- Latest available (Revised)
- Point in Time (25/11/2006)
- Original (As adopted by EU)
Commission Decision of 14 November 2003 laying down the animal health conditions and certification requirements for imports of molluscs, their eggs and gametes for further growth, fattening, relaying or human consumption (notified under document number C(2003) 4153) (Text with EEA relevance) (2003/804/EC) (repealed)
You are here:
- Decisions originating from the EU
- 2003 No. 804
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 01/01/20090.46 MB
- Revised 08/03/20070.38 MB
- Revised 25/11/20060.38 MB
- Revised 02/06/20050.38 MB
- Revised 31/08/20040.38 MB
- Revised 01/05/20040.32 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Decision of 14 November 2003 laying down the animal health conditions and certification requirements for imports of molluscs, their eggs and gametes for further growth, fattening, relaying or human consumption (notified under document number C(2003) 4153) (Text with EEA relevance) (2003/804/EC) (repealed) (Annexes only)
Version Superseded: 01/01/2009
Status:
Point in time view as at 25/11/2006.
Changes to legislation:
There are currently no known outstanding effects for the Commission Decision of 14 November 2003 laying down the animal health conditions and certification requirements for imports of molluscs, their eggs and gametes for further growth, fattening, relaying or human consumption (notified under document number C(2003) 4153) (Text with EEA relevance) (2003/804/EC) (repealed).![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
[F1ANNEX I U.K.
Textual Amendments
F1 Substituted by Commission Decision of 31 May 2005 amending Annex I to Decision 2003/804/EC laying down the animal health conditions and certification requirements for imports of molluscs, their eggs and gametes for further growth, fattening, relaying or human consumption (notified under document number C(2005) 1585) (Text with EEA relevance) (2005/409/EC).
Territories from which importation of certain species of live molluscs, their eggs and gametes intended for further growth, fattening, or relaying in European Community waters, or importation of live molluscs intended for further processing before human consumption is authorised
| Country | Territory | Comments | ||
|---|---|---|---|---|
| ISO code | Name | Code | Description | |
| CA | Canada | Live molluscs only for further processing before human consumption | ||
| MA | Morocco | Live molluscs only for further processing before human consumption | ||
| NZ | New Zealand | Live molluscs only for further processing before human consumption | ||
| TN | Tunisia | Live molluscs only for further processing before human consumption | ||
| TR | Turkey | Live molluscs only for further processing before human consumption | ||
| US | United States | US-01 Version 1/2005 |
| Live molluscs for further growth, fattening or relaying, and for further processing before human consumption] |
[F2ANNEX II] U.K.
Textual Amendments
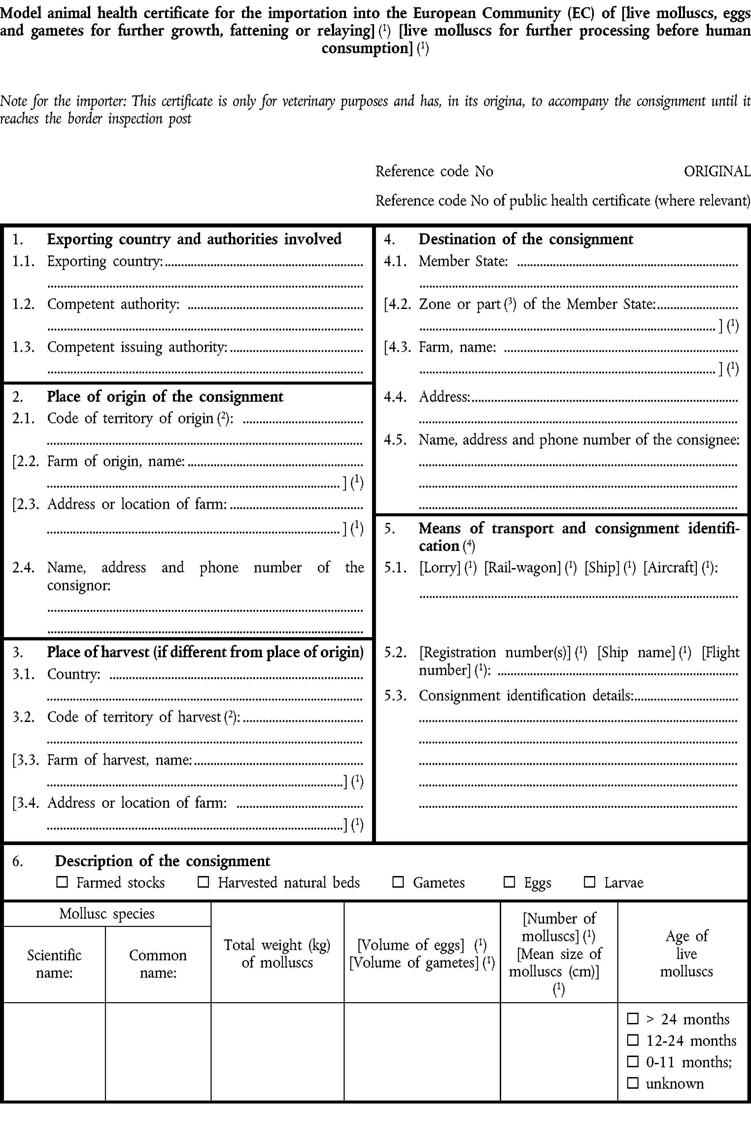
ANNEX IIIU.K.Explanatory notes for certification and labelling
The certificates shall be produced by the competent authorities of the exporting country, based on the appropriate model appearing in Annex II to this Decision taking into account the use to which the molluscs are to be put after the arrival to the EC.
Considering the status of the place of destination as regards Bonamia ostreae and Marteilia refringens in the EC Member State, the appropriate specific additional requirements shall be incorporated and completed in the certificate.
The original of each certificate shall consist of a single page, double-sided, or, where more than one page is required, it shall be in such a form that all pages form part of an integrated whole and are indivisible.
It shall, on the right-hand side of the top of each page, be marked as ‘original’ and bear a specific code number issued by the competent authority. All pages of the certificate shall be numbered — (page number) of (total number of pages).
The original of the certificate and the labels referred to in the model certificate shall be drawn up in at least one official language of the EC Member State in which the inspection at the border post shall be carried out and of the EC Member State of destination. However, these Member States may allow other languages, if necessary, accompanied by an official translation.
The original of the certificate must be completed on the day of loading the consignment for exportation to the European Community with an official stamp and signed by an official inspector designated by the competent authority. In doing so, the competent authority of the exporting country shall ensure that the principles of certification equivalent to those laid down in Council Directive 96/93/EC are followed.
The stamp, unless embossed, and the signature shall be in a colour different to that of the printing.
If for reasons of identification of the items of the consignment, additional pages are attached to the certificate, these pages shall be considered as forming part of the original and be signed and stamped by the certifying official inspector on each page.
The original of the certificate must accompany the consignment until it reaches the EC border inspection post.
The certificate shall be valid for 10 days from the date of issue. In the case of transport by ship, the time of validity is prolonged by the time of the journey at sea.
The molluscs, their eggs and gametes, shall not be transported together with other molluscs, eggs or gametes that, either are not destined to the European Community, or are of a lower health status. Furthermore, they must not be transported under any other conditions that alter their health status.
The possible presence of pathogens in the water is a relevant factor when considering the health status of molluscs. The certifying officer should therefore consider the following:
the ‘place of origin’ should be the localisation of the farm or harvested natural bed where the molluscs were reared reaching their commercial size relevant for the consignment covered by this certificate.
The ‘place of harvest’ should be the last place the molluscs were in contact with natural waters in the exporting country, like purification centres or intermediate storage places where molluscs are kept before exported to the Community.
F3ANNEX IVU.K. [F3Statements as regard live molluscs, their eggs and gametes intended for further growth, fattening, relaying or human consumption in the European Community to be issued by the competent authority at the border inspection post to complete the document referred to in the Annex to Decision 92/527/EEC]
Textual Amendments
[ F3. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F3. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F3. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .]
ANNEX VU.K.MINIMUM ANIMAL HEALTH CONDITIONS FOR THE APPROVAL OF ‘APPROVED IMPORT CENTRES’
A.General ProvisionsU.K.
1.Member States shall only approve centres and establishments as import centres for further processing of imported molluscs provided that the conditions at the import centre are such that risks of contamination of molluscs in Community waters via discharges or other waste, or by other means, with pathogens capable of causing significant abnormal mortality in molluscs are avoided.U.K.
[F42. Viable molluscs may only leave approved import centres if they are packaged and labelled to be presented for sale to the final consumer in accordance with Regulation (EC) No 853/2004.] U.K.
Textual Amendments
F4 Substituted by Commission Decision of 6 November 2006 amending Commission Decisions 2003/804/EC and 2003/858/EC, as regards certification requirements for live molluscs and live fish of aquaculture origin and products thereof intended for human consumption (notified under document number C(2006) 5167) (Text with EEA relevance) (2006/767/EC).
3.The minimum animal health conditions as laid down in part B of this Annex shall apply, in addition to the public health provisions laid down under Directive 91/492/EEC for any centres and establishments, including dispatch centres and purification centres, as well as to the health rules laid down by Community legislation concerning animal by-products not intended for human consumption.U.K.
B.Management ProvisionsU.K.
1.Approved import centres must be under the control and responsibility of the competent authority.U.K.
2.Approved import centres must have an efficient disease control, and monitoring system; in application of Directive 95/70/EC, cases of suspected disease and mortality shall be investigated by the competent authority; the necessary analysis and treatment must be carried out in consultation with and under the control of the competent authority, taking into consideration the requirement in Article 3(1)(a) of Directive 91/67/EEC.U.K.
3.Approved import centres must apply a management system, approved by the competent authority, including hygiene and disposal routines for transport, transport containers, facilities, and equipment. The guidelines laid down for disinfection of mollusc farms in the OIE International aquatic animal health code, sixth edition, 2003, Appendix 5.2.2, should be followed. The disinfectants used must be approved for the purpose by the competent authority and appropriate equipment must be available for cleaning and disinfection. Discharges of by-products and other waste materials including dead molluscs and their products must be carried out in accordance with Regulation (EC) No 1774/2002. The management system at the approved import centre shall be such that risks of contamination of molluscs in Community waters with pathogens capable of causing significant impact to mollusc stocks, in particular diseases referred to in Annex D to Directive 95/70/EC, are avoided.U.K.
4.Approved import centres must keep an updated record of observed abnormal mortality, and of all the live molluscs, eggs and gametes entering the centre and products leaving the centre including their source, their suppliers and their destination.U.K.
5.Approved import centres must be cleaned and disinfected regularly in accordance with the programme described in point 3 above.U.K.
6.Only authorised persons may enter approved import centres and must wear protective clothing including appropriate footwear.U.K.
Options/Help
Print Options
PrintThe Whole Decision
PrintThe Annexes only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources