Amendment of the Medical Devices Regulations 2002
6. After regulation 38, insert—
Applications for approval of coronavirus test devices
38A.—(1) A person may make an application to the Secretary of State under this regulation for approval of a coronavirus test device.
(2) An application must include such information as the Secretary of State may require for the purposes of exercising their functions under—
(a)paragraph (5); and
(b)regulation 38C.
(3) An application must be made through the gov.uk website.
(4) The Secretary of State may treat an application made before the coming into force of this regulation as an application made under this regulation, if it meets the requirements of paragraph (2).
(5) The Secretary of State must approve a coronavirus test device if the Secretary of State is satisfied on the basis of the information contained in the application that the coronavirus test device meets the requirements of regulation 38B.
(6) An approval granted under paragraph (5) is valid for a period of 5 years, beginning with the day on which it is granted.
(7) Nothing in this regulation shall be taken to prevent—
(a)the Secretary of State;
(b)a weights and measures authority in Great Britain; or
(c)a district council in Northern Ireland,
from exercising a duty under regulation 61 to enforce these Regulations.
Performance requirements for coronavirus test devices
38B.—(1) The requirements that a coronavirus test device must meet for the purposes of regulation 38A(5) are set out in paragraphs (2) to (6).
(2) A coronavirus test device must be able to be put into service in accordance with this Part.
(3) A coronavirus test device that is an antigen test must have—
(a)a level of sensitivity, using a 95% two-sided confidence interval, that is entirely above 60%;
(b)a level of specificity, using a 95% two-sided confidence interval, that is entirely above 93%.
(4) A coronavirus test device that is a direct molecular test must have—
(a)a level of sensitivity, using a 95% two-sided confidence interval, that is entirely above 70%;
(b)a level of specificity, using a 95% two-sided confidence interval, that is entirely above 93%.
(5) A coronavirus test device that is an extracted molecular test must have—
(a)a level of sensitivity, using a 95% two-sided confidence interval, that is entirely above 93%;
(b)a level of specificity, using a 95% two-sided confidence interval, that is entirely above 97%.
(6) Where a coronavirus test device is also intended to detect the presence of anything other than a viral antigen or viral ribonucleic acid (RNA) specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the requirements in paragraphs (2) to (5) apply only in relation to its performance in detecting the presence of that viral antigen or viral ribonucleic acid (RNA).
(7) In this regulation and in regulation 38C—
“antigen test” means an in vitro diagnostic medical device for the detection of the presence of a viral antigen specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2);
“direct molecular test” means an in vitro diagnostic medical device which—
is for the detection of the presence of viral ribonucleic acid (RNA) specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and
does not use a preliminary step of purification and concentration;
“extracted molecular test” means an in vitro diagnostic medical device which—
is for the detection of the presence of viral ribonucleic acid (RNA) specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and
uses a preliminary step of purification and concentration;
“sensitivity”, in relation to a coronavirus test device, means the proportion of true positives that are correctly identified by the test, calculated using the equation—
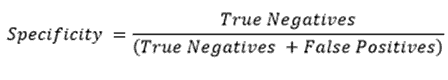
“specificity”, in relation to a coronavirus test device, means the proportion of true negatives that are correctly identified by the test, calculated using the equation—
Register of approved coronavirus test devices
38C.—(1) The Secretary of State must establish a register of coronavirus test devices which the Secretary of State has approved in accordance with regulation 38A.
(2) The Secretary of State must publish the register on the gov.uk website.
(3) The register must contain the following information in respect of each coronavirus test device—
(a)the name and address of the registered place of business of the person who made the application under regulation 38A;
(b)if the person who made the application was not the manufacturer, the name and address of the registered place of business of the manufacturer;
(c)the country in which the manufacturer is established;
(d)the name and address of the registered place of business of the UK responsible person or the manufacturer’s authorised representative having a registered place of business in Northern Ireland, if there is one in respect of the device;
(e)the name and description of the coronavirus test device;
(f)the date and version number of the instructions for use included in the application;
(g)whether the coronavirus test device is an antigen test, a direct molecular test, or an extracted molecular test;
(h)the date on which the coronavirus test device was approved in accordance with regulation 38A and the date on which that approval ceases to be valid.
(4) The register may contain such other information relating to the coronavirus test device and its intended use as the Secretary of State considers appropriate.”.