
a general description of the product;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product;
d
a list of the designated standards and other relevant technical specifications applied in full or in part (and where designated standards have been applied in part, the technical documentation must specify the parts which have been applied);
e
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements;
f
results of design calculations made and examinations carried out;
g
test reports.
5
The supporting evidence for the adequacy of the technical design solution referred to in sub-paragraph (2)(e) must—
a
mention any documents that have been used, in particular where the relevant designated standards or technical specifications have not been applied in full; and
b
include, where necessary, the results of tests carried out by the appropriate laboratory of the manufacturer, or by another testing laboratory on the manufacturer's behalf and under the manufacturer's responsibility.
Examination, etc. by approved body4
1
The approved body must examine the technical documentation and supporting evidence to assess the adequacy of the technical design of the product.
2
The approved body must—
a
verify that the specimen has been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards or technical specifications, as well as the elements which have been designed without applying the relevant provisions of those standards or specifications;
b
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards or technical specifications, these have been applied correctly;
c
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards or technical specifications have not been applied, the solutions adopted by the manufacturer meet the essential requirements covered by the standards or specifications; and
d
agree with the manufacturer on a location where the examinations and tests will be carried out.
Evaluation report5
The approved body must draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes. Without prejudice to its obligations vis-à vis the Secretary of State, the approved body may release the content of the report, in full or in part, only with the agreement of the manufacturer.
Type examination certificate6
1
Where the type meets the essential requirements that apply to the product concerned, the approved body must issue a type examination certificate to the manufacturer.
2
The certificate (which may have one or more annexes attached) must contain—
a
the name and address of the manufacturer;
b
the conclusions of the examination;
c
the conditions (if any) for its validity;
d
the necessary data for identification of the approved type; and
e
all relevant information to allow the conformity of manufactured products with the examined type to be evaluated and to allow for in-service control.
3
Where the type does not meet the essential requirements that apply to the product concerned, the approved body must refuse to issue a type examination certificate and must inform the applicant accordingly, giving detailed reasons for its refusal.
Changes7
1
The approved body must keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the essential requirements that apply to the product concerned and must determine whether such changes require further investigation. If so, the approved body must inform the manufacturer accordingly.
2
The manufacturer must inform the approved body that holds the technical documentation relating to the type examination certificate of all modifications to the approved type that may affect the conformity of the product with the essential requirements that apply to it or the conditions for validity of the certificate. Such modifications require additional approval in the form of an addition to the original type examination certificate.
Approved body's duties in respect of type examination certificates8
1
The approved body must inform the Secretary of State about the type examination certificates and any additions thereto which it has issued or withdrawn and must, periodically or upon request, make available to the Secretary of State a list of certificates and any additions thereto refused, suspended or otherwise restricted.
2
The approved body must inform the other approved bodies about the type examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted and, upon request, about such certificates and additions thereto which it has issued.
3
The approved body must, on request, provide the Secretary of State and other approved bodies with a copy of the type examination certificates and additions thereto which it has issued.
4
The approved body must, on request, provide the Secretary of State with a copy of the technical documentation and the results of the examinations carried out by the approved body.
5
The approved body must keep a copy of the type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of the certificate.
Manufacturer's duties in respect of type examination certificates9
The manufacturer must keep a copy of the type examination certificate, its annexes and additions together with the technical documentation at the disposal of the enforcing authority for 10 years after the product has been placed on the market.
MODULE CConformity to type based on internal production control
Conformity to type based on internal production control1
Conformity to type based on internal production control is the part of a conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 and 3; and
b
ensures and declares that the product concerned is in conformity with the type described in the type examination certificate and meets the essential requirements that apply to it M97.
Manufacturing2
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with—
a
the approved type described in the type examination certificate; and
b
the essential requirements that apply to it.
UK marking and declaration of conformity3
1
The manufacturer must affix the UK marking to each individual product thatis in conformity with the type described in the type examination certificate and meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
MODULE C1Conformity to type based on internal production control plus supervised product testing
Conformity to type based on internal production control plus supervised product testing1
Conformity to type based on internal production control plus supervised product testing is the part of a conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 to 4; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned—
i
is in conformity with the type described in the type examination certificate; and
ii
meets the essential requirements that apply to it.
Manufacturing2
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with—
a
the type described in the type examination certificate; and
b
the essential requirements that apply to it.
Product checks3
1
For each individual product manufactured, one or more tests on one or more specific aspects of the product must be carried out on the manufacturer's behalf in order to verify the product's conformity with the essential requirements that apply to it.
2
The tests must be carried out under the responsibility of an approved body chosen by the manufacturer.
3
The manufacturer must, under the responsibility of the approved body, affix the approved body's identification number to the product during the manufacturing process.
UK marking and declaration of conformity4
1
The manufacturer must affix the UK marking to each individual product thatis in conformity with the type described in the type examination certificate and meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
MODULE DConformity to type based on quality assurance of the production process
Conformity to type based on quality assurance of the production process1
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 and 5; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned—
i
is in conformity with the type described in the type examination certificate; and
ii
meets the essential requirements that apply to it.
Manufacturing2
The manufacturer—
a
must operate an approved quality system for production, final product inspection and testing of the products concerned as specified in paragraph 3; and
b
is subject to surveillance as specified in paragraph 4.
Quality system3
1
The manufacturer must lodge an application for assessment of the manufacturer's quality system with the approved body of the manufacturer's choice for the products concerned.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the product category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the type examination certificate.
3
The quality system must ensure that the products—
a
are in conformity with the type described in the type examination certificate; and
b
meet the essential requirements that apply to them.
4
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions.
5
The quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture and the frequency with which they will be carried out;
d
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned; and
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
6
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraph (3). The approved body must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard or technical specification.
7
For the purpose of the assessment referred to in sub-paragraph (6), the approved body must ensure that—
a
in addition to experience in quality management systems, the auditing team has at least one member with experience of evaluation in the relevant product field and product technology concerned and knowledge of the essential requirements that apply to the products;
b
the audit includes an assessment visit to the manufacturer's premises; and
c
the auditing team reviews the technical documentation referred to in sub-paragraph (2)(e) to verify the manufacturer's ability to identify the essential requirements that apply to the products and to carry out the necessary examinations with a view to ensuring compliance of the products with those requirements.
8
The approved body must notify its decision on whether the quality system satisfies the requirements referred to in sub-paragraph (3) to the manufacturer. The notification must contain the conclusions of the audit and the approved body's reasoned assessment.
9
The manufacturer must undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
10
The manufacturer must keep the approved body that approved the quality system informed of any intended change to the quality system, and if so informed, the approved body must evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (3) or whether a reassessment is necessary.
11
The approved body must notify the manufacturer of its decision. The notification must contain the conclusions of the examination and the approved body's reasoned assessment.
Surveillance under the responsibility of the approved body4
1
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
2
The manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and must provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body must provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity5
1
The manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3(1), the approved body's identification number to each individual product that is in conformity with the type described in the type examination certificate and meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
Manufacturer's duty to keep application, etc.6
The manufacturer must, for a period ending at least 10 years after the product has been placed on the market, keep at the disposal of the enforcing authority—
a
a copy of the application referred to in paragraph 3(1) including the information and documentation referred to in paragraph 3(2);
b
documents relating to any change to the quality system referred to in paragraph 3(10), as approved by the approved body;
c
the decisions and reports of the approved body referred to in paragraphs 3(11) and 4(3) and (4).
Approved body's duties in respect of quality system approvals7
1
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State a list of quality system approvals refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
MODULE EConformity to type based on product quality assurance
Conformity to type based on product quality assurance1
Conformity to type based on product quality assurance is that part of a conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 and 5; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned—
i
is in conformity with the type described in the type examination certificate; and
ii
meets the essential requirements that apply to it.
Manufacturing2
The manufacturer—
a
must operate an approved quality system for final product inspection and testing of the products concerned as specified in paragraph 3; and
b
is subject to surveillance as specified in paragraph 4.
Quality system3
1
The manufacturer must lodge an application for assessment of the manufacturer's quality system with the approved body of the manufacturer's choice for the products concerned.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the authorised representative's name and address;
b
a declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the product category envisaged;
d
the documentation concerning the quality system; and
e
the technical documentation of the approved type and a copy of the type examination certificate.
3
The quality system must ensure that the products—
a
are in conformity with the type described in the type examination certificate; and
b
meet the essential requirements that apply to them.
4
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions.
5
The quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned; and
d
the means of monitoring the effective operation of the quality system.
6
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraph (3). The approved body must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard or technical specification.
7
For the purpose of the assessment referred to in sub-paragraph (6), the approved body must ensure that—
a
in addition to experience in quality management systems, the auditing team has at least one member with experience of evaluation in the relevant product field and product technology concerned and knowledge of the essential requirements that apply to the products;
b
the audit includes an assessment visit to the manufacturer's premises; and
c
the auditing team reviews the technical documentation referred to in sub-paragraph (2)(e) to verify the manufacturer's ability to identify the essential requirements that apply to the products and to carry out the necessary examinations with a view to ensuring compliance of the products with those requirements.
8
The approved body must notify its decision on whether the quality system satisfies the requirements referred to in sub-paragraph (3) to the manufacturer. The notification must contain the conclusions of the audit and the approved body's reasoned assessment.
9
The manufacturer must undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
10
The manufacturer must keep the approved body that approved the quality system informed of any intended change to the quality system, and if so informed, the approved body must evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (3) or whether a reassessment is necessary.
11
The approved body must notify the manufacturer of its decision. The notification must contain the conclusions of the examination and the approved body's reasoned assessment.
Surveillance under the responsibility of the approved body4
1
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
2
The manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and must provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body must provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity5
1
The manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3(1), the approved body's identification number to each individual product that is in conformity with the type described in the type examination certificate and meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
Manufacturer's duty to keep application, etc.6
The manufacturer must, for a period ending at least 10 years after the product has been placed on the market, keep at the disposal of the enforcing authority—
a
a copy of the application referred to in paragraph 3(1) including the information and documentation referred to in paragraph 3(2);
b
documents relating to any change to the quality system referred to in paragraph 3(10), as approved by the approved body;
c
the decisions and reports of the approved body referred to in paragraphs 3(11) and 4(3) and (4).
Approved body's duties in respect of quality system approvals7
1
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn and, upon request, of quality system approvals which it has issued.
MODULE FConformity to type based on product verification
Conformity to type based on product verification1
Conformity to type based on product verification is the part of a conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2, 5(1) and 6; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned, which has been subject to the provisions of paragraph 3—
i
is in conformity with the type described in the type examination certificate; and
ii
meets the essential requirements that apply to it M98.
Manufacturing2
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with—
a
the approved type described in the type examination certificate; and
b
the essential requirements that apply to it.
Verification3
1
An approved body chosen by the manufacturer must carry out appropriate examinations and tests in order to check the conformity of the product with—
a
the approved type described in the type examination certificate; and
b
the essential requirements that apply to it.
2
The examinations and tests to check the conformity of the products with the essential requirements that apply to it must be carried out, at the choice of the manufacturer, either by—
a
examination and testing of every product as specified in paragraph 4; or
b
examination and testing of the products on a statistical basis as specified in paragraph 5.
Verification of conformity by examination and testing of every product4
1
All products must be individually examined, and appropriate tests set out in the relevant designated standard or technical specifications or equivalent tests must be carried out in order to verify conformity with the approved type described in the type examination certificate and with the essential requirements that apply to it. In the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out and must affix its identification number to each approved product or have it affixed under its responsibility.
3
The manufacturer must keep the certificates of conformity at the disposal of the enforcing authority for 10 years after the product has been placed on the market.
Statistical verification of conformity5
1
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure the homogeneity of each lot produced and must present the manufacturer's products for verification in the form of homogeneous lots.
2
A random sample must be taken from each lot by the approved body. All products in a sample must be individually examined, and appropriate tests set out in the relevant designated standard or technical specification or equivalent tests must be carried out in order to ensure their conformity with the essential requirements that apply to them and to determine whether the lot is to be accepted or rejected. In the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
3
If a lot is accepted, all products of the lot must be considered approved, except for those products from the sample that have been found not to satisfy the tests.
4
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out and must affix its identification number to each approved product or have it affixed under its responsibility.
5
The manufacturer must keep the certificates of conformity at the disposal of the enforcing authority for 10 years after the product has been placed on the market.
6
If a lot is rejected, the approved body or, if the approved body fails to do so, the Secretary of State must take appropriate measures to prevent that lot being placed on the market. In the event of the frequent rejection of lots, the approved body may suspend the statistical verification and take appropriate measures.
UK marking and declaration of conformity6
1
The manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3, the approved body's identification number to each individual product that is in conformity with the approved type described in the type examination certificate and meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
4
If the approved body referred to in paragraph 3 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the product.
Affixing of approved body's identification number during manufacturing process7
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the product during the manufacturing process.
Authorised representative8
Where the manufacturer appoints an authorised representative (see regulation 39), the obligations in paragraphs 2 and 5(1) must not form part of the authorised representative's mandate.
MODULE GConformity based on unit verification
Conformity based on unit verification1
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2, 3 and 5; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned, which has been subject to the provisions of paragraph 4, meets the essential requirements that apply to it.
Technical documentation2
1
The manufacturer must draw up the technical documentation and make it available to the approved body referred to in paragraph 4.
2
The technical documentation must—
a
make it possible to assess the product's conformity with the essential requirements that apply to it;
b
include an adequate analysis and assessment of the risks;
c
specify the essential requirements that apply to the product; and
d
cover, as far as relevant for the assessment, the design, manufacture and operation of the product.
3
The technical documentation must contain, where applicable, at least the following—
a
a general description of the product;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product;
d
a list of the designated standards and other relevant technical specifications, applied in full or in part (and where designated standards have been applied in part, the technical documentation must specify the parts which have been applied);
e
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements;
f
results of design calculations made and examinations carried out;
g
test reports.
4
The manufacturer must keep the technical documentation at the disposal of the enforcing authority for 10 years after the product has been placed on the market.
Manufacturing3
The manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with the essential requirements that apply to it.
Verification4
1
An approved body chosen by the manufacturer must carry out appropriate examinations and tests, set out in the relevant designated standard or technical specification or equivalent tests, to check the conformity of the product with the essential requirements that apply to it or have them carried out. In the absence of such a designated standard or technical specification the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out and must affix its identification number to the approved product or have it affixed under its responsibility.
3
The manufacturer must keep the certificates of conformity at the disposal of the enforcing authority for 10 years after the product has been placed on the market.
UK marking and declaration of conformity5
1
The manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 4, the approved body's identification number to each product that meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
MODULE HConformity based on full quality assurance
Conformity based on full quality assurance1
Conformity based on full quality assurance is the conformity assessment procedure whereby the manufacturer—
a
fulfils the obligations set out in paragraphs 2 and 5; and
b
ensures and declares on the manufacturer's sole responsibility that the product concerned meets the essential requirements that apply to it.
Manufacturing2
The manufacturer—
a
must operate an approved quality system for design, manufacture and final product inspection and testing of the product concerned as specified in paragraph 3; and
b
is subject to surveillance as specified in paragraph 4.
Quality system3
1
The manufacturer must lodge an application for assessment of the manufacturer's quality system with the approved body of the manufacturer's choice for the product concerned.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
the technical documentation for one model of each category of products intended to be manufactured, which must contain, where applicable, at least the following—
i
a general description of the product;
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product;
iv
a list of the designated standards and other relevant technical specifications applied in full or in part (and where designated standards have been applied in part, the technical documentation must specify the parts which have been applied);
v
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential requirements;
vi
results of design calculations made and examinations carried out;
vii
test reports;
c
the documentation concerning the quality system; and
d
a declaration that the same application has not been lodged with any other approved body.
3
The quality system must ensure that the products meet the essential requirements that apply to them.
4
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions.
5
The quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to design and product quality;
b
the technical design specifications, including standards, that will be applied and, where the relevant designated standards or technical specifications will not be applied in full, the means that will be used to ensure that the essential requirements that apply to the products will be met;
c
the design control and design verification techniques, processes and systematic actions that will be used when designing the products pertaining to the product category covered;
d
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
e
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
f
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned;
g
the means of monitoring the achievement of the required design and product quality and the effective operation of the quality system.
6
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in sub-paragraph (3). The approved body must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard or technical specification.
7
For the purpose of the assessment referred to in sub-paragraph (6), the approved body must ensure that—
a
in addition to experience in quality management systems, the auditing team has at least one member experienced as an assessor in the relevant product field and product technology concerned, and knowledge of the essential requirements that apply to the products;
b
the audit includes an assessment visit to the manufacturer's premises; and
c
the auditing team reviews the technical documentation referred to sub-paragraph (2)(b) to verify the manufacturer's ability to identify the essential requirements that apply to the products and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
8
The approved body must notify the manufacturer or the manufacturer's authorised representative of its decision on whether the quality system satisfies the requirements referred to in sub-paragraph (3). The notification must contain the conclusions of the audit and the approved body's reasoned assessment.
9
The manufacturer must undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
10
The manufacturer must keep the approved body that approved the quality system informed of any intended change to the quality system, and if so informed, the approved body must evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in sub-paragraph (3) or whether a reassessment is necessary.
11
The approved body must notify the manufacturer of its decision. The notification must contain the conclusions of the examination and the approved body's reasoned assessment.
Surveillance under the responsibility of the approved body4
1
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
2
The manufacturer must, for assessment purposes, allow the approved body access to the design, manufacture, inspection, testing and storage sites and must provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records as provided for by the design part of the quality system, such as results of analyses, calculations and tests; and
c
the quality records as provided for by the manufacturing part of the quality system, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and must provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits, the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body must provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity5
1
The manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3(1), the approved body's identification number to each individual product that meets the essential requirements that apply to it.
2
The manufacturer must draw up a declaration of conformity for each product model and keep it at the disposal of the enforcing authority for 10 years after the product has been placed on the market. The declaration of conformity must identify the product model for which it has been drawn up.
3
The manufacturer must make a copy of the declaration of conformity available to the enforcing authority upon request.
Manufacturer's duty to keep application, etc.6
The manufacturer must, for a period ending at least 10 years after the product has been placed on the market, keep at the disposal of the enforcing authority—
a
the technical documentation referred to in paragraph 3(2)(b);
b
the documentation concerning the quality system referred to in paragraph 3(2)(c);
c
documents relating to any change to the quality system referred to in paragraph 3(10), as approved by the approved body;
d
the decisions and reports of the approved body referred to in paragraphs 3(11) and 4(3) and (4).
Approved body's duties in respect of quality system approvals7
1
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn and, upon request, of quality system approvals which it has issued.
PART 2Amendment to Commission Implementing Regulation (EU) 2017/1
IntroductionI91055
Commission Implementing Regulation (EU) 2017/1 on procedures for watercraft identification under Directive 2013/53/EU of the European Parliament and of the Council on recreational and personal watercraft is amended in accordance with paragraphs 56 to 66.
Amendment to Article 2I62256
In Article 2 (definitions)—
a
for point (a) substitute—
a
‘UK national body’ means a body appointed by the Secretary of State for assigning the unique code of the manufacturer;
b
for point (c) substitute—
c
‘UK national register’ means the register held by or on behalf of the Secretary of State, where the unique code of manufacturer for manufacturers established in the United Kingdom is recorded;
c
for point (d) substitute—
d
‘UK third country register’ means the register held by or on behalf of the Secretary of State, where the unique code of manufacturer for manufacturers established outside of the United Kingdom is recorded;
d
omit point (e);
e
for point (f) substitute—
f
‘approved bodies’ register' means the register held by or on behalf of the Secretary of State where the post-construction assessment identification code is recorded.
Amendment to Article 3I62357
In Article 3 (watercraft identification number)—
a
in paragraph 1(b)—
i
for “the national authority of a Member State” substitute “
or on behalf of the Secretary of State
”
;
ii
omit the words from “however” to the end;
b
in paragraph 1(e) after “on the” insert “
United Kingdom
”
;
c
in paragraph 2 for “Point 2.1 of Annex I to Directive 2013/53/EU” substitute “
paragraph 2.1 of Schedule 1 to the Recreational Craft Regulations 2017 M99
”
.
Amendment to Article 4I62458
In Article 4 (assignment of the unique code of the manufacturer)—
a
in paragraph 1 for “national authority or national body of the Member State” substitute “
UK national body or, if none is designated, the Secretary of State
”
;
b
in paragraph 2—
i
for “national authority or national body of a Member State” substitute “
UK national body or the Secretary of State
”
;
ii
for “Union” substitute “
United Kingdom
”
.
Amendment to Article 5I62559
In Article 5 (national authority for assigning the unique code of the manufacturer)—
a
in the heading for “National authority” substitute “
UK national body
”
;
b
in paragraph 1 for “Each Member State shall designate the national authority or the” substitute “
The Secretary of State may designate the UK
”
;
c
omit paragraph 2;
d
after paragraph 2 insert—
3
In the absence of a designation under paragraph 1, the Secretary of State is responsible for assigning the unique code of the manufacturer.
Amendment to Article 6I62660
In Article 6 (procedure for assignment of the unique code of the manufacturer to a manufacturer established in a Union Member State)—
a
in the heading for “manufacturer established in a Union Member State” substitute “
manufacturer established in the United Kingdom
”
;
b
for paragraph 1 substitute—
1
A manufacturer established in the United Kingdom must, before placing a watercraft on the F336market of Great Britain, submit an application in English for the assignment of the unique code of the manufacturer, to the UK national body or, if none is designated, the Secretary of State.
c
in paragraph 2—
i
for “its Member State” substitute “
the United Kingdom
”
;
ii
for “a language” to the end, substitute “
English
”
;
d
in paragraph 3 for “national authority or national body” substitute “
UK national body or, if none is designated, the Secretary of State
”
;
e
in paragraph 4—
i
for “Each Member State” substitute “
The Secretary of State
”
;
ii
for “their national register” substitute “
the UK national register
”
;
iii
omit the second sentence.
Amendment to Article 7I62761
In Article 7 (procedure for assignment of the unique code of the manufacturer to a manufacturer established in a third country)—
a
in the heading and in paragraph 1, for “in a third country” substitute “
outside of the United Kingdom
”
;
b
in paragraph 1—
i
for “Union” substitute “
United Kingdom
”
;
ii
for “a language which can be easily understood by the authority where the application is introduced as determined by the authority” substitute “
English
”
;
iii
for “national authority” to the end substitute “
UK national body or, if none is designated, the Secretary of State
”
;
c
in paragraph 2—
i
after “a document” insert “
in English
”
;
ii
for “that the manufacturer is established” to the end substitute “
in which country the manufacturer is established
”
;
d
in paragraph 3—
i
for “national authority or the national body of the Member State” substitute “
UK national body or, if none is designated, the Secretary of State
”
;
ii
for “third country register” substitute “
UK third country register
”
;
iii
omit “to any Member State”;
e
omit paragraph 4;
f
in paragraph 5—
i
for “national authority or national body” substitute “
UK national body or, if none is designated, the Secretary of State
”
;
ii
omit the second sentence;
g
for paragraph 6 substitute—
6
When assigning the unique code of the manufacturer to a manufacturer established outside of the United Kingdom, the UK national body or, if none is designated, the Secretary of State shall register that code and the name and address of the manufacturer in the UK third country register.
Amendment to Article 8I62862
In Article 8 (procedure in case of post-construction assessment)—
a
in paragraph 1—
i
for “Articles 19 and 23 of Directive 2013/53/EU” substitute “
regulations 42, 43 and 48 of the Recreational Craft Regulations 2017
”
;
ii
for “notified” in the first place in which it occurs, substitute “
approved
”
;
iii
for “his” substitute “
its
”
;
iv
for “national authority of the Member State where the notified body is established” substitute “
Secretary of State
”
;
b
in paragraph 2 for “notified” substitute “
approved
”
in both places in which it occurs.
Omission of Article 9I62963
Omit Article 9 (fees).
Insertion of Article 9AI63064
After Article 9 (fees) insert—
Article 9ATransitional provision in relation to EU exit
1
In this Article, “pre-exit period” means the period beginning with 24 January 2017 and ending immediately before exit day.
2
Where during the pre-exit period—
a
a manufacturer has submitted an application to the national body of the United Kingdom for the assignment of the unique code of the manufacturer, in accordance with Article 6 as it had effect immediately before exit day; but
b
the unique code of the manufacturer has not been assigned,
that application is to be treated as having been submitted under Article 6 as it has effect on and after exit day.
F3373
Where during the pre-exit period the national body of the United Kingdom has assigned the unique code for a manufacturer, in accordance with Article 4 as it had effect immediately before IP completion day, that unique code is to be treated as if it were issued by the UK national body (or, if none is designated, the Secretary of State) in accordance with Article 4 as it has effect on and after IP completion day.
Omission of Chapter 4I3I63165
Omit Chapter 4 (final provisions).
I63266
Omit—
a
the words “This Regulation shall be binding” to the end;
b
“Done at Brussels, 3 January 2017”;
c
the signatory text.
SCHEDULE 29Amendment of the Radio Equipment Regulations 2017 and related amendments
PART 1Amendments to the Radio Equipment Regulations 2017
IntroductionI9111
The Radio Equipment Regulations 2017 are amended in accordance with paragraphs 2 to 54.
Amendment to regulation 2I6332
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “the 1987 Act” insert—
“approved body” has the meaning given to it in regulation 46 (approved bodies);
F309d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
before the definition of “conformity assessment body” insert—
“commencement date” means the date these regulations come into force;
h
after the definition of “conformity assessment body” insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 42 by regulation 10(1)(a) ( declaration of conformity);
“designated standard” has the meaning given to it in regulation 2A;
i
for the definition of “electromagnetic disturbance” substitute—
“electromagnetic disturbance” means any electromagnetic phenomenon which may degrade the performance of equipment; an electromagnetic disturbance may be electromagnetic noise, an unwanted signal or a change in the propagation medium itself;
j
omit the definition of “EU declaration of conformity”;
k
omit the definition of “European Commission”;
l
for the definition of “harmful interference” substitute—
“harmful interference” means interference which endangers the functioning of a radio navigation service or of other safety services or which otherwise seriously degrades, obstructs or repeatedly interrupts a radiocommunications service operating in accordance with the applicable international, European Community or national regulations;
m
omit the definition of “harmonised standard”;
n
before the definition of “importer” insert—
“the Implementing Regulation” means Commission Implementing Regulation (EU) 2017/1354 specifying how to present the information provided for in Article 10(10) of Directive 2014/53/EU of the European Parliament and of the Council;”;
F310o
for the definition of “importer” substitute—
importer” means a person who—
a
is established in the United Kingdom and places radio equipment from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places radio equipment on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
F311p
in the definition of “make available on the market” for “EU market” substitute “market of Great Britain”;
q
omit the definition of “national accreditation body”;
r
omit the definition of “notified body requirements”;
s
omit the definition of “Official Journal”;
F312t
in the definition of “place on the market” for “EU market” substitute “market of Great Britain”;
F313u
in the definition of “put into service” for “the EU” substitute “Great Britain”;
v
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
Omit paragraphs (3) and (6).
New regulation 2AI6353
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a reference to a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures;
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove the reference to a standard from publication referred to in paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to radio equipment to which these Regulations apply.
9
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies referred.
10
Regulations made under paragraph (9) are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 3I6364
In regulation 3 (scope)—
a
in paragraph (3), omit from “Directive” to “or”; and
b
in paragraph (4), omit from “Directive” to “or”.
Amendment to regulation 6I6375
In paragraph (1) of regulation 6 (essential requirements)—
a
in sub-paragraph (a), after “set out in” insert “
the Electrical Equipment (Safety) Regulations 2016
”
and omit from “Directive” to the end of the subparagraph, and
b
in sub-paragraph (b), after “set out in” insert “
the Electromagnetic Compatibility Regulations 2016
”
and omit from “Directive” to the end of the subparagraph.
Insertion of regulation 6AI6386
After regulation 6 insert—
Power to specify additional essential requirements6A
1
The Secretary of State may by regulations—
a
amend regulation 6 to add any of the matters listed in paragraph (2) as additional essential requirements; and
b
specify that only certain categories or classes of radio equipment are required to meet any additional essential requirements.
2
The matters that may be added as additional essential requirements are that the—
a
radio equipment interworks with accessories, in particular with common chargers;
b
radio equipment interworks via networks with other radio equipment;
c
radio equipment can be connected to interfaces of the appropriate type throughout the United Kingdom;
d
radio equipment does not harm the network or its functioning nor misuse network resources, thereby causing an unacceptable degradation of service;
e
radio equipment incorporates safeguards to ensure that the personal data and privacy of the user and of the subscriber are protected;
f
radio equipment supports certain features ensuring protection from fraud;
g
radio equipment supports certain features ensuring access to emergency services;
h
radio equipment supports certain features in order to facilitate its use by users with a disability;
i
radio equipment supports certain features in order to ensure that software can only be loaded into the radio equipment where the compliance of the combination of the radio equipment and software has been demonstrated.
3
Regulations made under paragraph (1)—
a
may make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate; and
b
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Amendment to regulation 8I6397
1
Regulation 8 (construction must allow operation in at least one Member State) is amended as follows.
2
In the heading for “in at least one Member State” substitute “
without infringement of requirements
”
.
3
In the regulation omit “in at least one Member State” and “in the relevant Member State or Member States”.
Amendment to regulation 10I6408
In regulation 10 (EU declaration of conformity and CE marking)—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)—
i
for “an EU” substitute “
a
”
; and
ii
for “(EU declaration of conformity)” substitute “
(declaration of conformity)
”
;
c
in paragraph (1)(b)—
i
for “CE” substitute “
UK
”
; and
ii
for “(CE marking)” substitute “
(UK marking)
”
;
d
in paragraph (2), omit “EU”; and
e
for paragraph (3) substitute—
3
Where radio equipment is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 11I6419
In regulation 11(retention of technical documentation and EU declaration of conformity), in the heading and in paragraph (a), omit “EU”.
Amendment to regulation 12I64210
In regulation 12 (identification of the radio equipment and manufacturer) in paragraph (3), for “competent national authority in the Member State in which it is to be made available to such end users” substitute “
enforcing authority
”
.
Amendment to regulation 13I64311
Regulation 13 (instructions and information to be included with the radio equipment) is amended as follows—
a
in paragraph (1) –
i
for sub-paragraph (a) substitute “
(a) are clear, legible and in easily understandable English,
”
;
ii
at the end of sub-paragraph (b) insert “
and
”
;
iii
at the end of sub-paragraph (c) for “, and” substitute “
.
”
; and
iv
omit sub-paragraph (d);
b
in paragraph (3), in each place in which it occurs, omit “EU”; and
c
omit paragraph (4).
Amendment to regulation 14I64412
In regulation 14 (information to be included where there are restrictions on putting into service or requirements for authorisation of use)—
a
in paragraph (1)—
i
omit “Member States and the”; and
ii
for “within a Member State” substitute “
in the United Kingdom
”
;
b
for paragraph (2)(b)—
i
for “Commission” substitute “
the
”
; and
ii
omit from “specifying” to the end;
c
in paragraph (3), for “on or after 8th August 2018” substitute “
on or after F338IP completion day ”
.
Amendment to regulation 15I64513
In regulation 15 (duty to take action in respect of radio equipment placed on the market which is considered not to be in conformity), in paragraph (2) omit “and the competent national authorities of any other Member State in which the manufacturer made the radio equipment available on the market,”.
Amendment to regulation 16I64614
In regulation 16 (provision of information and cooperation) for paragraph (1) substitute—
1
Following a request from the enforcing authority, the manufacturer must, within such reasonable period as the authority may specify, provide the authority concerned with all the information and documentation necessary to demonstrate that the radio equipment is in conformity with Part 2.
Amendment to regulation 17I64715
In regulation 17 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Insertion of regulations 18A, 18B and 18CI64816
After regulation 18 (monitoring) insert—
Provision of information on compliance of combinations of radio equipment and software18A
1
In this regulation “product” means a combination of radio equipment and software allowing such radio equipment to be used as intended.
2
The Secretary of State may by regulations make provision requiring a manufacturer of a product to provide the Secretary of State with information on the compliance of the product with the essential requirements.
3
Regulations under paragraph (2) may—
a
specify categories or classes of product for which a manufacturer must provide information on compliance;
b
include requirements as to—
i
the identification of the radio equipment and software intended to be used in combination;
ii
the results of conformity assessment carried out in accordance with regulation 41(conformity assessment procedures);
iii
the form the information must take;
c
make provision for the information on compliance to be made available to the enforcing authorities; and
d
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
4
Regulations made under paragraph (2) are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Power to amend R14(2)(b) and specify how information is to be presented18B
1
In this regulation “product” means radio equipment types that fall within categories of radio equipment affected by a low level of compliance with the essential requirements.
2
The Secretary of State may by regulations make provision requiring a manufacturer, before placing a product on the market, to—
a
register information on compliance with the essential requirements; and
b
affix to the product a registration number allocated by the Secretary of State.
3
Regulations made under paragraph (2) may specify—
a
the categories or classes of product in respect of which the manufacturer must register information;
b
that some or, where the Secretary of State considers necessary, all of the technical documentation listed in Schedule 5 must be registered;
c
that when setting out a registration process the Secretary of State must take the following matters into account—
i
whether the process includes a central system of registration by manufacturers;
ii
whether the process ensures appropriate control of access to information of a confidential nature; and
iii
whether the process allocates a registration number to each registered radio equipment type.
4
Regulations made under paragraph (2)—
a
may make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate; and
b
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Power to require registration of radio equipmentI91218C
1
The Secretary of State may by regulations—
a
amend regulation 14(2)(b);
b
amend the Implementing Regulation;
c
make provision specifying the manner and form in which information concerning any restrictions or putting into service or requirements for authorisation of use must be presented.
2
Regulations made under paragraph (1)—
a
may make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate; and
b
are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.”.
Amendment to regulation 19I64917
In regulation 19 (authorised representatives)—
a
in paragraph (1), for “within the EU” substitute “
in the United Kingdom
”
;
b
in paragraphs (3)(a) and (4)(c), omit “EU”; and
c
in paragraph (4)(c), for “CE” substitute “
UK
”
.
Amendment to regulation 21I65018
In regulation 21 (requirements which must be satisfied before an importer places radio equipment on the market)—
a
in paragraph (b), omit “in at least one Member State” and “in the relevant Member State or Member States”; and
b
in paragraph (d)(i) for “CE” substitute “
UK
”
.
Amendment to regulation 23I65119
Regulation 23 (information identifying importer) is amended as follows –
a
in paragraph (2), for “competent national authority in the Member State in which it is made available to such end-users” substitute “
enforcement authority
”
; and
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the radio equipment, or
b
before placing the radio equipment on the market, the importer sets out the information referred to in paragraph (1)-
i
on the packaging; or
ii
in a document accompanying the F340radio equipment.
Amendment to regulation 24I65220
In regulation 24 (instructions and safety information)—
a
in paragraph (1), for “in a language which can be understood by customers and other end users in the Member State in which the radio equipment is to be made available to such consumers and end-users” substitute “
that are clear, legible and in easily understandable English
”
; and
b
omit paragraph (2).
Amendment to regulation 27I65321
In regulation 27 (duty to take action in respect of radio equipment placed on the market which is considered not to be in conformity), in paragraph (2), omit “and the competent national authorities of any other Member State in which the importer made the radio equipment available on the market,”.
Amendment to regulation 28I65422
In regulation 28 (retention of technical documentation and EU declaration of conformity), in the heading to that regulation and in paragraph (a), omit “EU”.
Amendment to regulation 29I65523
In regulation 29 (provision of information and cooperation)—
a
in paragraph (1) omit “or a competent national authority of another Member State”; and
b
in paragraph (3)(b) for “authority concerned” substitute “
enforcing authority
”
.
Amendment to regulation 31I65624
In regulation 31 (requirements which must be satisfied before a distributor makes radio equipment available on the market)—
a
in paragraph (1)(a)(i), for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)(iii), for “in a language which can be easily understood by consumers and other end-users in the Member State in which the radio equipment is to be made available on the market” substitute “
which are clear, legible and in easily understandable English
”
;
c
in paragraph (1)(b)(i), for “in at least one Member State” substitute “
without infringement of requirements
”
; and
d
omit paragraph (2).
Amendment to regulation 34I65725
In regulation 34 (duty to take action in respect of radio equipment made available on the market which is not in conformity), in paragraph (2) omit “and the competent national authorities of the other Member States in which the distributor has made the radio equipment available on the market”.
Amendment to regulation 35I65826
In regulation 35 (provision of information and cooperation)—
a
in paragraph (1) omit “or a competent national authority of another Member State”; and
b
in paragraph (3)(b) for “authority concerned” substitute “
enforcing authority
”
; and
c
in paragraph (4) omit “a competent national authority of another Member State”.
New regulation 36AI65927
After regulation 36 insert—
Obligations which are met by complying with obligations in the Directive36A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(26);
c
“harmonised standard” has the meaning given to it in Article 2(18).
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing radio equipment on the market, the manufacturer—
a
ensures that the radio equipment has been designed and manufactured in accordance with the essential safety requirements set out in Article 3;
b
ensures that the conformity assessment procedure that applies to that radio equipment in accordance with Article 17 of the Directive has been carried out;
c
affixes a CE marking and where the conformity assessment procedure set out in Annex IV is applied, the notified body identification number, in accordance with Articles 19 and 20(1) to (3);
d
draws up the technical documentation in accordance with Article 21;
e
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
f
draws up an EU declaration of conformity, in accordance with Article 18; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 7, 9, 10(1) and (3) are to be treated as being satisfied;
b
regulations 2(2)(a), 10(2), 11, 19(3) and 39 apply subject to the modifications in paragraph (8);
c
Part 3 does not apply; and
d
regulation 63 does not apply.
4
Subject to paragraphs (6) and (7) paragraph (5) applies where, before placing radio equipment on the market, the importer ensures that—
a
the conformity assessment procedure that applies to that radio equipment in accordance with Article 17 has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex V; and
c
the radio equipment bears the CE marking referred to Article 19.
5
Where this paragraph applies—
a
the requirements of regulation 21(a) to (d) are to be treated as being satisfied; and
b
regulations 2(2)(a), 22(1), 25 and 28 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard as referred to in Article 16.
7
Where paragraph (6) applies paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to have carried out—
a
one of the conformity assessment procedures in Article 17(1) with respect to the essential requirements set out in Article 3(1); and
b
the conformity assessment procedure in Article 17(4) with respect to the essential requirements set out in Article 3(2) and (3).
8
Paragraph (9) applies where, before making radio equipment available on the market, a distributor ensures that the radio equipment bears the CE marking referred to in Article 19.
9
Where this paragraph applies—
a
regulation 31(a)(i) is satisfied; and
b
regulations 32(1) and 33 apply subject to the modifications in paragraph (10).
10
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (8)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements set out in Article 3;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard within the meaning of Article 2(18);
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the conformity assessment procedure that applies to the radio equipment in accordance with Article 17;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex V.
F343Expiry of regulations 36A and 36B36C
1
Subject to paragraph (2), regulation 36A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 36A—
a
any radio equipment which was placed on the market pursuant to regulation 36A may continue to be made available on the market on or after the expiry of regulation 36A;
b
any obligation to which a person was subject under regulation 36A in respect of any radio equipment placed on the market pursuant to regulation 36A continues to have effect after the expiry of regulation 36A, in respect of that equipment.
Qualifying Northern Ireland Goods36D
1
Where paragraph (2) applies radio equipment is to be treated as being in conformity with Part 2.
2
This paragraph applies where—
a
radio equipment—
i
is in conformity with Part 2, as that Part applies in Northern Ireland; and
ii
is qualifying Northern Ireland goods; and
b
an importer has complied with the obligations set out in paragraph (3).
3
The obligations referred to in paragraph (2)(b) are that, before placing the product on the market, the importer—
a
complies with regulation 23;
b
ensures that—
i
the relevant conformity assessment procedure has been carried out in relation to the product;
ii
the manufacturer has drawn up the technical documentation; and
iii
the product bears the CE marking;
4
In this regulation—
“CE marking” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“relevant conformity assessment procedure” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland;
“technical documentation” has the meaning given to it in regulation 2(1), as it applies in Northern Ireland.
Amendment to regulation 37I66028
Omit regulation 37 (translation of declaration of conformity).
Amendment to regulation 39I66129
In regulation 39 (prohibition on improper use of CE marking) in the heading to that regulation and in each place where it occurs, for “CE” substitute “
UK
”
.
Amendment to regulation 40I66230
In regulation 40 (presumption of conformity), paragraph (1)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal,”.
Amendment to regulation 41I66331
In regulation 41 (conformity assessment procedures)—
a
in paragraph (4)(b) omit “EU-”; and
b
in paragraphs (5) and (6)—
i
in each place where it occurs for “harmonised” substitute “
designated
”
; and
ii
omit “the references to which have been published in the Official Journal”.
Amendment to regulation 42I66432
In regulation 42 (EU declaration of conformity)—
a
in the heading, for “EU declaration” substitute “
Declaration
”
; and
b
in the opening words and paragraph (b), omit “EU”.
Amendment to regulation 43I66533
In regulation 43 (simplified EU declaration of conformity), in the heading and each place where it occurs, omit “EU”.
Amendment to regulation 44I66634
In regulation 44 (CE marking)—
F345a
for paragraph (1) substitute—
1
The UK marking must be affixed visibly, legibly and indelibly—
a
to the radio equipment or to its data plate, unless that is not possible or not warranted on account of the radio equipment; or
b
where paragraph (1A) applies—
i
to a label affixed to the radio equipment or its data plate; or
ii
to a document accompanying the radio equipment.
F344aa
after paragraph (1) insert—
1A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the radio equipment or its data plate; or
b
a document accompanying the radio equipment.
ab
for paragraph (2) substitute—
2
The UK marking must be affixed visibly and legibly—
a
to the radio equipment packaging; or
b
where paragraph (2A) applies—
i
to a label affixed to the radio equipment packaging; or
ii
a document accompanying the radio equipment packaging.
ac
after paragraph (2) insert—
2A
For a period of 24 months beginning with IP completion day, the UK marking may be affixed to—
a
a label affixed to the radio equipment packaging; or
b
a document accompanying the radio equipment packaging.
ad
in the heading and in paragraphs (3) to (5) for “CE” substitute “
UK
”
;
b
in all places where it occurs, for “notified body” substitute “
approved body
”
.
Amendment to regulation 45I66735
In regulation 45 (technical documentation)—
a
omit paragraph (3); and
b
in paragraph (4), for “paragraphs (1), (2) or (3)” substitute “
paragraphs (1) and (2)
”
.
Amendment to Part 4I66836
For Part 4 notification of conformity assessment bodies), substitute—
PART 4Approval of conformity assessment bodies
Approved bodies46
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 47 (approval of conformity assessment bodies); or
b
2
Paragraph (1) has effect subject to regulation 50 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Secretary of State had before F346IP completion day notified to the European Commission and the Member States of the European Union, in accordance with Article 22 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 46(1)(b) as it had effect immediately before F346IP completion day;
“approved body requirements” means the requirements set out in Schedule 8.
Approval of conformity assessment bodies47
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the radio equipment in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies48
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or that part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring49
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 47(6)(b); or
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval50
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 49(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 46 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 49(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 46.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies51
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 47; or
b
in respect of which body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 9.
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type-examination certificate referred to in Schedule 3; or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 44(5) (UK marking).
Subsidiaries and contractors52
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation, “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M100.
Register of approved bodies53
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body54
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 49; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 53.
Amendment to regulation 57I66937
In regulation 57 (enforcement powers) each place where it occurs, for “CE” substitute “
UK
”
.
Amendment to regulation 58I67038
In regulation 58 (exercise of enforcement powers), omit sub- paragraph (c).
Amendment to regulation 60I67139
In regulation 60 (enforcement action in respect of radio equipment which is not in conformity and which presents a risk)—
a
omit paragraphs (3), (4) and (7);
b
in paragraph (8), for “notifications under paragraphs (6) and (7), substitute “
notification under paragraph (6)
”
; and
c
in paragraph (8)(f)(ii), for “harmonised” substitute “
designated
”
.
Amendment to regulation 61I67240
Omit regulation 61 (EU safeguard procedure).
Amendment to regulation 62I67341
In regulation 62 (enforcement action in respect of radio equipment which is in conformity, but which presents a risk)—
a
omit paragraph (3); and
b
in paragraph (4), for “notifications referred to in paragraphs (2) and (3)” substitute “
notification referred to in paragraph (2)
”
.
Amendment to regulation 63I67442
In regulation 63 (enforcement action in respect of formal non-compliance)—
a
in paragraphs (1)(a), (1)(a)(ii), (1)(b)(ii) and (1)(c)(ii), in each place in which it occurs for “CE” substitute “
UK
”
;
b
in paragraph (1)(b), for “notified” substitute “
approved
”
; and
c
in paragraphs (1)(c) and (1)(c)(ii), in each place in which it occurs omit “EU”.
Amendment to regulation 77I67543
1
Regulation 77 (transitional provisions), is amended as follows.
2
In paragraph (a), for from “Directive” to the end of the paragraph substitute “
the Radio Equipment and Telecommunications Terminal Equipment Regulations 2000 M101
”
.
Transitional provision in relation to EU ExitI63444
After regulation 76 insert—
Transitional provision in relation to EU Exit76A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before F348IP completion day;
“product” means radio equipment to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 29 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M102, any obligation to which a person was subject under these Regulations as they had effect immediately before F348IP completion day, continues to have effect as it did immediately before F348IP completion day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the Member States of any matter; or
b
any obligation to take action outside of the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 41 as it had effect immediately before F348IP completion day in relation to that product,
that action has effect as if it had been done under regulation 41 as it has effect on and after F348IP completion day.
Amendment to regulation 78I67645
1
Regulation 78 (revocations and savings) is amended as follows.
2
For paragraph (2) substitute—
2
The Regulations referred to in paragraph (1) continue to apply, as if they had not been revoked, to any equipment placed on the market in accordance with those Regulations before the commencement date, subject to the modifications made in paragraph (2A).
3
Before paragraph (3), insert—
2A
The modifications referred to in paragraph (2) are as follows—
a
references to the Community are to be read as including the United Kingdom;
b
except where “Member State” first appears in regulation 14 (notified bodies), references to Member State are to be read as including the United Kingdom;
c
the references to European Union and EEA State in regulation 14 are both to be read as including the United Kingdom;
d
regulation 18A (duty of enforcement authority to inform Secretary of State of action taken), is to be read without the words “, with a view to this information being passed by her to the Commission.”;
e
Schedule 5 applies as if paragraph 6 were omitted.
Amendment to Schedule 1I67746
In Schedule 1 (radio equipment outside the scope of these regulations), for paragraph 2 substitute “
Marine equipment falling within the Merchant Shipping (Marine Equipment) Regulations 2016 M103
”
.
Amendment to Schedule 2I67847
In Schedule 2 (conformity assessment module A), in paragraph 4—
a
in the heading, and in subparagraphs (2) and (3), in each place where it occurs, omit “EU”; and
b
in the heading, and in subparagraph (1) in each place where it occurs, for “CE” substitute “
UK
”
.
Amendment to Schedule 3I67948
In Schedule 3 (conformity assessment modules B and C)—
a
in the first, second and fifth headings, and in paragraphs 1 and 2, for “EU-Type” substitute “
Type
”
;
b
in paragraphs 6(1) and (4), for “an EU-Type” substitute “
a Type
”
;
c
in each place in which it occurs in paragraphs, 3, 6(2), 6(3), 7(2) 8(1), 8(3), 9, 11, 12, and 13(1), for “EU-type” substitute “
Type
”
;
d
in each place in which it occurs in paragraphs 3(1), 3(2)(b), 4, 5, 6(1), 6(4), 7, and 8, for “notified” substitute “
approved
”
;
e
in paragraphs 3(2)(d) and 8(3) for “harmonised” substitute “
designated
”
;
f
in both places in which it occurs in paragraph 8(1) for “its notifying authority” substitute “
the Secretary of State
”
;
g
in paragraph 8(3)—
i
in the first place in which it occurs, for “Member States” substitute “
Secretary of State and the other approved bodies
”
;
ii
for “Member States, the European Commission” in the second sentence substitute “
Secretary of State
”
;
iii
for “Member States and the European Commission” in the third sentence substitute “
Secretary of State
”
; and
iv
omit “the references of which have been published in the Official Journal”;
h
in paragraph 9 for “national” substitute “
enforcing
”
;
i
in paragraph 13—
i
in the heading and in each place in which it occurs in sub-paragraph (1), for “CE” substitute “
UK
”
; and
ii
in the heading and in each place in which it occurs in sub-paragraphs (2) and (3), omit “EU”.
Amendment to Schedule 4I68049
In Schedule 4 (conformity assessment module H)—
a
in each place in which it occurs in paragraphs 3(1), 3(1)(d), 3(3), 3(9), 4(2), 4(3), 4(4), 5(1), 6, 7(1) and 7(2), as well as the heading to paragraph 4, for “notified” substitute “
approved
”
;
b
in paragraphs 3(2)(b) and 3(3)(b), for “harmonised” substitute “
designated
”
; and
c
in paragraph 5—
i
in the heading and in each place in which it occurs in paragraph (1), for “CE” substitute “
UK
”
;
ii
in the heading and in both places in which it occurs in sub-paragraph (2), omit “EU”; and
iii
in sub-paragraph (2) in both places in which it occurs for “national” and for “relevant” substitute “
enforcing
”
;
d
in paragraph 6, for “national” substitute “
enforcing
”
; and
e
in paragraph 7(1) in both places in which it occurs for “its notifying authority” substitute “
the Secretary of State
”
.
Amendment to Schedule 5I68150
In Schedule 5 (contents of technical documentation), in paragraph 1—
a
in subparagraph (d)—
i
in each place in which it occurs, for “harmonised” substitute “
designated
”
; and
ii
omit “the references of which have been published in the Official Journal”;
b
in subparagraph (e), omit “EU”;
c
in subparagraph (f)—
i
in both places in which it occurs, for “EU-type” substitute “
Type
”
; and
ii
for “notified” substitute “
approved
”
; and
d
in subparagraph (i), for “in at least one Member” substitute “
without infringement of requirements
”
.
Amendment to Schedule 6I68251
In Schedule 6 (EU declaration of conformity)—
a
in the heading and in paragraph 8 omit “EU”;
b
in the heading and subheading, for “declaration” substitute “
Declaration
”
;
c
in paragraph 5, for from “Union harmonisation legislation:” to “where applicable” substitute “
statutory requirements
”
;
d
in paragraph 6, for “harmonised” substitute “
designated
”
; and
e
in paragraph 7, for “EU-type” substitute “
Type
”
.
Amendments to Schedule 7I68352
In Schedule 7 (simplified EU declaration of conformity)—
a
in the heading and in paragraphs 1 and 3 omit “EU”; and
b
in paragraph 2, for “Directive 2014/53/EU” substitute “
the relevant statutory requirements
”
.
Amendment to Schedule 8I68453
In Schedule 8 (notified body requirements)—
a
in the heading and in paragraphs 5, 8, 9(c), 11(a) and 17, for “notified” substitute “
approved
”
;
b
in paragraph 11(c)—
i
for “harmonised” substitute “
designated
”
; and
ii
omit “, the Directive”;
c
in paragraph 11(d) for “EU-type” substitute “
Type
”
;
d
in paragraph 14 omit “and must satisfy the Secretary of State that it has”; and
e
in paragraph 17, for “under the Directive” substitute “
by the Secretary of State
”
.
Amendment to Schedule 9I68554
In Schedule 9 (operational obligations of notified bodies)—
a
for “notified” substitute “
approved
”
i
in the heading;
ii
in each place in which it occurs in paragraphs 7 and 9; and
iii
in the second place in which it occurs in paragraphs 12 and 13;
b
for “a notified” substitute “
an approved
”
in each place in which it occurs in paragraphs 1, 2, 5, 6, 8, 10, 11, 12, 13 and 14;
c
in all places in which it occurs in paragraphs 5, 8, 10 and 11, and in the first place in which it occurs in paragraph 6, for “an Eu-type” substitute “
a Type
”
;
d
in paragraph 6 in the second place in which it occurs and in all places in which it occurs in paragraphs 7 and 9 for “EU-Type” substitute “
Type
”
;
e
in paragraph 5, for “harmonised” substitute “
designated
”
;
f
in paragraph 10(b)—
i
in the first place in which it occurs for “notification” substitute “
approval
”
;
ii
in the second place in which it occurs for “notification” substitute “
approval of conformity assessment bodies
”
g
in paragraph 10(c), omit “or a competent national body of another Member State”;
h
in paragraph 12, for “the Directive” substitute “
these Regulations
”
; and
i
in paragraph 13, for “under the Directive” substitute “
by the Secretary of State
”
.
PART 2Amendment of Commission Implementing Regulation (EU) 2017/1354
I91355
Commission Implementing Regulation (EU) 2017/1354 specifying how to present the information provided for in Article 10(10) of Directive 2014/53/EU of the European Parliament and of the Council is amended in accordance with paragraphs 56 to 62.
I68656
In Article 1 and in both places in which it occurs in Article 2 for “Article 10(10) of Directive 2014/53/EU” substitute “
regulation 14 of the Radio Equipment Regulations 2017
”
.
I68757
In Article 1 for “at least one Member State” substitute “
the United Kingdom
”
.
I68858
In Article 2—
a
in paragraph 1(a) after “Annex I” insert “
, followed by the abbreviation “UK”
”
;
b
in paragraph 1(b)—
i
after “Requirements in” insert “
the UK
”
;
ii
omit from “, in a language” to the end;
c
in paragraph 2 for the words from “a language” to the end substitute—“English, the geographical areas in the United Kingdom where such restrictions or requirements exist, as well as the types of restrictions or requirements applicable”.
]I68959
Omit Article 3.
I69060
After Article 3 omit—
a
the words “This Regulation shall be binding” to the end;
b
“Done at Brussels, 20 July 2017”;
c
the signatory text.
I69161
In Annex I—
a
omit paragraph 3;
b
omit paragraph 5.
I69262
Omit Annex II.
SCHEDULE 30Amendment of the Identification and Traceability of Explosives Regulations (Northern Ireland) 2013
IntroductionI9141
The Identification and Traceability of Explosives Regulations (Northern Ireland) 2013 are amended as follows.
Amendment to regulation 5I6932
In regulation 5—
a
in paragraph (3)—
i
in the opening words for “that is not an EEA State” substitute “
other than the United Kingdom
”
,
ii
in sub-paragraph (a) for “an EEA State” substitute “
the United Kingdom
”
,
iii
in sub-paragraph (b) for “an EEA State” substitute “
the United Kingdom
”
,
iv
in sub-paragraph (c) for “either Great Britain or an EEA State other than the United Kingdom” substitute “
Great Britain
”
;
b
in paragraph (4)(b) omit “or an EEA State other than the United Kingdom”;
c
in paragraph (5)—
i
at the end of sub-paragraph (a) omit “and”,
ii
for sub-paragraph (b) substitute—
b
the importer must at the time of its application provide the Secretary of State with the details of any site code previously attributed to those explosives; and
c
the Secretary of State must attribute the code (which may be the same as the code previously attributed to the explosives) and inform the importer accordingly.
d
for paragraph (6) substitute—
6
Where this paragraph applies, the manufacturer must apply to the Health and Safety Executive for the Health and Safety Executive to attribute a code for the site where the explosives are manufactured.
Amendment to Schedule 1I6943
In Schedule 1, for paragraph 1(a)(ii)(aa) substitute—
aa
two letters identifying Northern Ireland, Great Britain or the EEA state (place of production or import);
SCHEDULE 31Amendment of the Making Available on the Market and Supervision of Transfers of Explosives Regulations (Northern Ireland) 2016
IntroductionI9151
The Making Available on the Market and Supervision of Transfers of Explosives Regulations (Northern Ireland) 2016 are amended as follows.
Amendment to regulation 2I6952
1
Regulation 2 (interpretation) is amended as follows—
2
In paragraph (1)—
a
omit the definition of “accreditation”;
b
omit the definition of “accreditation certificate”;
c
after the definition of “the 1993 Regulations” insert—
“approved body” has the meaning given to it in regulation 35 (approved bodies);
d
for the definition of “authorised representative” substitute—
“authorised representative” means—
a
a person who—
i
immediately before exit day was established in the United Kingdom or an EEA state; and was appointed by a manufacturer by written mandate to perform specified tasks for that manufacturer, in accordance with regulation 12, as it had effect immediately before exit day; and
ii
on or after exit day continues to be so established and appointed by the manufacturer to perform those tasks; or
b
a person who, on or after exit day is appointed in accordance with regulation 12;
e
omit the definition of “CE marking”;
f
omit the definition of “competent national authority”;
g
after the definition of “conformity assessment body” insert—
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 7;
h
after the definition of “the Department” insert—
“designated standard” has the meaning given to it in regulation 2A;
i
in the definition of “the Directive” at the end insert “
(as it has effect immediately before exit day)
”
;
j
omit the definition of “EU declaration of conformity”;
k
omit the definition of “harmonised standard”;
l
for the definition of “importer” substitute—
“importer”, in relation to civil explosives, means any person who—
a
is established in the United Kingdom; and
b
places a civil explosive from a country outside the United Kingdom on the market;
m
in the definition of “making available on the market” for “an EEA state” substitute “
the United Kingdom
”
;
n
omit the definition of “notified body requirements”;
o
in the definition of “place on the market”—
i
after “means” insert “
, apart from in regulation 45A,
”
;
ii
for “on the market in an EEA state” substitute “
on the United Kingdom market
”
;
p
for the definition of “relevant authority” substitute—
“relevant authority” means any public authority which has a function under these Regulations or a function under another enactment in relation to the security or traceability of civil explosives;
q
after the definition of “transfer” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4 of RAMS;
3
Omit paragraph (3).
Insertion of regulation 2AI6973
After regulation 2 insert—
Interpretation: designated standard2A
1
Subject to paragraphs (6) and (7), “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body for repeated or continuous application with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures;
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee of Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenlec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing a reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this regulation, a reference to a “product” is a reference to a civil explosive.
9
The Department may by regulations amend the list of recognised standardisation bodies in paragraph (3) to reflect any changes in the name or structure of those bodies made by the Secretary of State.
10
Regulations made under paragraph (9) are subject to negative resolution.
Amendment to regulation 4I6984
1
Regulation 4 (authorisation to transfer civil explosives) is amended as follows.
2
In paragraph (1) omit “for the place where the transfer will terminate”.
3
In paragraph (2) for “relevant authority” substitute “
relevant competent authority
”
.
4
In paragraph (5), in both places where it appears, for “the area of the EEA States” substitute “
the United Kingdom
”
.
5
After paragraph (7) insert—
7A
A recipient competent authority document issued under this regulation may be granted for such period as the competent authority determines and may be revoked by notice in writing by that authority on grounds of safety or security.
6
For paragraph (8) substitute—
8
In this regulation—
a
“competent authority” means the Chief Constable;
b
“recipient competent authority document” means a document issued in accordance with this regulation by the competent authority; and
c
“relevant competent authority” means—
i
in respect of a transfer or part of a transfer which takes place within Northern Ireland, the Chief Constable; and
ii
in respect of a transfer or part of a transfer which takes place in Great Britain, the body which discharges in Great Britain similar functions to those discharged by the Chief Constable under these Regulations in relation to Northern Ireland.
7
After paragraph (8) insert—
9
A transfer document issued under the Directive, which was valid immediately before exit day is deemed to be a valid recipient competent authority document for the purposes of this regulation after exit day, until such time as it expires or is withdrawn by a relevant competent authority.
Amendment to regulation 6I6995
Regulation 6 (technical documentation and conformity assessment) is amended as follows—
a
in paragraph (b)(i)—
i
for “32(a)” substitute “
32(2)(a)
”
;
ii
for “point 3(c) of Module B of Annex III to the Directive (as amended from time to time)” substitute “
paragraph 2(2)(c) of Part 1 (Module B) of Schedule 5
”
;
b
in paragraph (b)(ii)—
i
for “32(b)” substitute “
32(2)(b)
”
;
ii
for “point 2 of Module G of Annex III to the Directive (as amended from time to time)” substitute “
paragraph 46 of Part 6 (Module G) of Schedule 5
”
.
Amendment to regulation 7I7006
Regulation 7 (EU declaration of conformity and CE marking) is amended as follows—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
;
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a) omit “(EU declaration of conformity)”;
c
in paragraph (1)(b)—
i
for “CE” substitute “
UK
”
;
ii
omit “(CE marking)”;
d
for paragraph (3) substitute—
3
Where a civil explosive is subject to more than one enactment requiring a declaration of conformity to be drawn up, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 8I7017
In regulation 8 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 9I7028
In regulation 9 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Amendment to regulation 10I7039
In regulation 10 (traceability of certain civil explosives excluded from the scope of regulations 4, 5 and 6 of the Identification and Traceability of Explosives Regulations (Northern Ireland) 2013 (ITOER (NI) 2013)) for paragraph (4) substitute—
4
For a civil explosive that is to be made available on the market in Northern Ireland the contact details referred to in paragraph (1) must be provided in English.
Amendment to regulation 11I70410
For regulation 11 (instructions and safety information), substitute—
Instructions and safety information11
1
When placing a civil explosive on the market, a manufacturer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
2
Any labelling on the civil explosive must be clear, legible and in easily understandable English.
Amendment to regulation 12I70511
Regulation 12 (appointment of authorised representative by written mandate) is amended as follows—
a
in paragraph (1) after “appoint a person” insert “
established in the United Kingdom
”
;
b
in paragraph (2)(a) omit “EU”.
Amendment to regulation 14I70612
In regulation 14 (requirements which must be satisfied before an importer places a civil explosive on the market), in paragraph (1)(c)(i) for “CE” substitute “
UK
”
.
Amendment to regulation 16I70713
In regulation 16 (information identifying importer)—
a
after paragraph (1) insert—
1A
Paragraph (1) does not apply where the importer has imported the civil explosive from an EEA state F349or Switzerland and places it on the market within the period of eighteen months beginning with exit day, and before placing the civil explosive on the market, the importer sets out the information referred to in paragraph (1) in a document accompanying the civil explosive.
b
in paragraph (2) for “the market surveillance authority in the EEA State in which the civil explosive is to be made available to such end-users” substitute “
a relevant authority
”
.
Amendment to regulation 17I70814
For regulation 17 (instructions and safety information) substitute—
Instructions and safety information17
When placing a civil explosive on the market, an importer must ensure that it is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 18I70915
In regulation 18 (retention of technical documentation and EU declaration of conformity), in the heading to that regulation and in paragraph (a), omit “EU”.
Amendment to regulation 19I71016
In regulation 19 (duty to take action in respect of civil explosives placed on the market which are considered not to be in conformity), in paragraph (2) for “competent national authorities of any EEA State in which the manufacturer or importer made the civil explosive available on the market,” substitute “
market surveillance authority
”
.
Amendment to regulation 20I71117
In regulation 20 (provision of information and cooperation)—
a
in each place where it occurs, for “a competent national authority” substitute “
the market surveillance authority
”
; and
b
in paragraph (1)(b) for “in a language which can be easily understood by the authority” substitute “
in clear, legible and in easily understandable English
”
.
Amendment to regulation 22I71218
In regulation 22 (requirements which must be satisfied before a distributor makes a civil explosive available on the market), paragraph (1)(a) is amended as follows—
a
in sub-paragraph (i) for “CE” substitute “
UK
”
; and
b
for sub-paragraph (iii) substitute—
iii
is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 24I71319
In regulation 24 (duty to take action in respect of civil explosives made available on the market which are not in conformity), in paragraph (2) for “competent national authorities of any EEA State in which the distributor has made the civil explosive available on the market,” substitute “
market surveillance authority
”
.
Amendment to regulation 25I71420
In regulation 25 (provision of information and cooperation), in each place in which it occurs for “a competent national authority” substitute “
the enforcing authority
”
.
Revocation of regulation 28I71521
Omit regulation 28 (translation of declaration of conformity).
Amendment to regulation 30I71622
In regulation 30 (prohibition on improper use of CE marking), in the heading and in each place in which it occurs, for “CE” substitute “
UK
”
.
Insertion of regulation 30AI71723
After regulation 30 insert—
Obligations which are met by complying with obligations in the Directive30A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive;
b
“CE marking” has the meaning given to it in Article 2(24);
c
“harmonised standard” has the meaning given to it in Article 2(16).
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing a civil explosive on the market, the manufacturer—
a
ensures that the civil explosive has been designed and manufactured in accordance with the essential safety requirements set out in Annex II;
b
ensures that the relevant conformity assessment procedures that apply to that civil explosive in accordance with Article 20 have been carried out;
c
draws up the technical documentation referred to in Annex III;
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking, in accordance with Articles 22 and 23(1) to (5);
f
draws up an EU declaration of conformity, in accordance with Article 21; and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 5, 6, 7(1) and 7(3) are to be treated as being satisfied;
b
regulations 7(2), 8, 9(2), 12(2) and 30 apply subject to the modifications in paragraph (10); and
c
Schedule 2 paragraph 12 does not apply.
4
Subject to paragraphs (6) and (7), paragraph (5) applies where, before placing a civil explosive on the market, the importer ensures that—
a
the relevant conformity assessment procedures that apply to that explosive in accordance with Article 20 have been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III; and
c
the civil explosive bears the CE marking referred to in Article 23.
5
Where this paragraph applies—
a
the requirements of regulation 14(1)(a) to (c) are to be treated as being satisfied; and
b
regulations 13, 15(1), 18 and 26 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 19.
7
Where paragraph (6) applies paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to carry out one of the conformity assessment procedures set out in Article 20.
8
Paragraph (9) applies where, before making a civil explosive available on the market, a distributor ensures that the civil explosive bears the CE marking referred to in Article 23.
9
Where this paragraph applies—
a
regulation 22(1)(a)(i) is to be treated as being satisfied; and
b
regulations 23(1) and 26 apply subject to the modifications in paragraph (10).
10
The modifications referred to in sub-paragraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex II;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 20;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III.
Conformity assessment procedure obligation which is met by complying with the Directive30B
1
In this regulation any reference to an Article or an Annex is a reference to an Article or an Annex of the Directive.
2
Paragraph (3) applies where, prior to the manufacture of a civil explosive, the manufacturer ensures that the conformity assessment procedure that applies to that explosive in accordance with Article 20(a) has been carried out.
3
Where this paragraph applies—
a
any reference to “relevant conformity assessment procedure” in regulations 6(a), 7(1), 14(1)(a), 30(1)(b), 33(b) and 34(3) are to be read as including the conformity assessment procedure referred to in Article 20(a) of the Directive; and;
b
any reference to “technical documentation” in regulations 6(b), 8, 14(1)(b), 18(b), and in Schedule 2 Part 1 paragraph 12(1)(d) and Schedule 5 is to be read as including the technical documentation relating to the design of the civil explosive referred to in Annex III.
Amendment to regulation 31I71824
In regulation 31 (presumption of conformity), paragraph (1) is amended as follows—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal of the European Union,”.
Amendment to regulation 32I71925
For regulation 32 (conformity assessment procedures) substitute—
Conformity assessment procedures32
1
Assessment of conformity of a civil explosive is carried out by an approved body in accordance with the procedures set out in Schedule 5.
2
For the assessment of conformity of a civil explosive, the manufacturer must follow one of the following procedures set out in Schedule 5—
a
in Part 1 of Schedule 5, Type examination carried out by an approved body (Module B), and, at the choice of the manufacturer, one of the following procedures—
i
in Part 2 of Schedule 5, conformity to type based on internal production control plus supervised product checks at random intervals (Module C2);
ii
in Part 3 of Schedule 5, conformity to type based on quality assurance of the production process (Module D);
iii
in Part 4 of Schedule 5, conformity to type based on product quality assurance (Module E);
iv
in Part 5 of Schedule 5, conformity to type based on product verification (Module F);
b
in Part 6 of Schedule 5, conformity based on unit verification (Module G).
Amendment to regulation 33I72026
Regulation 33 (EU declaration of conformity) is amended as follows—
a
in the heading for “EU declaration” substitute “
Declaration
”
;
b
in the opening words omit “EU”;
c
in paragraph (b) for “Annex III to the Directive (as amended from time to time)” substitute “
Schedule 5
”
;
d
in paragraph (c) for “Annex IV to the Directive (as amended from time to time)” substitute “
Schedule 6
”
.
Amendment to regulation 34I72127
Regulation 34 (CE marking) is amended as follows—
a
in the heading for “CE” substitute “
UK
”
;
b
in paragraph (1) for “CE” substitute “
UK
”
;
c
in paragraph (2) for “CE” in both places it appears, substitute “
UK
”
;
d
in paragraph (3)—
i
for “CE” substitute “
UK
”
;
ii
for “notified” substitute “
approved
”
;
e
in paragraph (4) for “notified” in each place it appears, substitute “
approved
”
;
f
in paragraph (5) for “CE” substitute “
UK
”
.
Amendment to Part 3, Sub-Part CI72228
For Part 3, Sub-Part C (NOTIFICATION OF CONFORMITY ASSESSMENT BODIES) substitute—
SUB-PART C: APPROVAL OF CONFORMITY ASSESSMENT BODIES
Approved bodies35
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in regulation 36 (approval of conformity assessment bodies); or
b
immediately before exit day was a notified body in respect of which the Secretary of State had taken no action under regulation 41(1) or (2) as they had effect immediately before exit day to suspend or withdraw the body's status as a notified body.
2
Paragraph (1) has effect subject to regulation 39 (restriction, suspension or withdrawal of approval).
3
In this Sub-Part—
“notified body” means a body—
- a
which the Secretary of State had before exit day notified to the European Commission and to the other EEA states, in accordance with Article 24 of the Directive; and
- b
in respect of which no objections had been raised, as referred to in regulation 35(1)(b), as it had effect immediately before exit day;
“approved body requirements” means the requirements set out in Schedule 3.
Approval of conformity assessment bodies36
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second condition are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the civil explosives in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Secretary of State may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation, “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies37
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Secretary of State is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring38
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 36(6)(b); or
ii
in the case of an approved body which was a notified body immediately before exit day, in accordance with conditions set under regulation 36(6)(b) as it applied immediately before exit day; and
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval39
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement; or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 38(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under regulation 35 (approved bodies).
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in regulation 38(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under regulation 35.
3
In deciding what action is required under paragraph (1) or (2), the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2), the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for the Secretary of State and market surveillance authority for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies40
1
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 36; or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it must do so in accordance with Schedule 4 (operational obligations of approved bodies).
3
An approved body must make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Schedule 5 (conformity assessment procedures); or
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 34 (UK marking).
Subsidiaries and contractors41
1
An approved body may subcontract specific conformity assessment activities or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M104.
Register of approved bodies42
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) must be made publicly available.
UK national accreditation body43
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 38; and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 42.
Transitional provision in relation to EU ExitI69629
After regulation 45 (transitional provisions) insert—
Transitional provision in relation to EU Exit45A
1
In this regulation—
“pre-exit period” means the period beginning with 20th April 2016 and ending immediately before exit day;
“product” means a civil explosive to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 31 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M105, any obligation to which a person was subject under these Regulations as they had effect immediately before exit day, continues to have effect as it did immediately before exit day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 6 as it had effect immediately before exit day in relation to that product,
that action has effect as if it had been done under regulation 6 as it had effect on and after exit day.
Amendment to Schedule 1I72330
In Schedule 1 at the beginning omit “(This Schedule reproduces, with minor modifications, the provision of Annex II to the Directive)”.
Amendments to Schedule 2I72431
Schedule 2 is amended as follows—
a
in paragraph 2 for “the Directive” substitute “
these Regulations
”
;
b
omit paragraph 4(c);
c
in paragraph 9(2) for “notified body” substitute “
approved body
”
;
d
omit paragraph 9(4);
e
omit paragraph 9(7);
f
in paragraph 9(8)—
i
for “The notices in sub-paragraphs (6) and (7)” substitute “
The notice in sub-paragraph (6)
”
;
ii
in head (f)(ii) for “harmonised” substitute “
designated
”
;
g
in paragraph 9(10) for “competent national authority” substitute “
relevant authority
”
;
h
omit paragraph 10 (EU safeguarding procedures);
i
omit paragraph 11(3);
j
in paragraph 11(4) for “The notices referred to in sub-paragraphs (2) and (3)” substitute “
The notice referred to in sub-paragraph (2)
”
;
k
in paragraph 12—
i
in sub-paragraphs (1)(a), (1)(b) and (1)(c), for “CE marking” substitute “
UK marking
”
in each place it appears;
ii
in sub-paragraph (1)(b) for “a notified body” substitute “
an approved body
”
;
iii
in sub-paragraph (1)(b) “the notified body” substitute “the approved body”; and
iv
in sub-paragraph (1)(c) for “EU declaration of conformity” substitute “
declaration of conformity
”
in each place it appears.
Amendment to Schedule 3I72532
Schedule 3 is amended as follows—
a
in the heading, for “Notified” substitute “
Approved
”
;
b
in paragraph 8 for “notified” substitute “
approved
”
;
c
in paragraph 11(c)—
i
for “harmonised” substitute “
designated
”
;
ii
omit “and of the Directive”;
d
in paragraph 17—
i
for “notified” substitute “
approved
”
;
ii
for “under the Directive” substitute “
by the Secretary of State
”
.
Amendments to Schedule 4I72633
Schedule 4 is amended as follows—
a
in the heading for “Notified” substitute “
Approved
”
;
b
in paragraph 1, for “A notified” substitute “
An approved
”
;
c
in paragraph 2, for “A notified” substitute “
An approved
”
;
d
in paragraph 3, for “A notified” substitute “
An approved
”
;
e
in paragraph 4, for “A notified” substitute “
An approved
”
;
f
in paragraph 5—
i
for “a notified” substitute “
an approved
”
;
ii
for “harmonised” substitute “
designated
”
;
g
in paragraph 6, for “a notified” substitute “
an approved
”
;
h
in paragraph 7, for “notified” substitute “
approved
”
in both places it appears;
i
in paragraph 8, for “a notified” substitute “
an approved
”
;
j
in paragraph 9, for “notified” substitute “
approved
”
;
k
in paragraph 10—
i
for “A notified” substitute “
An approved
”
;
ii
in sub-paragraph (b)—
aa
for “notification” in the first place it appears substitute “
approval
”
;
bb
in the second place it appears omit “(notification)”;
iii
in sub-paragraph (d) for “notification” substitute “
approval
”
;
l
in paragraph 11, for “A notified” substitute “
An approved
”
;
m
in paragraph 12—
i
for “A notified” substitute “
An approved
”
;
ii
for “notified under the Directive” substitute “
approved under these Regulations
”
;
n
in paragraph 13—
i
for “A notified” substitute “
An approved
”
;
ii
for “any notified body” substitute “
any approved body
”
;
iii
for “under the Directive” substitute “
by the Secretary of State
”
.
Insertion of Schedule 5 and Schedule 6I72734
After Schedule 4 insert—
SCHEDULE 5CONFORMITY ASSESSMENT PROCEDURES
PART 1TYPE EXAMINATION (MODULE B)
1
1
Type examination (Module B) is a conformity assessment procedure in which an approved body examines the technical design of an explosive and verifies and attests that the technical design of the explosive meets the requirements of these Regulations that apply to it.
2
Type examination must be carried out as an assessment of the adequacy of the technical design of the explosive through—
a
examination of the technical documentation and supporting evidence referred to in paragraph 2; and
b
examination of a specimen of the production envisaged which is representative of the complete product (combination of production type and design type).
2
1
A manufacturer must lodge an application for Type examination (Module B) with an approved body of the manufacturer's choice.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
the technical documentation;
d
the specimens representative of the production envisaged, and any further specimens requested by the approved body if needed for carrying out the test programme;
e
the supporting evidence for the adequacy of the technical design solution; this supporting evidence must—
i
mention any documents that have been used, in particular where the relevant designated standards have not been applied in full;
ii
include, where necessary, the results of tests carried out in accordance with other relevant technical specifications by the appropriate laboratory of the manufacturer, or by another testing laboratory on the manufacturer's behalf and under the manufacturer's responsibility.
3
The technical documentation referred to in paragraph 2(2)(c) must—
a
make it possible to assess the explosive's conformity with the applicable requirements of these Regulations and must include an adequate analysis and assessment of any risks;
b
specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the explosive;
c
contain, wherever applicable, at least the following elements—
i
a general description of the explosive;
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the explosive;
iv
a list of the designated standards applied in full or in part (where applicable specifying the parts which have been applied);
v
where designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements, including a list of other relevant technical specifications applied to meet the essential safety requirements;
vi
the results of design calculations made and examinations carried out;
vii
test reports.
4
1
The approved body must examine the technical documentation and supporting evidence in respect of an explosive to assess the adequacy of the technical design of the explosive.
2
For each of the specimens examined, the approved body must—
a
verify that the specimen—
i
has been manufactured in conformity with the technical documentation;
ii
identifies the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
b
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
c
carry out, or arrange the carrying out of, appropriate examinations and tests to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential safety requirements;
d
agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body must draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes and, without prejudice to the approved body's obligations in relation to the Secretary of State, the approved body may disclose the content of that report, in full or in part, only with the agreement of the manufacturer.
6
1
Where the type meets the applicable requirements of these Regulations, the approved body must issue a Type examination certificate to the manufacturer, which must contain—
a
the name and address of the manufacturer;
b
the conclusions of the examination;
c
the conditions (if any) for its validity;
d
the necessary data for the identification of the approved type;
e
all relevant information to allow the conformity of manufactured explosives with the examined type to be evaluated and to allow for in-service control.
2
The Type examination certificate referred to in sub-paragraph (1)—
a
may have one or more annexes attached;
b
must be accompanied by the descriptions and drawings necessary for identification of the approved type.
3
Where the type does not satisfy the applicable requirements of these Regulations, the approved body must refuse to issue a Type examination certificate and must inform the applicant accordingly, giving detailed reasons for its refusal.
7
An approved body must keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations, and must determine whether such changes require further investigation and, if so, the approved body must inform the manufacturer accordingly.
8
A manufacturer must inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the explosive with the essential safety requirements or the conditions for validity of that certificate; such modifications require additional approval in the form of an addition to the original Type examination certificate.
9
1
Each approved body must inform the Secretary of State of all Type examination certificates and any additions thereto which it has issued or withdrawn, and must, periodically or upon request, make available to the Secretary of State the list of such certificates and any additions thereto refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of all Type examination certificates and any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and must, upon request, inform the other approved bodies of such certificates and additions thereto which it has issued.
3
The other approved bodies and the Secretary of State may obtain from the approved body a copy of—
a
the Type examination certificates and additions thereto;
b
the technical documentation and the results of the examinations carried out by the approved body.
4
An approved body must keep a copy of the Type examination certificate, its annexes and additions, as well as the file containing the technical documentation including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
5
A manufacturer must keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
10
A manufacturer's authorised representative (if any) may lodge the application referred to in paragraph 2 and fulfil the obligations set out in paragraphs 8 and 9(5), provided that they are specified in the mandate by which they were appointed under regulation 12.
PART 2CONFORMITY TO TYPE BASED ON INTERNAL PRODUCTION CONTROL PLUS SUPERVISED PRODUCT CHECKS AT RANDOM INTERVALS (MODULE C2)
11
Conformity to type based on internal production control plus supervised product checks at random intervals (Module C2) is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 12 to 14, and it is solely the manufacturer's responsibility to ensure and declare that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing12
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosives with the type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Product checks13
1
The approved body chosen by the manufacturer must carry out product checks or have them carried out at random intervals determined by that body, in order to verify the quality of the internal checks on the explosive, taking into account, amongst other things, the technological complexity of the explosives and the quantity of production.
2
The approved body must ensure that—
a
it takes an adequate sample of the final product on site before its placing on the market; and
b
the sample is examined and appropriate tests as identified by the relevant parts of the designated standards, or equivalent tests set out in other relevant technical specifications, are carried out to check the conformity of the explosive with the type described in the Type examination certificate and with the relevant requirements of these Regulations.
3
Where a sample does not conform to the acceptable quality level, the approved body must take appropriate measures.
4
The acceptance sampling procedure to be applied is intended to determine whether the manufacturing process of the explosive performs within acceptable limits, with a view to ensuring conformity of the explosive.
5
The manufacturer must, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking and declaration of conformity14
1
A manufacturer must affix the UK marking to each individual explosive that is in conformity with the type described in the Type examination certificate and which satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market; the declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authority upon request.
Authorised representative15
A manufacturer's obligations set out in paragraph 14 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 12.
PART 3CONFORMITY TO TYPE BASED ON QUALITY ASSURANCE OF THE PRODUCTION PROCESS (MODULE D)
16
Conformity to type based on quality assurance of the production process (Module D) is a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 17 and 23, and it is solely the manufacturer's responsibility to ensure and declare that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing17
A manufacturer must operate an approved quality system for production, final product inspection and testing of the explosives specified in paragraph 18, and which is subject to surveillance as specified in paragraph 22.
Quality system18
1
A manufacturer must lodge an application for assessment of the manufacturer's quality system with an approved body of the manufacturer's choice.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the explosive category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
19
1
The quality system must ensure that the explosives are in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations that apply to them.
2
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions.
3
The quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used;
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out;
d
quality records, such as inspection reports and test data, calibration data, and qualification reports on the personnel concerned;
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
20
1
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in paragraph 19 and, where applicable, it must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
2
The audit team appointed by the approved body to carry out the audit in sub-paragraph (1) (“the audit”) must have experience in quality management systems, with at least one member of the team having experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations.
3
The audit must include an assessment visit to the manufacturer's premises.
4
The audit team must review the technical documentation referred to in paragraph 18(2)(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the explosive with those requirements.
5
The decision of the approved body must be notified to the manufacturer and must contain the conclusions of the audit and a reasoned assessment of the decision.
21
1
A manufacturer must—
a
fulfil the obligations arising out of the quality system as approved and maintain it in an adequate and efficient state; and
b
keep the approved body that has approved the quality system informed of any intended change to the quality system.
2
Where the approved body is notified by a manufacturer of any proposed change to the quality system the approved body must—
a
evaluate such proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 19 or whether a reassessment is necessary; and
b
notify the manufacturer of its decision and, that notification must contain the conclusions of the examination and a reasoned assessment of the decision.
Surveillance under the responsibility of the approved body22
1
The approved body must carry out surveillance, the purpose of which is to ensure that a manufacturer fulfils the obligations arising out of the approved quality system.
2
A manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide the approved body with all necessary information including, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data, and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to ensure that a manufacturer maintains and applies the quality system and, following each audit, must provide the manufacturer with an audit report.
4
The approved body may pay unexpected visits to a manufacturer; during such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly; and following such a visit the approved body must provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking and declaration of conformity23
1
A manufacturer must affix the UK marking, and, under the responsibility of the approved body referred to in paragraph 18(1), the latter's identification number to each individual explosive that is in conformity with the type described in the Type examination certificate and which satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market; the declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authority upon request.
24
A manufacturer must, for a period of 10 years beginning on the day on which the explosive has been placed on the market, keep at the disposal of the relevant authority—
a
the documentation referred to in paragraph 18(2);
b
any information relating to the change referred to in paragraph 21(1)(b) and 21(2), as approved;
c
the decisions and reports of the approved body referred to in paragraphs 21, 22(3) and 22(4).
25
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
26
Each approved body must inform other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative27
A manufacturer's obligations set out in paragraphs 18(1), 18(2), 21(1)(b), 21(2), 23 and 24 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 12.
PART 4CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE (MODULE E)
28
Conformity to type based on product quality assurance (Module E) is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 29 and 34, and it is solely manufacturer's responsibility to ensure and declare that the explosives concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing29
A manufacturer must operate an approved quality system for final product inspection and testing of the explosives concerned as specified in paragraphs 30 and 31 and, which must be subject to surveillance as specified in paragraph 33.
Quality system30
1
A manufacturer must lodge an application for assessment of the manufacturer's quality system with an approved body of the manufacturer's choice for the explosives concerned.
2
The application must include—
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, the name and address of the authorised representative;
b
a written declaration that the same application has not been lodged with any other approved body;
c
all relevant information for the explosive category envisaged;
d
the documentation concerning the quality system;
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system must ensure compliance of the explosives with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
4
All the elements, requirements and provisions adopted by the manufacturer must be documented in a systematic and orderly manner in the form of written policies, procedures and instructions; this quality system documentation must permit a consistent interpretation of the quality programmes, plans, manuals and records and, it must, in particular, contain an adequate description of—
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality;
b
the examinations and tests that will be carried out after manufacture;
c
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned;
d
the means of monitoring the effective operation of the quality system.
31
1
The approved body must assess the quality system to determine whether it satisfies the requirements referred to in paragraph 30(3) and (4) and, where applicable, it must presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of a relevant designated standard.
2
The audit team, appointed by the approved body to carry out the audit under sub-paragraph (1) (“the audit”) must have experience in quality management systems and have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations.
3
The audit must include an assessment visit to the manufacturer's premises.
4
The audit team must review the technical documentation referred to in paragraph 30(2)(e), in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the explosive with those requirements.
5
The decision of the approved body must be notified to the manufacturer and, the notification must contain the conclusions of the audit and the reasoned assessment for the decision.
32
1
A manufacturer must—
a
fulfil the obligations arising out of the quality system as approved and maintain it in an adequate and efficient state; and
b
keep the approved body that has approved the quality system informed of any intended change to the quality system.
2
Where the approved body is notified by a manufacturer of any proposed change to the quality system the approved body must—
a
evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 30(3) and (4) or whether a reassessment is necessary; and
b
notify the manufacturer of its decision and, that notification must contain the conclusions of the examination and the reasoned assessment for the decision.
Surveillance under the responsibility of the approved body33
1
The approved body must carry out surveillance, the purpose of which is to ensure that a manufacturer fulfils the obligations arising out of the approved quality system.
2
A manufacturer must, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and must provide it with all necessary information, in particular—
a
the quality system documentation;
b
the quality records, such as inspection reports and test data, calibration data and qualification reports on the personnel concerned.
3
The approved body must carry out periodic audits to ensure that a manufacturer maintains and applies the quality system and, following each audit, must provide the manufacturer with an audit report.
4
The approved body may pay unexpected visits to the manufacturer; during such visits the approved body may carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly and, following such a visit, the approved body must provide the manufacturer with a visit report and, if tests have been carried out, a test report.
UK marking and declaration of conformity34
1
A manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 30(1), the latter's identification number to each individual explosive that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
3
A copy of the declaration of conformity must be made available to the relevant authority upon request.
35
A manufacturer must, for a period of 10 years, beginning on the day on which the explosive has been placed on the market, keep at the disposal of the relevant authority—
a
the documentation referred to in paragraph 30(1) and 30(2);
b
the information relating to the change referred to in paragraph 32, as approved;
c
the decisions and reports of the approved body referred to in paragraphs 32(2), 33(3) and 33(4).
36
1
Each approved body must inform the Secretary of State of quality system approvals issued or withdrawn, and must, periodically or upon request, make available to the Secretary of State the list of quality system approvals refused, suspended or otherwise restricted.
2
Each approved body must inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
Authorised representative37
A manufacturer's obligations set out in paragraphs 30(1), 30(2), 32(1)(b), 34 and 35 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 12.
PART 5CONFORMITY TO TYPE BASED ON PRODUCT VERIFICATION (MODULE F)
38
Conformity to type based on product verification (Module F) is the part of a conformity assessment procedure whereby a manufacturer fulfils the obligations laid down in paragraphs 39, 42(1) and 43, and it is solely the manufacturer's responsibility to ensure and declare that the explosives concerned, which have been subject to examinations and tests under paragraph 40, are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing39
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosives with the approved type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Verification40
1
An approved body chosen by the manufacturer must carry out appropriate examinations and tests in order to check the conformity of the explosives with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
2
The examinations and tests to check the conformity of the explosives with the appropriate requirements must be carried out, at the choice of the manufacturer, either—
a
by examination and testing of every product as specified in paragraph 41; or
b
by examination and testing of the explosives on a statistical basis as specified in paragraph 42.
Verification of conformity by examination and testing of every product41
1
All explosives must be individually examined and appropriate tests in the relevant designated standard or equivalent tests in other relevant technical specifications must be carried out in order to verify conformity with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out, and must affix its identification number to each approved explosive or have it affixed under its responsibility.
3
A manufacturer must keep the certificates of conformity available for inspection by the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
Statistical verification of conformity42
1
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure the homogeneity of each lot produced, and must present the manufacturer's explosives for verification in the form of homogeneous lots.
2
The approved body must take a random sample from each lot; all explosives in a sample must be individually examined and appropriate tests set out in the relevant designated standards, or equivalent tests set out in other relevant technical specifications, must be carried out in order to verify their conformity with the approved type described in the Type examination certificate and with the applicable requirements of these Regulations and to determine whether the lot is accepted or rejected; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
3
If a lot is accepted, all explosives of the lot must be considered approved, except for those explosives from the sample that have been found not to satisfy the tests.
4
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out, and must affix its identification number to each approved explosive or have it affixed under its responsibility.
5
A manufacturer must keep the certificates of conformity at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
6
If a lot is rejected, the approved body, or enforcing authority, must take appropriate measures to prevent the placing on the market of that lot and, in the event of the frequent rejection of lots the approved body may suspend statistical verification and take appropriate measures.
UK marking and declaration of conformity43
1
A manufacturer must affix the UK marking, and, under the responsibility of the approved body referred to in paragraph 40(1), the latter's identification number to each individual explosive confirming that the explosive is in conformity with the approved type described in the Type examination certificate and that it satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity for each explosive type and keep it at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market and, such a declaration of conformity must identify the explosive type for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authority upon request.
4
If the approved body referred to in paragraph 40(1) agrees, and under its responsibility, the manufacturer may affix the approved body's identification number to the explosives.
5
If the approved body referred to in paragraph 40(1) agrees and under its responsibility, a manufacturer may affix the approved body's identification number to the explosives during the manufacturing process.
Authorised representative44
A manufacturer's obligations under this Part of this Schedule may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 12, but an authorised representative may not fulfil the manufacturer's obligations set out in paragraphs 39 and 42(1).
PART 6CONFORMITY BASED ON UNIT VERIFICATION (MODULE G)
45
Conformity based on unit verification (Module G) is the conformity assessment procedure whereby a manufacturer fulfils the obligations laid down in paragraphs 46, 47 and 49, and it is solely the manufacturer's responsibility to ensure and declare that the explosive concerned, which has been subject to the provisions of paragraph 48, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation46
1
A manufacturer must establish the technical documentation and make it available to the approved body referred to in paragraph 48; the documentation must make it possible to assess the explosive's conformity with the relevant requirements and must include an adequate analysis and assessment of any risks.
2
The technical documentation must specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the explosive and, wherever applicable, the technical documentation must contain at least the following elements—
a
a general description of the explosive;
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies and circuits;
c
descriptions and explanations necessary for the understanding of the drawings and schemes and the operation of the explosive;
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential safety requirements of these Regulations, including a list of other relevant technical specifications applied; and in the case of partly applied designated standards, the technical documentation must specify the parts which have been applied;
e
results of design calculations made and examinations carried out; and
f
test reports.
3
A manufacturer must keep the technical documentation at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
Manufacturing47
A manufacturer must take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured explosive with the applicable requirements of these Regulations.
Verification48
1
An approved body chosen by the manufacturer must carry out, or have carried out, appropriate examinations and tests set out in the relevant designated standards, or equivalent tests set out in other relevant technical specifications, to check the conformity of the explosive with the applicable requirements of these Regulations; in the absence of such a designated standard, the approved body concerned must decide on the appropriate tests to be carried out.
2
The approved body must issue a certificate of conformity in respect of the examinations and tests carried out and must affix its identification number to the approved explosive, or have it affixed under its responsibility.
3
A manufacturer must keep the certificates of conformity at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market.
UK marking and declaration of conformity49
1
A manufacturer must affix the UK marking and, under the responsibility of the approved body referred to in paragraph 48, the latter's identification number to each explosive that satisfies the applicable requirements of these Regulations.
2
A manufacturer must draw up a written declaration of conformity and keep it at the disposal of the relevant authority for 10 years beginning on the day on which the explosive has been placed on the market and, the declaration of conformity must identify the explosive for which it has been drawn up.
3
A copy of the declaration of conformity must be made available to the relevant authority upon request.
Authorised representative50
A manufacturer's obligations set out in paragraphs 46(3) and 49 may be fulfilled by the manufacturer's authorised representative (if any), on the manufacturer's behalf and under the manufacturer's responsibility, provided that they are specified in the mandate by which they were appointed under regulation 12.
SCHEDULE 6DECLARATION OF CONFORMITY
Declaration of conformity (No XXXX) M106
1
No … (product, type, batch or serial number):
2
Name and address of the manufacturer and, where applicable, the manufacturer's authorised representative:
3
This declaration of conformity is issued under the sole responsibility of the manufacturer.
4
Object of the declaration (identification of product allowing traceability):
5
The object of the declaration described above is in conformity with the relevant statutory requirements:
6
References to the relevant designated standards used or references to the other technical specifications in relation to which conformity is declared:
7
The approved body … (name, number) performed … (description of intervention) and issued the certificate:
8
Additional information:
Signed for and on behalf of: (place and date of issue): (name, function) (signature):
SCHEDULE 32Amendment of the Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations (Northern Ireland) 2017
IntroductionI9161
The Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations (Northern Ireland) 2017 are amended in accordance with paragraphs 2 to 41.
Amendment to regulation 2I7282
1
Regulation 2 (interpretation) is amended as follows.
2
In paragraph (1)—
a
in the definition of the “1994 Directive” at the end insert “
(as it has effect immediately before exit day)
”
;
b
after the definition of the “1996 Regulations” insert—
“approved body” has the meaning given to it in regulation 42;
c
omit the definition of “accreditation certificate”;
d
in the definition of “attestation of conformity”—
i
omit “EU”; and
ii
for “CE” substitute “
UK
”
;
e
for the definition of “authorised representative” substitute—
“authorised representative” means—
a
a person who—
i
immediately before exit day was established in the United Kingdom or an EEA state and was appointed by a manufacturer by written mandate to perform specified tasks for that manufacturer, in accordance with regulation 17; and
ii
on or after exit day continues to be so established and appointed by the manufacturer to perform those tasks; or
b
a person who, on or after exit day, is appointed in accordance with regulation 17 as it had effect immediately before exit day;
f
omit the definition of “CE Marking”;
g
omit the definition of “competent national authority”;
h
after the definition of “conformity assessment” insert—
“conformity assessment activities” means any activities connected with conformity assessment including calibration, testing, certification and inspection;
i
after the definition of “conformity assessment body” insert—
“conformity assessment procedure” means a procedure referred to in regulation 39 (conformity assessment procedures);
“declaration of conformity” means a declaration of conformity required to be drawn up in accordance with regulation 7(1)(a) (declaration of conformity and UK marking);
“designated standard” has the meaning given to it in regulation 2A;
j
for the definition of “equipment category” substitute—
“equipment category” means the classification of equipment, within each equipment group, specified in Schedule 1A to these Regulations;
k
in the definition of “equipment-group I” for “as set out in Annex I to the ATEX Directive (as amended from time to time)” substitute “
as set out in Schedule 1A to these Regulations
”
;
l
in the definition of “equipment-group II” for “as set out in Annex I to the ATEX Direction (as amended from time to time)” substitute “
as set out in Schedule 1A to these Regulations
”
;
m
omit the definition of “EU declaration of conformity”;
n
omit the definition of “European Commission”;
o
omit the definition of “harmonised standard”;
p
for the definition of “importer” substitute—
“importer” means any person who—
a
is established in the United Kingdom; and
b
places a product from a country outside of the United Kingdom on the market;
q
in the definition of “make available on the market” for “EU” substitute “
United Kingdom
”
;
r
omit the definition of “national accreditation body”;
s
omit the definition of “notified body requirements”;
t
omit the definition of “Official Journal”;
u
in the definition of “place on the market” for “EU” substitute “
United Kingdom
”
;
v
in the definition of “putting into service” omit “within the EU market”;
w
after the definition of “technical specification” insert—
“UK marking” means the marking in the form set out in Annex 2 of RAMS;
“UK national accreditation body” means the body appointed by the Executive in accordance with Article 4 of RAMS.
3
After paragraph (1) insert—
1A
Schedule 1A reproduces the provisions of Annex I to the ATEX Directive with amendments to correct deficiencies in retained EU law.
1B
A reference to a provision of Schedule 1A is a reference to the equivalent provision of Annex I to the ATEX Directive as set out in Schedule 1A.
1C
Schedule 3A reproduces the provisions of Annexes III to IX to the ATEX Directive with amendments to correct deficiencies in retained EU law
1D
A reference to any provision of Schedule 3A is a reference to the equivalent provision of Annexes III to IX as set out in Schedule 3A.
4
Omit paragraph (3).
5
Omit paragraph (6).
Insertion of regulation 2AI7303
After regulation 2 insert—
Designated standard2A
1
Subject to paragraphs (6) and (7), in these Regulations a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, service or system, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product, service or system as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product, service or system.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph (1)(b), the Secretary of State shall have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State shall have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
The Secretary of State may by regulations amend paragraph (3) to reflect any changes in the name or structure of the recognised standardisation bodies.
9
Regulations made under paragraph (8) are to be made by statutory instrument.
10
A statutory instrument containing regulations made under paragraph (8) is subject to annulment in pursuance of a resolution in the House of Commons.
Amendment to regulation 3I7314
In regulation 3(3) (scope) for paragraph (g) substitute—
g
products connected with the production of trade in arms, munitions and war material;
Amendment to regulation 6I7325
In regulation 6 (technical documentation and conformity assessment) for paragraph (b) substitute—
b
draw up the technical documentation referred to—
i
for a product in respect of which the conformity assessment procedure in regulation 39(1)(a) is being carried out, in paragraph 3(c) of Part 1 of Schedule 3A to these Regulations;
ii
for a product in respect of which the conformity assessment procedure in regulation 39(1)(b) is being carried out, in paragraph 3(c) of Part 1 of Schedule 3A to these Regulations;
iii
for a product in respect of which the conformity assessment procedure in regulation 39(1)(c) is being carried out, in paragraph 2 of Part 6 of Schedule 3A to these Regulations;
iv
for a product in respect of which the conformity assessment procedure in regulation 39(1)(d) is being carried out, in paragraph 2 of Part 7 of Schedule 3A to these Regulations.
Amendment to regulation 7I7336
Regulation 7 (EU declaration of conformity and CE marking) is amended as follows—
a
in the heading to that regulation—
i
for “EU declaration” substitute “
Declaration
”
; and
ii
for “CE” substitute “
UK
”
;
b
in paragraph (1)(a) omit “EU”;
c
in paragraph (1)(b) for “CE” substitute “
UK
”
each time it occurs;
d
in paragraphs (2), (4) and (5) omit “EU”;
e
for paragraph (6) substitute—
6
Where a product is subject to more than one enactment requiring the drawing up of a declaration of conformity, the manufacturer shall draw up a single declaration of conformity which identifies each enactment by its title.
Amendment to regulation 8I7347
In regulation 8 (retention of technical documentation and EU declaration of conformity) and in the heading of that regulation omit “EU”.
Amendment to regulation 9I7358
In regulation 9 (compliance procedures for series production), in paragraph (2)(b)—
a
for “harmonised” substitute “
designated
”
;
b
omit “EU”.
Amendment to regulation 13I7369
In regulation 13 (information identifying manufacturer), for paragraph 3 substitute—
3
The information specified in paragraph (1) must be in a language which can be easily understood by end users and the market surveillance authority.
Amendment to regulation 14I73710
For regulation 14 (instructions and safety information) substitute—
Provision of instructions and safety information14
When placing a product on the market, a manufacturer must ensure that a product is accompanied by instructions and safety information in clear, legible and easily understandable English.
Amendment to regulation 15I73811
In regulation 15 (duty to take action in respect of a product placed on the market which is considered not to be in conformity), in paragraph (2) omit “, and the competent national authorities of any other Member State in which the manufacturer made the product available on the market,”.
Amendment to regulation 17I73912
In regulation 17 (authorised representatives)—
a
in paragraph (1) for “EU” substitute “
United Kingdom
”
;
b
in paragraph (4)(a) omit “EU”.
Amendment to regulation 19I74013
In regulation 19 (requirements which must be satisfied before an importer places a product on the market)—
a
in paragraph (1)(c)(i) for “CE” substitute “
UK
”
;
b
in paragraph (1)(c)(ii) omit “EU”;
c
in paragraph (2)(c) for “14(1) (instructions and safety information)” substitute “
14 (provision of instructions and safety information)
”
.
Amendment to regulation 21I74114
Regulation 21 (information identifying importer) is amended as follows—
a
in paragraph (2) for “by the competent national authority in the Member State in which it is to be made available to end-users” substitute “
the enforcing authority
”
;
b
for paragraph (3) substitute—
3
Paragraph (1) does not apply where—
a
either—
i
it is not possible to set out the information referred to in paragraph (1) on the product, or
ii
the importer has imported the product from an EEA state F350or Switzerland and places it on the market within the period of 18 months beginning with exit day, and
b
before placing the product on the market, the importer sets out the information referred to in paragraph (1)—
i
on the packaging; or
ii
in a document accompanying the product.
Amendment to regulation 22I74215
For regulation 22 (instructions and safety information) substitute—
Provision of instructions and safety information22
When placing a product on the market, an importer shall ensure that the product is accompanied by instructions and safety information that are clear, legible and in easily understandable English.
Amendment to regulation 25I74316
In regulation 25 (duty to take action in respect of a product placed on the market which is considered not to be in conformity), in paragraph (2) omit “, and the competent national authorities of any other Member State in which the importer made the product available on the market,”.
Amendment to regulation 27I74417
In regulation 27 (retention of technical documentation and EU declaration of conformity) and in the heading to that regulation omit “EU”.
Amendment to regulation 29I74518
In regulation 29 (requirements which must be satisfied before a distributor makes a product available on the market)—
a
in paragraph (1)(a)(i) for “CE” substitute “
UK
”
;
b
in paragraph (1)(a)(ii) omit “EU”;
c
for paragraph (1)(a)(iv) substitute—
iv
is accompanied by instructions and safety information that are clear, legible and in easily understandable English;
Amendment to regulation 32I74619
In regulation 32 (duty to take action in respect of products made available on the market which are not in conformity), in paragraph (2) omit “, and the competent national authorities of the other Member States in which the distributor has made the product available on the market,”.
Amendment to regulation 36I74720
In regulation 36 (prohibition on improper use of CE marking) in each place in which it occurs, and in the heading, for “CE” substitute “
UK
”
.
Insertion of regulations 36A and 36BI74821
After regulation 36 insert—
Obligations which are met by complying with obligations in the ATEX Directive36A
1
In this regulation—
a
any reference to an Article or an Annex is a reference to an Article or an Annex of the ATEX Directive;
b
“CE marking” has the meaning given to it in Article 2(26); and
c
“harmonised standard” has the meaning given to in in Article 2(18).
2
Subject to paragraphs (6) and (7), paragraph (3) applies where, before placing the product on the market, the manufacturer—
a
ensures that the product has been designed and manufactured in accordance with the essential safety requirements set out in Annex II;
b
ensures that the relevant conformity assessment procedures that apply to that product in accordance with Article 13(1) and (2) have been carried out;
c
draws up the technical documentation referred to in Annexes III to IX;
d
ensures that the records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the inscriptions in accordance with Articles 15 and 16(1) to (4);
f
draws up an EU declaration of conformity, in accordance with Article 14; and
g
ensures that the declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of regulations 5, 6, 7(1), (3) and 7(6) are to be treated as being satisfied;
b
regulations 2(a), 7(6), 8, 9(2), 17(4) 36 and 59 apply subject to the modifications in paragraph (10);
c
Part 3 does not apply; and
d
regulation 57 does not apply.
4
Subject to paragraphs (6) and (7), paragraph (5) applies where, before placing a product on the market, the importer ensures that—
a
the relevant conformity assessment procedure referred to in Article 13 has been carried out;
b
the manufacturer has drawn up the technical documents relevant to the conformity assessment procedure followed; and
c
the product bears the CE marking and inscriptions referred to in point 1.0.5 of Annex II.
5
Where this paragraph applies—
a
the requirements of regulation 19(1)(a) to (c) are to be treated as being satisfied; and
b
regulations 2(a), 18, 23 and 27 apply subject to the modifications in paragraph (10).
6
This paragraph applies where there is no designated standard or part of a designated standard which corresponds exactly to a harmonised standard or part of a harmonised standard referred to in Article 12.
7
Where paragraph (6) applies, paragraphs (2)(b) and (4)(a) are to be treated as requiring the manufacturer to carry out—
a
the conformity assessment procedure set out in Article 13(1)(b); and
b
the relevant conformity assessment procedure that applies to that product in accordance with Article 13(2).
8
Paragraph (9) applies where, before making a product available on the market, a distributor ensures that the product bears the CE marking and inscriptions referred to in point 1.0.5 of Annex II.
9
Where this paragraph applies—
a
regulation 29(1)(a)(i) is to be treated as being satisfied; and
b
regulations 2(a), 30 and 31(1) apply subject to the modifications in paragraph (10).
10
The modifications referred to in subparagraphs (3)(b), (5)(b) and (9)(b) are that—
a
any reference to “declaration of conformity” is to be read as a reference to the EU declaration of conformity;
b
any reference to “UK marking” is to be read as a reference to the CE marking;
c
any reference to “essential safety requirements” is to be read as a reference to the essential safety requirements referred to in Annex II;
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “relevant conformity assessment procedure” is to be read as a reference to the relevant conformity assessment procedures referred to in Article 13;
f
any reference to “technical documentation” is a reference to the technical documentation referred to in Annexes III to IX.
Conformity assessment procedure obligation which is met by complying with the ATEX Directive36B
1
In this regulation any reference to an Article or Annex is a reference to an Article or an Annex of the ATEX Directive;
2
Paragraph (3) applies where, prior to the manufacture of a product, the manufacturer ensures that the conformity assessment procedure that applies to that product in accordance with Annex III as referred to in Article 13(1)(a) and (b) has been carried out.
3
Where this paragraph applies—
a
any requirement to follow the Type examination set out in Part 1 of Schedule 3A in regulation 39 is to be treated as being satisfied;
b
any reference to “relevant conformity assessment procedure” in regulations 6(a), 7(1), 19(a), 36(1)(b), 40(c) and 41(3) is to be read as including the conformity assessment procedure set out in Annex III as referred to in Article 13(1)(a) and (b); and
c
any reference to “technical documentation” in regulations 6(b), 8, 19(b) and 27 is to be read as including the technical documentation relating to the design of the product referred to in Annex III.
Omission of regulation 37I74922
Omit regulation 37 (translation of declaration of conformity).
Amendment to regulation 38I75023
In regulation 38 (presumption of conformity), in paragraph (1)—
a
for “harmonised” substitute “
designated
”
; and
b
omit “the reference to which has been published in the Official Journal”.
Amendment to regulation 39I75124
In regulation 39 (conformity assessment procedures)—
a
for paragraph (1)(a) substitute—
a
for equipment-groups I and II, equipment-categories M1 and 1, the manufacturer shall follow either—
i
the Type-examination set out in Part 1 of Schedule 3A, in conjunction with either the procedure set out in—
aa
Part 2 of Schedule 3A, or
bb
Part 3 of Schedule 3A; or
ii
the conformity based on unit verification referred to in Part 7 of Schedule 3A;
b
for paragraph (1)(b) substitute—
b
for equipment-groups I and II, equipment-categories M2 and 2, the manufacturer shall follow—
i
for internal combustion engines and electrical equipment in these groups and categories the Type examination set out in Part 1 of Schedule 3A, in conjunction with either the procedure set out in either Part 4 or Part 5 of Schedule 3A unless subparagraph (1)(d) applies;
ii
for other equipment in these groups and categories the procedures set out in Part 6 of Schedule 3A unless subparagraph (1)(d) applies;
c
for paragraph (1)(c) substitute—
c
for equipment group II, equipment-category 3, the procedure relating to internal production control referred to in Part 6 of Schedule 3A unless subparagraph (1)(d) applies;
d
for paragraph (1)(d) substitute—
d
for equipment groups I and II, instead of the procedures referred to in paragraphs (1)(a), (b) and (c), the manufacturer may follow conformity based on unit verification referred to in Part 7 of Schedule 3A.
e
in paragraph (2) omit “or (d)”;
f
in paragraph (3)(a)(i) for “CE” substitute “
UK
”
;
g
in paragraph (3)(a)(ii) omit “EU”;
h
in paragraph (4) for “Annex VIII to the ATEX Directive (as amended from time to time)” substitute “
Part 6 of Schedule 3A
”
;
i
in paragraph (5), omit “in the Member State concerned”;
j
in paragraph (6) for “Member State” substitute “
designated state
”
.
Amendment to regulation 40I75225
Regulation 40 (EU declaration of conformity) is amended as follows—
a
in the heading and in the body of the regulation, omit “EU”;
b
in paragraph (c) for “Annexes III to IX to the ATEX Directive (as amended from time to time)” substitute “
Schedule 3A
”
.
Amendment to regulation 41I75326
In regulation 41 (CE marking)—
a
in the heading, and the regulation, for “CE” substitute “
UK
”
in each place in which it occurs;
b
for “notified body” substitute “
approved body
”
in each place in which it occurs.
Amendment to Part 4I75427
For Part 4, substitute—
PART 4APPROVAL OF CONFORMITY ASSESSMENT BODIES
Approved bodies42
1
An approved body is a conformity assessment body which—
a
has been approved by the Executive pursuant to the procedure set out in regulation 43 (approval of conformity assessment bodies); or
b
immediately before exit day was a notified body in respect of which the Executive has taken no action under regulation 46(1) or (2) as they had effect immediately before exit day.
2
Paragraph (1) has effect subject to regulation 46 (restriction, suspension or withdrawal of approval).
3
In this Part—
“notified body” means a body—
- a
which the Executive had before exit day notified to the European Commission and the member States of the European Union, in accordance with Article 17 of the ATEX Directive; and
- b
in respect of which no objections had been raised as referred to in regulation 42(1)(b) as it had effect immediately before exit day;
“approved body requirements” means the requirements set out in Schedule 2.
Approval of conformity assessment bodies43
1
The Executive may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Executive to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of products in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate, or
ii
the documentary evidence necessary for the Executive to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Executive is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph (4), the Executive may accept an accreditation certificate, provided in accordance with paragraph (3)(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that applies for approval, the Executive may—
a
have regard to any other matter which appears to the Executive to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this regulation “accreditation certificate” means a certificate, issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
Presumption of conformity of approved bodies44
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such standard), the Executive is to presume that the conformity assessment body meets the approved body requirements covered by that standard (or part of that standard).
2
The presumption in paragraph (1) is rebuttable.
Monitoring45
The Executive shall monitor each approved body with a view to verifying that the body—
a
continues to meet the approved body requirements;
b
meets any conditions set—
i
in accordance with regulation 43(6)(b), or
ii
in the case of an approved body which was a notified body immediately before exit day, in accordance with regulation 43(6)(b) as it applied immediately before exit day; and
c
carries out its functions in accordance with these Regulations.
Restriction, suspension or withdrawal of approval46
1
Where the Executive determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in regulation 45(b),
the Executive shall restrict, suspend or withdraw the body's status as an approved body under regulation 42 (approved bodies).
2
Where the Executive determines that an approved body no longer meets a condition referred to in regulation 45(b), the Executive may restrict, suspend or withdraw the body's status as an approved body under regulation 42.
3
In deciding what action is required under paragraph (1) or (2) the Executive shall have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph (1) or (2) the Executive shall—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Executive regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Executive has taken action in respect of an approved body under paragraph (1) or (2), or where an approved body has ceased its activity, the approved body shall, at the request of the Executive—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Executive, or
b
keep its files relating to the activities it has undertaken as an approved body available for the Executive and market surveillance authorities for a period of 10 years from the date they were created.
6
The activities undertaken by an approved body referred to in paragraph (5) include any activities that the body has undertaken as a notified body.
Operational matters in relation to approved bodies47
1
Subject to the terms of its appointment, an approved body shall carry out the conformity assessment activities and procedures—
a
in respect of which the body's approval was given under regulation 43 (approval of conformity assessment bodies), or
b
in respect of which the body's notification as a notified body was made.
2
Where an approved body carries out a conformity assessment procedure, it shall do so in accordance with Schedule 3.
3
An approved body shall make provision for a manufacturer to be able to make an appeal against a refusal by the approved body—
a
to issue a Type examination certificate referred to in Part 1 of Schedule 3A;
b
to affix, or cause to be affixed, the body's identification number pursuant to regulation 41 (UK marking).
Subsidiaries and contractors48
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meet the approved body requirements;
b
the body has informed the Executive that it is satisfied that the subcontractor or subsidiary meet those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body shall, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Executive all relevant documents concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this regulation “subsidiary” has the meaning given to it in section 1159 of the Companies Act 2006 M107;
Register of approved bodies49
1
The Executive shall—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body notification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph (1) shall be made publicly available.
UK national accreditation body50
The Executive may authorise the UK national accreditation body to carry out the following activities on behalf of the Executive—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with regulation 45(monitoring); and
c
compiling and maintaining the register of approved bodies, in accordance with regulation 49(register of approved bodies).
Amendment to regulation 54I75528
In regulation 54 (exercise of enforcement powers) omit paragraph (c).
Amendment to regulation 56I75629
Regulation 56 (enforcement action in respect of products which are not in conformity and which present a risk) is amended as follows—
a
in paragraph (2) for “notified” substitute “
approved
”
;
b
in paragraph (3) for “shall inform the European Commission, Great Britain and the other Member states” substitute “
shall inform Great Britain
”
;
c
in paragraph (5) for “shall notify the European Commission, Great Britain and the other Member States” substitute “
shall notify Great Britain
”
;
d
in subparagraph (6)(f)(ii) for “harmonised” substitute “
designated
”
.
Amendment to regulation 57I75730
Omit regulation 57 (EU safeguard procedure).
Amendment to regulation 58I75831
In regulation 58 (enforcement action in respect of products which are in conformity, but present a risk), in paragraph (2) for “the European Commission, Great Britain and the other Member States” substitute “
Great Britain
”
.
Amendment to regulation 59I75932
Regulation 59 (enforcement action in respect of formal non-compliance) is amended as follows—
a
in paragraphs (1)(a) and (1)(c)(ii) for “CE” substitute “
UK
”
in each place in which it occurs;
b
in paragraph (1)(b) for “a notified” substitute “
an approved
”
; and
c
in paragraph (1)(c) omit “EU” in each place in which it occurs.
Amendment to regulation 71I76033
In regulation 71 (transitional provisions) omit paragraph (2).
I72934
After regulation 71 insert—
Transitional provision in relation to EU Exit71A
1
In this regulation—
“pre-exit period” means the period beginning with the commencement date and ending immediately before exit day;
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 32 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M108, any obligation to which a person was subject under these Regulations as they had effect immediately before exit day, continues to have effect as it did immediately before exit day, in relation to that product.
3
Paragraph (2) does not apply to—
a
any obligation of any enforcing authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the market in respect of that product.
4
Where during the pre-exit period—
a
a product has not been placed on the market; and
b
a manufacturer has taken any action under regulation 38 (Presumption of conformity) as it had effect immediately before exit day in relation to that product;
that action has effect as if it had been done under regulation 38 as it had effect on and after exit day.
Amendment to regulation 72I76135
Regulation 72(consequential amendments, revocations and savings) is amended as follows—
a
for paragraph (3) after “Equipment and Protective Systems Intended for use in Potentially Explosive Atmospheres (Amendment) Regulations (Northern Ireland) 2008” insert “
subject to the modifications in paragraph (3A)
”
;
b
after paragraph (3), insert—
3A
The modifications in the 1996 Regulations referred to in paragraph (3) are as follows—
a
references to the Community shall be read as including the United Kingdom;
b
references to a member State shall be read as including the United Kingdom;
c
references to a “notified body” shall be read as “approved body” as defined in The Equipment and Protective Systems Intended for Use in Potentially Explosive Atmospheres Regulations (Northern Ireland) 2017 M109.
Amendment to Schedule 1I76236
Schedule 1 (essential health and safety requirements) is amended as follows—
a
in paragraph 5(1)(b) for “CE marking (see Annex II RAMS)” substitute “
UK marking;
”
;
b
at paragraph 13(2)—
i
for “other European Union legislation” substitute “
any other enactment
”
;
ii
for “European Union legislation” substitute “
specific enactment
”
.
Insertion of Schedule 1AI76337
After Schedule 1 insert—
SCHEDULE 1ACriteria determining the classification of equipment-groups into categories (Annex I to the ATEX Directive)
1
Equipment group I
a
Equipment category M 1 comprises equipment designed and, where necessary, equipped with additional special means of protection to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a very high level of protection.
Equipment in this category is intended for use in underground parts of mines as well as those parts of surface installations of such mines endangered by firedamp and/or combustible dust.
Equipment in this category is required to remain functional, even in the event of rare incidents relating to equipment, with an explosive atmosphere present, and is characterised by means of protection such that:
either, in the event of failure of one means of protection, at least an independent second means provides the requisite level of protection,
or the requisite level of protection is assured in the event of two faults occurring independently of each other.
Equipment in this category must comply with the supplementary requirements referred to in paragraph 30 of Schedule 1.
b
Equipment category M 2 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a high level of protection.
Equipment in this category is intended for use in underground parts of mines as well as those parts of surface installations of such mines likely to be endangered by firedamp and/or combustible dust.
This equipment is intended to be de-energised in the event of an explosive atmosphere.
The means of protection relating to equipment in this category assure the requisite level of protection during normal operation and also in the case of more severe operating conditions, in particular those arising from rough handling and changing environmental conditions.
Equipment in this category must comply with the supplementary requirements referred to in paragraph 31 of Schedule 1.
2
Equipment-group II
a
Equipment category 1 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and ensuring a very high level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by mixtures of air and gases, vapours or mists or by air/dust mixtures are present continuously, for long periods or frequently.
Equipment in this category must ensure the requisite level of protection, even in the event of rare incidents relating to equipment, and is characterised by means of protection such that:
either, in the event of failure of one means of protection, at least an independent second means provides the requisite level of protection,
or the requisite level of protection is assured in the event of two faults occurring independently of each other.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 32 and 33 of Schedule 1.
b
Equipment category 2 comprises equipment designed to be capable of functioning in conformity with the operational parameters established by the manufacturer and of ensuring a high level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by gases, vapours, mists or air/dust mixtures are likely to occur occasionally.
The means of protection relating to equipment in this category ensure the requisite level of protection, even in the event of frequently occurring disturbances or equipment faults which normally have to be taken into account.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 34 and 35 of Schedule 1.
c
Equipment category 3 comprises equipment designed to be capable of functioning in conformity with the operating parameters established by the manufacturer and ensuring a normal level of protection.
Equipment in this category is intended for use in areas in which explosive atmospheres caused by gases, vapours, mists, or air/dust mixtures are unlikely to occur or, if they do occur, are likely to do so only infrequently and for a short period only.
Equipment in this category ensures the requisite level of protection during normal operation.
Equipment in this category must comply with the supplementary requirements referred to in paragraphs 36 and 37 of Schedule 1.
Amendment to Schedule 2I76438
Schedule 2 (notified body requirements) is amended as follows—
a
in the heading and in paragraphs 6, 9, 12(a) and 18 substitute each “
notified
”
for “approved”;
b
for “a notified body” substitute “
an approved body
”
in every place in which it occurs;
c
in paragraph 3 for “regulation 43 (notification)” substitute “
regulation 43 (approval of conformity assessment bodies)
”
;
d
in paragraph 10(b) for “a notified” substitute “
an approved
”
;
e
in paragraph 12(c) for “harmonised standards and of the ATEX Directive” substitute “
designated standards
”
; and
f
in paragraph 18 for “under the ATEX Directive” substitute “
by the Executive
”
.
Amendment to Schedule 3I76539
In Schedule 3 (operational obligations of notified bodies) is amended as follows—
a
in the shoulder reference for “Regulation 49” substitute “
Regulation 47
”
;
b
in the heading and in paragraphs 7 and 9 for “notified body” substitute “
approved body
”
;
c
in all places in which it occurs (other than where stated in paragraph (b)) for “a notified body” substitute “
an approved body
”
;
d
in paragraphs 10(b) and (c) for “regulation 44” substitute “
regulation 43 (approval of conformity assessment bodies)
”
;
e
in paragraph 10(c) for “notification” substitute “
approval
”
;
f
in paragraph 12 for “bodies notified under the ATEX Directive” substitute “
bodies approved under these Regulations
”
;
g
in paragraph 13 for “notified body coordination group established under the ATEX Directive” substitute “
approved body coordination group established by the Executive
”
.
Insertion of Schedule 3AI76640
After Schedule 3 insert—
SCHEDULE 3AConformity Assessment Procedures (Annexes III to IX to the ATEX Directive)
PART 1
TYPE EXAMINATION1
Type examination is the part of a conformity assessment procedure in which an approved body examines the technical design of a product and verifies and attests that the technical design of the product meets the requirements of these Regulations that apply to it.
2
Type examination shall be carried out with the examination of a specimen, representative of the production envisaged, of the complete product (production type).
3
The manufacturer shall lodge an application for Type examination with a single approved body of his choice.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
a written declaration that the same application has not been lodged with any other approved body,
c
the technical documentation. The technical documentation shall make it possible to assess the product's conformity with the applicable requirements of these Regulations and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
i
a general description of the product,
ii
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.,
iii
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
iv
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
v
results of design calculations made, examinations carried out, etc., and
vi
test reports,
d
the specimens representative of the production envisaged. The approved body may request further specimens if needed for carrying out the test programme.
4
The approved body shall:
4
examine the technical documentation, verify that the specimen(s) have been manufactured in conformity with the technical documentation, and identify the elements which have been designed in accordance with the applicable provisions of the relevant designated standards, as well as the elements which have been designed in accordance with other relevant technical specifications;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the manufacturer has chosen to apply the solutions in the relevant designated standards, these have been applied correctly;
4
carry out appropriate examinations and tests, or have them carried out, to check whether, where the solutions in the relevant designated standards have not been applied, the solutions adopted by the manufacturer applying other relevant technical specifications meet the corresponding essential health and safety requirements of these Regulations;
4
agree with the manufacturer on a location where the examinations and tests will be carried out.
5
The approved body shall draw up an evaluation report that records the activities undertaken in accordance with paragraph 4 and their outcomes. Without prejudice to its obligations vis-à-vis the Executive, the approved body shall release the content of that report, in full or in part, only with the agreement of the manufacturer.
6
Where the type meets the requirements of these Regulations that apply to the product concerned, the approved body shall issue a Type examination certificate to the manufacturer. That certificate shall contain the name and address of the manufacturer, the conclusions of the examination, the conditions (if any) for its validity and the necessary data for identification of the approved type. The Type examination certificate may have one or more annexes attached.
The Type examination certificate and its annexes shall contain all relevant information to allow the conformity of manufactured products with the examined type to be evaluated and to allow for in-service control.
Where the type does not satisfy the applicable requirements of these Regulations, the approved body shall refuse to issue a Type examination certificate and shall inform the applicant accordingly, giving detailed reasons for its refusal.
7
The approved body shall keep itself apprised of any changes in the generally acknowledged state of the art which indicate that the approved type may no longer comply with the applicable requirements of these Regulations and shall determine whether such changes require further investigation. If so, the approved body shall inform the manufacturer accordingly.
The manufacturer shall inform the approved body that holds the technical documentation relating to the Type examination certificate of all modifications to the approved type that may affect the conformity of the product with the essential health and safety requirements of these Regulations or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original Type examination certificate.
8
Each approved body shall inform the Executive concerning the Type examination certificates and/or any additions thereto which it has issued or withdrawn, and shall, periodically or upon request, make available to the Secretary of State the list of such certificates and/or any additions thereto refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies concerning the Type examination certificates and/or any additions thereto which it has refused, withdrawn, suspended or otherwise restricted, and, upon request, concerning such certificates and/or additions thereto which it has issued.
Great Britain may, on request, obtain a copy of the Type examination certificates and/or additions thereto. On request, Great Britain may obtain a copy of the technical documentation and the results of the examinations carried out by the approved body. The approved body shall keep a copy of the Type examination certificate, its annexes and additions, as well as the technical file including the documentation submitted by the manufacturer, until the expiry of the validity of that certificate.
9
The manufacturer shall keep a copy of the Type examination certificate, its annexes and additions together with the technical documentation at the disposal of the national authorities for 10 years after the product has been placed on the market.
10
The manufacturer's authorised representative may lodge the application referred to in paragraph 3 and fulfil the obligations set out in paragraphs 7 and 9, provided that they are specified in the mandate.
PART 2CONFORMITY TO TYPE BASED ON QUALITY ASSURANCE OF THE PRODUCTION PROCESS
1
Conformity to type based on quality assurance of the production process is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5, and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for production, final product inspection and testing of the products concerned as specified in paragraph 3 and shall be subject to surveillance as specified in paragraph 4.
Quality system3
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the products concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well,
b
a written declaration that the same application has not been lodged with any other approved body,
c
all relevant information for the product category envisaged,
d
the documentation concerning the quality system,
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure that the products are in conformity with the type described in the Type examination certificate and comply with the requirements of these Regulations that apply to them.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality,
b
the corresponding manufacturing, quality control and quality assurance techniques, processes and systematic actions that will be used,
c
the examinations and tests that will be carried out before, during and after manufacture, and the frequency with which they will be carried out,
d
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc., and
e
the means of monitoring the achievement of the required product quality and the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in paragraph 3.1(e) to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation,
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model, other than a component and keep it at the disposal of the national authorities for 10 years after the product other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
6
The manufacturer shall, for a period ending 10 years after the product has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved,
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Executive of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Executive the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended, withdrawn or otherwise restricted, and, upon request, of quality system approvals which it has issued.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 3CONFORMITY TO TYPE BASED ON PRODUCT VERIFICATION
1
Conformity to type based on product verification is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5 and ensures and declares on his sole responsibility that the products concerned, which have been subject to the provisions of paragraph 3, are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the approved type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Verification3
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests in order to check the conformity of the products with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
The examinations and tests to check the conformity of the products with the appropriate requirements shall be carried out by examination and testing of every product as specified in paragraph 4.
Verification of conformity by examination and testing of every product4
4
All products shall be individually examined, and appropriate tests set out in the relevant designated standard(s) and/or equivalent tests set out in other relevant technical specifications, shall be carried out in order to verify conformity with the approved type described in the Type examination certificate and with the appropriate requirements of these Regulations.
In the absence of such a designated standard, the approved body concerned shall decide on the appropriate tests to be carried out.
4
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to each approved product or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity available for inspection by the national authorities for 10 years after the product has been placed on the market.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3, the latter's identification number to each individual product other than a component that is in conformity with the approved type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model other than a component and keep it at the disposal of the national authorities, for 10 years after the product, other than a component, has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
If the approved body referred to in paragraph 3 agrees and under its responsibility, the manufacturer may also affix the approved body's identification number to the products other than components.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
6
If the approved body agrees and under its responsibility, the manufacturer may affix the approved body's identification number to the products during the manufacturing process.
Authorised representative7
The manufacturer's obligations may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate. An authorised representative may not fulfil the manufacturer's obligations set out in paragraph 2.
PART 4CONFORMITY TO TYPE BASED ON INTERNAL PRODUCTION CONTROL PLUS SUPERVISED PRODUCT TESTING
1
Conformity to type based on internal production control plus supervised product testing is the part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 4, and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured products with the type described in the Type examination certificate and with the requirements of these Regulations that apply to them.
Product checks3
For each individual product manufactured one or more tests on one or more specific aspects of the product shall be carried out by the manufacturer or on his behalf, in order to verify conformity with the type described in the Type examination certificate and with the corresponding requirements of these Regulations. The tests shall be carried out under the responsibility of an approved body, chosen by the manufacturer.
The manufacturer shall, under the responsibility of the approved body, affix the approved body's identification number during the manufacturing process.
UK marking, declaration of conformity and attestation of conformity4
4
The manufacturer shall affix the UK marking to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for a product model other than a component and keep it at the disposal of the national authorities for 10 years after the product, other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
4
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative5
The manufacturer's obligations set out in paragraph 4 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 5CONFORMITY TO TYPE BASED ON PRODUCT QUALITY ASSURANCE
1
Conformity to type based on product quality assurance is that part of a conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2 and 5 and ensures and declares on his sole responsibility that the products concerned are in conformity with the type described in the Type examination certificate and satisfy the requirements of these Regulations that apply to them.
Manufacturing2
The manufacturer shall operate an approved quality system for final product inspection and testing of the products concerned as specified in paragraph 3 and shall be subject to surveillance as specified in paragraph 4.
Quality System3
3
The manufacturer shall lodge an application for assessment of his quality system with the approved body of his choice, for the products concerned.
The application shall include:
a
the name and address of the manufacturer and, if the application is lodged by the authorised representative, his name and address as well
b
a written declaration that the same application has not been lodged with any other approved body,
c
all relevant information for the product category envisaged,
d
the documentation concerning the quality system, and
e
the technical documentation of the approved type and a copy of the Type examination certificate.
3
The quality system shall ensure compliance of the products with the type described in the Type examination certificate and with the applicable requirements of these Regulations.
All the elements, requirements and provisions adopted by the manufacturer shall be documented in a systematic and orderly manner in the form of written policies, procedures and instructions. The quality system documentation shall permit a consistent interpretation of the quality programmes, plans, manuals and records.
It shall, in particular, contain an adequate description of:
a
the quality objectives and the organisational structure, responsibilities and powers of the management with regard to product quality,
b
the examinations and tests that will be carried out after manufacture,
c
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.,
d
the means of monitoring the effective operation of the quality system.
3
The approved body shall assess the quality system to determine whether it satisfies the requirements referred to in paragraph 3.2.
It shall presume conformity with those requirements in respect of the elements of the quality system that comply with the corresponding specifications of the relevant designated standard.
In addition to experience in quality management systems, the auditing team shall have at least one member with experience of evaluation in the relevant product field and product technology concerned, and knowledge of the applicable requirements of these Regulations. The audit shall include an assessment visit to the manufacturer's premises. The auditing team shall review the technical documentation referred to in paragraph 3.1(e) in order to verify the manufacturer's ability to identify the relevant requirements of these Regulations and to carry out the necessary examinations with a view to ensuring compliance of the product with those requirements.
The decision shall be notified to the manufacturer. The notification shall contain the conclusions of the audit and the reasoned assessment decision.
3
The manufacturer shall undertake to fulfil the obligations arising out of the quality system as approved and to maintain it so that it remains adequate and efficient.
3
The manufacturer shall keep the approved body that has approved the quality system informed of any intended change to the quality system.
The approved body shall evaluate any proposed changes and decide whether the modified quality system will continue to satisfy the requirements referred to in paragraph 3.2 or whether a reassessment is necessary.
It shall notify the manufacturer of its decision. The notification shall contain the conclusions of the examination and the reasoned assessment decision.
Surveillance under the responsibility of the approved body4
4
The purpose of surveillance is to make sure that the manufacturer duly fulfils the obligations arising out of the approved quality system.
4
The manufacturer shall, for assessment purposes, allow the approved body access to the manufacture, inspection, testing and storage sites and shall provide it with all necessary information, in particular:
a
the quality system documentation,
b
the quality records, such as inspection reports and test data, calibration data, qualification reports on the personnel concerned, etc.
4
The approved body shall carry out periodic audits to make sure that the manufacturer maintains and applies the quality system and shall provide the manufacturer with an audit report.
4
In addition, the approved body may pay unexpected visits to the manufacturer. During such visits the approved body may, if necessary, carry out product tests, or have them carried out, in order to verify that the quality system is functioning correctly. The approved body shall provide the manufacturer with a visit report and, if tests have been carried out, with a test report.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 3.1, the latter's identification number to each individual product other than a component that is in conformity with the type described in the Type examination certificate and satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity for each product model, other than a component and keep it at the disposal of the national authorities for 10 years after the product other than a component has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
5
The manufacturer shall draw up a written attestation of conformity for each component model and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component model for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
6
The manufacturer shall, for a period ending 10 years after the product has been placed on the market, keep at the disposal of the national authorities:
a
the documentation referred to in paragraph 3.1,
b
the information relating to the change referred to in paragraph 3.5, as approved,
c
the decisions and reports of the approved body referred to in paragraphs 3.5, 4.3 and 4.4.
7
Each approved body shall inform the Executive of quality system approvals issued or withdrawn, and shall, periodically or upon request, make available to the Executive the list of quality system approvals refused, suspended or otherwise restricted.
Each approved body shall inform the other approved bodies of quality system approvals which it has refused, suspended or withdrawn, and, upon request, of quality system approvals which it has issued.
Authorised representative8
The manufacturer's obligations set out in paragraphs 3.1, 3.5, 5 and 6 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 6INTERNAL PRODUCTION CONTROL
1
Internal production control is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 4, and ensures and declares on his sole responsibility that the products concerned satisfy the requirements of these Regulations that apply to them.
Technical documentation2
The manufacturer shall establish the technical documentation. The documentation shall make it possible to assess the product's conformity to the relevant requirements and shall include an adequate analysis and assessment of the risk(s).
The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
a
a general description of the product,
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
e
results of design calculations made, examinations carried out, etc., and
f
test reports.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure compliance of the manufactured products with the technical documentation referred to in paragraph 2 and with the requirements of these Regulations that apply to them.
UK marking, declaration of conformity and attestation of conformity4
4
The manufacturer shall affix the UK marking to each individual product other than a component that satisfies the applicable requirements of these Regulations.
4
The manufacturer shall draw up a written declaration of conformity for a product model other than a component and keep it together with the technical documentation at the disposal of the national authorities for 10 years after the product, other than a component, has been placed on the market. The declaration of conformity shall identify such product model for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product other than a component.
4
The manufacturer shall draw up a written attestation of conformity for each component model and keep it together with the technical documentation at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative5
The manufacturer's obligations set out in paragraph 4 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
PART 7CONFORMITY BASED ON UNIT VERIFICATION
1
Conformity based on unit verification is the conformity assessment procedure whereby the manufacturer fulfils the obligations laid down in paragraphs 2, 3 and 5, and ensures and declares on his sole responsibility that the product concerned, which has been subject to the provisions of paragraph 4, is in conformity with the requirements of these Regulations that apply to it.
Technical documentation2
2
The manufacturer shall establish the technical documentation and make it available to the approved body referred to in paragraph 4. The documentation shall make it possible to assess the product's conformity with the relevant requirements and shall include an adequate analysis and assessment of the risk(s). The technical documentation shall specify the applicable requirements and cover, as far as relevant for the assessment, the design, manufacture and operation of the product. The technical documentation shall contain at least the following elements:
a
a general description of the product,
b
conceptual design and manufacturing drawings and schemes of components, sub-assemblies, circuits, etc.,
c
descriptions and explanations necessary for the understanding of those drawings and schemes and the operation of the product,
d
a list of the designated standards applied in full or in part and, where those designated standards have not been applied, descriptions of the solutions adopted to meet the essential health and safety requirements of these Regulations, including a list of other relevant technical specifications applied. In the event of partly applied designated standards, the technical documentation shall specify the parts which have been applied,
e
results of design calculations made, examinations carried out, etc., and
f
test reports.
2
The manufacturer shall keep the technical documentation at the disposal of the relevant national authorities for 10 years after the product has been placed on the market.
Manufacturing3
The manufacturer shall take all measures necessary so that the manufacturing process and its monitoring ensure conformity of the manufactured product with the applicable requirements of these Regulations.
Verification4
An approved body chosen by the manufacturer shall carry out appropriate examinations and tests, set out in the relevant designated standards and/or equivalent tests set out in other relevant technical specifications, to check the conformity of the product with the applicable requirements of these Regulations, or have them carried out. In the absence of such a designated standard the approved body concerned shall decide on the appropriate tests to be carried out.
The approved body shall issue a certificate of conformity in respect of the examinations and tests carried out and shall affix its identification number to the approved product, or have it affixed under its responsibility.
The manufacturer shall keep the certificates of conformity at the disposal of the national authorities for 10 years after the product has been placed on the market.
UK marking, declaration of conformity and attestation of conformity5
5
The manufacturer shall affix the UK marking and, under the responsibility of the approved body referred to in paragraph 4, the latter's identification number to each product other than a component that satisfies the applicable requirements of these Regulations.
5
The manufacturer shall draw up a written declaration of conformity and keep it at the disposal of the national authorities for 10 years after the product, other than a component has been placed on the market. The declaration of conformity shall identify such product for which it has been drawn up.
A copy of the declaration of conformity shall accompany every product, other than a component.
5
The manufacturer shall draw up a written attestation of conformity and keep it at the disposal of the national authorities for 10 years after the component has been placed on the market. The attestation of conformity shall identify the component for which it has been drawn up. A copy of the attestation of conformity shall accompany every component.
Authorised representative6
The manufacturer's obligations set out in paragraphs 2.2 and 5 may be fulfilled by his authorised representative, on his behalf and under his responsibility, provided that they are specified in the mandate.
Amendment to Schedule 6I76741
Schedule 6 (EU Declaration of Conformity) is amended as follows—
a
omit “EU” from the heading;
b
in paragraph 5, for “Union harmonisation legislation” substitute “
statutory requirements
”
;
c
in paragraph 6, for “harmonised” substitute “
designated
”
;
d
in paragraph 7, for “notified” substitute “
approved
”
.
SCHEDULE 33Amendment of Regulation (EC) No 765/2008
IntroductionI9171
Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9 July 2008 setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93 is amended in accordance with paragraphs 2 to 38.
Amendments to Chapter 1I7682
In Article 1—
a
in paragraph 3, for “from third countries”, substitute “
F351entering the market of Great Britain
”
; and
b
for paragraph 4 substitute—
4
This Regulation provides the requirements as to the form of the UK marking.
I7693
In Article 2—
a
in the text before paragraph 1 omit “shall”;
b
in paragraphs 1 to 3, 6, 7, 12 to 15 and 17 for “shall mean” substitute “
means
”
;
c
in paragraph 1—
F352i
for “Community market” substitute “
market of Great Britain
”
;
ii
at the end, insert “
and related expressions must be construed accordingly
”
;
d
in paragraph 2—
F353i
for “Community market” substitute “
market of Great Britain
”
;
ii
at the end, insert “
and related expressions must be construed accordingly
”
;
F354e
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
f
for paragraph 5 substitute—
5
“importer” means any person established in the United Kingdom who places a product from a country outside of the United Kingdom on the market;
g
omit paragraphs 8 and 9;
h
for paragraph 10 substitute—
10
“accreditation” means an attestation by a national accreditation body conveying formal recognition that a conformity assessment body is competent to carry out a specific conformity assessment activity;
i
for paragraph 11 substitute—
11
“UK national accreditation body” means the body appointed by the Secretary of State in accordance with Article 4;
j
omit paragraph 16;
k
in paragraph 17 for “the relevant Community harmonisation legislation” substitute “
any relevant enactment;
”
;
l
for paragraph 18 substitute—
18
“market surveillance authority” means an authority responsible for carrying out market surveillance in the United Kingdom;
m
for paragraph 19 substitute—
19
“the free circulation procedure” means the procedure set out in Schedule 1 to the Taxation (Cross-border Trade) Act 2018 M110;
n
for paragraph 20 substitute—
20
“conformity marking” means a marking, such as the UK marking, by which the manufacturer indicates that a product is in conformity with the applicable requirements of any enactment providing for the affixing such a marking;
o
for paragraph 21 substitute—
21
“relevant enactment” means any retained EU law F355, as it applies in Great Britain, derived from an EU instrument harmonising the conditions for the marketing of products in the EU;
p
after paragraph 21 insert—
22
“UK marking” means the marking in the form set out in Annex 2.
Amendments to Chapter 2I7704
In Article 3 for “shall apply” substitute “
applies
”
.
I7715
For Article 4 substitute—
Article 4UK national accreditation body
1
The Secretary of State must by regulations appoint a single UK national accreditation body.
2
The appointment of the UK national accreditation body under regulation 3 of the Accreditation Regulations 2009 M111 is to be treated on and after F356IP completion day as having been made in pursuance of the duty on the Secretary of State to appoint a UK national accreditation body set out in paragraph 1.
3
The UK national accreditation body must perform its functions in accordance with the provisions of this Chapter.
4
Regulations made under this Article may—
a
terminate the appointment of a UK national accreditation body; and
b
appoint a different body as the UK national accreditation body
where the Secretary of State considers that a UK national accreditation body is not performing its functions in accordance with this Chapter.
5
Regulations made under this Article must establish procedures for the resolution of appeals against accreditation decisions made by the appointed UK national accreditation body.
6
Regulation 5 of the Accreditation Regulations 2009 is to be treated as meeting the requirements of paragraph 5.
7
The UK national accreditation body must operate on a not-for-profit basis.
8
The UK national accreditation body must not—
a
offer or provide any activities or services that are provided by conformity assessment bodies,
b
provide consultancy services; and
c
own shares in, or otherwise have any financial or managerial interest in, a conformity assessment body.
9
The UK national accreditation body must establish and maintain appropriate structures to ensure—
a
consultation on its activities with interested parties; and
b
responses of interested parties to consultation are adequately taken into account.
10
The Secretary of State must ensure that the UK national accreditation body has the appropriate financial and personnel resources for the proper fulfilment of its tasks, including—
a
the fulfilment of special tasks such as activities related to international accreditation cooperation; and
b
activities that are required to support public policy and which are not self-financing.
11
Regulations made under this Article must be made by statutory instrument.
12
Regulations made under this Article may make such transitional, transitory F357, consequential or saving provision as the Secretary of State considers appropriate.
13
A statutory instrument containing regulations made under this Article is subject to annulment in pursuance of a resolution of either House of Parliament.
I7726
In Article 5—
a
in paragraph 1—
i
for “A national accreditation body shall” substitute “
The UK national accreditation body must
”
;
ii
for “the national accreditation body shall” substitute “
the UK national accreditation body must
”
;
b
omit paragraph 2;
c
in paragraph 3—
i
for “National accreditation bodies shall” substitute “
The UK national accreditation body must
”
;
ii
for “they have” substitute “
it has
”
;
d
in paragraph 4—
i
for “a national accreditation body” substitute “
the UK national accreditation body
”
;
ii
for “that national accreditation body shall” substitute “
the UK national accreditation body must
”
;
e
omit paragraph 5.
I7737
In Article 6—
a
in paragraph 1 for “National accreditation bodies shall” substitute “
The UK national accreditation body must
”
;
b
omit paragraphs 2 and 3.
I7748
Omit Article 7.
I7759
In Article 8—
a
in the heading, for “national accreditation bodies” substitute “
the UK national accreditation body
”
;
b
in the words before paragraph 1, for “A national accreditation body shall” substitute “
The UK national accreditation body must
”
;
c
in each paragraph for “shall” substitute “
must
”
;
d
in paragraph 5 for “relevant Community or national legislation” substitute “
any enactment
”
.
I77610
In Article 9—
a
in paragraph 1—
i
for “a national accreditation body” substitute “
the UK national accreditation body
”
;
ii
for “Member State concerned shall” substitute “
Secretary of State must
”
;
iii
for “shall ensure” substitute “
must ensure
”
;
iv
omit “, and shall inform the Commission thereof”;
b
in paragraph 2—
i
for “Member States shall” substitute “
The Secretary of State must
”
;
ii
for “their national accreditation bodies” substitute “
the UK national accreditation body
”
;
iii
for “they fulfil” substitute “
it fulfils
”
;
c
omit paragraph 3;
d
in paragraph 4—
i
for “National accreditation bodies shall” substitute “
The UK national accreditation body must
”
;
ii
for “they have” substitute “
it has
”
.
I77711
For Article 10 substitute—
Article 10Peer evaluation
1
The UK national accreditation body must—
F358a
ensure that it is evaluated in accordance with the requirements of this Article by a body which is approved by the Secretary of State;
b
set out the results of the evaluation in a report;
c
make that report publicly available; and
d
provide a copy of that report to the Secretary of State.
2
The evaluation referred to in paragraph 1(a) must—
a
be based on evaluation criteria and procedures—
i
agreed between the national accreditation body and the body undertaking the evaluation; and
ii
approved by the Secretary of State; and
b
evaluate whether the UK national accreditation body meets the requirements of Article 8.
3
The procedures referred to in paragraph 2(a) must include provision for appeal by the UK national accreditation body against the results of the evaluation.
4
The report paragraph (1)(c) must include the evaluation criteria and procedures referred to in paragraph (2)(a).
5
The first report under paragraph (1) must be made publicly available before the end of the period of five years beginning on F359IP completion day.
6
After the first report, reports made under this Article are to be made publicly available at intervals not exceeding five years
I77812
Omit Articles 11 to 14.
Amendment to Chapter 3I77913
After “CHAPTER III”, in the heading—
a
omit “COMMUNITY” in the first place it occurs;
F360b
for “COMMUNITY MARKET” substitute “
MARKET OF GREAT BRITAIN
”
.
I78014
In Article 15—
a
in paragraphs 1, 2 and 4 before “26”, insert “
22 and
”
;
b
in paragraphs 1 and 2, for “Community harmonisation legislation”, substitute “
any relevant enactment
”
;
c
in paragraphs 1 and 5 omit “shall”;
d
in paragraph 2 for “shall apply” substitute “
applies
”
;
e
in paragraph 3—
i
for “Directive 2001/95/EC” substitute “
the General Product Safety Regulations 2005 M112
”
;
ii
for “shall” substitute “
does
”
;
f
in paragraph 4 for “shall mean” substitute “
means
”
;
g
in paragraph 5—
i
for “Community legislation” in the first place it occurs, substitute “
any relevant enactment
”
;
ii
for “Community legislation does” substitute “
enactments do
”
.
I78115
In Article 16—
a
omit paragraph 1;
b
in paragraph 2—
i
after “Market surveillance”, insert “
authorities
”
;
ii
for “shall” substitute “
must
”
;
iii
for “Community harmonisation legislation” substitute “
any relevant enactment
”
in both places in which it occurs;
iv
for “, the Commission and the other Member States”, substitute “
and the Secretary of State
”
;
c
for paragraph 3 substitute—
3
The Secretary of State must ensure that there is a national market surveillance infrastructure and programme so that effective measures may be taken in relation to any product subject to any relevant enactment.
d
in paragraph 4—
i
for “shall” in the first place it occurs substitute “
must
”
;
ii
for “Community harmonisation legislation”, substitute “
any relevant enactment
”
;
iii
omit “shall” in the second place it occurs.
I78216
After Article 16, in the heading, after “SECTION 2” for “Community market” substitute “
Market
”
.
I78317
In Article 17—
a
omit paragraph 1;
b
in paragraph 2, for “Member States shall” substitute “
The Secretary of State must take appropriate measures to
”
.
I78418
In Article 18—
a
in the heading, omit “of the Member States”;
b
omit paragraph 1;
c
in paragraph 2—
i
for “Member States shall” substitute “
The Secretary of State must
”
;
ii
in sub-paragraph (a) for “Community harmonisation legislation” substitute “
any relevant enactment
”
;
d
for paragraph 3, substitute—
3
The Secretary of State may by regulations make such provision as the Secretary of State considers appropriate to ensure that market surveillance authorities have the powers necessary for the proper performance of their duties.
The Secretary of State must entrust market surveillance authorities with the resources and knowledge necessary for the proper performance of their duties.
Regulations made under this paragraph must be made by statutory instrument.
A statutory instrument containing regulations under this paragraph may not be made unless a draft of the instrument has been laid before, and approved by a resolution of, each House of Parliament.
e
for paragraph 4, substitute—
4
Market surveillance authorities must exercise their powers proportionately.
f
for paragraph 5, substitute—
5
The Secretary of State must establish, implement and periodically update the United Kingdom's market surveillance programme setting out the principles as to how market surveillance is to be organised and covering the sectors in which market surveillance is conducted in the United Kingdom. The Secretary of State must make this programme available to the public by way of electronic communication and, where appropriate, by other means.
g
in paragraph 6—
i
for “Member States” substitute “
The Secretary of State
”
;
ii
for “shall” substitute “
must
”
in each place it occurs;
iii
omit “their”;
iv
after “activities” insert “
by market surveillance authorities
”
; and
v
omit “be communicated to the other Member States and the Commission and”.
I78519
In Article 19—
a
in paragraphs 1, 2, 4 and 5 for “shall” substitute “
must
”
in each place it occurs;
b
in paragraph 2 omit “within their territories”;
c
for paragraph 3 substitute—
3
Where a market surveillance authority decides to withdraw a product manufactured in the United Kingdom, it must inform the economic operator concerned at the address indicated on the product in question or in the documentation accompanying the product.
d
for paragraph 5, substitute—
5
Market surveillance authorities must observe confidentiality where necessary in order to protect commercial secrets or to preserve personal data pursuant to national legislation, subject to the requirements that:
a
information be made public under this Regulation to the fullest extent necessary in order to protect the users in the United Kingdom;
b
the protection of confidentiality shall not prevent the dissemination to market surveillance authorities of information relevant to ensuring the effectiveness of market surveillance activities.
I78620
In Article 20—
a
in paragraph 1—
i
for “Member States shall”, substitute, “Market surveillance authorities must”;
ii
for “their”, in the second place it occurs, substitute “
the
”
; and
iii
for “Commission” substitute “
Secretary of State
”
;
b
in paragraph 2 for “shall”—
i
in the first place it occurs substitute “
must
”
;
ii
in the second place it occurs substitute “
does
”
.
I78721
In Article 21—
a
in paragraph 1—
i
for “Member States shall”, substitute “
Market surveillance authorities must
”
; and
ii
for “the relevant Community harmonisation legislation”, substitute “
any relevant enactment
”
;
b
in paragraph 2—
i
for “shall” substitute “
must
”
in both places it occurs;
ii
after “informed of the”, insert “
legal
”
; and
iii
omit “under the law of the Member State concerned”; and
c
in paragraph 3—
i
for “shall” substitute “
must
”
in each place it occurs;
ii
for “the relevant Community harmonisation legislation”, substitute “
any of the relevant enactments
”
.
d
in paragraph 4 for “shall” substitute “
must
”
.
I78822
In Article 22—
a
for the heading and paragraph 1, substitute—
Notification of serious risk”1
Where a market surveillance authority takes or intends to take a measure in accordance with Article 20 it must immediately notify the Secretary of State of that measure.
b
in paragraphs 2 and 3 for “shall” substitute “
must
”
in each place it occurs;
c
in paragraph 2—
i
for “Member States” substitute “
the market surveillance authority
”
;
ii
for “Commission” substitute “
the Secretary of State
”
;
d
in paragraph 3 omit “national”;
e
in paragraph 4 from “and information” to the end of that paragraph, substitute “
authority must notify the Secretary of State through the database containing information relating to market surveillance and product safety established by regulation 33(A1) of the General Product Safety Regulations 2005 M113
”
.
I78923
Omit Articles 23 to 25.
I79024
In Article 26—
a
in paragraph 1—
i
omit “, promoting and facilitating access to European systems”;
ii
omit “The Commission shall, in cooperation with Member States, develop appropriate programmes for that purpose.”
b
omit paragraph 2.
I79125
After “SECTION 3”, in the heading—
a
after “of” insert “
imported
”
;
b
omit “entering the Community market”.
I79226
In Article 27—
a
for the heading and paragraph 1, substitute—
Controls of imported products1
The authorities in charge of the control of products entering F361Great Britain must carry out appropriate checks on the characteristics of products on an adequate scale, in accordance with the principles set out in Article 19(1), before those products are discharged from the free-circulation procedure.
b
in paragraph 2—
i
omit “in a Member State”;
ii
for “shall” substitute “
must
”
;
c
in paragraph 3—
i
for “shall” substitute “
must
”
in both places it occurs;
ii
for “release of a product for free circulation on the Community market”, substitute “
the discharge of a product from the free-circulation procedure
”
; and
iii
in point (b), for “the relevant Community harmonisation legislation”, substitute “
any relevant enactment
”
;
iv
in point (c), for “the CE”, substitute “
a conformity
”
;
d
in paragraph 4 for “shall” substitute “
must
”
;
e
omit paragraph 5.
I79327
In Article 28—
a
in the heading for “Release” substitute “Discharge;
b
in paragraph 1—
i
for “release” in each place it occurs substitute “
discharge
”
;
ii
for “shall” substitute “
must
”
;
iii
for “released” substitute “
discharged from the free-circulation procedure
”
; and
c
in paragraph 2—
i
for “Community harmonisation legislation”, substitute “
any relevant enactment
”
; and
ii
for “shall be released”, substitute “
must be discharged from the free-circulation procedure
”
iii
for “release” substitute “
discharge
”
.
I79428
In Article 29—
a
in paragraphs 1 and 2—
i
for “the market surveillance authorities find” substitute “
a market surveillance authority finds
”
in both places it occurs;
ii
for “they”, substitute “
it
”
in both places it occurs;
b
in paragraph 1—
i
for “shall” substitute “
must
”
in each place it occurs;
ii
for “release for free circulation”, substitute “
discharge from the free-circulation procedure
”
.
c
in paragraph 2—
i
for “Community harmonisation legislation”, substitute “
any relevant enactment
”
;
ii
for “shall” substitute “
must
”
in both places it occurs;
iii
for “authorities” substitute “
authority
”
;
iv
for “release the product for free circulation”, substitute “
discharge the product from the free-circulation procedure
”
; and
v
for “release for free circulation”, substitute “
discharge from the free-circulation procedure
”
;
d
in paragraph 3—
i
for “release for free circulation”, substitute “
the free-circulation procedure
”
;
ii
for “shall” substitute “
must
”
;
e
in paragraph 4 for “Member States'” substitute “
Market surveillance
”
;
f
in paragraph 5 for “shall” substitute “
must
”
.
Amendment to Chapter 4I79529
For Chapter 4, substitute—
CHAPTER 4UK MARKING
Article 30
1
The Secretary of State must publish the form of the UK marking as set out in Annex 2 on the Gov.uk website (https://www.gov.uk) M114.
2
Annex 2 has effect.
3
Any reference to the UK marking in any enactment is a reference to the UK marking in the form set out in Annex 2 and published in accordance with paragraph 1.
4
A requirement in any enactment to affix the UK marking is a requirement to affix the UK marking in accordance with the requirements in Annex 2.
5
The UK marking must not be affixed to a product unless an enactment provides for its affixing.
6
An economic operator must not affix any other marking, sign or inscription which is likely to mislead any person as to the meaning or form of the UK marking.
7
An economic operator must not affix any other marking if the visibility, legibility and meaning of the UK marking would be impaired as a result.
8
Where the UK marking is affixed to a product in breach of paragraph 4, the UK marking is affixed in a false or misleading manner and Article 27(3)(c) applies.
Omission of Chapter 5I79630
Omit Chapter 5.
Amendments to Chapter 6I79731
Omit Article 38.
I79832
Omit Article 40.
I79933
Omit Article 41.
I80034
Omit Article 42.
I80135
In Article 43 omit the words from “references to the repealed Regulation” to the end.
I80236
Omit Article 44.
I80337
After Article 44, omit—
a
the words “This Regulation shall be binding” to the end;
b
“Done at Strasbourg, 9 July 2008”; and
c
the signature text.
Omission of Annex 1I80438
Omit Annex 1.
Substitution of Annex 2I80539
For Annex 2 substitute—
ANNEX 2UK marking
1
The UK marking consists of the initials “UKCA” taking the following form—
2
Where the UK marking is reduced or enlarged, the proportions given in the graduated drawing in paragraph 1 must be respected.
3
Where an enactment does not require specific dimensions, the UK marking must be at least 5 millimetres high.
SCHEDULE 34Amendment of Regulation (EC) No 1223/2009 and related amendments
IntroductionI9181
Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November on cosmetic products (recast) is amended in accordance with paragraphs 2 to 28.
Amendment of Article 1I8062
In Article 1 (scope and objective) omit “internal”.
Amendment of Article 2I8073
In Article 2 (definitions), in paragraph 1—
a
in point (d) (manufacturer)—
i
omit “natural or legal”;
ii
for “his” substitute “
their
”
;
b
in point (e) (distributor)—
i
omit “natural or legal”;
ii
omit “Community”;
c
in point (g) (making available on the market)—
F295i
for “Community market” substitute “
market of Great Britain
”
;
ii
at the end insert “
and related expressions are to be construed accordingly
”
;
d
e
for point (i) (importer) substitute—
F298i
“importer” means a person who—
aa
is established in the United Kingdom and places a cosmetic product from a country outside of the United Kingdom on the market; or
bb
is established in Northern Ireland and places a cosmetic product on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
f
omit point (j) (harmonised standard);
g
in point (s) omit the last sentence;
h
after point (s) insert—
t
‘Regulation (EC) No 1272/2008’ means Regulation (EC) 1272/2008 of the European Parliament and of the Council of 16th December 2008 on classifications, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) 1907/2006;
u
‘EU Regulation (pre-exit)’ means Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November on cosmetic products (recast) M115, as it has effect immediately before F301IP completion day;
v
‘Enforcement Regulations’ means the Cosmetic Products Enforcement Regulations 2013 M116;
F299va
‘CMR’ means carcinogenic, mutagenic or toxic for reproduction;
w
‘competent authority’ has the meaning given to it in regulation 4 of the Enforcement Regulations;
x
‘enforcement authority’ has the meaning given to it in regulation 2(1) of the Enforcement Regulations;
y
‘finished cosmetic product’ means the cosmetic product in its final formulation, as placed on the market and made available to the end user, or its prototype;
F300ya
‘historic animal testing data’ means data from any animal testing that was carried out before the date on which such testing was prohibited in accordance with Article 18 of the EU Regulation (pre-exit);
z
‘prototype’ means a first model or design that has not been produced in batches, and from which the finished cosmetic product is copied or finally developed;
za
“the transitory period” means the period of 90 days beginning on the day after the day on which F302IP completion day falls.
i
for paragraph (3) substitute—
3
1
Subject to subparagraphs (6) and (7), in this Regulation a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of subparagraph (1), a “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a cosmetic product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this regulation a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with subparagraph (1)(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with subparagraph (1)(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
Amendment of Article 3I8084
In Article 3 (safety), in point (a) for “Directive 87/357/EEC” substitute “
the Food Imitations (Safety) Regulations 1989 M117
”
.
Substitution of Article 4I8095
For Article 4 (responsible person) substitute—
Article 4Responsible person
1
A cosmetic product may not be placed on the market unless there is a responsible person established in the United Kingdom in respect of the cosmetic product.
2
Subject to paragraphs 6 and 7, a manufacturer of a cosmetic product is the responsible person in respect of that product where—
a
the manufacturer is established in the United Kingdom; and
b
the cosmetic product—
i
is manufactured in the United Kingdom; and
ii
after manufacture but prior to placing on the market is not exported and imported back into the United Kingdom.
3
Where paragraph 4 applies the manufacturer must ensure that—
a
there is a person established in the United Kingdom designated by written mandate as the responsible person in respect of the cosmetic product; and
b
that person has agreed in writing to be the responsible person in respect of that cosmetic product.
4
This paragraph applies where—
a
a manufacturer of a cosmetic product is established in a country outside the United Kingdom; and
b
the cosmetic product—
i
is manufactured in the United Kingdom; and
ii
after manufacture but prior to placing on the market is not exported and imported back into the United Kingdom.
5
Subject to paragraphs 6 and 7, any importer placing a cosmetic product on the market is the responsible person in respect of that cosmetic product.
6
An importer or a manufacturer established in the United Kingdom may by written mandate designate a person established in the United Kingdom as the responsible person.
7
Where the person designated by the importer or the manufacturer under paragraph 6 accepts the designation in writing, that person is the responsible person.
8
A distributor is the responsible person in respect of a cosmetic product where that distributor—
a
places a product on the market under the distributor's name or trademark; or
b
modifies a product already placed on the market in such a way that compliance with the applicable requirements may be affected.
Amendment of Article 5I8106
In Article 5 (obligations of responsible persons)—
F362ia
at the beginning of paragraph 1 insert “
Subject to Article 5A
”
;
a
in paragraphs 2 and 3 omit “national”;
b
in paragraph 2 omit the words from “of the Member States” (in the first place in which it occurs) to “accessible”;
c
in paragraph 3 omit “, in a language which can be easily understood by that authority”;
d
after paragraph 3 insert—
4
The information and documentation referred to in paragraph 3 must be in English.
I921F4Insertion of Article 5A6
After Article 5 insert—
Article 5A
Obligations of responsible persons established in Northern Ireland1
Where paragraph 3 applies, a responsible person is to be treated as complying with Articles 3, 8, 10 to 12, 14 to 18, 19(1), (2) and (5) and 20 to 24.
2
Where paragraph 4 applies, a responsible person is to be treated as complying with Articles 8, 10 to 12, 14 to 18, 19(1), (2) and (5) and 20 to 24.
3
This paragraph applies where—
a
the responsible person—
i
is established in Northern Ireland;
ii
is a responsible person for the purposes of EU Regulation (Northern Ireland);
iii
has complied with the obligations of a responsible person under Article 5 of EU Regulation (Northern Ireland); and
iv
when submitting information under Article 13 the responsible person at the same time gives notice to the Secretary of State confirming the matters in points (i) to (iii); and
b
the cosmetic product is qualifying Northern Ireland goods.
4
This paragraph applies where—
a
the responsible person is a person—
i
to which Article 2(i)(bb) applies; and
ii
who gives notice to the Secretary of State when submitting information under Article 13 that a responsible person for the purposes of EU Regulation (Northern Ireland) has complied with the obligations of a responsible person under Article 5 of EU Regulation (Northern Ireland); and
b
the cosmetic product is qualifying Northern Ireland goods.
5
In this Article—
“EU Regulation (Northern Ireland)” means Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30th December 2008 on cosmetic products (recast), as it has effect by virtue of the Protocol on Ireland/Northern Ireland in the withdrawal agreement.
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018.
Amendment of Article 6I8117
In Article 6 (obligations of distributors)—
a
in paragraph 3—
i
omit “national”;
ii
omit “of the Member States in which they made the product available”;
b
in paragraph 5—
i
omit “national”;
ii
omit “, in a language which can be easily understood by that authority”;
c
after paragraph 5 insert—
6
The information and documentation referred to in paragraph 5 must be in English
Amendment of Article 8I8128
In Article 8 (good manufacturing practice), in paragraph 2—
a
for “harmonised” substitute “
designated
”
;
b
omit “, the references of which have been published in the Official Journal of the European Union”.
Omission of Article 9I8139
Omit Article 9 (free movement).
Amendment of Article 10I81410
In Article 10 (safety assessment)—
a
in paragraph 1 omit the words from “The first subparagraph shall” to “referred to in Article 32(2).”;
b
in paragraph 2 for “a Member State” substitute “
the Secretary of State
”
;
c
in paragraph 3—
i
for the words from “shall comply with” to “study” substitute “
must comply with the Good Laboratory Practice Regulations 1999
”
M118
;
ii
before “international standards” omit “other”;
iii
for “Commission or the ECHA” substitute “
Secretary of State
”
.
Amendment of Article 11I81511
In Article 11 (product information file)—
a
in point (e) for “his” substitute “
their
”
;
b
for paragraph 3 substitute—
3
The responsible person must make the product information file readily accessible to a competent authority in an electronic or other format at the address notified in accordance with Article 13 as the address at which the product information file is kept.
c
for paragraph 4 substitute—
4
The information contained in the product information file must be in English
Amendment of Article 12I81612
In Article12, in paragraph 2—
a
omit “In the absence of any applicable Community legislation,”;
b
for “harmonised” substitute “
designated
”
;
c
omit “, the references of which have been published in the Official Journal of the European Union”.
Substitution of Article 13I81713
For Article 13 (notification) substitute—
Article 13Notification
1
Before placing a cosmetic product on the market, the responsible person must submit by electronic means the following information to the Secretary of State—
a
the category of cosmetic product and its name or names, enabling its specific identification;
b
the name of the responsible person;
c
the address at which the product information file in respect of the cosmetic product is kept;
d
the contact details of a natural person to contact in the case of urgency;
e
where applicable, the following information—
i
presence of substances in the form of nanomaterials;
ii
the identification including the chemical name (IUPAC) and other descriptors as specified in point 2 of the Preamble to Annexes 2 to 6 to this Regulation; and
iii
the reasonably foreseeable exposure conditions;
f
the name and the Chemicals Abstracts Service (CAS) or EC number of substances classified as F363CMR substances of category 1A or 1B under Regulation (EC) No 1272/2008;
g
the frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties.
2
When a cosmetic product is placed on the market, the responsible person must notify to the Secretary of State the original labelling and, where reasonably legible, a photograph of the corresponding packaging
3
Paragraph 4 applies in relation to a cosmetic product where prior to F364IP completion day—
a
the cosmetic product has been supplied on the market of the United Kingdom or the market of any EEA state for distribution, consumption or use in the course of a commercial activity (whether in return for payment or free of charge); and
b
a responsible person designated under Article 4 of the EU Regulation (pre-exit) has complied with Article 13 of that Regulation in relation to that product.
4
Where this paragraph applies—
a
if the cosmetic product is placed on the market at any time before the expiry of the transitory period, subject to subparagraph (b), paragraphs 1 and 2 are to have effect as if they required the information specified in those paragraphs before the end of the transitory period;
b
paragraph 1 is to be treated as being satisfied in respect of the cosmetic product and paragraph 2 does not apply in respect of that product where—
i
before the expiry of the transitory period, the responsible person for the cosmetic product submits to the Secretary of State by electronic means the information set out in points (a) to (d) and (g) of paragraph 1; and
ii
when submitting that information, the responsible person at the same time gives notice confirming the matters set out in paragraph 3 in relation to the cosmetic product;
c
if at any time a request is made to the responsible person by the Secretary of State in accordance with paragraphs 5 and 6, the responsible person must comply with the request within the period specified in the request.
5
Where the Secretary of State considers it necessary for the purposes of reducing a risk to human health, the Secretary of State may request that a responsible person submits the information referred to in paragraph 1(e) to (f) in relation to a cosmetic product to which paragraph 4 applies.
6
When making a request under paragraph 5 the Secretary of State must specify a period—
a
within which the responsible person must respond; and
b
which is reasonable and commensurate with the nature of the risk presented by the product.
7
The Secretary of State must make the following information available in relation to a cosmetic product to all other competent authorities—
a
the information referred to in paragraph 1(a) to (f); and
b
the information referred to in paragraph 2.
8
Competent authorities may only use the information referred to in paragraph 7 for the purposes of market surveillance, market analysis, evaluation and consumer information in the context of Articles 25 to 27.
9
The Secretary of State must without delay make the following information available to poison centres or similar bodies established in the United Kingdom—
a
the information referred to in paragraph 1; and
b
the information referred to in paragraph 2
10
Those poison centres and similar bodies may only use that information for the purposes of medical treatment.
11
Where any information provided under this Article in relation to a cosmetic product changes, the responsible person must provide an update by electronic means to the Secretary of State without delay.
Amendment of Article 14I81814
In Article 14 (restrictions for substances)—
a
in paragraph 1(c)(i)—
i
at the beginning insert “Subject to point (iii);
ii
omit “except for hair colouring products referred to in paragraph 2”;
b
after point (c)(ii) insert—
iii
“point (c)(i) does not apply to hair colouring products;”;
c
omit paragraph 2.
Substitution of Article 15I81915
For Article 15 (substances classified as CMR substances) substitute—
Article 15Substances classified as CMR substances
F3651
A cosmetic product must not contain a substance classified as a CMR substance of category 1A, 1B or 2 under Regulation (EC) No 1272/2008, unless the substance is included in any of Annexes 3 to 6.
F3662
Where a CMR substance of category 1A or 1B is permitted for use in cosmetic products, specific labelling in order to avoid misuse of the cosmetic product must be provided in accordance with Article 3 of this Regulation, taking into account possible risks linked to the presence of hazardous substances and the routes of exposure.
Substitution of Article 16I82016
For Article 16 (nanomaterials) substitute—
Article 16Nanomaterials
1
The provisions of this Article do not apply to nanomaterials used as colourants, UV-filters or preservatives that are regulated under Article 14.
2
A cosmetic product containing nanomaterials must be notified in accordance with paragraph 3.
3
To meet the requirements of paragraph 2, the information set out in paragraph 4 must be submitted by electronic means—
a
to the Secretary of State;
b
by the responsible person; and
c
at least six months prior to the cosmetic product being placed on the market.
4
The information referred to in paragraph 3 must contain—
a
the identification of the nanomaterial including its chemical name (IUPAC) and other descriptors as specified in point 2 of the Preamble to Annexes 2 to 6 to this Regulation;
b
the specification of the nanomaterial including size of particles and chemical properties;
c
an estimate of the quantity of nanomaterials contained in cosmetic products intended to be placed on the market per year;
d
except where paragraph 13 applies, the toxicological profile of the nanomaterial;
e
the safety data of the nanomaterial relating to the category of cosmetic product, as used in such products;
f
the reasonably foreseeable exposure conditions.
5
Paragraph 6 applies in relation to a cosmetic product containing nanomaterials where prior to F367IP completion day—
a
the cosmetic product has been supplied on the market of the United Kingdom or the market of any EEA state for distribution, consumption or use in the course of a commercial activity (whether in return for payment or free of charge); and
b
a responsible person designated under Article 4 of the EU Regulation (pre-exit) has complied with Article 16 of that Regulation in relation to that product.
6
Where this paragraph applies—
a
if the cosmetic product containing nanomaterials is placed on the market at any time before the expiry of the transitory period, subject to subparagraph (b) paragraphs 2 and 3 are to have effect as if they required the information specified in paragraph 4 before the end of the transitory period; and
b
paragraphs 2 and 3 are to be treated as being satisfied in respect of the cosmetic product where—
i
before the end of the transitory period, the responsible person for the cosmetic product submits to the Secretary of State by electronic means the information set out in paragraph 4; and
ii
when submitting that information, the responsible person at the same time gives notice confirming the matters set out in paragraph 5 in relation to the cosmetic product;
c
if at any time a request is made to the responsible person by a competent authority in accordance with paragraphs 9 and 10, the responsible person must comply with the request within the period specified in the request.
7
Paragraph 8 applies in relation to a cosmetic product containing nanomaterials where—
a
prior to F367IP completion day a responsible person designated under Article 4 of the EU Regulation (pre-exit) has complied with the requirements of Article 16 of that Regulation in relation to that product; and
b
the period between the day on which F367IP completion day falls and the day on which the person designated under Article 4 of the EU Regulation (pre-exit) complied with Article 16 of that Regulation is less than six months.
8
Where this paragraph applies—
a
paragraphs 2 and 3 are to be treated as being satisfied where—
i
a period of 7 months has elapsed between the day on which the responsible person designated under Article 4 of the EU Regulation (pre-exit) complied with Article 16 of that Regulation and the day on which the responsible person places the cosmetic product on the market;
ii
before the expiry of the transitory period, the responsible person for that cosmetic product submits to the Secretary of State the information set out in paragraph 4; and
iii
when submitting that information, the responsible person at the same time gives notice confirming the matters set out in paragraph 7; and
b
if at any time a request is made to the responsible person by a competent authority in accordance with paragraphs 9 and 10, the responsible person must comply with the request within the period specified in the request.
9
Where a competent authority has concerns regarding the safety of a nanomaterial, the competent authority may request that a responsible person submits the following information to the competent authority—
a
which nanomaterials are used in a cosmetic product; and
b
the reasonably foreseeable exposure conditions.
10
When a competent authority makes a request under paragraph 9, the competent authority must specify a period—
a
within which the responsible person must respond; and
b
which is reasonable and commensurate with the nature of the concerns held by the competent authority.
11
Where paragraph 12 applies, the information set out in paragraph 4 may be provided by the person designated in accordance with that paragraph on behalf of the responsible person.
12
This paragraph applies where—
a
the responsible person designates another person by written mandate to meet the notification requirements under this Article in respect of a cosmetic product on that responsible person's behalf (“the designated person”);
b
the designated person accepts the designation in writing; and
c
the responsible person informs the Secretary of State of the name and address of that designated person.
13
The Secretary of State may provide a reference for the toxicological profile and that reference may be provided in the place of the information referred to in paragraph 4(d)
Substitution of Article 18I82117
For Article 18 (animal testing) substitute—
Article 18Animal testing
1
F368Except as provided in paragraph 1A, no cosmetic product may be placed on the market—
a
where the final formulation of the product has been the subject of animal testing in order to meet the requirements of this Regulation;
b
where the ingredients or combinations of ingredients of the product have been the subject of animal testing in order to meet the requirements of this Regulation.
F3691A
Paragraph 1 does not prevent the use of historic animal testing data in order to meet the requirements of this Regulation.
2
No animal testing of finished cosmetic products may take place in the United Kingdom in order to meet the requirements of this Regulation.
3
No animal testing of ingredients or combinations of ingredients may take place in the United Kingdom in order to meet the requirements of this Regulation.
Amendment of Article 19I82218
In Article 19 (labelling)—
a
in paragraph 1 point (a) for “his” substitute “
their
”
;
b
after point (a) of paragraph 1 insert—
ab
for a period of two years beginning on the day after the day on which F370IP completion day falls, point (a) is to be treated as satisfied where the requirements of Article 19(1)(a) of the EU Regulation (pre-exit) are complied with;
c
in paragraph 4 for the words from “Member” to “rules” substitute “
the requirements of regulation 5(1) and (2) of the Enforcement Regulations apply
”
;
d
in paragraph 5 for the words from “shall be” to “user” substitute “
must meet the requirements of regulation 5(3) of the Enforcement Regulations
”
;
e
in paragraph 6 for “provided for” substitute “
referred to
”
.
Amendment of Article 20I82319
In Article 20 (product claims)—
a
for paragraph 2 substitute—
2
A responsible person must ensure that the wording of any claim in relation to a cosmetic product is in compliance with the common criteria set out in the Annex to Commission Regulation (EU) No 655/2013 of 10th July 2013 laying down common criteria for the justification of claims used in relation to cosmetic products.
b
in paragraph 3 for “his” substitute “the manufacturer's”.
Amendment of Article 22I82420
In Article 22 (in-market control)—
a
in the first and second paragraphs for “Member States shall” substitute “
enforcement authorities must
”
;
b
for “They shall” substitute “
Enforcement authorities must
”
;
c
for the third paragraph substitute—
The Secretary of State must entrust other enforcement authorities with the resources and knowledge necessary for the proper performance of their duties.
d
omit the fourth paragraph.
Amendment of Article 23I82521
In Article 23 (communication of serious undesirable effects)—
a
in paragraph 1—
i
for “competent authority” substitute “
Secretary of State
”
;
ii
omit the words from “of the Member State” to “occurred”;
b
in point (a) for “him”—
i
in the first place in which it occurs substitute “
the responsible person or the distributor
”
;
ii
in the second place in which it occurs substitute “
that responsible person or distributor
”
'
c
in point (c) for “him” substitute “
that responsible person or distributor
”
;
d
for paragraph 2 substitute—
2
The Secretary of State must immediately inform all other competent authorities of any information notified to the Secretary of State under paragraph 1.
e
for paragraph 3 substitute—
3
Where a distributor reports serious undesirable effects of a cosmetic product to the Secretary of State, the Secretary of State must immediately inform the responsible person.
f
for paragraph 4 substitute—
4
Where end users or health professionals report serious undesirable effects of a cosmetic product to any competent authority that is not the Secretary of State, that competent authority must immediately inform the Secretary of State who must then immediately inform the responsible person.
Where end users or health professionals report serious undesirable effects of a cosmetic product to the Secretary of State, the Secretary of State must immediately inform all other competent authorities and the responsible person.
Amendment of Article 24I82622
In Article 24 (information on substances)—
a
for “the competent” substitute “
a competent
”
;
b
omit the words from “of a Member” to “market”;
c
for “he” substitute “
the responsible person
”
.
Amendment of Article 25I82723
In regulation 25 (non-compliance by responsible person)—
a
in paragraph 1 omit “Without prejudice to paragraph 4,”
b
omit paragraph 2;
c
in paragraph 3 omit “throughout the Community”;
d
omit paragraph 4;
e
in paragraph 5 omit the subparagraph after point (b);
f
for paragraph 6 substitute—
6
In the event of serious risks to human health, a competent authority which has taken measures under paragraph 5 must inform all other competent authorities of the measures taken.
g
for paragraph 7 substitute—
7
For the purposes of paragraph 6 the database provided for in regulation 33(A1) of the General Product Safety Regulations 2005 (S.I. 2005/1803) must be used
Amendment to Article 27I82824
In Article 27 (safeguard clause)—
a
in paragraph 1 for “a competent authority” substitute “
an enforcement authority
”
b
for paragraph 2 substitute—
2
An enforcement authority which is not the Secretary of State must obtain authorisation from the Secretary of State by requesting the authorisation in accordance with regulation 11 of the Enforcement Regulations prior to taking provisional measures under this Article.
c
in paragraph 3—
i
for “Commission shall” substitute “
Secretary of State must
”
;
ii
for “it shall” substitute “
the Secretary of State must
”
;
iii
for the words from “the interested” to “SCCS” substitute “
any person the Secretary of State considers has an interest in the measure
”
;
d
for paragraph 4 substitute—
4
Where the provisional measures are justified the Secretary of State must give authorisation to the enforcement authority to take those measures.
e
omit paragraph 5.
Amendment of Article 28I82925
In Article 28 (good administrative practice)—
a
in paragraph 1—
i
for “him” substitute “
that responsible person
”
;
ii
omit “of the Member State concerned”;
b
in paragraph 2 for “his” substitute “
their
”
.
Omission of Chapter 9I83026
Omit Chapter 9.
Substitution of Chapter 10I83127
For Chapter 10, substitute—
CHAPTER 10POWERS AND FURTHER DUTIES OF THE SECRETARY OF STATE
Article 30Power to amend Articles
1
Where the Secretary of State considers it necessary to do so to take technical progress into account, the Secretary of State may by regulations amend—
a
point (k) of Article 2(1) (nanomaterials);
b
paragraphs 1, 2 and 6 to 12 of Article 13 (notification) to add requirements; or
c
paragraphs 3, 4 and 11 to 13 of Article 16 (nanomaterials) to add requirements.
2
The Secretary of State may by regulations amend paragraph 3 of Article 2(2) to reflect any changes in the name or structure of the recognised standardisation bodies.
F3713
Where the conditions in paragraph 4 are met, the Secretary of State may by regulations amend Article 16(1) to extend the provisions of Article 16 to nanomaterials used as colourants, UV-filters or preservatives that are regulated under Article 14.
4
The conditions referred to in paragraph 3 are that the Secretary of State considers that it is necessary to do so in view of—
a
safety concerns raised by a competent authority; or
b
scientific or technical evidence that there are safety concerns relating to colourants, UV filters or preservatives regulated under Article 14.
5
The Secretary of State may amend Article 14(1)(c) to extend its scope to hair colouring products.
Article 31Power to amend the annexes
1
The Secretary of State may by regulations amend—
a
Annex 1 where the Secretary of State considers there is sufficient scientific evidence that it is necessary to do so to ensure the safety of cosmetic products;
b
Annexes 2 to 6 where the Secretary of State considers that there is sufficient scientific evidence that there is a potential risk to human health arising from the use of a substance in a cosmetic product;
c
Annexes 2 or 3 where the Secretary of State considers that there is insufficient data to be able to determine whether there is a potential risk to human health;
d
Annexes 3 to 6 and 8 where the Secretary of State considers that there is sufficient scientific evidence that it is necessary to do so to take technical progress into account;
e
Annex 4 to extend its scope to hair colouring products.
F372f
Annex 2 to add a substance classified as a CMR substance of category 1A, 1B or 2 under Regulation (EC) No 1272/2008;
g
Annexes 3 to 6—
i
to allow a substance classified as a CMR substance of category 2 under Regulation (EC) No 1272/2008 to be used in cosmetic products where the Secretary of State considers that there is sufficient scientific evidence that the substance is safe for use in cosmetic products;
ii
to allow a substance classified as a CMR substance of category 1A or 1B under Regulation (EC) No 1272/2008 to be used in cosmetic products where the conditions in point (h) are met;
iii
to make provision as to labelling in order to implement Article 15(2);
h
the conditions referred to in point (g)(ii) are that—
i
the CMR substance complies with the food safety requirements as defined in Regulation (EC) No 178/2002 of the European Parliament and of the Council of January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety;
ii
an analysis of alternative substances has been undertaken and concluded that there are no suitable alternative substances available;
iii
an application to the Secretary of State is made for a particular use of the product category with a known exposure;
iv
the Secretary of State considers that there is sufficient scientific evidence that the CMR substance has been evaluated and found safe for use in cosmetic products; and
v
the evaluation referred to in point (iv) took into account exposure to the product and overall exposure to the CMR substance from other sources, particularly for vulnerable population groups
Article 32Procedure for making regulations
1
Regulations made under Articles 30 or 31 may—
a
make different provisions for different cases; and
b
make such supplementary, transitional, transitory, consequential or saving provision as the Secretary of State considers appropriate.
3
Regulations made under Articles 30 or 31 are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
Article 33Further duties of the Secretary of State
1
The Secretary of State must establish and operate a database containing information relating to cosmetic products which have been made available on the market.
2
The Secretary of State must publish guidance to enable undertakings to comply with the requirements in Annex 1.
3
Before publishing guidance referred to in paragraph 1, the Secretary of State must—
a
consult such persons as the Secretary of State considers have an interest in the guidance;
b
consider how the guidance can be made accessible to business with fewer than 250 members of staff.
4
The Secretary of State must publish the reference to a glossary of common ingredient names and the glossary must be easily accessible and free to use M119.
Amendment to the Preamble to Annexes 2 to 6I83228
In paragraph 2, after “1907/2006” insert “
of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restrictions of Chemicals establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC
”
.
PART 3Amendment of the Cosmetic Products Enforcement Regulations 2013
I91929
The Cosmetic Products Enforcement Regulations 2013 M120 are amended in accordance with paragraphs 30 to 40.
Amendment to regulation 2I83330
In regulation 2 (interpretation)—
a
in the definition of “the EU Cosmetics Regulation” omit “EU”;
b
in the definition of “officer” omit “EU”;
c
omit paragraph (2);
d
in paragraph (3) omit “EU” in both places in which it occurs.
Amendment to regulation 3I83531
In regulation 3 (revocation and savings)—
a
in paragraph (2)(a) after “apply” insert “
subject to the modification in paragraph 3,
”
;
b
in paragraph (2)(b) omit “EU”;
c
after paragraph (2)(b) insert—
c
enforcement authorities must keep information received under regulations 17 or 19 of the 2008 Regulations until 11th July 2020;
d
a responsible person under those Regulations must keep the information collected under regulation 16 of those Regulations until 11th July 2020.
d
after paragraph (2) insert—
3
The modification referred to in paragraph (2)(a) is that any reference to “EEA” is to be read as including the United Kingdom.
Insertion of regulation 3AI83432
After regulation 3, insert—
Transitional provisions in relation to EU Exit3A
1
In this regulation—
“pre-exit period” means the period beginning with 11 July 2013 and ending immediately before F373IP completion day;
“product” means a cosmetic product to which these Regulations apply.
2
Subject to paragraph (3), where a product was made available on the market during the pre-exit period, despite the amendments made by Schedule 34 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M121—
a
b
enforcement authorities continue to be under an obligation to enforce the obligations referred to in paragraph (a).
3
Paragraph (2) does not apply to—
a
any obligation of any competent authority to inform the European Commission or the member States of any matter; or
b
any obligation to take action outside of the United Kingdom in respect of that product.
Amendment to regulation 4I83633
In regulation 4 (competent authority)—
a
in paragraph (1)—
i
omit “Subject to paragraph (2)”; and
ii
omit “EU”;
b
omit paragraph (2);
c
in paragraph (3) omit “Notwithstanding paragraph (2),”.
Amendment to regulations 5 to 8 and 10I83734
In regulations 5 to 8 and 10 each place in which it occurs and in the heading to regulation 8 omit “EU”.
Omission of regulation 9I83835
Omit regulation 9.
Amendment to regulation 10I83936
In regulation 10 (notification to the Secretary of State) omit the words from “,which is required” to “member States”.
Amendment to regulation 11I84037
In regulation 11 for “regulation 9” substitute “
Article 27(2) of the Cosmetics Regulation
”
.
Amendment to regulations 12 to 15, 17, 19 to 21I84138
In regulations 12 to 15, 17 and 19 to 21 in each place in which it occurs omit “EU”.
Amendment to regulation 26I84239
In regulation 26 in paragraphs (1) and (3) after “these Regulations” insert “
and the Cosmetic Regulation
”
.
Amendment to Schedule 3I84340
In Schedule 3 (sampling and testing) omit “EU” in each place in which it occurs.
PART 4Amendment to EU tertiary legislation
Amendment to Commission Regulation (EU) No 655/2013I92041
Commission Regulation (EU) No 655/2013 of 10 July 2013 laying down common criteria for the justification of claims used in relation to cosmetic products is amended in accordance with paragraphs 42 and 43.
Amendment to Article 2I84442
In Article 2—
a
in the first place in which it occurs, after “1223/2009” insert “of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast);
b
in the second place in which it occurs for “Regulation (EC) No 1223/2009” substitute “
that Regulation
”
.
Amendment to the AnnexI84543
In the Annex—
a
in paragraph 1(1) for “within the Union” substitute “
within the meaning of regulation 4 of the Cosmetic Products Enforcement Regulations 2013 or under Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast) M122 (as it has effect in EU law)
”
;
b
in paragraph 6(3) for “relevant Member States” substitute “
the United Kingdom or relevant parts of the United Kingdom
”
.
SCHEDULE 35Amendment of Regulation (EU) 2016/425 and the Personal Protective Equipment (Enforcement) Regulations 2018
PART 1Amendment of subordinate legislation
Amendment of the Personal Protective Equipment (Enforcement) Regulations 2018I8461
1
The Personal Protective Equipment (Enforcement) Regulations 2018 are amended as follows.
2
In regulation 1—
a
in paragraph (2), at the appropriate place, insert the following F45definition—
“Regulation 2016/425 (pre-exit)” means Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment and repealing Council Directive 89/686/EEC, as it had effect immediately before F47IP completion day;
F46...
b
in paragraph (3)—
i
after “In these Regulations”, insert “
(unless otherwise stated)
”
;
ii
in sub-paragraph (a), omit “unless otherwise stated”;
iii
in sub-paragraph (b), after “paragraph of an Article” in both places insert “
, Chapter
”
;
c
in paragraph (4), after “EU Regulation 2016/425” in the second place it occurs, insert “
unless otherwise stated
”
.
3
In regulation 2—
F48a
at the beginning of paragraph (4) insert “
Subject to the modifications made in paragraph (4A),
”
F49b
after paragraph (4), insert—
4A
The modifications referred to in paragraph (4) are as follows—
a
any reference to “Community” is to be read as including the United Kingdom;
b
any reference to “Member State” is to be read as including the United Kingdom;
c
in Schedule 7—
i
in paragraph 5—
aa
omit from “The Commission” to “conducted”;
bb
before “file shall be held” insert
“ manufacturer's technical ”;ii
in paragraph 6, omit from “An inspection body” to the end;
d
in Schedule 10, in paragraph 2, omit from “with a view” to “the Commission
F50c
in paragraph (5), at the end, insert “
of Regulation 2016/425 (pre-exit) or a declaration of conformity set out in paragraphs 7 or 8 of Annex IX
”
;
F51d
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
e
in paragraph (6), for “(4) and (5)” substitute “
(4) to (F525)
”
.
4
After regulation 2, insert—
Obligations which are met by complying with obligations in Regulation 2016/425 (pre-exit)2A
1
In this regulation, “harmonised standard” has the meaning in Article 3(10) of Regulation 2016/425 (pre-exit).
2
Paragraph (3) applies where before placing PPE on the market, the manufacturer—
a
ensures that the PPE has been designed and manufactured in accordance with the applicable essential health and safety requirements set out in Annex II of Regulation 2016/425 (pre-exit);
b
carries out the applicable conformity assessment procedure referred to in Article 19 of Regulation 2016/425 (pre-exit), or has it carried out;
c
draws up the technical documentation referred to in Annex III of Regulation 2016/425 (pre-exit);
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in, or translated into, English;
e
affixes a CE marking in accordance with Articles 16 and 17(1) to (4) of Regulation 2016/425 (pre-exit);
f
draws up an EU declaration of conformity, in accordance with Article 15 of Regulation 2016/425 (pre-exit); and
g
ensures that the EU declaration of conformity is prepared in, or translated into, English.
3
Where this paragraph applies—
a
the requirements of Articles 8(1) and (2), 15, 16, 17 and 19 are to be treated as being satisfied;
b
Articles 8(3), (4), (7) and (8), 9(2) and regulation 7(1) apply, subject to the modifications in paragraph (8);
c
Article 41 does not apply.
4
Paragraph (5) applies, where before placing PPE on the market, the importer ensures that—
a
the applicable conformity assessment procedure referred to in Article 19 of Regulation 2016/425 (pre-exit) has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III of Regulation 2016/425 (pre-exit); and
c
the PPE bears the CE marking in accordance with Articles 16 and 17(1) to (4) of Regulation 2016/425 (pre-exit).
5
Where this paragraph applies—
a
the requirements in Article 10(2), to ensure that—
i
the appropriate conformity assessment procedure referred to in Article 19 has been carried out;
ii
the manufacturer has drawn up the technical documentation;
iii
the PPE bears the UK marking,
are to be treated as being satisfied; and
b
the second subparagraph of Article 10(2), Article 10(4), (5) and (8), and regulation 7(1) apply, subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before placing PPE on the market, a distributor ensures that the PPE bears the CE marking in accordance with Articles 16 and 17(1) to (4) of Regulation 2016/425 (pre-exit).
7
Where this paragraph applies—
a
the requirement for the distributor to verify that the PPE bears the UK marking, referred to in Article 11(2), is to be treated as being satisfied; and
b
(excluding the requirement mentioned in sub-paragraph (a)), Article 11(2), 11(3) and regulation 7(1) apply, subject to the modifications in paragraph (8).
8
The modifications referred to in subparagraphs (3)(b), (5)(b) and (7)(b) are that—
a
any reference to a “declaration of conformity” is to be read as a reference to an EU declaration of conformity, referred to in Article 15 of Regulation 2016/425 (pre-exit);
b
any reference to point 1.4 of Annex II is to be read as a reference to point 1.4 of Annex II of Regulation 2016/425 (pre-exit);
c
any reference to “essential health and safety requirements” is to be read as a reference to the essential health and safety requirements referred to in Annex II of Regulation 2016/425 (pre-exit);
d
any reference to “designated standard” is to be read as a reference to a harmonised standard;
e
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III of Regulation 2016/425 (pre-exit);
f
in regulation 7(1), any reference to a numbered Article is to be read as a reference to the equivalent Article of Regulation 2016/425 (pre-exit).
Conformity assessment procedure obligation which is met by complying with Regulation 2016/425 (pre-exit)2B
1
Paragraph (2) applies where—
a
PPE is classified under Article 18 of Regulation 2016/425 (pre-exit) as falling within risk category II or risk category III, as set out in Annex I to Regulation 2016/425 (pre-exit); and
b
prior to manufacture of that PPE, the manufacturer ensures that the conformity assessment procedure set out in Annex V to Regulation 2016/425 (pre-exit), and referred to in Article 19(b) and (c) of Regulation 2016/425 (pre-exit) as EU type-examination, has been carried out in accordance with Article 19(b) or (c).
2
Where this paragraph applies—
a
the requirement in Article 19(b) or (c) to follow the conformity assessment procedure referred to in those provisions as type-examination, and set out in Annex V, is to be treated as being satisfied;
b
any reference to “conformity assessment procedure” in Articles 8(2) and 10(2) is to be read as including the conformity assessment procedure referred to in Article 19(b) and (c) of Regulation 2016/425 (pre-exit) as EU type-examination;
c
any reference to “technical documentation” in Articles 8(2), 8(3), 10(2) and 10(8) is to be read as including the technical documentation relating to the design of the PPE as referred to in Annex V to Regulation 2016/425 (pre-exit).
F53Expiry of regulations 2A and 2B2C
1
Subject to paragraph (2), regulation 2A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 2A—
a
any PPE which was placed on the market pursuant to regulation 2A may continue to be made available on the market on or after the expiry of regulation 2A;
b
any obligation to which a person was subject under regulation 2A in respect of PPE placed on the market pursuant to regulation 2A continues to have effect after the expiry of regulation 2A, in respect of that PPE.
3
Subject to paragraph (4), regulation 2B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 2B in relation to a product prior to the expiry of regulation 2B, regulation 2B continues to apply in respect of that product where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to that certificate to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after the conformity assessment procedure referred to in regulation 2B(1)(b) has been carried out in relation to that PPE, in accordance with Article 19(b) or (c) of Regulation 2016/425 (pre-exit).
Qualifying Northern Ireland Goods2D
1
In this regulation—
“EU Regulation 2016/425 (Northern Ireland)” means Regulation (EU) No. 2016/425 of March 2016 of March 2016 of the European Parliament and of the Council on personal protective equipment, repealing Council Directive 89/686/EEC, as it has effect by virtue of the Protocol on Ireland/Northern Ireland in the EU withdrawal agreement;
“applicable conformity assessment procedure” means the conformity assessment procedure applicable to the PPE in accordance with Article 19 of EU Regulation 2016/245 (Northern Ireland);
“CE marking” has the meaning given to it in Article 3(18) of EU Regulation 2016/425 (Northern Ireland);
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” means the documentation referred to in Annex III of Regulation 2016/425 (Northern Ireland).
2
Where paragraph (3) applies—
a
PPE is to be treated as being in conformity with the essential safety requirements within the meaning given in EU Regulation 2016/425; and
b
each relevant economic operator is to be treated as having complied or as complying with the obligations imposed on them under Chapter II of EU Regulation 2016/425.
3
This paragraph applies where—
a
PPE is—
i
in conformity with the essential requirements within the meaning given in EU Regulation 2016/245 (Northern Ireland); and
ii
qualifying Northern Ireland goods; and
b
each relevant economic operator has complied or is complying with the obligations imposed on them under Chapter II of EU Regulation 2016/425 (Northern Ireland); and
c
an importer has complied with the obligations set out in paragraph (4).
4
The obligations referred to in paragraph (3)(c) are that, before placing the PPE on the market, the importer—
a
complies with Article 10(3) of EU Regulation 2016/425;
b
ensures that—
i
the applicable conformity assessment procedure has been carried out in relation to the PPE;
ii
the manufacturer has drawn up the technical documentation; and
iii
the PPE bears the CE marking.
5
In regulation 6, for the words from “or other matter within its knowledge” to the end, substitute “
in accordance with Chapter 6
”
.
6
In regulation 7(1), in both places, for “CE” substitute “
UK
”
.
7
In regulation 12(1), omit “at national level”.
F548
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F549
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Consequential amendments to subordinate legislationI8472
1
The following consequential amendments are made to subordinate legislation.
2
In the Personal Protective Equipment at Work Regulations 1992 M123, in regulation 4(5)(b), omit “(OJ No L81, 31.3.2016, p51)”.
3
In the Control of Lead at Work Regulations 2002 M124, in regulation 6(11), omit “(OJ No L81, 31.3.2016, p51)”.
4
In the Control of Substances Hazardous to Health Regulations 2002 M125, in regulation 7(12), omit “(OJ No L81, 31.3.2016, p51)”.
F2905
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F2916
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7
In the Control of Noise at Work Regulations 2005 M126, in regulation 7(5), omit “(OJ No L81, 31.3.2016, p51)”.
F2928
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
9
In Schedule 5B to the REACH Enforcement Regulations 2008 M127, in paragraph 5, omit “(OJ No L81, 31.3.2016, p51)”.
10
In the Control of Asbestos Regulations 2012 M128, in regulation 11(6), omit “(OJ No L81, 31.3.2016, p51)”.
F29311
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12
In the Ionising Radiations Regulations 2017 M129, in regulation 10(3), omit “(OJ No L81, 31.3.2016, p51)”.
F29413
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
PART 2Amendment of retained direct EU legislation
Amendment of Regulation (EU) 2016/425I8483
1
Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment and repealing Council Directive 89/686/EEC is amended as follows.
2
In Article 1—
a
for “on the free movement of” substitute “
concerning
”
;
b
for “Union” substitute “
United Kingdom
”
.
3
In Article 2(2)(d), for “Member States” substitute “
the United Kingdom
”
.
4
In Article 3—
F24a
in points (2) and (3) for “Union market” substitute “
market of Great Britain
”
;
F25b
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
c
for point (6), substitute—
F266
importer” means a person who—
a
is established in the United Kingdom and places PPE from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places PPE on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
d
omit points (10) to (12), and (17) and (18);
e
at the end, insert—
19
‘approved body’ has the meaning given in Article 20;
20
‘designated standard’ has the meaning given in Article 7A;
21
‘enforcement authority’ means a person enforcing this Regulation under regulation 4 of the Personal Protective Equipment (Enforcement) Regulations 2018 (S.I. 2018/390);
22
‘UK Marking’ means the marking in the form set out in Annex 2 of Regulation (EC) 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products, and repealing Regulation (EEC) 339/93;
23
‘UK national accreditation body’ means the body appointed by the Secretary of State in accordance with Article 4 of Regulation (EC) 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products, and repealing Regulation (EEC) 339/93;
24
‘Regulation 2016/425 (pre-exit)’ means Regulation (EU) 2016/425 of the European Parliament and of the Council on personal protective equipment and repealing Council Directive 89/686/EEC, as it had effect immediately before F27IP completion day;
25
In this Regulation, references to “the market surveillance authority” are to be construed in accordance with regulation 3 of the Personal Protective Equipment (Enforcement) Regulations 2018.
5
Omit Article 6.
6
In Article 7—
a
for the heading, substitute— “
Making available, putting into service and exhibition at trade fairs, etc
”
;
b
in paragraph 1, for “Member States shall not impede” substitute “
Nothing in this Regulation impedes
”
;
c
in paragraph 2, for “Member States shall not prevent” substitute “
nothing in this Regulation prevents
”
.
7
After Article 7, insert—
Article 7ADesignated standard
1
Subject to paragraphs 6 and 7, in this Regulation, a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State, by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph 1, “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions; and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures;
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this Article, a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph 1(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph 1(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this Article, a reference to a “product” is a reference to PPE to which this Regulation applies.
9
The Secretary of State may, by regulations, amend paragraph 3 to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph 9 must be made by statutory instrument, subject to annulment in pursuance of a resolution of either House of Parliament.
8
In Article 8—
a
in paragraph 2, in the second subparagraph (beginning “Where compliance of”)—
i
omit “EU”;
ii
for “CE” substitute “
UK
”
;
b
in paragraph 3, omit “EU”;
c
in paragraph 4, for “harmonised” substitute “
designated
”
;
d
in paragraph 6, for “market surveillance authorities” substitute “
the market surveillance authority
”
;
e
in paragraph 7, for the words from “in a language” to the end, substitute “
and that they are clear, legible and in easily understandable English
”
;
f
in paragraph 8, omit “EU” in both places;
g
in paragraph 9, for the words from “competent national authorities” to “on the market”, substitute “
enforcement authority
”
;
h
in paragraph 10, for “a competent national authority” substitute “
the enforcement authority
”
.
9
In Article 9—
a
in paragraph 1, after “appoint” insert “
a person established in the United Kingdom as their
”
, and omit “an”;
b
in paragraph 2—
i
in point (a)—
aa
omit “EU” and “national”;
bb
for “authorities” substitute “
authority
”
;
ii
in point (b), for “a competent national authority” substitute “
the enforcement authority
”
;
iii
in point (c), for “competent national authorities” substitute “
enforcement authority
”
.
10
In Article 10—
a
in paragraph 2—
i
in the first subparagraph (beginning “Before placing PPE”), for “CE” substitute “
UK
”
;
ii
in the second subparagraph (beginning “Where an importer”), for “authorities” substitute “
authority
”
;
b
in paragraph 3—
i
omit the words from “or, where” to the end of the first sentence;
ii
for “authorities” substitute “
authority
”
;
iii
after “and market surveillance authorities” insert—
The obligation set out in this paragraph 3 to indicate information on the PPE does not apply where—
a
either—
i
it is not possible to indicate that information on the PPE, or
b
before placing the PPE on the market, the importer sets out the information referred to in this paragraph 3 on the packaging of the PPE or in a document accompanying the PPE.
c
in paragraph 4, for the words from “in a language which” to the end, substitute “
and that they are clear, legible and in easily understandable English
”
;
d
in paragraph 7, for the words from “competent national authorities” to “available on the market” substitute “
enforcement authority
”
;
e
in paragraph 8—
i
omit “EU”;
ii
for “authorities” in the first place it occurs, substitute “
authority
”
;
iii
for “those authorities” substitute “
that authority
”
;
f
in paragraph 9, for “a competent national authority” substitute “
the enforcement authority
”
.
11
In Article 11—
a
in paragraph 2—
i
in the first subparagraph (beginning “Before making PPE”)—
aa
for “CE” substitute “
UK
”
;
bb
for the words from “in a language which” to “available on the market” substitute “
and that they are clear, legible and in easily understandable English
”
;
ii
in the second subparagraph (beginning “Where a distributor”), for “authorities” at the end, substitute “
authority
”
;
b
in paragraph 4, for the words from “competent national authorities” to “on the market” substitute “
enforcement authority
”
;
c
in paragraph 5, for “a competent national authority” substitute “
the enforcement authority
”
.
12
In Article 13, (in the first sentence), for “authorities” substitute “
authority
”
.
13
For Article 14, substitute—
Article 14Presumption of conformity of PPE
1
PPE which is in conformity with a designated standard or part thereof shall be presumed to be in conformity with the essential health and safety requirements set out in Annex II covered by that standard or part thereof.
2
The presumption in paragraph 1 is rebuttable.
14
In Article 15—
a
in the heading, and in paragraphs 1, 2 and 4, omit “EU”;
b
in paragraph 2, for the words from “translated into the language” to the end, substitute “
in English
”
;
c
for paragraph 3, substitute—
3
Where PPE is subject to more than one enactment requiring a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
15
In Article 16, and in the heading to that Article, for “CE” substitute “
UK
”
.
16
In Article 17—
F31aa
before paragraph 1 insert—
A1
Paragraph 1 is subject to paragraph 1A.
ab
after paragraph 1 insert—
1A
For a period of 24 months beginning with IP completion day, paragraph 1 does not apply where the UK marking is affixed to—
a
a label affixed to the PPE; or
b
a document accompanying the PPE.
a
in paragraphs 1 to 4, and in the heading, for “CE” substitute “
UK
”
;
b
in paragraph 3, for “notified”, in both places, substitute “
approved
”
;
c
in paragraph 4, for “notified” substitute “
approved
”
;
d
omit paragraph 5.
17
In Article 19(b) and (c), omit “EU”.
18
In the heading to Chapter V for “Notification” substitute “
Approval
”
.
19
For Article 20, substitute—
Article 20Approved bodies
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in Article 21 (approval of conformity assessment bodies); or
b
immediately before F32IP completion day was a notified body in respect of which the Secretary of State had taken no action under Article 30 of Regulation 2016/425 (pre-exit), to suspend or withdraw the body's status as a notified body.
2
Paragraph 1 has effect subject to Article 30 (restriction, suspension or withdrawal of approval).
3
In this Chapter—
“notified body” means a body which—
- a
the Secretary of State had, before F32IP completion day, notified to the European Commission and the member States of the European Union in accordance with Article 20 of Regulation 2016/425 (pre-exit); and
- b
in respect of which no objections had been raised, as referred to in Article 28(5) of Regulation 2016/425 (pre-exit);
“approved body requirements” means the requirements set out in Article 24.
20
For Article 21, substitute—
Article 21Approval of conformity assessment bodies
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent;
iii
the category of PPE in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph 4, the Secretary of State may accept an accreditation certificate provided in accordance with paragraph 3(b), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this Article, “accreditation certificate” means a certificate issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
21
For Article 22, substitute—
Article 22UK national accreditation body
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with Article 23; and
c
compiling and maintaining the register of approved bodies, in accordance with Article 29.
22
For Article 23 substitute—
Article 23Monitoring obligations
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet—
i
the approved body requirements; and
ii
any conditions set by the Secretary of State under Article 21(6)(b); and
b
carries out its functions in accordance with this Regulation.
23
In Article 24—
a
for the heading, substitute— “
Approved body requirements
”
;
b
in paragraph 1, for “notification” substitute “
approval
”
;
c
in paragraph 2, for “under the national law of a Member State” substitute “
in the United Kingdom
”
;
d
in paragraphs 4 (in the second subparagraph), and 7(a), for “notified” substitute “
approved
”
;
e
in paragraph 6—
i
for “notified”, in the first two places where it occurs, substitute “
approved
”
;
ii
in point (b), for “a notified body” substitute “
an approved body
”
;
f
in paragraph 7(c)—
i
for “harmonised” substitute “
designated
”
;
ii
for “Union harmonisation legislation and of national legislation” substitute “
this Regulation and any other relevant United Kingdom legislation
”
;
g
in paragraph 9, for “liability is assumed by the Member State in accordance with national law, or the Member State itself” substitute “
the Secretary of State
”
;
h
in paragraph 10—
i
for “national” substitute “
United Kingdom
”
;
ii
for “competent authorities of the Member State in which its activities are carried out” substitute “
enforcement authority
”
;
i
in paragraph 11—
i
for “the notified” substitute “
any approved
”
;
ii
for “under Article 36” substitute “
by the Secretary of State
”
.
24
For Article 25, substitute —
Article 25Presumption of conformity of approved bodies
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such a standard), the Secretary of State must presume that the conformity assessment body meets the approved body requirements covered by that standard (or the part of that standard).
2
The presumption in paragraph 1 is rebuttable.
25
For Article 26, substitute—
Article 26Subsidiaries of, and subcontracting by approved bodies
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this Article, “subsidiary” has the meaning given to it by section 1159 of the Companies Act 2006 M130.
26
Omit Articles 27 and 28.
27
For Article 29, substitute—
Article 29Identification numbers and register of approved bodies
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph 1 must be made publicly available.
28
For Article 30, substitute—
Article 30Restriction, suspension or withdrawal of approval
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in Article 21(6)(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under Article 21.
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in Article 21(6)(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under Article 21.
3
In deciding what action to take under paragraph 1 or 2, the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph 1 or 2, the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph 1 or 2, or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for inspection by the Secretary of State and market surveillance authority for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph 5 include any activities that the body has undertaken as a notified body.
29
Omit Article 31.
30
In Article 32—
a
in the heading, and in paragraph 5, for “notified” substitute “
approved
”
;
b
in paragraph 1, for “Notified” substitute “
Approved
”
;
c
after paragraph 1 insert—
1A
Subject to the terms of its appointment an approved body must carry out the conformity assessment activities and procedures, in respect of which—
a
the body's approval was given under Article 21; or
b
the body's notification as a notified body was made.
d
in paragraphs 3 and 4, for “a notified” substitute “
an approved
”
;
e
in paragraph 3 for “harmonised” substitute “
designated
”
.
31
In Article 33—
a
for “Notified” substitute “
Approved
”
; and
b
in the heading, for “notified” substitute “
approved
”
.
32
In Article 34—
a
in the heading, for “notified” substitute “
approved
”
;
b
in paragraph 1—
i
for—
aa
“Notified” substitute “Approved”;
bb
“notifying authority” substitute “Secretary of State”;
ii
in point (b), for “notification” substitute “
their approval
”
;
iii
in point (c), for “market surveillance authorities” substitute “
the market surveillance authority
”
;
iv
in point (d), for “notification” substitute “
approval
”
;
c
in paragraph 2—
i
for “Notified” in the first place it occurs, substitute “
Approved
”
;
ii
for “the other bodies notified” substitute “
other approved bodies
”
.
33
Omit Article 35 and 36.
34
For the heading to Chapter VI, substitute— “
MARKET SURVEILLANCE AND CONTROL OF PPE ENTERING THE UNITED KINGDOM MARKET
”
.
35
In Article 37—
a
for the heading, substitute— “
Market surveillance and control of PPE entering the United Kingdom market
”
;
b
for “Article 15(3) and Articles 16 to 29” substitute “
Articles 15(3), 16 to 22 and 26 to 29
”
.
36
In Article 38—
a
in the heading, omit “at national level”;
b
in paragraph 1—
i
in the first subparagraph (beginning “Where the market”)—
aa
for “authorities of one Member State have” substitute “
authority has
”
;
bb
for “they” substitute “
the authority
”
;
cc
in the last sentence, for “authorities” substitute “
authority
”
,
ii
in the second subparagraph (beginning “Where, in the course of the evaluation”)—
aa
for “authorities find” substitute “
authority finds
”
;
bb
for “they” in both places, substitute “
the authority
”
;
iii
in the third subparagraph (beginning “The market surveillance authorities”)—
aa
for “authorities”, substitute “
authority
”
;
bb
for “notified” substitute “
approved
”
;
c
omit paragraph 2;
d
in paragraph 3, omit “throughout the Union”;
e
in paragraph 4—
i
in the first subparagraph (beginning “Where the relevant”)—
aa
for “authorities”, substitute “
authority
”
;
bb
omit “provisional”;
cc
for “their national market” substitute “
the market
”
;
ii
omit the second subparagraph (beginning “The market surveillance authorities shall”);
f
omit paragraphs 5 to 8.
37
Omit Article 39.
38
In Article 40—
a
in paragraph 1, for “a Member State” substitute “
the enforcement authority
”
;
b
in paragraph 2, omit “throughout the Union”;
c
omit paragraphs 3 to 5.
39
In Article 41—
a
in paragraph 1—
i
in the first sentence, for “a Member State” substitute “
the enforcement authority
”
;
ii
in points (a) and (b), for “CE” substitute “
UK
”
;
iii
in point (c), for “notified” substitute “
approved
”
;
iv
in point (d), omit “EU”;
b
in paragraph 2, for “Member State concerned” substitute “
enforcement authority
”
.
40
For Article 42 substitute—
Article 42Regulation making powers
1
In order to take into account technical progress and knowledge or new scientific evidence with respect to the category of a specific risk, the Secretary of State may, by regulations, amend Annex I by reclassifying the risk from one category to another.
2
Regulations made under paragraph 1 must be made by statutory instrument, subject to annulment in pursuance of a resolution of either House of Parliament.
3
Any power to make regulations under this Article includes power to make—
a
different provision for different purposes;
b
consequential, supplementary, transitional or transitory provision or savings.
41
Omit Articles 43 to 46.
42
For Article 47, substitute—
Article 47Transitional provision in relation to EU exit
1
In this Article, “pre-exit period” means the period beginning with 21 April 2018 and ending immediately before F23IP completion day.
2
Subject to paragraph 3, where PPE was made available on the market during the pre-exit period, despite the amendments made by Schedule 35 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M131, any obligation to which a person was subject under Regulation 2016/425 (pre-exit), continues to have effect as it did immediately before F23IP completion day, in relation to that PPE.
3
Paragraph 2 does not apply to—
a
any obligation of the enforcement authority to inform the European Commission or the member States of any matter, or
b
any obligation to take action outside of the United Kingdom in relation to that PPE.
4
Where during the pre-exit period—
a
PPE has not been placed on the market; and
b
a manufacturer has taken any action under Article 8(2) of Regulation 2016/425 (pre-exit) with respect to carrying out the applicable conformity assessment procedure referred to in Article 19 of that Regulation,
that action has effect as if it had been done under Article 8(2) of this Regulation (with respect to carrying out the applicable conformity assessment procedure referred to in Article 19 of this Regulation).
43
Omit Article 48.
44
After Article 48, omit—
a
the words “This Regulation shall be binding” to the end;
b
“Done at Strasbourg, 9 March 2016”;
c
the signatory text.
45
In Annex II (Essential Health and Safety Requirements)—
a
in paragraph 1.4—
i
in point (i), for “Union harmonisation” substitute “
relevant United Kingdom
”
;
ii
in point (j), for “notified” substitute “
approved
”
;
iii
in point (k), for “harmonised” substitute “
designated
”
;
iv
in point (l), omit “EU”;
v
in the sentence after point (l), (beginning “The information referred”), omit “EU”.
b
in paragraph 2.12—
i
in the first subparagraph, (beginning “Where PPE bears”)—
aa
omit “harmonised”;
bb
for the words from “a language easily” to the end, substitute “
English
”
;
c
in paragraph 3.5, for “by Directive 2003/10/EC of the European Parliament and of the Council” substitute “
in the Control of Noise at Work Regulations 2005 (S.I. 2005/1643) and the Control of Noise at Work Regulations (Northern Ireland) 2006 (S.R. 2006 No.1)
”
M132
.
46
In Annex III—
a
in point (f), for “harmonised” in both places, substitute “
designated
”
;
b
in point (g), for “harmonised” substitute “
designated
”
.
47
In Annex IV—
a
in the heading to paragraph 4—
i
for “CE” substitute “
UK
”
;
ii
omit “EU”;
b
in paragraph 4.1, for “CE” substitute “
UK
”
;
c
in paragraph 4.2, omit “EU”, in each place it occurs.
48
In Annex V—
a
in the heading, and in the headings to paragraphs 3, 4, 6 and 7, omit “EU”;
b
in paragraph 1, for “a notified” substitute “
an approved
”
;
c
in paragraphs 1, 2, 3, 6.2, 6.3, 7.7, and 9, omit “EU”;
d
in paragraphs 7.2, 7.4, 7.5, 7.6, and 8, omit “EU”, in each place it occurs;
e
in paragraphs 4, 6.1, 6.2, 6.4, 7.2 and 7.7, for “notified” substitute “
approved
”
;
f
in paragraph 3, 5, 7.1, 7.4, 7.5, 7.6 and 8, for “notified”, in each place it occurs, substitute “
approved
”
;
g
in paragraph 4, for “harmonised”, in each place it occurs, substitute “
designated
”
;
h
in paragraph 5, for “notifying authorities” substitute “
Secretary of State
”
;
i
in paragraphs 6.1 and 6.4, for “an EU” substitute “
a
”
;
j
in paragraph 6.2(e) and 7.6(b), for “harmonised” substitute “
designated
”
;
k
in paragraph 8—
i
in the first subparagraph (beginning “Each notified body shall inform its notifying”), for “its notifying authority”, in both places, substitute “
the Secretary of State
”
;
ii
in the third subparagraph (beginning “The Commission”)—
aa
for “The Commission, the Member States”, substitute “
The Secretary of State
”
;
bb
for “On a reasoned request, the Commission and the Member States may” substitute “
The Secretary of State may on request
”
.
49
In Annex VI—
a
in paragraphs 1 and 2, and in the heading to paragraph 3, omit “EU”;
b
in paragraph 3, omit “EU”, in each place it occurs;
c
in paragraph 3.1 and in the heading to paragraph 3, for “CE” substitute “
UK
”
;
50
In Annex VII—
a
in paragraphs 1, 2, 4.1, 4.2, 4.3, 4.4, 4.6 and 6.1, and in the heading to paragraph 6, omit “EU”;
b
in paragraph 3, in each place it occurs—
i
for “notified” substitute “
approved
”
;
ii
omit “EU”;
c
in paragraphs 4.1, 4.2, 4.3, 4.4, 4.6, 5.1 and 6.1, for “notified” substitute “
approved
”
;
d
in paragraph 4.3, for “harmonised” substitute “
designated
”
;
e
in paragraph 4.6, for “notifying authority” substitute “
Secretary of State
”
;
f
in paragraph 5.3, for “notified”, in both places, substitute “
approved
”
;
g
in the heading to paragraph 6, and in paragraph 6.1, for “CE” substitute “
UK
”
;
h
in paragraph 6.2, omit “EU”, in each place it occurs.
51
In Annex VIII—
a
in paragraphs 1, 3.2, 3.6 and 5.1, and in the heading to paragraph 5, omit “EU”;
b
in paragraphs 3.1 and 5.2, omit “EU”, in each place it occurs;
c
in paragraphs 3.3, 4.2, 4.3, 5.1 and 6, and in the heading to paragraph 4, for “notified” substitute “
approved
”
;
d
in paragraphs 3.1, 3.5, 3.6, 4.4 and 7, for “notified”, in each place it occurs, substitute “
approved
”
;
e
in paragraph 3.3, for “harmonised” substitute “
designated
”
;
f
in the heading to paragraph 5, and in paragraph 5.1, for “CE” substitute “
UK
”
;
g
in paragraph 5.2—
i
in the first subparagraph (beginning “The manufacturer shall”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
;
h
in paragraph 6, for “national authorities” substitute “
enforcement authority
”
;
i
in paragraph 7, for “its notifying authority”, in both places, substitute “
the Secretary of State
”
.
52
In Annex IX—
a
in the heading, omit “EU”;
b
in paragraph 5, for “Union harmonisation legislation” substitute “
statutory requirements
”
;
c
in paragraph 6, for “harmonised” substitute “
designated
”
;
d
in paragraphs 7 and 8, for “notified” substitute “
approved
”
;
e
in paragraph 7, omit “EU” in both places it occurs.
SCHEDULE 36Amendment of Regulation (EU) 2016/426 and the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018
PART 1Amendment of subordinate legislation
Amendment of the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018I8491
1
The Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018 are amended as follows.
2
In regulation 1—
a
in paragraph (2), at the appropriate place, insert—
Regulation 2016/426 (pre-exit)” means Regulation (EU) 2016/426 of the European Parliament and of the Council on appliances burning gaseous fuels and repealing Directive 2009/142/EC as it had effect immediately before F374IP completion day;
b
in paragraph (3)—
i
after “In these Regulations”, insert “
(unless otherwise stated)
”
;
ii
in sub-paragraph (a), omit “unless otherwise stated”;
iii
in sub-paragraph (b), after “paragraph of an Article” in both places insert “
, Chapter
”
;
c
in paragraph (4), after “EU Regulation 2016/426” in the second place it occurs, insert “
unless otherwise stated
”
.
3
In regulation 2—
a
in paragraph (4), for “The” substitute “
Subject to the modifications made in paragraph (4A), the
”
;
b
after paragraph (4), insert—
4A
The modifications referred to in paragraph (4) are as follows—
a
any reference to the “Community” is to be read as including the United Kingdom;
b
any reference to “member State” is to be read as though the United Kingdom were a member State;
c
regulation 10(6) is to be read as if the words from “and, on request made by it” to the end were omitted;
d
regulation 13(2) is to be read as if sub-paragraph (b) were omitted;
e
regulation 15(2) is to be read as if sub-paragraph (b) were omitted.
4
After regulation 2, insert—
Obligations which are met by complying with obligations in Regulation 2016/426 (pre-exit)2A
1
In this regulation, “harmonised standard” has the meaning given in Article 2(23) of Regulation 2016/426 (pre-exit).
2
Paragraph (3) applies where before placing an appliance or fitting on the market, or using an appliance for their own purposes, the manufacturer—
a
ensures that the appliance or fitting has been designed and manufactured in accordance with the essential requirements set out in Annex I to Regulation 2016/426 (pre-exit);
b
carries out the applicable conformity assessment procedure referred to in Article 14 of Regulation 2016/426 (pre-exit), or has it carried out;
c
draws up the technical documentation referred to in Annex III to Regulation 2016/426 (pre-exit);
d
ensures that the technical documentation and other records and correspondence relating to the conformity assessment procedures are prepared in or translated into English;
e
affixes a CE marking and the inscriptions provided for in Annex IV of Regulation 2016/426 (pre-exit), in accordance with Articles 16, 17(1) to (4) and 18 of Regulation 2016/426 (pre-exit);
f
draws up an EU declaration of conformity, in accordance with Article 15 of Regulation 2016/426 (pre-exit); and
g
ensures that the EU declaration of conformity is prepared in or translated into English.
3
Where this paragraph applies—
a
the requirements of Articles 7(1) and (2) and 14 to 18 are to be treated as being satisfied;
b
the requirement in Article 7(5) to ensure that appliances and fittings bear inscriptions, is to be treated as being satisfied;
c
Articles 7(3), (4) and (7), 8(2) and point 1.7 of Annex I (referred to in Article 7(7)) and regulations 7(1) and 8(3)(a) apply subject to the modifications in paragraph (8); and
d
Article 40 does not apply.
4
Paragraph (5) applies where before placing an appliance or fitting on the market, the importer ensures that—
a
the applicable conformity assessment procedure referred to in Article 14 of Regulation 2016/426 (pre-exit) has been carried out;
b
the manufacturer has drawn up the technical documentation referred to in Annex III of Regulation 2016/426 (pre-exit); and
c
the appliance or fitting bears the CE marking in accordance with Articles 16 and 17(1) to (4) of Regulation 2016/426 (pre-exit).
5
Where this paragraph applies—
a
the requirements in the first or second subparagraph of Article 9(2), as applicable, to ensure that—
i
the appropriate conformity assessment procedure referred to in Article 14 has been carried out;
ii
the manufacturer has drawn up the technical documentation; and
iii
the appliance or fitting bears the UK marking,
are to be treated as being satisfied;
b
the third subparagraph of Article 9(2), Article 9(5) and (8), and regulations 7(1) and 8(3)(b) apply subject to the modifications in paragraph (8); and
c
in relation to fittings, the second subparagraph of Article 9(2) (other than those requirements treated as being satisfied), point 1.7 of Annex I (referred to in that subparagraph) and the second subparagraph of Article 9(4) also apply subject to the modifications in paragraph (8).
6
Paragraph (7) applies where, before placing an appliance or fitting on the market, a distributor ensures that the appliance or fitting bears the CE marking in accordance with Articles 16 and 17(1) to (4) of Regulation 2016/426 (pre-exit).
7
Where this paragraph applies—
a
the requirement in the first or second subparagraph of Article 10(2), as applicable, for the distributor to verify that the appliance or fitting bears the UK marking, is to be treated as being satisfied;
b
the third subparagraph of Article 10(2), Article 10(3) and regulation 7(1) apply subject to the modifications in paragraph (8); and
c
in relation to fittings, the second subparagraph of Article 10(2) (other than that requirement treated as being satisfied) and point 1.7 of Annex I (referred to in that subparagraph) also apply subject to the modifications in paragraph (8).
8
The modifications referred to in paragraphs (3)(c), (5)(b) and (c), and (7)(b) and (c) are that—
a
any reference to a “declaration of conformity” is to be read as a reference to an EU declaration of conformity, referred to in Article 15 of Regulation 2016/426 (pre-exit);
b
any reference to “essential requirements” is to be read as a reference to the essential requirements set out in Annex I of Regulation 2016/426 (pre-exit);
c
any reference to “designated standard” is to be read as a reference to a harmonised standard;
d
any reference to “technical documentation” is a reference to the technical documentation referred to in Annex III to Regulation 2016/426 (pre-exit);
e
in regulations 7(1) and 8(3), any reference to a numbered Article is to be read as a reference to the equivalent Article of Regulation 2016/426 (pre-exit).
Conformity assessment procedure obligation which is met by complying with Regulation 2016/426 (pre-exit)2B
1
Paragraph (2) applies where, before placing an appliance or fitting on the market the manufacturer ensures that its conformity with Regulation 2016/426 (pre-exit) has been assessed by means of the conformity assessment procedure set out in point 1 of Annex III to Regulation 2016/426 (pre-exit) and referred to in Article 14(2) of Regulation 2016/426 (pre-exit) as EU type-examination, in accordance with that Article.
2
Where this paragraph applies—
a
the requirement in Article 14(2) that the conformity of appliances and fittings with Regulation EU 2016/426 be assessed by means of the type-examination set out in point 1 of Annex III is to be treated as being satisfied;
b
any reference to “conformity assessment procedure” in Articles 7(2) and 9(2) (first and second subparagraphs) is to be read as including the conformity assessment procedure referred to in Article 14(2) of Regulation 2016/426 (pre-exit) as EU type-examination;
c
any reference to “technical documentation” in Articles 7(2), 7(3), 9(2) (first and second subparagraphs) and 9(8) is to be read as including the technical documentation relating to the design of the appliance or fitting as referred to in point 1 of Annex III to Regulation 2016/426 (pre-exit).
F375Expiry of regulations 2A and 2B2C
1
Subject to paragraph (2), regulation 2A ceases to have effect at the end of the period of 12 months beginning with IP completion day.
2
Notwithstanding the expiry of regulation 2A—
a
any appliance or fitting which was placed on the market pursuant to regulation 2A may continue to be made available on the market on or after the expiry of regulation 2A;
b
any obligation to which a person was subject under regulation 2A in respect of any appliance or fitting placed on the market pursuant to regulation 2A continues to have effect after the expiry of regulation 2A, in respect of that appliance or fitting.
3
Subject to paragraph (4), regulation 2B ceases to have effect at the end of the period of 12 months beginning with IP completion day.
4
Where a conformity assessment procedure has been completed pursuant to regulation 2B in relation to a product prior to the expiry of regulation 2B, regulation 2B continues to apply in respect of that product where—
a
the manufacturer arranges for the EU-Type examination certificate and any annexes to be transferred to an approved body;
b
the approved body referred to in sub-paragraph (a) accepts responsibility for the EU-Type examination certificate; and
c
the approved body issues a Type-examination certificate relying, or relying in part, on any examinations or tests undertaken prior to the issue of the EU-Type examination certificate.
5
In paragraph (4) “EU-Type examination certificate” means a certificate issued after the conformity assessment procedure referred to in regulation 2B(1) has been carried out in relation to that appliance or fitting, in accordance with Article 14(2) of Regulation 2019/426 (pre-exit).
Qualifying Northern Ireland Goods2D
1
In this regulation—
“EU Regulation 2016/426 (Northern Ireland)” means Regulation (EU) No. 2016/426 of the European Parliament and of the Council on appliances burning gaseous fuels, repealing Council Directive 2009/142/EC, as it has effect by virtue of the Protocol on Ireland/ Northern Ireland in the EU withdrawal agreement;
“applicable conformity assessment procedure” means the conformity assessment procedure applicable to the appliance or fitting in accordance with Article 14 of EU Regulation 2016/426 (Northern Ireland);
“CE marking” has the meaning given to it in Article 2(31) of EU Regulation 2016/426 (Northern Ireland);
“qualifying Northern Ireland goods” has the meaning given to it in regulations made under section 8C(6) of the European Union (Withdrawal) Act 2018;
“technical documentation” means the documentation referred to in Annex III of Regulation 2016/426 (Northern Ireland).
2
Where paragraph (3) applies—
a
an appliance or fitting is to be treated as being in conformity with the essential safety requirements within the meaning given in EU Regulation 2016/426; and
b
each relevant economic operator is to be treated as having complied or as complying with the obligations imposed on them under Chapter II of EU Regulation 2016/426.
3
This paragraph applies where—
a
the appliance or fitting is—
i
in conformity with the essential requirements within the meaning given in EU Regulation 2016/246 (Northern Ireland); and
ii
qualifying Northern Ireland goods; and
b
each relevant economic operator has complied or is complying with the obligations imposed on them under Chapter II of EU Regulation 2016/426 (Northern Ireland); and
c
an importer has complied with the obligations set out in paragraph (4).
4
The obligations referred to in paragraph (4)(c) are that, before placing the appliance or fitting on the market, the importer—
a
complies with Article 9(3) of EU Regulation 2016/426;
b
ensures that—
i
the applicable conformity assessment procedure has been carried out in relation to the appliance or fitting;
ii
the manufacturer has drawn up the technical documentation; and
iii
the appliance or fitting bears the CE marking.
5
In regulation 6, for the words from “or other matter within its knowledge” to the end, substitute “
in accordance with Chapter 5
”
.
6
In regulation 7(1)(e), for “CE” substitute “
UK
”
.
7
In regulation 8(3), omit “EU” in both places in which it occurs.
8
In regulation 12(1), omit “at national level”.
PART 2Amendment of retained direct EU legislation
Amendment of Regulation (EU) 2016/426I8502
1
Regulation (EU) 2016/426 of the European Parliament and of the Council on appliances burning gaseous fuels and repealing Directive 2009/142/EC is amended as follows.
2
In Article 1—
a
in paragraph 2(b), for “Member States in their communication pursuant to” substitute “ the Secretary of State under”;
b
in paragraph 4, for “acts of Union harmonisation legislation” substitute “
enactments
”
;
c
in paragraph 5, for “a measure adopted pursuant to Article 15 of Directive 2009/125/EC” substitute “
an implementing measure within the meaning given in the Ecodesign for Energy-Related Products Regulations 2010 M133
”
;
d
omit paragraph 6.
3
In Article 2—
F34a
in points (14) and (15) for “Union market” substitute “
market of Great Britain
”
;
F35aa
in point (16) for “the Union” substitute “
Great Britain
”
;
F36b
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
c
for point (19), substitute—
F3719
“importer” means a person who—
a
is established in the United Kingdom and places an appliance or fitting from a country outside of the United Kingdom on the market; or
b
is established in Northern Ireland and places an appliance or fitting on the market that has been supplied to them for distribution, consumption or use in the course of a commercial activity, whether in return for payment or free of charge, from an EEA state;
d
omit points (23) to (25), and (30) to (31);
e
after point (31), insert—
32
‘approved body’ has the meaning given in Article 19;
33
‘designated standard’ has the meaning given in Article 6A;
34
‘enforcement authority’ means a person enforcing this Regulation under regulation 4 of the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018 (S.I. 2018/389);
35
‘UK marking’ means the marking in the form set out in Annex 2 of Regulation (EC) 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) 339/93;
36
‘UK national accreditation body’ means the body appointed by the Secretary of State in accordance with Article 4 of Regulation (EC) 765/2008 of the European Parliament and of the Council setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) 339/93;
37
‘Regulation 2016/426 (pre-exit)’ means Regulation (EU) 2016/426 of the European Parliament and of the Council on appliances burning gaseous fuels and repealing Directive 2009/142/EC, as it had effect immediately before F38IP completion day;
38
In this Regulation, references to “the market surveillance authority” are to be construed in accordance with regulation 3 of the Gas Appliances (Enforcement) and Miscellaneous Amendments Regulations 2018.
4
Omit Article 3(3).
5
For Article 4, substitute—
Article 4Gas supply conditions
1
The Secretary of State must publish information, in accordance with Annex II, about the types of gas and corresponding supply pressures of gaseous fuels used in the United Kingdom.
2
The information that is published under paragraph 1, may be—
a
published in such form and manner as the Secretary of State considers appropriate;
b
reviewed by the Secretary of State at any time, and if it is revised following such a review, the Secretary of State must publish revised information as soon as reasonably practicable following that review.
3
Where the Secretary of State considers it necessary to do so to take account of any technical developments with regard to gas supply conditions, the Secretary of State may by regulations amend the content of the information regarding gas supply conditions as set out in Annex II.
4
Before making regulations under this Article, the Secretary of State must consult such persons as the Secretary of State considers appropriate.
5
Where regulations are made under this Article, the Secretary of State must, as soon as reasonably practicable after those regulations come into force, publish revised information in accordance with Annex II as amended by those regulations.
6
Regulations made under this Article may—
a
make different provisions for different cases; and
b
make such supplemental, consequential and transitional provisions as the Secretary of State considers appropriate.
7
Regulations made under this Article are to be made by statutory instrument subject to annulment in pursuance of a resolution of either House of Parliament.
6
In Article 6—
a
for the heading, substitute— “
Making available, putting into service and exhibition at trade fairs, etc
”
;
b
in paragraphs 1 and 2—
i
for “Member States shall not” substitute “
Nothing in this Regulation prohibits, restricts or impedes
”
;
ii
omit “prohibit, restrict or impede”;
c
in paragraph 3, for “Members States shall not prevent” substitute “
nothing in this Regulation prevents
”
.
7
After Article 6, insert—
Article 6ADesignated standard
1
Subject to paragraphs 6 and 7, in this Regulation a “designated standard” means a technical specification which is—
a
adopted by a recognised standardisation body, for repeated or continuous application, with which compliance is not compulsory; and
b
designated by the Secretary of State, by publishing the reference to the standard and maintaining that publication in a manner the Secretary of State considers appropriate.
2
For the purposes of paragraph 1, “technical specification” means a document that prescribes technical requirements to be fulfilled by a product, process, service or system and which lays down one or more of the following—
a
the characteristics required of a product, including—
i
levels of quality, performance, interoperability, environmental protection, health, safety or dimensions, and
ii
the requirements applicable to the product as regards the name under which the product is sold, terminology, symbols, testing and test methods, packaging, marking or labelling and conformity assessment procedures; and
b
production methods and processes relating to the product, where these have an effect on the characteristics of the product.
3
For the purposes of this Article, a “recognised standardisation body” means any one of the following organisations—
a
the European Committee for Standardisation (CEN);
b
the European Committee for Electrotechnical Standardisation (Cenelec);
c
the European Telecommunications Standards Institute (ETSI);
d
the British Standards Institution (BSI).
4
When considering whether the manner of publication of a reference is appropriate in accordance with paragraph 1(b), the Secretary of State must have regard to whether the publication will draw the standard to the attention of any person who may have an interest in the standard.
5
Before publishing the reference to a technical specification adopted by the British Standards Institution, the Secretary of State must have regard to whether the technical specification is consistent with technical specifications adopted by the other recognised standardisation bodies.
6
The Secretary of State may remove from publication the reference to a standard which has been published in accordance with paragraph 1(b).
7
Where the Secretary of State removes the reference to a standard from publication, that standard is no longer a designated standard.
8
In this Article, a reference to a “product” is a reference to an appliance or fitting to which this Regulation applies.
9
The Secretary of State may, by regulations, amend paragraph 3 to reflect any changes in the name or structure of the recognised standardisation bodies.
10
Regulations made under paragraph 9 must be made by statutory instrument, subject to annulment in pursuance of a resolution of either House of Parliament.
8
In Article 7—
a
in paragraph 2, in the second subparagraph (beginning “Where compliance of”)—
i
for “an EU” substitute “
a
”
;
ii
for “CE” substitute “
UK
”
;
b
in paragraph 3, omit “EU”;
c
in paragraph 4, for “harmonised” substitute “
designated
”
;
d
in paragraph 6, in the first and second subparagraphs, for “market surveillance authorities” substitute “
market surveillance authority
”
;
e
in paragraph 7—
i
in the first and second subparagraphs, for the words from “in a language which” to the end, substitute “
that are clear, legible and in easily understandable English
”
;
ii
in the second and third subparagraphs, omit “EU”;
f
in paragraph 8, for the words from “competent national authorities” to “available on the market”, substitute “
enforcement authority
”
;
g
in paragraph 9, for “a competent national authority” substitute “
the enforcement authority
”
.
9
In Article 8—
a
in paragraph 1, after “appoint” insert “
a person established in the United Kingdom as their
”
, and omit “an”;
b
in paragraph 2—
i
in point (a)—
aa
omit “EU”;
bb
for “national market surveillance authorities” substitute “
the market surveillance authority
”
;
ii
in point (b), for “a competent national authority” substitute “
the enforcement authority
”
;
iii
in point (c), for “competent national authorities” substitute “
enforcement authority
”
.
10
In Article 9—
a
in paragraph 2—
i
in the first and second subparagraphs, for “CE” substitute “
UK
”
;
ii
in the second subparagraph omit “EU”;
iii
in the third subparagraph (beginning “Where an importer”), for “authorities” substitute “
authority
”
;
b
in paragraph 3—
i
in the first and second subparagraphs—
aa
omit the words from “or, where” to the end of the first sentence;
bb
for “authorities” substitute “
authority
”
;
ii
after the second subparagraph insert—
The obligation set out in the first and second subparagraphs of this paragraph 3 to indicate information on the appliance or fitting does not apply where—
a
either—
i
it is not possible to indicate that information on the appliance or fitting, or
b
before placing the appliance or fitting on the market, the importer sets out the information referred to in the first and second subparagraphs on the packaging of the appliance or fitting or in a document accompanying the appliance or fitting.
c
in paragraph 4—
i
in the first and second subparagraphs, for the words from “in a language which” to the end, substitute “
that are clear, legible and in easily understandable English
”
;
ii
in the second subparagraph omit “EU”;
d
in paragraph 7, for the words from “competent national authorities” to “available on the market” substitute “
enforcement authority
”
;
e
in paragraph 8—
i
omit “EU”;
ii
for “authorities” in the first place it occurs, substitute “
authority
”
;
iii
for “those authorities” substitute “
that authority
”
;
f
in paragraph 9, for “a competent national authority” substitute “
the enforcement authority
”
.
11
In Article 10—
a
in paragraph 2—
i
in the first subparagraph (beginning “Before making an appliance”)—
aa
for “CE” substitute “
UK
”
;
bb
for the words from “in a language which” to “made available on the market” substitute “
that are clear, legible and in easily understandable English
”
;
ii
in the second subparagraph (beginning “Before making a fitting”)—
aa
for “CE” substitute “
UK
”
;
bb
omit “EU”;
cc
for the words from “in a language which” to “Member State concerned” substitute “
that are clear, legible and in easily understandable English
”
;
iii
in the third subparagraph (beginning “Where a distributor”), for “authorities” at the end, substitute “
authority
”
;
b
in paragraph 4, for the words from “competent national authorities” to “on the market” substitute “
enforcement authority
”
;
c
in paragraph 5, for “a competent national authority” substitute “
the enforcement authority
”
.
12
In Article 12 (in the first sentence), for “authorities” substitute “
authority
”
.
13
For Article 13, substitute—
Article 13Presumption of conformity of appliances and fittings
1
Appliances and fittings which are in conformity with a designated standard or part thereof shall be presumed to be in conformity with the essential requirements set out in Annex I covered by that standard or part thereof.
2
The presumption in paragraph 1 is rebuttable.
14
In Article 14—
a
in paragraph 2, omit “EU”;
b
in paragraph 4—
i
for the words from “an official language of the Member State” to “established” to the end, substitute “
English
”
;
ii
for “that body” substitute “
the approved body
”
.
15
In Article 15—
a
in the heading, and in paragraphs 1, 5 and 6, omit “EU”;
b
in paragraph 2—
i
omit “EU”;
ii
for the words from “translated into the language” to the end, substitute “
prepared in or translated into English
”
;
c
in paragraph 3—
i
omit “EU” in both places;
ii
for the words from “a language which” to the end, substitute “
English
”
;
d
for paragraph 4, substitute—
4
Where an appliance or a fitting is subject to more than one enactment requiring a declaration of conformity, the manufacturer must draw up a single declaration of conformity which identifies each enactment by its title.
16
In Article 16, and in the heading to that Article, for “CE” substitute “
UK
”
.
17
In Article 17—
F42aa
before paragraph 1 insert——
A1
Paragraph 1 is subject to paragraph (1A).
ab
after paragraph 1 insert—
1A
For a period of 24 months beginning with IP completion day, paragraph 1 does not apply where the UK marking is affixed visibly, legibly and indelibly to—
a
a label affixed to the appliance and fitting or their data plate; or
b
a document accompanying the appliance and fitting or their data plate.
a
in the heading, and in paragraphs 1, 2 and 4, for “CE” substitute “
UK
”
;
b
in paragraph 3—
i
for “CE”, in both places, substitute “
UK
”
,
ii
for “notified”, in both places, substitute “
approved
”
;
c
omit paragraph 5.
18
In the heading to Chapter IV for “Notification of Conformity Assessment Bodies” substitute “
Approval of Conformity Assessment Bodies
”
.
19
For Article 19, substitute—
Article 19Approved bodies
1
An approved body is a conformity assessment body which—
a
has been approved by the Secretary of State pursuant to the procedure set out in Article 20 (approval of conformity assessment bodies); or
b
immediately before F43IP completion day was a notified body in respect of which the Secretary of State had taken no action under Article 29 (changes to notifications) of Regulation 2016/426 (pre-exit), to suspend or withdraw the body's status as a notified body.
2
Paragraph 1 has effect subject to Article 29 (restriction, suspension or withdrawal of approval).
3
In this Chapter—
“notified body” means a body—
- a
which the Secretary of State had, before F43IP completion day, notified to the European Commission and the member States of the European Union in accordance with Article 19 of Regulation 2016/426 (pre-exit); and
- b
in respect of which no objections had been raised, as referred to in Article 27(5) of Regulation 2016/426 (pre-exit);
“approved body requirements” means the requirements set out in Article 23.
20
For Article 20 substitute—
Article 20Approval of conformity assessment bodies
1
The Secretary of State may approve only those conformity assessment bodies that qualify for approval.
2
A conformity assessment body qualifies for approval if the first and second conditions below are met.
3
The first condition is that the conformity assessment body has applied to the Secretary of State to become an approved body and that application is accompanied by—
a
a description of—
i
the conformity assessment activities that the conformity assessment body intends to carry out;
ii
the conformity assessment procedure in respect of which the conformity assessment body claims to be competent; and
iii
the category of appliance or fitting in respect of which the conformity assessment body claims to be competent; and
b
either—
i
an accreditation certificate; or
ii
the documentary evidence necessary for the Secretary of State to verify, recognise and regularly monitor the conformity assessment body's compliance with the approved body requirements.
4
The second condition is that the Secretary of State is satisfied that the conformity assessment body meets the approved body requirements.
5
For the purposes of paragraph 4, the Secretary of State may accept an accreditation certificate provided in accordance with paragraph 3(b)(i), as sufficient evidence that the conformity assessment body meets the approved body requirements.
6
When deciding whether to approve a conformity assessment body that qualifies for approval, the Secretary of State may—
a
have regard to any other matter which appears to the Secretary of State to be relevant; and
b
set conditions that the conformity assessment body must meet.
7
For the purposes of this Article, “accreditation certificate” means a certificate issued by the UK national accreditation body, attesting that a conformity assessment body meets the approved body requirements.
21
For Article 21 substitute—
Article 21UK national accreditation body
The Secretary of State may authorise the UK national accreditation body to carry out the following activities on behalf of the Secretary of State—
a
assessing whether a conformity assessment body meets the approved body requirements;
b
monitoring approved bodies in accordance with Article 22; and
c
compiling and maintaining the register of approved bodies, in accordance with Article 28.
22
For Article 22 substitute—
Article 22Monitoring obligations
The Secretary of State must monitor each approved body with a view to verifying that the body—
a
continues to meet—
i
the approved body requirements;
ii
any conditions set by the Secretary of State under Article 20(6)(b); and
b
carries out its functions in accordance with this Regulation.
23
In Article 23—
a
for the heading, substitute— “
Approved Body Requirements
”
;
b
in paragraph 1, for “notification” substitute “
approval
”
;
c
in paragraph 2, for “under the national law of a Member State” substitute “
in the United Kingdom
”
;
d
in paragraphs 4, in the second subparagraph, and 7(a), for “notified” substitute “
approved
”
;
e
in paragraph 6—
i
for “notified” in the first two places in which it occurs, substitute “
approved
”
;
ii
in point (b), for “a notified body” substitute “
an approved body
”
;
f
in paragraph 7(c)—
i
for “harmonised” substitute “
designated
”
;
ii
for “Union harmonisation legislation and of national legislation” substitute “
this Regulation and any other relevant United Kingdom legislation
”
;
g
in paragraph 9, for “liability is assumed by the State in accordance with national law, or the Member State itself” substitute “
the Secretary of State
”
;
h
in paragraph 10—
i
for “national” substitute “
United Kingdom
”
;
ii
for “competent authorities of the Member State in which its activities are carried out” substitute “
enforcement authority
”
;
i
in paragraph 11—
aa
for “the notified” substitute “
any approved
”
;
bb
for “pursuant to Article 35” substitute “
by the Secretary of State
”
.
24
For Article 24 substitute —
Article 24Presumption of conformity of approved bodies
1
Where a conformity assessment body demonstrates its conformity with the criteria laid down in a designated standard (or part of such a standard), the Secretary of State must presume that the conformity assessment body meets the approved body requirements covered by that standard (or the part of that standard).
2
The presumption in paragraph 1 is rebuttable.
25
For Article 25 substitute—
Article 25Subsidiaries of, and subcontracting by, approved bodies
1
An approved body may subcontract specific conformity assessment activities, or use a subsidiary to carry out such activities provided—
a
the body is satisfied that the subcontractor or subsidiary meets the approved body requirements;
b
the body has informed the Secretary of State that it is satisfied that the subcontractor or subsidiary meets those requirements; and
c
the economic operator for whom the activities are to be carried out has consented to the activities being carried out by that person.
2
The approved body which subcontracts specific conformity assessment activities or uses a subsidiary to carry out such activities remains responsible for the proper performance of those activities (irrespective of where the subcontractor or subsidiary is established).
3
Where an approved body subcontracts, or uses a subsidiary to carry out, a specific conformity assessment activity, the approved body must, for a period of 10 years beginning on the day on which the activity is first carried out, keep available for inspection by the Secretary of State all relevant documentation concerning—
a
the assessment of the qualifications of the subcontractor or the subsidiary; and
b
the conformity assessment activity carried out by the subcontractor or subsidiary.
4
In this Article, “subsidiary” has the meaning given to it by section 1159 of the Companies Act 2006 M134.
26
Omit Articles 26 and 27.
27
For Article 28, substitute—
Article 28Identification numbers and register of approved bodies
1
The Secretary of State must—
a
assign an approved body identification number to each approved body; and
b
compile and maintain a register of—
i
approved bodies;
ii
their approved body identification numbers;
iii
the activities for which they have been approved; and
iv
any restrictions on those activities.
2
The register referred to in paragraph 1 must be made publicly available.
28
For Article 29, substitute—
Article 29Restriction, suspension or withdrawal of approval
1
Where the Secretary of State determines that an approved body—
a
no longer meets an approved body requirement, or
b
is failing to fulfil its obligations under these Regulations, other than a condition referred to in Article 20(6)(b),
the Secretary of State must restrict, suspend or withdraw the body's status as an approved body under Article 20.
2
Where the Secretary of State determines that an approved body no longer meets a condition referred to in Article 20(6)(b), the Secretary of State may restrict, suspend or withdraw the body's status as an approved body under Article 20.
3
In deciding what action to take under paragraph 1 or 2, the Secretary of State must have regard to the seriousness of the non-compliance.
4
Before taking action under paragraph 1 or 2, the Secretary of State must—
a
give notice in writing to the approved body of the proposed action and the reasons for it;
b
give the approved body an opportunity to make representations to the Secretary of State regarding the proposed action within a reasonable period from the date of the notice; and
c
consider any such representations made by the approved body.
5
Where the Secretary of State has taken action in respect of an approved body under paragraph 1 or 2, or where an approved body has ceased its activity, the approved body must, at the request of the Secretary of State—
a
transfer its files relating to the activities it has undertaken as an approved body to another approved body or to the Secretary of State; or
b
keep its files relating to the activities it has undertaken as an approved body available for inspection by the Secretary of State and market surveillance authority for a period of 10 years from the date they were created.
6
The activities undertaken as an approved body referred to in paragraph 5 include any activities that the body has undertaken as a notified body.
29
Omit Article 30.
30
In Article 31—
a
in the heading, and in paragraph 5, for “notified” substitute “
approved
”
;
b
in paragraph 1, for “Notified” substitute “
Approved
”
;
c
after paragraph 1 insert—
1A
Subject to the terms of its appointment, an approved body must carry out the conformity assessment activities and procedures in respect of which —
a
the body's approval was given under Article 20; or
b
the body's notification as a notified body was made.
d
in paragraphs 3 and 4, for “a notified” substitute “
an approved
”
;
e
in paragraph 3 for “harmonised” substitute “
designated
”
.
31
In Article 32—
a
for “Notified” substitute “
Approved
”
; and
b
in the heading, for “notified” substitute “
approved
”
.
32
In Article 33—
a
in the heading, for “notified” substitute “
approved
”
;
b
in paragraph 1—
i
for—
aa
“Notified” substitute “Approved”;
bb
“notifying authority” substitute “Secretary of State”;
ii
in point (b), for “notification” substitute “
their approval
”
;
iii
in point (c), for “market surveillance authorities” substitute “
the market surveillance authority
”
;
iv
in point (d), for “notification” substitute “
approval
”
;
c
in paragraph 2—
i
for “Notified” in the first place it occurs, substitute “
Approved
”
;
ii
for “the other bodies notified” substitute “
other approved bodies
”
.
33
Omit Article 34 and 35.
34
For the heading to Chapter V, substitute— “
MARKET SURVEILLANCE AND CONTROL OF APPLIANCES AND FITTINGS ENTERING F44THE MARKET OF GREAT BRITAIN
”
.
35
In Article 36—
a
for the heading, substitute— “
Market surveillance and control of appliances and fittings entering the United Kingdom market
”
;
b
for “Article 15(3) and Articles 16 to 29” substitute “
Articles 15(3), 16 to 22 and 26 to 29
”
.
36
In Article 37—
a
in the heading, omit “at national level”;
b
in paragraph 1—
i
in the first subparagraph (beginning “Where the market”)—
aa
for “authorities of one Member State have” substitute “
authority has
”
;
bb
for “they” substitute “
the authority
”
;
cc
in the last sentence, for “authorities” substitute “
authority
”
;
ii
in the second subparagraph (beginning “Where, in the course of the evaluation”)—
aa
for “authorities find” substitute “
authority finds
”
;
bb
for “they” in both places, substitute “
the authority
”
;
iii
in the third subparagraph (beginning “The market surveillance authorities”)—
aa
for “authorities”, substitute “
authority
”
;
bb
for “notified” substitute “
approved
”
;
c
omit paragraph 2;
d
in paragraph 3, omit “throughout the Union”;
e
in paragraph 4—
i
in the first subparagraph (beginning “Where the relevant”)—
aa
for “authorities”, substitute “
authority
”
;
bb
omit “provisional”;
cc
for “their national market” substitute “
the market
”
;
ii
omit the second subparagraph (beginning “The market surveillance authorities shall”);
f
omit paragraphs 5 to 8.
37
Omit Article 38.
38
In Article 39—
a
in paragraph 1, for “a Member State” substitute “
the enforcement authority
”
;
b
in paragraph 2, omit “throughout the Union”;
c
omit paragraphs 3 to 5.
39
In Article 40—
a
in paragraph 1—
i
in the first sentence, for “a Member State” substitute “
the enforcement authority
”
;
ii
in points (a) and (b), for “CE” substitute “
UK
”
;
iii
in point (d), for “notified” substitute “
approved
”
;
iv
in points (e) and (f), omit “EU”;
b
in paragraph 2, for “Member State concerned” substitute “
enforcement authority
”
.
40
Omit Articles 41 to 43.
41
For Article 44, substitute—
Article 44Transitional provision in relation to EU exit
1
In this Article, “pre-exit period” means the period beginning with 21 April 2018 and ending immediately before F33IP completion day.
2
Subject to paragraph 3, where an appliance or fitting was made available on the market during the pre-exit period, despite the amendments made by Schedule 36 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 M135, any obligation to which a person was subject under Regulation 2016/426 (pre-exit), continues to have effect as it did immediately before F33IP completion day, in relation to that appliance or fitting.
3
Paragraph 2 does not apply to—
a
any obligation of the enforcement authority to inform the European Commission or the member States of any matter, or
b
any obligation to take action outside of the United Kingdom in respect of that appliance or fitting.
4
Where during the pre-exit period—
a
an appliance or fitting has not been placed on the market; and
b
a manufacturer has taken any action under Article 14 of Regulation 2016/426 (pre-exit) in relation to that appliance or fitting,
that action has effect as if it had been done under Article 14 of this Regulation.
42
Omit Articles 45 and 46.
43
After Article 46, omit—
a
the words “This Regulation shall be binding” to the end;
b
“Done at Strasbourg, 9 March 2016”;
c
the signatory text.
44
In Annex I—
a
in paragraph 1.7, omit “EU”;
b
in paragraph 3.1.7—
i
for “Directive 2014/53/EU of the European Parliament and of the Council” substitute “
the Radio Equipment Regulations 2017 M136
”
;
ii
for “Directive 2014/35/EU of the European Parliament and of the Council” substitute “
the Electrical Equipment (Safety) Regulations 2016 M137
”
;
c
in paragraph 3.1.8—
i
for “Directive 2014/53/EU” substitute “
the Radio Equipment Regulations 2017
”
ii
for “Directive 2014/30/EU of the European Parliament and of the Council” substitute “
the Electromagnetic Compatibility Regulations 2016 M138
”
.
45
In Annex II—
a
in the heading, omit “of the Member States communications”, and after “Conditions” insert “
to be published under Article 4
”
;
b
in paragraph 1, for the words “The communications” to “the following content” substitute “
The Secretary of State must provide the following content in the information that is published under Article 4
”
.
46
In Annex III—
a
in the heading at paragraph 1 and in paragraphs 1.1, 1.2, 1.3, 1.3.1(c)(8), 1.3.2(a) in both places, 1.6 in the second subparagraph, 1.7 in the second subparagraph in both places, 1.8 in each place and 1.9, omit “EU”;
b
in paragraph 1.1, for “a notified” substitute “
an approved
”
;
c
in paragraph 1.3, for “a single notified” substitute “
a single approved
”
;
d
in paragraphs 1.3.1(b), 1.3.1(d), 1.3.2 in both places, 1.4, 1.5 in both places, 1.6 in the first and fourth subparagraphs, 1.7 in each place, and 1.8 in each place, for “notified” substitute “
approved
”
;
e
in paragraph 1.3.1—
i
in point (c)(4) in each place it occurs, and in point (e), for “harmonised” substitute “
designated
”
;
ii
in point (c)(4), omit the words from “the references of” to “Union”;
f
in paragraphs 1.4.2, 1.4.3 and 1.4.4, for “harmonised” substitute “
designated
”
;
g
in paragraph 1.5, for “notifying authorities” substitute Secretary of State”;
h
in paragraph 1.6, in the first subparagraph (beginning “Where the appliance”), and in the fourth subparagraph (beginning “Where the type”), for “an EU” substitute “
a
”
;
i
in paragraph 1.8—
i
in the first subparagraph (beginning “Each notified body shall inform its”), for “its notifying authority” in both places, substitute “
the Secretary of State
”
,
ii
in the third subparagraph (beginning “The Commission”)—
aa
for “The Commission, the Member States” substitute “
The Secretary of State
”
;
bb
for “Commission and the Member States” substitute “
Secretary of State
”
;
j
in paragraph 1.9, for “national authorities” substitute “
enforcement authority
”
;
k
in paragraphs 2.1, 2.2, 2.4.1 and 2.4.2 in each place, and in the heading at paragraph 2.4, omit “EU”;
l
in paragraph 2.3—
i
in the first subparagraph (beginning “A notified body”)—
aa
for “A notified” substitute “
An approved
”
;
bb
for “the notified”, in both place it occurs, substitute “
the approved
”
;
cc
for “harmonised” substitute “
designated
”
;
ii
in the third subparagraph (beginning “The manufacturer shall”), for “notified”, in both places, substitute “
approved
”
;
m
in the heading at paragraph 2.4 and in paragraph 2.4.1, for “CE” substitute “
UK
”
;
n
in paragraph 2.4.2—
i
in the first subparagraph (beginning “The manufacturer shall”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
;
o
in paragraphs 3.1, 3.3.1(e), 3.3.2 in the first subparagraph, 3.5.1 and 3.5.2 in each place, and in the heading at paragraph 3.5, omit “EU”;
p
in paragraphs 3.3.1 in both places, 3.3.3 in the first subparagraph, 3.3.5 in both places, 3.4.2, 3.4.3, 3.4.4 in each place, 3.5.1, 3.6(c), 3.7 in each place, and in the heading at paragraph 3.4, for “notified” substitute “
approved
”
;
q
in paragraph 3.3.3, in the second subparagraph (beginning “It shall presume”), for “harmonised” substitute “
designated
”
;
r
in the heading at paragraph 3.5 and in paragraph 3.5.1, for “CE” substitute “
UK
”
;
s
in paragraph 3.5.2—
i
in the first subparagraph (beginning “The manufacturer shall”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
;
t
in paragraph 3.6, in the first sentence, for “national authorities” substitute “
enforcement authority
”
;
u
in paragraph 3.7, in the first subparagraph (beginning “Each notified body shall inform its”) for “its notifying authority”, in both places, substitute “
the Secretary of State
”
;
v
in paragraphs 4.1, 4.3.1(e), 4.3.2 in the first sentence, 4.5.1, 4.5.2 in each place, and in the heading at paragraph 4.5, omit “EU”;
w
in paragraphs 4.3.1 in both places, 4.3.3 in the first subparagraph, 4.3.5 in both places, 4.4.2, 4.4.3, 4.4.4 in each place it occurs, 4.5.1, 4.6(c), and 4.7 in each place it occurs, and in the heading at paragraph 4.4, for “notified” substitute “
approved
”
;
x
in paragraph 4.3.3, in the second subparagraph (beginning “It shall presume”), for “harmonised” substitute “
designated
”
;
y
in the heading at paragraph 4.5 and in paragraph 4.5.1, for “CE” substitute “
UK
”
;
z
in paragraph 4.5.2—
i
in the first subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
;
aa
in paragraph 4.6, in the first sentence, for “national authorities” substitute “
enforcement authority
”
;
bb
in paragraph 4.7, in the first subparagraph (beginning “Each notified body shall inform its”), for “its notifying authority” in both places it occurs, substitute “
the Secretary of State
”
;
cc
in paragraphs 5.1, 5.2, 5.3 in the first subparagraph, 5.4.1 in the first subparagraph, 5.6.1 and 5.6.2 in each place, and in the heading at paragraph 5.6, omit “EU”;
dd
in paragraph 5.3, in the first subparagraph (beginning “A notified body”), for “A notified” substitute “
An approved
”
;
ee
in paragraphs 5.4.1 in both places and 5.5.2 in both places, for “harmonised” substitute “
designated
”
;
ff
in paragraphs 5.4.1 in the second subparagraph, 5.4.2 in the first subparagraph, 5.5.2, 5.5.3, 5.5.4 in the second subparagraph, 5.5.5 in both places, 5.6.1, 5.6.2 in the third subparagraph in both places and 5.7 in both places, for “notified” substitute “
approved
”
;
gg
in paragraph 5.4.2, in the second subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
hh
in paragraph 5.5.4, in the third subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
ii
in paragraph 5.5.5, for “competent authority” substitute “
enforcement authority
”
;
jj
in the heading at paragraph 5.6 and in paragraph 5.6.1, for “CE” substitute “
UK
”
;
kk
in paragraph 5.6.2—
i
in the first subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
;
ll
in paragraphs 6.2, 6.2.2 in the first subparagraph and in point (c), 6.4 in the second and third subparagraphs, and 6.5.1, for “notified” substitute “
approved
”
;
mm
in paragraph 6.2.1(d)—
i
for “harmonised” in each place it occurs, substitute “
designated
”
;
ii
omit the words from “the references of which” to “Union”;
nn
in paragraphs 6.2.2(a) in both places and 6.5.2 in each place, and in the heading at paragraph 6.5, omit “EU”;
oo
in paragraph 6.2.2 in the second subparagraph (beginning “The manufacturer”), for “relevant national authorities” substitute “
enforcement authority
”
;
pp
in paragraph 6.4—
i
in the first subparagraph (beginning “A notified body”)—
aa
for “A notified” substitute “
An approved
”
;
bb
for “harmonised” in both places, substitute “
designated
”
;
cc
for “the notified” substitute “
the approved
”
;
ii
in the fourth subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
in the heading at paragraph 6.5 and in paragraph 6.5.1, for “CE” substitute “
UK
”
;
rr
in paragraph 6.5.2—
i
in the first subparagraph (beginning “The manufacturer”), for “national authorities” substitute “
enforcement authority
”
;
ii
in the second subparagraph (beginning “A copy of”), for “relevant authorities” substitute “
enforcement authority
”
.
47
In Annex IV, in the first sentence, for “CE” substitute “
UK
”
;
48
In Annex V—
a
in the heading omit “EU”;
b
in paragraph 5—
i
for “Union harmonisation legislation” substitute “
statutory requirements
”
;
ii
omit “(reference to the other Union acts applied)”;
c
in paragraph 6, for “harmonised” substitute “
designated
”
;
d
in paragraph 7, for “notified” substitute “
approved
”
;
49
Omit Annex VI.
SCHEDULE 37Revocation of retained direct EU and EEA legislation
GeneralI8511
The following are revoked—
a
Council Regulation (EC) No. 2679/98 of 7 December 1998 on the functioning of the internal market in relation to the free movement of goods among the Member States;
F376b
Regulation (EU) 2019/515 of the European Parliament and of the Council of 19 March 2019 on the mutual recognition of goods lawfully marketed in another Member State and repealing Regulation (EC) No 764/2008;
c
Decision (EC) No. 768/2008 of the European Parliament and of the Council of 9 July 2008 on a common framework for the marketing of products and repealing Council Decision 93/465/EEC;
d
Regulation (EU) No. 1025/2012 of the European Parliament and of the Council of 25 October 2012 on European standardisation, amending Council Directives 89/686/EEC and 93/15/EEC and Directives 94/9/EC, 94/25/EC, 95/16/EC, 97/23/EC, 98/34/EC, 2004/22/EC, 2007/23/EC, 2009/23/EC and 2009/105/EC of the European Parliament and of the Council and repealing Council Decision 87/95/EEC and Decision No 1673/2006/EC of the European Parliament and of the Council;
e
the following provisions of Part 2 of Annex II to the EEA Agreement—
i
paragraphs 2, 3d, and 3f of Chapter XIX; and
ii
paragraph 1 of Chapter XX.


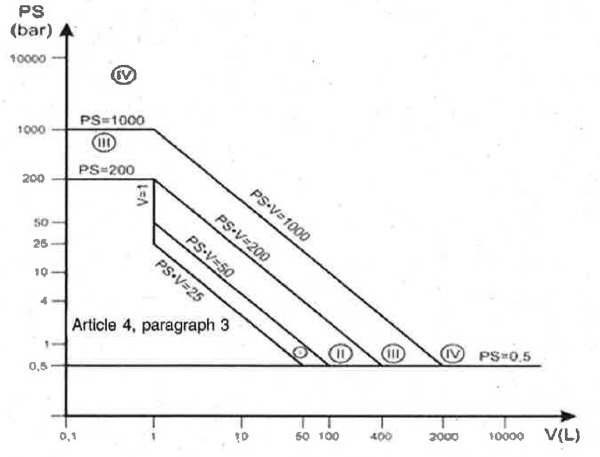

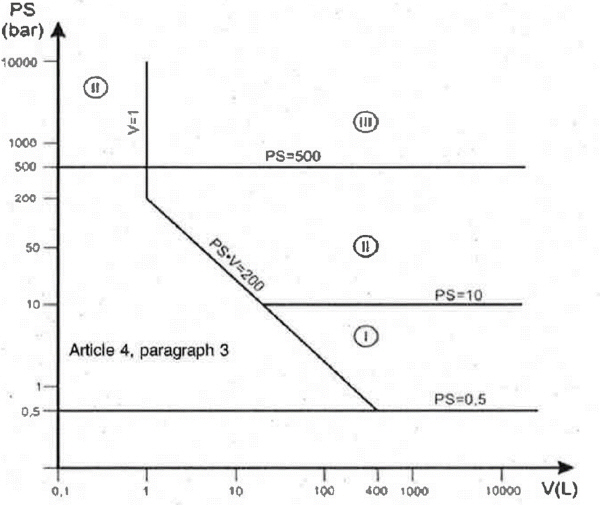
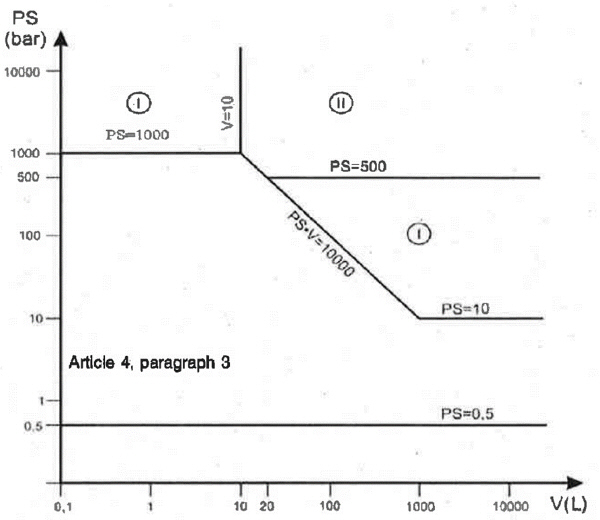






2018 c. 16.