ANNEX IU.K.METHOD FOR THE DETECTION AND DIAGNOSIS OF THE RING ROT BACTERIUM, CLAVIBACTER MICHIGANENSIS (Smith) Davis et al. ssp. SEPEDONICUS (Spieckermann et Kotthof) Davis et al. IN BATCHES OF POTATO TUBERS
1. Removal of heel-end cores U.K.
1.1.Wash 200 tubers in running tap water and remove the epidermis around the heel end of each tuber using a regularly disinfected scalpel or potato peeler; disinfection may be achieved by dipping the peeler in 70 % ethanol and flaming.U.K.
1.2.Carefully remove conical tissue cores from the heel ends with a knife or potato peeler. Keep the excess non-vascular tissue to a minimum. Once removed, heel ends should be processed within 24 hours (see paragraph 3) or conserved at -20oC for no longer than two weeks.U.K.
2. Visual examination for ring rot symptoms U.K.
After removal of heel ends, cut each tuber transversely and observe for the presence of ring rot symptoms.
Squeeze the tubers and look for expression of macerated tissues from the vascular tissue.
The earliest symptoms are a slight glassiness or translucence of the tissue without softening round the vascular system, particularly near the heel end. The vascular ring at the heel end may be slightly darker in colour than normal. The first readily identifiable symptom is one whereby the vascular ring has a yellowish coloration and when the tuber is gently squeezed, pillars of cheese-like material emerge from the vessels. This exudation contains millions of bacteria. Browning of the vascular tissue may develop at this stage. At first, these symptoms may be restricted to one part of the ring, not necessarily close to the heel end and may gradually extend to the whole ring. As the infection progresses, destruction of the vascular tissue occurs; the outer cortex may become separated from the inner cortex. In advanced stages of infection, cracks appear on the surface of the tuber, which are often reddish-brown at their margins. Secondary fungal or bacterial invasion may mask the symptoms and it may be difficult, if not impossible, to distinguish advanced ring rot symptoms from other tuber rots.
3. Preparation of samples for Gram staining, immunofluorescence staining (IF) and eggplant test U.K.
3.1.Homogenize the heel ends until complete maceration has just been achieved in a diluent known to be non-toxic to Corynebacterium sepedonicum (for example, 0,05 M phosphate buffered saline (PBS) pH 7,0) at a temperature of less than 30oC; the addition of a non-toxic deflocculant is advisable and non-toxic antifoam agent may be needed (Appendices 1 and 2). Excessive maceration should be avoided.U.K.
3.2.Extract bacteria from the homogenate by one of the methods as follows():U.K.
A.
(a)
Centrifuge at not more than 180 g for 10 minutes.
(b)
Centrifuge supernatant at not less than 4 000 g for 10 minutes. Decant and discard the supernatant.
B.
(a)
Allow the macerate to stand for 30 minutes for the tissue debris to settle. Decant the supernatant.without disturbing the sediment.
(b)
Filter the supernatant through filter paper (Whatman No 1) held in a sintered glass filter (No 2 = 40-100|J.m) using a water vacuum pump. Collect the filtrate in a centrifuge tube. Wash the filter with sterile PBS to a maximum filtrate volume of 35 ml.
(c)
Centrifuge filtrate at not less than 4 000 g for 20 minutes.
3.3.Suspend the pellet in sterile 0,01 M phosphate buffer pH 7,2 (Appendix 2) to give a total volume of approximately 1 ml. Divide in two equal parts and retain one part for reference purposes by freezing at - 20 oC() or by lyophilization. Divide the other part into halves using one half for the IF test and Gram stain and the other for the eggplant test.U.K.
3.4.It is imperative that all positive C. sepedonicum controls and samples are treated separately to avoid contamination. This applies to IF slides and to eggplant tests.U.K.
4. Gram staining U.K.
4.1.Prepare Gram stains for all pellet dilutions (5.2.1) and for any cut tubers (2) which show glassiness, rotting or other suspect symptoms. Samples should be taken from the edge of diseased tissues.U.K.
4.2.Prepare Gram stains for known C. sepedonicum cultures and, if possible, for naturally infected tissue (5.1).U.K.
4.3.Determine which samples contain typical Gram positive coryneform cells. In general, C. sepedonicum cells are 0,8 to l,2μm long and 0,4 to 0,60μm wide.U.K.
An appropriate staining procedure is given in Appendix 3.
Preparations from natural infections or recently isolated cultures often show a predominance of coccoid rods which are usually slightly smaller than cells from oder agar cultures. On most culture media, C. sepedonicum cells are pleomorphic coryneform rods and may give a variable Gram reaction. Cells are single, in pairs with characteristic ‘elbows’ typical of bending division, and occasionally in irregular groups often referred to as palisades and Chinese letters.
5. Scheme for IF-testing U.K.
5.1.Use antiserum to a known strain of C. sepedonicum — ATCC 33113 (NCPPB 2137), or NCPPB 2140. This should have an IF titre of more than 1:600. Include one PBS control on the test slide to determine whether the fluorescein isothiocyanate anti-rabbit immunoglobulin conjugate (FITC) combines non-specifically with bacterial cells. Corynebacterium sepedonicum (ATCC 33113 (NCPPB 2137), NCPPB 2140) should be used as homologous antigen controls on a separate slide. Naturally infected tissue (maintained by lyophilization or freezing at - 20oC) should be used where possible as a similar control on the same slide (Figure 2).U.K.
5.2. Procedure U.K.
5.2.1.Prepare three serial ten fold dilutions (101, 102, 103) of the final pellet in distilled water (Figure 1).U.K.
5.2.2.Pipette a measured standard volume sufficient to cover the window (approximately 25 μl) of each pellet dilution or C. sepedonicum suspension (approximately 106 cells/ml) to windows of a multispot slide as shown in Figure 1.U.K.
Figure 1
Sample and PBS control slide

Figure 2
Positive control slide

5.2.4.Cover appropriate windows which C. sepedonicum antiserum at the recommended dilutions, 0,01 M PBS pH 7,2 (Appendix 2), as shown in Figure 1. (Use PBS for the FITC control.) The working dilution of the antiserum should be approximately half that of the IF titre. If other antiserum dilutions are to be included, separate slides should be prepared for each dilution to be used.U.K.
5.2.5.Incubate in a humid chamber at ambient temperature for 30 minutes.U.K.
5.2.6.Rinse carefully with 0,01 M PBS pH 7,2. Wash for five minutes in three changes of 0,01 M PBS pH 7,2.U.K.
5.2.7.Carefully remove excess moisture.U.K.
5.2.8.Cover each window with FITC conjugate at the same dilution used to determine the titre and incubate in a dark humid chamber at ambient temperature for 30 minutes.U.K.
5.2.9.Rinse and wash as before.U.K.
5.2.10.Apply approximately 5 to 10 μl of 0,1 M phosphate buffered glycerine pH 7,6 (or a similar mountant with a pH not less than 7,6) to each window and cover with a coverglass (Appendix 2).U.K.
5.2.11.Examine with a microscope fitted with an epifluorescent light source and filters suitable for working with FITC. A magnification of 400 to 1 000 is suitable. Scan replicated windows across two diameters at right angles and around the window perimeters.U.K.
Observe for fluorescing cells in the positive controls and determine the titre. Observe for fluorescing cells in the FITC/PBS control window and, if absent, proceed to the test windows. Determine in a minimum of 10 microscope fields the mean number of morphologically typical fluorescing cells per field and calculate the number per ml of undiluted pellet (Appendix 4).
There are several problems inherent to the immunofluorescence test.
Background populations of fluorescing cells with atypical morphology and cross reacting saprophytic bacteria with size and morphology similar to Clavibacter michiganensis ssp. sepedonicus are likely to occur in potato pellets. Consider only fluorescing cells with typical size and morphology.
Because of the possibility of cross-reactions, samples with a positive immunoflorescence test should be retested using a different antiserum.
The technical limit of detection of this method is situated between 103 and 104 cells per ml of undiluted pellet. Samples with counts of IF typical cells at the detection limit are usually negative for C. m. ssp. sepedonicus but may be committed to eggplant testing.
A negative immunofluorescence test is identified for any sample where morphologically typical fluorescing cells are not found. The samples shall be considered as ‘not contaminated’ with Clavibacter michiganensis ssp. sepedonicus.
The eggplant test is not required.
A positive immunofluorescence test is identified for any sample where morphologically typical fluorescing cells are found.
Samples for which a positive immunofluorescence test have been identified with both antisera shall be considered as ‘potentially contaminated’ with Clavibacter michiganensis ssp. sepedonicus.
The eggplant test is required for all samples considered as potentially contaminated.
6. Eggplant test U.K.
For cultural details, see Appendix 5.
6.1.Distribute the pellet from 3.3 between at least 25 eggplants at leaf stage 3 (Appendix 5) by one of the methods given below (6.2, 6.3 or 6.4).U.K.
6.2. Slit inoculation I U.K.
6.2.1.Support each pot horizontally (a block of expanded polystyrene with a piece 5 cm deep x 10 cm wide x 15 cm long, removed from one surface (Figure 3) is adequate for a 10 cm pot). A strip of sterile aluminium foil should be placed between the stem and the block for each sample tested. The plant may be held in place by a rubber band around the block.U.K.
6.2.2.Using a scalpel, make a longitudinal or slightly diagonal cut 0,5 to 1,0 cm long and approximately three quarters of the stem diameter deep, between the cotyledons and the first leaf.U.K.
6.2.3.Hold the slit open with the scalpel blade point and paint the inoculum into it using an eyeliner or fine artist's brush charged with the pellet. Distribute the remainder of the pellet between the eggplants.U.K.
6.2.4.Seal the cut with sterile vaseline from a 2 ml syringe barrel.U.K.
Figure 3

6.3. Slit inoculation II U.K.
6.3.1.Holding the plant between two fingers, pipette a drop (approximately 5 to 10 µl) of the suspended pellet on the stem between the cotyledons and the first leaf.U.K.
6.3.2.Using a sterile scalpel, make a diagonal (at an angle of approximately 5o) slit, 1,0 cm long and approximately 2/3 of the stem thickness deep, starting the cut from the pellet drop.U.K.
6.3.3.Seal the cut with sterile vaseline from a syringe barrel.U.K.
6.4. Syringe inoculation U.K.
6.4.1.Do not water eggplants for one day prior to inoculation to reduce turgor pressure.U.K.
6.4.2.Inoculate the eggplant stems just above the cotyledons using a syringe fitted with a hypodermic needle (not less than 23G). Distribute the pellet between the eggplants.U.K.
6.5.Inoculate 25 plants with a known C. sepedonicum culture and, where possible, naturally infected tuber tissue (5.1) by the same inoculation method (6.2, 6.3 or 6.4).U.K.
6.6.Inoculate 25 plants with sterile 0,05 M PBS by the same inoculation method (6.2, 6.3 or 6.4).U.K.
6.7.Incubate the plants in appropriate conditions (Appendix 5) for 40 days. Examine regularly for symptoms after eight days. Count the number of plants showing symptoms. C. sepedonicum causes leaf wilting in eggplants which may commence as a marginal or interveinal flaccidity. Wilted tissue may initially appear dark green or mottled but turns paler before becoming necrotic. Interveinal wilts often have a greasy water-soaked appearance. Necrotic tissue often has a bright yellow margin. Plants are not necessarily killed; the longer the period before symptoms develop, the greater the chance of survival. Plants may outgrow the infection. Susceptible young eggplants are much more sensitive to low populations of C. sepedonicum than are older plants, hence the necessity to use plants at or just before leaf stage 3.U.K.
Wilts may also be induced by populations of other bacteria or fungi present in the tuber tissue pellet. These include Erwinia carotovora, subsp. carotovora and E. carotovora subsp. atroseptica, Phoma exigua var. foveata, as well as large populations of saprophytic bacteria. Such wilts can be distinguished from those caused by C. sepedonicum since whole leaves or whole plants wilt rapidly.
6.8.Prepare a Gram stain (4) for all batches of eggplants showing symptoms, using sections of wilted leaf tissue and stem tissue from plants and isolate on to suitable nutrient media (7). Surface disinfect the eggplant leaves and sterns by wiping with 70 % ethanol.U.K.
6.9.Under certain circumstances, in particular where growing conditions are not optimal, it may be possible for C. sepedonicum to exist as a latent infection within eggplants even after incubation for 40 days. Such infections may possibly result in stunting and lack of vigour in the inoculated plants. If the IF test is considered positive, it may be considered necessary to test further. It is, therefore, essential to compare the growth rates of all eggplant test plants with the sterile 0,05 M PBS inoculated controls and to monitor the environmental conditions of the glasshouse.U.K.
Recommendations for further testing are as follows:
6.9.1.
excise the stems above the inoculation site and remove the leaves;
6.9.2.
macerate the stems in 0,05 M PBS pH 7,0, as in 3.1 to 3.2;
6.9.3.
use half of the pellet to perform a Gram stain (4) and an IF test (5);
6.9.4.
use the other half to perform a further eggplant test (6) if the Gram stain and/or IF tests are positive. Use a known C. sepedonicum culture and sterile 0,05M PBS controls. If symptoms are not observed in the subsequent test, the sample must be considered negative.
7. Isolation of C. sepedonicum U.K.
Diagnosis can only be confirmed if C. sepedonicum is isolated and so identified (8). Although C. sepedonicum is a fastidious organism, it can be isolated from symptomatic tissue. However, it may be outgrown by rapidly growing saprophytic bacteria and, therefore, isolations directly from the tuber tissue pellet (3.3) are not recommended. Eggplants provide an excellent selective enrichment medium for the growth of C. sepedonicum and also provide an excellent confirmatory host test.
Isolations should be made from all symptomatic potato tubers and eggplants (4, 6). Maceration of eggplant stems when necessary should be carried out as in 3. and 6.9.
7.1.Streak suspensions on to one of the following media: (formulae are given in Appendix 6):U.K.
nutrient dextrose agar (for subculture only),
yeast peptone glucose agar,
nutrient yeast dextrose agar,
yeast extract mineral salts agar.
Incubate at 21 oC for up to 20 days.
C. sepedonicum is slow-growing, usually producing pin-point, cream, domed colonies within 10 days.
Re-streak to establish purity.
Growth rates are improved with subculture. Typical colonies are creamy-white or ivory, rounded, smooth, raised, convex-domed, mucoid-fluidal, with entire edges and usually 1 to 3 mm in diameter.
Identification U.K.
Many Gram positive coryneform bacteria, with colonial characters similar to those of C. sepedonicum, may be isolated from healthy or diseased potatoes and eggplants. In this context C. sepedonicum must be identified by the following tests:
IF test (5.1),
eggplant test,
nutrition and physiological tests (Appendix 7),
oxidation/fermentation test (O/F),
oxidase test,
growth at 37oC,
urease production,
aesculin hydrolysis,
starch hydrolysis,
tolerance of 7 % sodium chloride solution,
indole test,
catalase test,
H28 production,
citrate utilization,
gelatin hydrolysis
acid from: glycerol, lactose, rhamnose and salicin,
Gram stain.
All tests should include a known C. sepedonicum control. Nutritional and physiological tests should be made using inocula from nutrient agar subcultures. Morphological comparisons should be made from nutrient dextrose agar cultures.
For the IF test, cell populations should be adjusted to 106 cells/ml. The IF titre should be similar to that of the known C. sepedonicum culture.
For the eggplant test cell populations should be adjusted to c 107 cells/ml. Eggplant tests should be made using 10 plants for each of the test organisms, again using known C. sepedonicum culture and sterile water controls; with pure cultures typical wilting should be obtained within 20 days but plants not showing symptons after this time should be incubated for a total of 30 days at temperatures conducive to eggplant growth but not exceeding 30oC (Appendix 5). If after 30 days symptoms are not present, the culture cannot be confirmed as being a pathogenic form of Corynebacterium sepedonicum.
| Test | C. sepedonicum |
| O/F | Inert or weakly oxidative |
| Oxidase | - |
| Catalase | + |
| Nitrate reduction | - |
| Urease activity | - |
| H2S production | - |
| Indole production | - |
| Citrate utilization | - |
| Starch hydrolysis | - or weak |
| Growth at 37o | - |
| Growth in 7 % NaCl | - |
| Gelatin hydrolysis | - |
| Aesculin hydrolysis | + |
| Acid from: | |
| — Glycerol | - |
| — Lactose | - or weak |
| — Rhamnose | - |
| — Salicin | |
Appendix 1FORMULATION OF MACERATING FLUID RECOMMENDED BY LELLIOTT AND SELLAR, 1976
| D C silicone antifoam MS A compound (Hopkins & Williams Ltd, Cat. No 9964-25, Chadwell Heath, Essex, England) | 10 ml |
| Lubrol W flakes (ICI Ltd) | 0,5 g |
| Tetra-sodium pyrophosphate | 1 g |
| 0,05 M phosphate buffered saline pH 7,0 (Appendix 2) | 1 litre |
Appendix 2BUFFERS
0,05 M phosphate buffered saline pH 7,0 U.K.
This buffer can be used for tuber tissue maceration (2.1)
| Na2HPO4 | 4,26 g |
| KH2PO4 | 2,72 g |
| NaCl | 8,0 g |
| Distilled water to | 1 litre |
0,01 M phosphate buffered saline pH 7,2 U.K.
This buffer is used for diluting antisera and washing IF slides
| Na2HPO4 12 H2O | 2,7 g |
| NaH2PO4 2 H2O | 0,4 g |
| NaCl | 8,0 g |
| Distilled water to | 1 litre |
0,1 M phosphate buffered glycerine pH 7,6 U.K.
This buffer is used as a mountant to enhance fluorescence in the IF test
| Na2HPO4 12 H2O | 3,2 g |
| NaH2PO4 2 H2O | 0,15 g |
| Glycerol | 50 ml |
| Distilled water | 100 ml |
Appendix 3GRAM STAIN PROCEDURE (HUCKER'S MODIFICATION) (DOETSCH, 1981)
Crystal violet solution U.K.
Dissolve 2 g crystal violet in 20 ml 95 % ethanol.
Dissolve 0,8 g ammonium oxalate in 80 ml distilled water.
Mix the two solutions.
Lugol's iodine U.K.
| Iodine | 1 g |
| Potassium iodide | 2 g |
| Distilled water | 300 ml. |
Grind the solids together in a pestle and mortar. Add to the water and stir to dissolve in a closed container.
Safranin counterstain solution U.K.
Stock solution:
| Safranin O | 2,5 g |
| 95 % ethanol | 100 ml. |
Mix and store.
Dilute: 1:10 to obtain a working solution.
Staining procedure U.K.
1.Prepare smears, air dry and heat fix.U.K.
2.Flood slide with crystal violet solution for one minute.U.K.
3.Wash briefly with tap water.U.K.
4.Flood with Lugol's iodine for one minute.U.K.
5.Wash with tap water and blot dry.U.K.
6.Decolourize with 95 % ethanol, added dropwise, until no further colour is removed or immerse with gentle agitation for 30 seconds.U.K.
7.Wash in tap water and blot dry.U.K.
8.Flood with safranin solution for 10 seconds.U.K.
9.Wash with tap water and blot dry.U.K.
Gram positive bacteria stain violet-blue; Gram negative bacteria stain pink-red.
Appendix 4DETERMINATION OF POPULATION OF IF-POSITIVE CELLS
Surface area (S) of window of multispot slide
Where D = diameter of window. Surface area(s) of objective field
where d = diameter of field.
Calculate d either by direct measurement or from the following formule:
| where | i = field coefficient (depends upon ocular type and varies from 8 to 24),
K = tube coefficient (1 or 1,25),
G = magnification of 100 ×, 40 × etc) objective,
|
from (2) 
from (3) 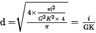 (4)
(4)
Count the number of typical fluorescent cells per field (c).
Calculate the number of typical fluorescent cells per window (C).
Calculate the number of typical fluorescent cells per ml pellet (N)
| where | y = volume of pellet on window,
|
| where | F = pellet dilution factor.
|
Appendix 5EGGPLANT CULTURE
Sow seeds of eggplant ( Solarium melongena cv. Black Beauty) in pasteurized seed compost. Transplant seedlings with fully expanded cotyledons (10 to 14 days) into pasteurized potting compost.
Use eggplants at leaf stage 3 when two, but not more than three, leaves are fully uncurled.
Eggplants should be grown in a glasshouse with the following environmental conditions:
| day length: | 14 hours or natural day length if greater; |
| temperature: day: | 21 to 24oC, |
| night: | 15oC. |
NB: C. sepedonicum will not grow at temperatures >30oC. If night temperatures do not fall to 15oC, chromophore damage (silvery necrosis) may occur.U.K.
Root damage caused by sciarid larvae can be overcome by the application of an appropriate insecticide.
Eggplant cv. Black Beauty may be obtained from:
1.
AB Hammenhögs Frö,
270 50 Hammenhög,
Sweden;
2.
HURST Seeds Ltd,
Avenue Road,
Witham,
Essex CM8 2DX,
England;
3.
ASGRO Italia Sp A,
Corso Lodi, 23,
Milan;
4.
KÜPPER
Mitteldeutsche Samen GmbH,
Hessenring 22,
D-37269 Eschwege
Appendix 6MEDIA FOR GROWTH AND ISOLATION OF C. SEPEDONICUM
Nutrient agar (NA) U.K.
Difco bacto nutrient agar in distilled water at manufacturer's rate. Sterilize by autoclaving at 121 oC for 15 minutes.
Nutrient dextrose agar (NDA) U.K.
Difco bacto nutrient agar containing 1 % D( + ) glucose (monohydrate). Sterilize by autoclaving at 115 oC for 20 minutes.
Yeast peptone glucose agar (YPGA) U.K.
| Difco bacto yeast extract (No 0127) | 5 g |
| Difco bacto peptone (No 0118) | 5 g |
| D( + )-glucose (monohydrate) | 10 g |
| Difco bacto purified agar (No 0560) | 15 g |
| Distilled water | 1 litre |
Sterilize 0,5 litre volumes of medium by autoclaving at 115 oC for 20 minutes.
Yeast extract mineral salts medium (YGM) U.K.
| Difco bacto yeast extract | 2,0 g |
| D( + )-glucose (monohydrate) | 2,5 g |
| K2HPO4 | 0,25 g |
| KH2PO4 | 0,25 g |
| MgSO4. 7H2O | 0,1 g |
| MnSO4. H2O | 0,015 g |
| NaCl | 0,05 g |
| FeSO4. 7H2O | 0,005 g |
| Difco bacto purified agar | 18 g |
| Distilled water | 1 litre |
Sterilize 0,5 litre volumes of medium by autoclaving at 115 oC for 20 minutes.
Appendix 7NUTRITIONAL AND PHYSIOLOGICAL TESTS FOR THE IDENTIFICATION OF C. SEPEDONICUM
All media should be incubated at 21 oC and examinated after six days. If no growth has occurred, incubate for up to 20 days.
— Oxidative and fermentative test (Hugh & Leifson), 1953) - O/F-test.U.K.
Basal medium:
| KCl | 0,2 g |
| MgSO4. 7H2O | 0,2 g |
| NH4H2PO4 | 1,0 g |
| Difco bacto peptone | 1,0 g |
| Difco bacto purified agar | 3,0 g |
| D( + )-glucose (monohydrate) | 10,0 g |
| Bromothymol blue | 0,03 g |
| Distilled water | 1 litre |
Mix and adjust to pH 7,0 to 7,2 with 1N KOH.
Dispense in Pyrex culture tubes 16 mm x 100 mm (12 ml capacity) in 5 ml and 10 ml volumes.
Sterilize by autoclaving at 115 oC for 10 minutes.
Stab inoculate 5 ml and 10 ml tubes for each culture. Aseptically add 1 to 2 ml sterile liquid paraffin to the 10 ml tube. Incubate.
Positive reaction:
| Tube | Colour | Interpretation |
|---|
| Open | Yellow | | Fermentative |
| Closed | Yellow |
| Open | Yellow | | Oxidative |
| Closed | Blue-green |
| Open | Greenish | | Oxidative or inert |
| Closed | Blue-green |
— Oxidase test (Kovacs, 1956)U.K.
Kovacs'oxidase reagent:
1 % aqueous solution of tetramethyl paraphenylenediamine dihydrochloride (BDH No 30386) in distilled water.
This reagent should be freshly made up in 1 ml volumes or can be stored in a brown glass bottle at 5 oC for 1 to 4 weeks.
Place a drop of reagent on filter paper in a clean Petri dish. Immediately rub some of the test culture from nutrient agar using a platinum loop.
Positive reaction: development of purple coloration within 10 seconds. Cultures with times of 10 to 30 seconds are weakly positive.
NB: It is essential to use a platinum loop and NA cultures, since traces of iron or high sugar content in the growth medium may give false positive results.U.K.
— Acid production from lactose, rhamnose, salicin, glycerol U.K.
Prepare Hugh & Leifson's O/F medium without the glucose. Distribute into 5 ml volumes in tubes. Sterilize by autoclaving at 115 oC for 10 minutes. To the molten base at 45 oC, aseptically add 0,5 ml of filter sterilized 10 % aqueous solutions of either glycerol, lactose, rhamnose or salicin. Mix carefully.
Positive reaction: colour change from blue-green to yellow indicates production of acid.
— Catalase test U.K.
Place a drop of hydrogen peroxide (30 volume) onto a clean slide, and emulsify with a loopful of culture using a platinum loop.
Positive reaction: production of oxygen bubbles in the drop indicates the presence of catalase.
— Nitrate reductase activity and denitrification (Bradbury, 1970)U.K.
Culture medium:
| KNO3 (nitrite free) | 1 g |
| Difco bacto yeast extract | 1 g |
| K2HPO4 | 5 g |
| Distilled water | 1 litre |
Dispense into 10 ml volumes in 20 ml bottles. Sterilize by autoclaving at 121 oC for 15 minutes.
Reagent A:
| H2SO4 | 8g |
| 5N acetic acid | 1 litre |
Reagent B:
| naphthylamine | 5 g |
| 5N acetic acid | 1 litre |
Inoculate the nitrate medium in duplicate. Test after 10 and 20 days, by adding one drop of Lugol's iodine, 0,5 ml reagent A and 0,5 ml reagent B. If medium does not turn reddish, add approximately 50 mg zinc powder. Observe the colour reaction.
Positive reaction:
| Colour reaction |
|---|
| Stage 1 | Stage 2 |
|---|
| No reduction of nitrate | colourless | red |
| Reduction of nitrate as far as nitrite (nitrate reductase only) | red | — |
| Reduction of nitrate beyond nitrite (denitrification — nitrate and nitrite reductase) | colourless | colourless |
— Urease production (Lelliott, 1966)U.K.
Basal medium:
| Oxoid urea agar base (CM53) | 2,4 g |
| Distilled water | 95 ml |
Sterilize by autoclaving at 115 oC for 20 minutes. Cool the molten base to 50 oC and aseptically add 5 ml of filter-sterilized 40 % aqueous urea solution (Oxoid SR20). Mix well.
Distribute into 6 ml volumes in sterile tubes (16. x 100 mm) and allow to set as slopes with a good butt.
Positive reaction: the yellow-orange medium develops a cherry red or magenta pink coloration if urease activity has occurred.
Utilization of citrate (Christensen) (Skerman, 1967)U.K.
| Citrate agar base (Merck 2503) | 23 g |
| Distilled water | 1 litre |
Mix and dissolve by heating. Dispense into 6 ml volumes as for urea medium. Sterilize by autoclaving at 121 oC for 15 minutes and allow to set as slopes.
Positive reaction: citrate utilization is indicated by a change in the colour of the medium from orange to red.
— Hydrogen sulphide production (Ramamurthi, 1959) U.K.
Medium:
| Difco bacto tryptone (No 0123) | 10 g |
| K2HPO4 | 1 g |
| NaCl | 5 g |
| Distilled water | 1 litre. |
Dissolve and dispense into 6 ml volumes in 16 x 100 mm tubes. Sterilize by autoclaving at 115 oC for 10 minutes.
Inoculate and aseptically suspend a lead acetate paper (Merck 9511) from the lip if the tube. Hold in place with the cap. Incubate for up to 20 days.
Positive reaction: H2S production from tryptone is indicated by the development of a black-brown coloration of the test paper.
— Indole production (Ramamurthi, 1959)U.K.
Medium:
As for H2S test.
Remove the lead acetate paper and add 1 to 2 ml of diethyl ether and shake gently. Allow the layers to separate (five minutes). Add 0,5 ml Kovacs' reagent (Merck 9293) carefully down the inside of the sloped tube.
Positive reaction: the presence of indole is indicated by the development of a red colour in the yellow layer between the ether and aqueous fractions.
— Growth ar 37 oC (Ramamurthi, 1959)U.K.
Medium:
| Difco bacto nutrient broth (No 0003) | 8 g |
| Distilled water | 1 litre |
Mix, dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Inoculate and incubate at 37 oC.
Positive reaction: look for growth.
— Growth in 7 % sodium chloride (Ramamurthi, 1959)U.K.
Medium:
| Difco bacto nutrient broth | 8g |
| NaCl | 70 g |
| Distilled water | 1 litre |
Mix, dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Positive reaction: look for growth.
— Gelatin hydrolysis (Lelliott, Billing & Hayward, 1966)U.K.
Medium:
| Difco bacto gelatine (No 0143) | 120 g |
| Distilled water | 1 litre |
Mix, dissolve by heating and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Positive reaction: liquefaction of gelating even when held at 5 oC for 30 minutes.
—Starch hydrolysisU.K.
Medium:
| Difco bacto nutrient agar (molten) | 1 litre |
| Difco bacto soluble starch (No 0178) | 2 g |
Mix, sterilize by autoclaving at 115 oC for 10 minutes.
Pour plates. Spot inoculate the plates.
After good growth has occurred (10 to 20 days), remove part of the growth and flood with Lugol's iodine.
Positive reaction: starch hydrolysis is indicated by clear zones under or around the bacterial growth; the remainder of the medium stains purple.
— Aesculin hydrolase activity (Sneath & Collins, 1974)U.K.
Medium:
| Difco bacto peptone | 10 g |
| Aesculin | 1 g |
| Ferric citrate (Merck 3862) | 0,05 g |
| Sodium citrate | 1 g |
| Distilled water | 1 litre |
Mix to dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 115 oC for 10 minutes.
The medium is clear but has a bluish fluorescence.
Positive reaction: aesculin hydrolysis is indicated by the development of a brown colour together with a disappearance of fluorescence. This can be checked using an ultraviolet lamp.
REFERENCES U.K.
Bradbury, J. F., 1970. Isolation and preliminary study of bacteria from plants. Rev. PI. Path., 49, 213-218.
Dinesen, I. G., 1984. The extraction and diagnosis of Corynebacterium sepedonicum from diseased potato tubers. EPPO Bull. 14 (2), 147-152.
Doetsch, R. N., 1981. Determinative methods of light microscopy. In: Manual of methods for general bacteriology, American Society for Microbiology, Washington, 21-23.
Hugh, R. and Leifson, F., 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative bacteria. J. Bact., 66, 24-26.
Janse, J. D. and J. Van Vaerenbergh. The interpretation of the EC method for the detection of latent ring rot infections (Corynebacterium sepedonicum) in potato. EPPO Bull., No 17, 1987, pp. 1-10.
Kovacs, N., 1956. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature, Lond., 178, 703.
Lelliott, R. A., 1966. The plant pathogenic coryneform bacteria. J. appl. Bact., 29, 114-118.
Lelliott, R. A., E. Billing and A. C. Hayward, 1966. A determinative scheme for the fluorescent plant pathogenic pseudomonads J. appl. Bact., 29, 470-489.
Lelliott, R. A. and P. W., Sellar, 1976. The detection of latent ring rot (Corynebacterium sepedonicum (Spiek. et Kotth.) Skapt. et Burkh.) in potato stocks. EPPO Bull., 6 (2), 101-106.
Ramamurthi, C. S., 1959. Comparative studies on some Gram-positive phytopathogenic bacteria and their relationship to the Corynebacteria. Mem. Cornell agric. Exp. Sta., 366, 52 pp.
Skerman, V. B. D., 1967. A guide to the identification of the genera of bacteria. 2nd ed., William and Wilkins Company, Baltimore.
Sneath, P. H. A. and V. G. Collins, 1974. A study in test reproductibility between laboratories: report of Pseudomonas working party. Antonie van Leeuwenhoek, 40, 481-527.
ANNEX IIU.K.
1.For each suspected occurrence for which a positive immunofluorescence test has been identified according to the method set out in Annex I, and confirmation or refutation by completion of the said method is awaited, there should be the retention and appropriate conservation of:U.K.
all tubers or plants sampled, wherever possible, and
any remaining extract and additional prepared immunofluorescence slides, until the completion of the said method.
2.In the case of positive confirmation of the organism, there should be retention and appropriate conservation of:U.K.
the material specified in paragraph 1, and
a sample of the infected eggplant material inoculated with the tuber or plant extract, and
the isolated culture of the organism,
until at least one month after the notification procedure under Article 5 (2).
ANNEX IIIU.K.
1.The elements to be considered in the determination of the extent of probable contamination under Article 5 (1) (b), shall include:U.K.
tubers or plants grown at a place of production designated as contaminated under Article 5(1) (a),
place(s) of production or premises with some production link to the tubers or plants, designated as contaminated under Article 5 (1) (a), including those sharing production equipment and facilities directly or through a common contractor,
tubers or plants produced in the place(s) of production referred to in the previous indent, or present in such place(s) of production during the period when the tubers or plants designated as contaminated under Article 5 (1) (a) were present on the premises or places of production referred to in the first indent,
central stores handling potatoes from the above places of production,
any machinery, vehicle, vessel, store, or units thereof, and any other objects including packaging material, that may have come into contact with the tubers or plants designated as contaminated under Article 5 (1) (a), during the previous 12 months or wherever appropriate,
any tubers or plants stored in, or in contact with, any of the structures or objects listed in the previous indent, prior to the cleansing and disinfection of such structures and objects, and
shall include, as a result of the testing under Article 6, tubers or plants with the same clonal origin as tubers or plants designated to be contaminated under Article 5 (1) (a) and for which investigations indicate contamination is probable.
2.The elements to be considered in the determination of the possible spread under Article 5 (1) (c) shall include:U.K.
the proximity of other places of production growing potatoes or other host plants,
the commonality of seed potato stocks.
3.The details of the notification referred to in the first subparagraph of Article 5 (2) shall include:U.K.
for any potato consignment or lot designated as contaminated, the certificates prescribed in Articles 7 or 8 of Directive 77/93/EEC, the passport or registration number, as appropriate,
the variety name for seed potato stocks, and where possible in all other cases,
a description of the elements of the designated contamination and zone demarcation,
the availability of extract, prepared immunofluorescent slides, infected eggplant material and an isolated culture of the organism from the test in which positive confirmation of the organism has been made.
ANNEX IVU.K.
1.The officially supervised measures referred to in Article 7 (1) for the disposal of tubers or plants designated to be contaminated under Article 5 (1) (a) shall be:U.K.
use for industrial processing through direct and immediate delivery to a processing plant with appropriate waste disposal facilities for which it has been established that there is no identifiable risk of the organism spreading, and with a system of disinfection of storage areas and departing vehicles, or
other measures, provided that it has been established that there is no identifiable risk of the organism spreading; such measures to be notified to the Commission and to the other Member States.
2.The appropriate use or disposal of tubers or plants determined as probably contaminated under Article 5 (1) (b) and referred to in Article 7 (2), under the control of the responsible official bodies of the Member States, shall be:U.K.
use as ware potatoes intended for consumption, packed ready for direct delivery and use without repacking, and intended for such direct delivery and use, or
use as ware potatoes intended for industrial processing, and intended for direct and immediate delivery to a processing plant with appropriate waste disposal and disinfection facilities, or
some other use or disposal, provided that it is established that there is no identifiable risk of the organism spreading.
3.The appropriate methods for cleansing and disinfecting of the objects referred to in Article 7 (3) shall be those for which it has been established that there is no identifiable risk of the organism spreading and shall be employed under the supervision of the responsible official bodies of the Member States.U.K.
4.The series of measures to be implemented by Member States within the demarcated zone established under Article 5 (1) (c) and referred to in Article 7 (4) shall include:U.K.
4.1.
in places of production designated as contaminated under Article 5(1) (a):
(a)
in a field designated to be contaminated under Article 5 (1) (a), either
(i)
during at least the three growing years following the year of the designated contamination,
measures shall be taken to eliminate volunteer potato plants and other naturally found host plants of the organism, and
no potato tubers, plants or true seeds, or other naturally found host plants of the organism, or crops for which there is an identified risk of the organism surviving or spreading, shall be planted until the field is found free from volunteer potato plants for at least two consecutive growing years,
in the first potato cropping season following the period specified in the preceding indent, officially certified seed potatoes shall be planted for ware production only and an official survey, as detailed in Article 2 (1), shall be conducted;
in the potato cropping season succeeding that referred to in the previous indent and following an appropriate rotation cycle, officially certified seed potatoes shall be planted for either seed or ware production and an official survey as detailed in Article 2 (1), shall be conducted; or
(ii)
during the four growing years following that of the designated contamination,
measures shall be taken to eliminate volunteer potato plants and other naturally found host plants of the organism, and
the field shall be laid to, and maintained either, in bare fallow or in permanent pasture with frequent close cutting or intensive grazing,
in the first potato cropping season following the period specified in the preceding indent, officially certified seed potatoes shall be planted for either seed or ware production and an official survey, as detailed in Article 2 (1), shall be conducted;
(b)
in other fields:
in the growing year following the designated contamination:
either no potato tubers, plants or true seeds or other naturally found host plants of the organism shall be planted, and measures shall be taken to eliminate volunteer potato plants, as appropriate, or
officially certified seed potatoes may be planted for ware production only, on the condition that the responsible official bodies are satisfied that the risk of volunteer potato plants and other naturally found host plants of the organism have been eliminated,
for, at least the two growing years following that specified in the preceding indent, only officially certified seed potatoes shall be planted, for either seed or ware production,
in each of the growing years referred to in the previous indents measures shall be taken to eliminate volunteer potato plants and naturally found host plants of the organism, and an official survey as detailed in Article 2 (1), shall be conducted,
where officially certified seed potatoes are planted for ware production in the growing year following the designated contamination, the growing crop shall be inspected at appropriate times and volunteer potato plants shall be tested for the organism;
(c)
immediately following the designation of contamination under Article 5(1) (a) and in each of the subsequent growing years up to and including the first permissible potato cropping season on the field(s) designated as contaminated, as detailed in paragraph (a), all machinery and storage facilities on the place of production and involved in potato production shall be cleansed and disinfected as appropriate and using appropriate methods, as specified in 3;
(d)
in those production systems where complete replacement of the growing medium is possible,
no tubers, plants or true seeds shall be planted unless the production unit has been subjected to officially supervised measures to eliminate the organism and remove any potato or other solanaceous material, including, at least, a complete change in growing medium and cleansing and disinfection of the production unit, and all equipment, and, subsequently has been granted approval for potato production by the responsible official bodies, and
potato production shall be from officially certified seed potatoes, or from mini-tubers or micro-plants derived from tested sources;
4.2.
within the demarcated zone, without prejudice to the measures detailed under 4.1, the Member States shall:
(a)
immediately, and for at least three growing seasons, after the designated contamination:
ensure supervision by their responsible official bodies of premises growing, storing or handling potato tubers, together with premises which operate potato machinery under contract,
require cleansing and disinfection of machinery and stores on such premises, as appropriate, and using appropriate methods, as specified under 3,
require the planting of certified seed only for all potato crops within that zone,
require the separate handling of harvested seed stocks to those of ware on all premises within the zone,
conduct an official survey as detailed in Article 2 (1);
(b)
establish a programme, where appropriate, for the replacement of all seed potato stocks over an appropriate period of time.
The measures implemented under 4.2, along with the registration numbers of producers, collective warehouses and dispatching centres within the demarcated zone, shall be notified annually to the other Member States and to the Commission.











