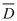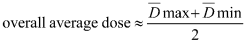- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Gwreiddiol (a wnaed Fel)
The Food Irradiation (Scotland) Regulations 2009
You are here:
- Offerynnau Statudol yr Alban
- 2009 No. 261
- Schedules only
- Dangos Graddfa Ddaearyddol(e.e. Lloegr, Cymru, Yr Alban aca Gogledd Iwerddon)
- Dangos Llinell Amser Newidiadau
Changes to legislation:
There are outstanding changes not yet made by the legislation.gov.uk editorial team to The Food Irradiation (Scotland) Regulations 2009. Any changes that have already been made by the team appear in the content and are referenced with annotations.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. Changes and effects are recorded by our editorial team in lists which can be found in the ‘Changes to Legislation’ area. Where those effects have yet to be applied to the text of the legislation by the editorial team they are also listed alongside the legislation in the affected provisions. Use the ‘more’ link to open the changes and effects relevant to the provision you are viewing.
Changes and effects yet to be applied to :
- sch. 3 heading words substituted by S.S.I. 2019/52 reg. 6(4) (This amendment not applied to legislation.gov.uk. Reg. 6(4) substituted immediately before IP completion day by S.S.I. 2020/372, regs. 1(2)(a), 5(3))
Changes and effects yet to be applied to the whole Instrument associated Parts and Chapters:
- Blanket amendment words substituted by S.I. 2011/1043 art. 3-6 8-10
Regulations 3(2) and (3), 5(3)(b)(ii) and Schedule 2 Part 1 paragraphs 1 and 2
SCHEDULE 1METHOD OF MEASUREMENT OF IRRADIATION
(This Schedule sets out (with a correction in paragraph 1(5)M1) the provisions of Annex III to Directive 1999/2/EC of the European Parliament and of the Council on the approximation of the laws of the Member States concerning foods and food ingredients treated with ionising radiationM2)
Marginal Citations
M1The Directive omits the word “average” after “overall”.
M2O.J. No. L 66, 13.3.1999, p.16.
PART 1Dosimetry
Overall average absorbed dose
1.—(1) It can be assumed for the purpose of the determination of the wholesomeness of foodstuffs treated with an overall average dose of 10 kGy or less, that all radiation chemical effects in that particular dose range are proportional to that dose.
| where | M = the total mass of the treated sample |
| p = the local density at the point (x,y,z) | |
| d = the local absorbed dose at the point (x,y,z) | |
| dV = dx dy dz, the infinitesimal volume element which in real cases is represented by the volume fractions. | |
(3) The overall average absorbed dose can be determined directly for homogeneous products or for bulk goods of homogeneous apparent density by distributing an adequate number of dosimeters strategically and at random throughout the volume of the goods. From the dose distribution determined in this manner an average can be calculated which is the overall average absorbed dose.
(4) If the shape of the dose distribution curve throughout the product is well determined, the positions of minimum and maximum dose are known. Measurements of the distribution of dose in these two positions in a series of samples of the product can be used to give an estimate of the overall average dose.
PART 2Procedures
2.—(1) Before routine irradiation of a given category of foodstuffs begins at a radiation facility, the locations of the minimum and maximum doses are determined by making dose measurements throughout the product volume. These validation measurements must be carried out a suitable number of times (e.g. 3-5) in order to make allowance for variations in product density or geometry.
(2) Measurements must be repeated whenever the product, its geometry or the irradiation conditions are changed.
(3) During the process, routine dose measurements are carried out in order to ensure that the dose limits are not exceeded. Measurements should be carried out by placing dosimeters at the positions of the maximum or minimum dose, or at a reference position. The dose at the reference position must be quantitatively linked to the maximum and minimum dose. The reference position should be located at a convenient point in or on the product, where dose variations are low.
(4) Routine dose measurements must be carried out on each batch and at regular intervals during production.
(5) In cases where flowing, non-packaged goods are irradiated, the locations of the minimum and maximum doses cannot be determined. In such a case it is preferable to use random dosimeter sampling to ascertain the values of these dose extremes.
(6) Dose measurements should be carried out by using recognised dosimetry systems, and the measurements should be traceable to primary standards.
(7) During irradiation, certain facility parameters must be controlled and continuously recorded. For radionuclide facilities the parameters include product transport speed or time spent in the radiation zone and positive indication for correct position of the source. For accelerator facilities, the parameters include product transport speed and energy level, electron current and scanner width of the facility.
Regulations 3(1), 4(2), 5(2)(b) and (3)(a), 6(1)(b)(ii), and 7(a)(i) and (ii)(aa)
SCHEDULE 2LICENCES
PART 1Grant of Licence
Application for licence
1. A person seeking a licence to irradiate food (“the applicant”) shall apply by sending to the Agency an application in writing containing—
(a)the applicant's name;
(b)the applicant's address;
(c)the address of the facility at which the applicant proposes to irradiate food;
(d)details of any licence or registration under any other legislation which enables the applicant to use ionising radiation at the facility in circumstances where, but for that licence or registration, that use would be unlawful;
(e)a description of each food which the applicant proposes to irradiate which is sufficient to show that it falls within a permitted category of food;
(f)in respect of each food described pursuant to sub-paragraph (e)—
(i)the purpose for which the applicant proposes to irradiate the food and how that would benefit consumers;
(ii)the method by which the applicant will ensure that the food is in a suitably wholesome state before irradiation;
(iii)the overall average dose, maximum dose and minimum dose of ionising radiation which the applicant proposes to apply to the food;
(iv)the method (including instrumentation and frequency) by which the applicant proposes to measure any dose of ionising radiation and the dosimetry standard which the applicant proposes to use to calibrate the dose meters used to measure it;
(v)whether or not the applicant proposes to irradiate the food in packaging in contact with the food and, if so, the packaging which the applicant proposes to use; and
(vi)whether or not the applicant proposes to apply temperature control to the food while irradiating it and, if so, the temperature at which the applicant proposes to keep the food during the application of temperature control;
(g)in respect of each food described under sub-paragraph (e), particulars demonstrating that the irradiation will be in conformity with Schedule 1 and the Joint FAO/WHO Codex Alimentarius Commission Recommended International Code of Practice for the operation of irradiation facilities used for the treatment of foods (in this Part of this Schedule referred to as “the Code of Practice”), reference FAO/WHO/CAC, vol XV, edition 1 M3;
(h)a plan of the layout of the facility, details of its design and construction and a statement of the practices which the applicant proposes to apply, including—
(i)the proposed method of irradiating food;
(ii)the type of radiation to be used;
(iii)the proposed methods of business control and organisation, including the minimum qualifications (whether they are formal or are derived from skill, training or experience) of persons who will be involved in applying the practices;
(i)the identity and qualifications of the person who has been designated to be responsible for compliance with the conditions necessary for application of the practices referred to in sub-paragraph (h) and that person's position within the applicant's management structure;
(j)the date from which the applicant wishes the licence to run; and
(k)any other particulars which the applicant wishes the Agency to consider in deciding whether to grant a licence.
Marginal Citations
M3A copy of the Code of Practice may be obtained from the Codex Alimentarius Commission, Food and Agriculture Organisation of the United Nations, Vialle della Terme di Caracalla, 0010, Rome.
Consideration of the application
2. The Agency may grant a licence where it is satisfied that—
(a)the facility specified in the application satisfies the requirements of the Code of Practice;
(b)the food described in the application falls within a permitted category;
(c)there is a reasonable technological need;
(d)the irradiation would present no health hazard and would be carried out under the conditions described in the application;
(e)the irradiation would be of benefit to the consumer;
(f)the irradiation would not be used as a substitute for hygiene and health practices or for good manufacturing or agricultural practice;
(g)the purposes of irradiation are only—
(i)to reduce the incidence of food-borne disease by destroying pathogenic organisms;
(ii)to reduce spoilage of foodstuffs by retarding or arresting decay processes and destroying spoilage organisms;
(iii)to reduce loss of foodstuffs by premature ripening, germination or sprouting; or
(iv)to rid foodstuffs of organisms harmful to plants or plant products;
(h)where the purposes of irradiation include reducing the incidence of food-borne disease by destroying pathogenic organisms, the applicant will use microbiological criteria in deciding whether to irradiate food;
(i)there is no significant risk that the applicant may irradiate food which for microbiological reasons cannot comply with food safety requirements, or cannot comply without being irradiated;
(j)every method specified under paragraph 1(f)(ii) is adequate to enable the applicant to ensure that the food is in a suitably wholesome state before irradiation;
(k)the overall average dose specified under paragraph 1(f)(iii) in relation to each description of food is consistent with each purpose specified in respect of that description of food under paragraph 1(f)(i);
(l)the method and standard specified under paragraph 1(f)(iv)—
(i)comply with Schedule 1; and
(ii)eliminate any significant risk that the overall average dose, measured by that method, will deviate significantly from the overall average dose as defined under paragraph 1 of Schedule 1;
(m)the factors specified under paragraph 1(f) eliminate any significant risk that food, irradiated in any packaging specified under paragraph 1(f)(v), and at any temperature specified under paragraph 1(f)(vi), will fail to comply with food safety requirements; and
(n)the practices and qualifications specified in the statement under paragraph 1(h) are adequate for ensuring that the requirements of these Regulations and of any conditions of the licence will not be breached.
3. Where the Agency believes that—
(a)it ought to take account of the practical operation of the facility before it finally determines the application; and
(b)it would not prejudice safety if food was irradiated at the facility for the time being,
it may grant a licence for a period, or further period, not exceeding 6 months in total to enable it to take such account.
Refusal of application
4.—(1) Where the Agency refuses to grant a licence, it shall give to the applicant a statement in writing of its reasons for doing so and shall invite the applicant to make representations to it in writing within 28 days after the statement is sent.
(2) After considering any such representations, the Agency—
(a)may, if satisfied as to the matters specified in paragraph 2, grant a licence; or
(b)shall give to the applicant a statement in writing of its reasons for continuing to refuse the licence.
Duration
5.—(1) Subject to sub-paragraph (2), a licence continues in effect unless cancelled or suspended in accordance with the provisions of Part 5 or surrendered by the licensee to the Agency.
(2) A licence under paragraph 3 shall continue in effect until—
(a)the expiration of the period for which it was granted; or
(b)the refusal by the Agency of a licence on its final determination of the application,
unless cancelled or suspended in accordance with the provisions of Part 5 or surrendered by the licensee to the Agency.
PART 2Contents of Licence
Contents of licence
6. Every licence must contain—
(a)the name of the licensee;
(b)the address of the licensed facility;
(c)a licence number;
(d)a description of each food to which the licence applies;
(e)the date from which the licence is to run; and
(f)in the case of a licence under Part 1, paragraph 3, the date of its expiry,
and may contain conditions.
PART 3Requirements and prohibitions to be observed by a licensee
7.—(1) A licensee must only irradiate food—
(a)to which the licence applies; and
(b)at the licensed facility.
(2) A licensee must not irradiate any food received from another person unless the following particulars are attached to or accompany the food when it is received—
(a)a description of the food and the name and address of its consignor;
(b)a reference by which the food, or any batch, lot or consignment of food of the same description within which food falls, can be identified;
(c)if the food is received from its owner for the purposes of irradiation—
(i)the name and address of its owner; and
(ii)the reason why its owner wants it to be irradiated; and
(d)a statement as to whether the food or any part of it has previously been irradiated.
8. A licensee must keep—
(a)all food which awaits irradiation at the licensed facility, on a part of the facility, which is separated by a wall or barrier from any part of the facility where food which has been irradiated is kept; and
(b)all food which is either awaiting irradiation or has been irradiated, on parts of the facility, which are separated by a wall or barrier from any part of the facility on which other food is kept in the course of the business.
9.—(1) A licensee must not irradiate food in combination with any chemical treatment having the same purpose as irradiating it.
(2) Subject to sub-paragraph (3), a licensee must not irradiate food which, or any part of which, has previously been irradiated.
(3) The removal of food from, and its return to, the facility where irradiation takes place does not constitute a breach of sub�??paragraph (2) where they form part of a continuous process required by the design and construction of that facility.
10. A licensee must number each batch of food irradiated by the licensee and, where any of the food has been received from another person, do so in such a way that the number can be linked to the reference specified in paragraph 7(2)(b).
11. A licensee must only irradiate food with—
(a)gamma rays from the radionuclide 60Co;
(b)gamma rays from the radionuclide 137Cs;
(c)X-rays generated from machine sources operated at or below an energy level of 5 MeV; or
(d)electrons generated from machine sources operated at or below an energy level of 10 MeV.
12. A licensee must only irradiate food by proper irradiation.
13. A licensee must maintain such controls as are necessary to at all times ensure that irradiation is consistent with the method of measurement specified under paragraph 1(f)(iv) of Part 1.
14. A licensee must record, in relation to each batch of food irradiated by the licensee, the following information—
(a)in the case of a radionuclide facility—
(i)in relation to each source configuration of ionising radiation available for use in the facility, such information as to its position as shows whether and, if so, when the batch of food was exposed to it; and
(ii)either—
(aa)the speed at which the batch travels through the facility and the route which the batch travels while passing through it; or
(bb)the time which the batch spends in the radiation zone; and
(b)in the case of a machine source—
(i)its energy level;
(ii)its electron current;
(iii)its scanner width;
(iv)the characteristics of its beam;
(v)unless it has a scattering device, the frequency with which its beam scans the batch; and
(vi)the speed at which the batch travels through the facility.
15.—(1) A licensee must record for each batch of food irradiated by the licensee—
(a)the nature and quantity of food in the batch;
[F1(b)its batch number;]
(c)the name and address of each consignor and consignee of food within the batch;
(d)the date on which the batch was irradiated;
(e)any microbiological information relating to food within the batch;
(f)the type of packaging in contact with the food in the batch during irradiation;
(g)where temperature control has been applied while irradiating the food, the temperature of the food in the batch immediately before irradiation;
(h)the maximum, minimum and overall average dose of ionising radiation applied to the batch;
(i)the type of ionising radiation used;
(j)the data used for control of the irradiation including—
(i)the positioning of dose meters within the batch and the doses of ionising radiation recorded by them;
(ii)previous tests used for the purpose of validating that positioning; and
(iii)the method (including instrumentation and frequency) used for measuring the doses of ionising radiation applied during the irradiation, and in the previous tests, and the dosimetry standard used to calibrate the meters used to measure them.
(2) A licensee must not consign food irradiated by the licensee to another person unless it is accompanied by—
(a)the licensee's name;
(b)the licensee's licence number;
(c)the information specified in sub-paragraph (1)(a) to (d); and
(d)the overall average dose required by sub�??paragraph (1)(h).
Textual Amendments
F1Sch. 2 para. 15(1)(b) substituted (20.10.2010) by The Food Irradiation (Scotland) Amendment Regulations 2010 (S.S.I. 2010/328), regs. 1, 4
16. A licensee must keep the information required by paragraphs 14 and 15(1) to be recorded for 5 years, even if the licensee ceases meanwhile to be licensed.
17. A licensee shall send to the Agency by the last day of February each year a return in writing in respect of the previous calendar year containing—
(a)the licensee's name;
(b)the licensee's licence number;
(c)the year to which the return relates;
(d)a description of each food which the licensee has irradiated during the year; and
(e)the quantity, by volume or weight, of each such food.
PART 4Variation of Licence
18. Subject to paragraph 19, the Agency may at the request or with the consent of the licensee vary any condition of the licence.
19.—(1) Subject to sub-paragraph (2), the Agency shall not agree a variation which permits any act or omission a proposal for which would, had it been made in the application for a licence, have caused the Agency to refuse to grant the licence under paragraph 2 of Part 1.
(2) For the purposes of sub-paragraph (1), the Agency, in considering whether to vary a licence, shall treat all scientific knowledge which it has at that time as if it had had it at the time it granted the licence.
PART 5Cancellation and Suspension of Licence
20. If the Agency considers that circumstances exist which, had it foreseen them (and possessed the same scientific knowledge it does now) at the time, it would have refused under paragraph 2 of Part 1 to grant a licence, it may give notice to the licensee—
(a)explaining why it would have refused to grant the licence; and
(b)informing the licensee that, unless the licensee persuades it in writing not to do so within a period of twenty-eight days after the sending of the notice, or such longer period as it may allow, it will cancel the licence.
21. If by the expiration of the 28 day period or of any longer period allowed under paragraph 20(b) the Agency is not persuaded to the contrary, it shall give notice in writing to the licensee that the licence is cancelled from a date specified in the notice and shall state in the notice why it is not so persuaded; but if so persuaded it shall notify the licensee accordingly.
22. If cancelled, the licence shall cease to have effect on the date specified in the notice.
23.—(1) If the Agency considers that unless the licence is suspended there will or may be a risk of injury to health it may give notice in writing to the licensee suspending the licence from a date specified in the notice and, subject to sub-paragraphs (2) and (3), the licence shall have no effect for the purpose of these Regulations during that period.
(2) Subject to sub-paragraph (3), where notice is given suspending the licence—
(a)the suspension shall cease to have effect at the expiration of three days after notice of the suspension has been served on the licensee unless notice has in the meantime been served on the licensee under paragraph 20; but
(b)if notice has in the meantime been served on the licensee under paragraph 20 the suspension shall continue until either—
(i)the Agency decides not to cancel the licence; or
(ii)the licence terminates.
(3) The Agency may, if it considers that in the absence of suspension there will not be a risk of injury to health, by further notice in writing to the licensee withdraw the notice suspending the licence.
PART 6Other Provisions Concerning Licences
24. The Agency shall publish in the Edinburgh Gazette notice of—
(a)each licence granted;
(b)each suspension of a licence;
(c)each cancellation of a licence; and
(d)each agreed variation of the terms of a licence.
25. Any notice so published shall specify—
(a)the name of the licensee or former licensee;
(b)the licensed or formerly licensed facility; and
(c)the licence number,
and shall state in outline the effect of the matter to which it relates.
26. Except as provided by section 43 of the Act (continuance on death), a licence is not transferable.
Regulations 3(1) and 5(1)(b)(i)
SCHEDULE 3[F2[F3List of Facilities in Member States and the United Kingdom]
Textual Amendments
F2Sch. 3 substituted (20.10.2010) by The Food Irradiation (Scotland) Amendment Regulations 2010 (S.S.I. 2010/328), reg. 1, sch. 1
F3Sch. 3 heading substituted (31.12.2020) by S.S.I. 2019/52, reg. 6(4) (as substituted by The Food and Feed (EU Exit) (Scotland) (Amendment) Regulations 2020 (S.S.I. 2020/372), regs. 1(2)(a), 5(3))
| Official reference number | Name and address |
|---|---|
| 2110/91/0004 | Sterigenics |
| SA Zoning Industriel | |
| B-6220 Fleurus | |
| Belgium | |
| 01/23.05.2008 | BULGAMMA SOPHARMA Ltd |
| Iliensko Shosse Str.16 | |
| Sofia | |
| Bulgaria | |
| IR-02-CZ | BIOSTER a.s. |
| Tejny 621 | |
| 664- 71 Veverska Bityska | |
| Czech Republic | |
| SN 01 | Gamma-Service |
| Produktbestrahlung GmbH | |
| Juri—Gagarin Strasse 15 | |
| D-01454 Radeberg | |
| Germany | |
| BY FS 01/2001 | Isotron Deutschland GmbH |
| Kesselbodenstrasse 7 | |
| D-85391 Allershausen | |
| Germany | |
| NRW-GM 01 and NRW-GM 02 | BSG Beta-Gamma-Service GmbH & Co. KG |
| Fritz-Kotz-Str.16 | |
| D-51674 Wiehl | |
| Germany | |
| D-BW-X-01 | Beta-Gamma-Service GmbH & Co. KG |
| John-Deere-Strasse 3 | |
| D-76646 Bruchsal | |
| Germany | |
| 500001/CU | Ionmed Esterilización, SA |
| Santiago Rusiñol 12. Madrid | |
| Antigua Cntra Madrid-Valencia KM 83.7. | |
| Tarancón | |
| Cuenca | |
| Spain | |
| 5.00002/B | ARAGOGAMMA S.A. |
| Salvador Mundi 11, bajo. | |
| 08017 Barcelona | |
| Carretera Granoolers a Cardedeu km 3,5. | |
| 08520 Les Franqueses dêsVallés | |
| Barcelona | |
| Spain | |
| 13055 F | Isotron France SAS |
| Rue Jean Queillau | |
| Marché des Arnavaux | |
| F-13014 Marseille Cedex 14 | |
| France | |
| 01 142 F | Ionisos SA |
| Zone Industrielle les Chartinières | |
| F-01120 Dagneux | |
| France | |
| 72 264 F | Ionisos SA |
| Zone Industrielle de l’Aubrée | |
| F-72300 Sablé-sur-Sarthe | |
| France | |
| 85 182 F | Ionisos SA |
| ZI Montifaud | |
| F-85700 Pouzauges | |
| France | |
| 10 093 F | Ionisos SA |
| Zone Industrielle | |
| F-10500 Chaumesnil | |
| France | |
| EU-AIF-04-2002 | AGROSTER Besugárzó Részvénytársaság |
| Budapest X | |
| Jászberényi út 5 | |
| H-1106 | |
| Hungary | |
| RAD 1/04 IT | GAMMARAD ITALIA SPA |
| Via Marzabotto, 4 | |
| Minerbio (BO) | |
| Italy | |
| GZB/VVB-991393 Ede | Isotron Nederland BV |
| Morsestraat 3 | |
| 6716AH Ede | |
| Netherlands | |
| GZB/VVB-991393 Etten-Leur | Isotron Nederland BV |
| Soevereinsestraat 2 | |
| 4879NN Etten-Leur | |
| Netherlands | |
| GIS-HZ-4434-W.-3/MR/03 | Institute of Nuclear Chemistry and Technology |
| 16 Dorodna Str. | |
| 03-195 Warsaw | |
| Poland | |
| GIS-HZ-4434-W.-2/MR/03 | Institute of Applied Radiation Chemistry |
| Technical University of Lodz | |
| 15 Wróblewskiego Str. | |
| 39-590 Lodz | |
| Poland | |
| RG016/2008 | Multipurpose Irradiation Facility |
| IRASM Technological Irradiations Department | |
| Horia Hulubei National Institute for Research | |
| and Development of Physics and Nuclear | |
| Engineering | |
| Atomistilor Street No. 407 | |
| PO Box MG-6 | |
| Magurele. Ilfov County | |
| Romania | |
| EW/04 | Isotron Limited |
| Moray Road | |
| Elgin Industrial Estate | |
| Swindon | |
| Wilts SN2 8XS | |
| United Kingdom] |
Regulation 5(1)(b)(ii)
SCHEDULE 4[F4LIST OF [F5APPROVED] FACILITIES IN COUNTRIES OUTSIDE THE [F5UNITED KINGDOM AND] EUROPEAN UNION
Textual Amendments
F4Sch. 4 substituted (20.10.2010) by The Food Irradiation (Scotland) Amendment Regulations 2010 (S.S.I. 2010/328), reg. 1, sch. 2
F5Words in sch. 4 heading inserted (31.12.2020) by The Food and Feed Safety and Hygiene (EU Exit) (Scotland) (Amendment) Regulations 2019 (S.S.I. 2019/52), regs. 1(1), 6(5); 2020 c. 1, Sch. 5 para. 1(1)
| Official reference number | Name and address |
|---|---|
| EU-AIF 01-2002 | HEPRO Cape (Pty) Ltd |
| 6 Ferrule Avenue | |
| Montague Gardens | |
| Milnerton 7441 | |
| Western Cape | |
| Republic of South Africa | |
| EU-AIF 02-2002 | Gammaster South Africa (Pty) Ltd |
| PO Box 3219 | |
| 5 Waterpas Street | |
| Isando Extension 3 | |
| Kempton Park 1620 | |
| Johannesburg | |
| Republic of South Africa | |
| EU-AIF 03-2002 | Gamwave (Pty) Ltd |
| PO Box 26406 | |
| Isipingo Beach | |
| Durban 4115 | |
| Kwazulu-Natal | |
| Republic of South Africa | |
| EU-AIF 05-2004 | Gamma-Pak As |
| Yünsa Yolu N: 4 0SB | |
| Cerkezköy/TEKIRDAG | |
| TR-59500 | |
| Turkey | |
| EU-AIF 06-2004 | Studer Agg Werk Hard |
| Hogenweidstrasse 2 | |
| Däniken | |
| CH-4658 | |
| Switzerland | |
| EU-AIF 07-2006 | Thai Irradiation Centre |
| Thailand Institute of Nuclear Technology | |
| (Public Organisation) | |
| 37 Moo 3, TECHNOPOLIS | |
| Klong 5, Klong Luang | |
| Pathumthani 12120 | |
| Thailand | |
| EU-AIF 08-2006 | Isotron (Thailand) Ltd |
| Bangpakong Industrial Park (Amata Nakorn) | |
| 700/465 Moo 7, Tambon Donhuaroh, | |
| Amphur Muang, | |
| Chonburi 20000 | |
| Thailand | |
| EU-AIF 09-2010 | Board of Radiation and Isotope Technology |
| Department of Atomic Energy | |
| BRIT/BARC Vashi Complex | |
| Sector 20, Vashi | |
| Navi Mumbai – 400 705 (Maharashtra) | |
| India | |
| EU-AIF 10-2010 | Board of Radiation and Isotope Technology |
| ISOMED | |
| Bhabha Atomic Research Centre | |
| South Site Gate, Refinery Road | |
| Next to TATA Power Station, Trombay | |
| Mumbai – 400 085 (Maharashtra) | |
| India | |
| EU-AIF 11-2010 | Microtrol Sterilisation Services Pvt. Ltd |
| Plot No. 14 Bommasandra- Jigani Link Road | |
| Industrial Area | |
| KIADB, Off Hosur Road | |
| Hennagarra Post | |
| Bengalooru – 562 106 (Karnataka) | |
| India] |
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Schedules only
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i Deddfwyd neu y’i Gwnaed): Mae'r wreiddiol fersiwn y ddeddfwriaeth fel ag yr oedd pan gafodd ei deddfu neu eu gwneud. Ni wnaed unrhyw newidiadau i’r testun.
Gweler y wybodaeth ychwanegol ochr yn ochr â’r cynnwys
Rhychwant ddaearyddol: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Dangos Llinell Amser Newidiadau: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Nodyn Gweithredol
Mae Nodyn Gweithredol yn nodi datganiad byr o ddiben Offeryn Statudol yr Alban ac yn rhoi gwybodaeth am ei amcan polisi a goblygiadau polisi. Ei nod yw gwneud yr Offeryn Statudol yr Alban yn hygyrch i ddarllenwyr nad oes ganddynt gymhwyster cyfreithiol ac maent yn cyd-fynd ag unrhyw Offeryn Statudol yr Alban neu Offeryn Statudol Drafft yr Alban a gyflwynwyd yn fanwl gerbron Senedd yr Alban o Orffennaf 2005 ymlaen.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel deddfwyd fersiwn a ddefnyddiwyd am y copi print
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Llinell Amser Newidiadau
This timeline shows the different points in time where a change occurred. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. The first date in the timeline will usually be the earliest date when the provision came into force. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). This date is our basedate. No versions before this date are available. For further information see the Editorial Practice Guide and Glossary under Help.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel gwnaed fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill