- Latest available (Revised)
- Point in Time (01/01/2007)
- Original (As adopted by EU)
Council Directive of 15 October 1990 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs (90/539/EEC) (repealed)
You are here:
- Directives originating from the EU
- 1990 No. 539
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 01/01/20100.44 MB
- Revised 03/09/20080.52 MB
- Revised 13/11/20070.53 MB
- Revised 01/09/20070.53 MB
- Revised 01/01/20070.46 MB
- Revised 12/12/20060.46 MB
- Revised 01/05/20040.45 MB
- Revised 05/06/20030.44 MB
- Revised 01/01/20020.50 MB
- Revised 01/10/20000.46 MB
- Revised 23/11/19990.45 MB
- Revised 01/01/19950.44 MB
- Revised 20/01/19940.44 MB
- Revised 29/07/19920.36 MB
- Revised 25/06/19920.42 MB
- Revised 19/08/19910.42 MB
- Revised 23/07/19910.49 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Council Directive of 15 October 1990 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs (90/539/EEC) (repealed) (Annexes only)
Version Superseded: 01/09/2007
Status:
EU Directives are published on this site to aid cross referencing from UK legislation. Since IP completion day (31 December 2020 11.00 p.m.) no amendments have been applied to this version.
ANNEX IU.K.
[F1The national reference laboratories for avian diseases are as follows:
| AT | AGES: Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH — Institut für veterinärmedizinische Untersuchungen Mödling (Austrian Agency for Health and Consumer Protection-Institute for veterinary investigations Mödling) Robert Koch-Gasse 17 A-2340 Mödling Tel.: +43 (0) 505 55-38112 Fax: +43 (0) 505 55-38108 E-mail: vetmed.moedling@ages.at |
| BE | CODA — CERVA — VAR Veterinary and Agrochemical Research Centre Groeselenberg 99 B-1180 Brussels |
| [F2BG | Национален диагностичен научноизследователски ветеринарномедицински институт ‘ Проф. д-р Георги Павлов ’ , Национална референтна лаборатория ‘ Нюкясълска болест и Инфлуенца А по птиците ’ , бул. ‘ Пенчо Славейков ’ 15, София 1606 (National Diagnostic Veterinary Research Institute ‘ Prof. Dr. Georgi Pavlov ’ , National Reference Laboratory for Newcastle Disease and Avian Influenza A, 15, Pencho Slaveykov Blvd., 1606 Sofia)] |
| CY | State Veterinary Laboratory Veterinary Services 1417 Athalassa Nicosia |
| CZ | State Veterinary Institute Praha Sídlištní 136/24 165 03 Praha 6 |
| DE | Friedrich-Loeffler-Institut Bundesforschungsinstitut für Tiergesundheit Boddenblick 5a 17493 Greifswald-Insel Riems Tel.: +49 383 51-7-0 Fax: +49 383 51-7-151 |
| DK | Danish Institute for Food and Veterinary Research, Dpt. of Poultry, Fish and Fur Animals, Hangoevej 2, DK-8200 Aarhus N |
| EE | Veterinaar- ja Toidulaboratoorium Väike-Paala 3, 11415 Tallinn, Estonia Tel.: +372 603 58 10 Faks: +372 603 58 11 E-post: tallinn@vetlab.ee |
| ES | Laboratorio Central de Sanidad Animal de Algete Carretera de Algete, km 8 Algete 28110 (Madrid) Tel.: +34 916 290 300 Fax: +34 916 290 598 E-mail: lcv@mapya.es |
| FI | Finnish Food Safety Authority Animal Diseases and Food Safety Research Mustialankatu 3 FI-00790 Helsinki, Finland E-mail: info@evira.fi Tel.: +358 20 772 003 (exchange) Fax: +358 20 772 4350 |
| FR | Laboratoire d’études et de recherches avicoles, porcines et piscicoles AFSSA site de Ploufragan/Brest — LERAPP BP 53 22440 Ploufragan |
| GB | Veterinary Laboratories Agency New Haw, Addlestone, Weybridge Surrey KT15 3NB, UK Tel. (44-1932) 341111 Fax (44-1932) 347046 |
| GR | Centre of Thessaloniki Veterinary Institutions, 80, 26th October Street, GR-546 27 Thessaloniki Tel.: 2310785104 |
| HU | Országos Állategészségügyi Intézet (Central Veterinary Institute) H-1581 Budapest 146., Pf. 2. Tel.: +36-1-460-6300, +36-1-460-6317 Fax: +36-1-222-6070 |
| IE | Virology Division Central Veterinary Research Laboratory Department of Agriculture and Food Laboratories Backweston Campus Stacumny Lane Celbridge Co. Kildare |
| IT | Centro di Referenza Nazionale per l’influenza aviare e la malattia di New Castle e Centro di Referenza Nazionale per le Salmonellosi c/o Istituto zooprofilattico sperimentale delle Venezie, V.le dell'Università, 10-35020 Legnaro (Pd) |
| LT | National Veterinary Laboratory (Nacionalinė veterinarijos laboratorija) J. Kairiūkščio 10 LT-08409 Vilnius |
| LU | CODA — CERVA — VAR Veterinary and Agrochemical Research Centre Groeselenberg 99 B-1180 Brussels |
| LV | Nacionālais diagnostikas centrs (National Diagnostic Centre) Lejupes iela 3, Rīga, LV-1076 Tel.: +371 7620526 Fax: +371 7620434 E-mail: ndc@ndc.gov.lv |
| MT | National Veterinary Laboratory, Marsa |
| NL | Centraal Instituut voor Dierziekte Controle CIDC-Lelystad Hoofdvestiging: Houtribweg 39 Nevenvestiging: Edelhertweg 15 Postbus 2004 8203 AA Lelystad |
| PL | Laboratory Department of Poultry Diseases Państwowy Instytut Weterynaryjny – Państwowy Instytut Badawczy Al. Partyzantów 57, 24-100 Puławy Tel.: +48.81.886 30 51 Fax: +48.81.886 25 95 E-mail: sekretariat@piwet.pulawy.pl |
| PT | Laboratório Nacional de Investigação Veterinária (LNIV) Estrada de Benfica, 701 P-1549-011 Lisboa |
| [F2RO | Institutul de Diagnostic și Sănătate Animală Strada Dr. Staicovici nr. 63, sector 5 Codul 050557, București] |
| SE | Statens Veterinärmedicinska Anstalt Department of Virology SE-751 89 Uppsala Tel (46-18) 674000 Fax (46-18) 674467 Department of Bacteriology SE-751 89 Uppsala Tel (46-18) 674000 Fax (46-18) 309162 |
| SI | Univerza v Ljubljani Veterinarska fakulteta Nacionalni veterinarski inštitut Gerbičeva 60, SI-1000 Ljubljana |
| SK | Štátny veterinárny a potravinový ústav, Botanická 15, 842 52 Bratislava] |
The national reference laboratories for avian diseases listed in point 1 are responsible in each Member State for coordinating the diagnostic methods provided for in this Directive. To this end:
they may supply approved laboratories with the reagents needed for diagnostic testing;
they monitor the quality of all the reagents used by the approved laboratories;
they organize periodic comparative tests.
Textual Amendments
F1 Substituted by Commission Decision of 5 December 2006 amending Council Directives 64/432/EEC, 90/539/EEC, 92/35/EEC, 92/119/EEC, 93/53/EEC, 95/70/EC, 2000/75/EC, 2001/89/EC, 2002/60/EC and Decision 2001/618/EC as regards lists of national reference laboratories and State institutes (notified under document number C(2006) 5856) (Text with EEA relevance) (2006/911/EC).
ANNEX IIU.K.APPROVAL OF ESTABLISHMENTS
CHAPTER IU.K.General rules
In order to be approved by the competent authority for the purposes of intra-Community trade, establishments must:
satisfy the conditions as regards facilities and operation set out in Chapter II;
apply and adhere to a disease surveillance programme approved by the competent central veterinary authority, taking into account the requirements of Chapter III;
provide every facility for the carrying out of the operations listed in (d);
be subject to the supervision of the competent veterinary authority within the context of an organized form of animal health monitoring. Such monitoring will include in particular:
at least one inspection visit per year by the official veterinarian, supplemented by checks to verify the application of hygiene measures and the operation of the establishment in accordance with the conditions in Chapter II,
the recording by the farmer of all the information necessary for the continuous monitoring of the health status of the establishment by the competent veterinary authority;
contain only poultry as defined in Article 2 (1).
The competent authority will give each establishment which complies with the conditions laid down in point 1 a distinguishing approval number, which may be the same as that given pursuant to Regulation (EEC) No 2782/75.
CHAPTER IIU.K.Facilities and operation
Pedigree breeding, breeding and rearing establishments
Facilities
The siting and layout of the facilities must be compatible with the type of production pursued, ensuring that the introduction of disease can be prevented or, if an outbreak occurs, that it can be controlled. If an establishment houses several species of poultry, there must be a dear separation between them.
The facilities must provide good conditions of hygiene and allow health monitoring to be carried out.
The equipment must be compatible with the type of production pursued, and allow cleansing and disinfection of the facilities and of vehicles for transporting poultry and eggs at the most suitable spot.
Rearing
Rearing techniques must be based as far as possible on the ‘protected rearing’ principle and on the ‘all-in/all-out’ principle. Cleansing, disinfection and depopulation must be carried out between batches.
Pedigree-breeding, breeding and rearing establishments must house only poultry:
from the establishment itself, and/or
from other pedigree-breeding, breeding or rearing establishments in the Community approved in accordance with Article 6 (a), and/or
imported from third countries in accordance with this Directive.
Hygiene rules must be drawn up by the management of the establishment; personnel must wear appropriate working clothing and visitors protective clothing.
Buildings, pens and equipment must be kept in good repair.
Eggs must be collected several times a day, and must be clean and be disinfected as soon as possible.
The farmer must notify the authorized veterinarian of any variation in production performance or any other sign suggesting the presence of a contagious poultry disease. As soon as disease is suspected, the authorized veterinarian must send the samples needed for making or confirming the diagnosis to an approved laboratory.
A flock history, register or data medium must be kept for each flock for at least two years after the disposal of the flock and must show.
arrivals and departures,
production performance,
morbidity and mortality with causes,
any laboratory tests and the results thereof,
the place of origin of the poultry,
the destination of eggs.
Where a contagious poultry disease occurs, the results of laboratory tests must be communicated immediately to the authorized veterinarian.
Hatcheries
Facilities
A hatchery must be physically and operationally separate from rearing facilities. The layout must be such as to allow the various functional units listed below to be kept separate:
egg storage and grading,
disinfection,
pre-incubation,
hatching,
preparation and packaging of goods for dispatch.
Buildings must be protected against birds coming from outside and rodents; floors and walls must be of hard-wearing, impervious and washable materials; natural or artificial lighting and air flow and temperature systems must be of an appropriate type; provision must be made for the hygienic evacuation of waste (eggs and chicks).
Equipment must have smooth and waterproof surfaces.
Operation
Operation must be based on a one-way circuit for eggs, mobile equipment and personnel.
Hatching eggs must be:
from Community pedigree breeding or breeding establishments approved in accordance with Article 6 (a),
imported from third countries in accordance with this Directive.
Hygiene rules must be drawn up by the management of the establishment; personnel must wear appropriate working clothing and visitors protective clothing.
Buildings and equipment must be kept in good repair.
The following must be disinfected:
eggs, between the time of their arrival and the incubation process,
the incubators, regularly,
hatchers and equipment, after the hatching of each batch.
A programme of microbiological quality control must be used to assess the health status of the hatchery.
The farmer must notify the authorized veterinarian of any variation in production performance or any other sign suggesting the presence of a contagious poultry disease. As soon as contagious disease is suspected, the authorized veterinarian must send the samples needed for making or confirming the diagnosis to an approved laboratory and inform the competent veterinary authority, which will decide on appropriate measures to be taken.
A flock history, register or data medium for the hatchery must be kept for at least two years showing, if possible by flock:
the origin of the eggs and their arrival date,
hatching yields,
any abnormalities,
any laboratory tests and the results thereof,
details of any vaccination programmes,
the number and the destination of incubated eggs which have not hatched,
the destination of day-old chicks.
Where a contagious poultry disease occurs, the results of laboratory tests must be communicated immediately to the authorized veterinarian.
CHAPTER IIIU.K.Disease surveillance programme
Without prejudice to health measures and to Articles 13 and 14, disease surveillance programmes must, as a minimum, comprise surveillance of the infections and species listed below.
Salmonella pullorum, Salmonella gallinarum and Salmonella arizonae infections
Species concerned
S. pullorum et gallinarum: fowls, turkeys, guinea fowls, quails, pheasants, partridges and ducks.
S. arizonae: turkeys.
Disease surveillance programme
Serological and/or bacteriological tests must be used to determine whether an infection is present.
Samples for testing must be taken, as appropriate, from blood, second-grade chicks, down or dust taken from hatchers, swabs taken from the walls of the hatchery, litter or water from a drinker.
When blood samples are taken from a flock for serological testing for S. pullorum or S. arizonae, prevalence of infection in the country concerned and its past incidence in the establishment must be allowed for in determining the number of samples to be taken.
Flocks must be inspected during each laying period at the best time for detecting the disease.
Mycoplasma gallisepticum and Mycoplasma meleagridis infections
Species concerned
Mycoplasma gallisepticum: fowls and turkeys.
Mycoplasma meleagridis: turkeys.
Disease surveillance programme
Presence of infection must be tested by serological and/or bacteriological testing and/or by the presence of air sacculitis lesions in day-old chicks and turkey poults.
Samples for testing must be taken, as appropriate, from blood, day-old chicks and turkey poults, sperm, or swabs taken from the trachea, the cloaca or air sacs.
Tests for detecting M. gallisepticum or M. meleagridismust be performed on a representative sample in order to allow continous surveillance of the infection during rearing and laying, i.e. just before the start of laying and every three months thereafter.
Results and measures to be taken
If there are no reactors, the test is deemed negative. Otherwise, the flock is suspect and the measures specified in Chapter IV must be applied to it.
In the case of holdings which consist of two or more separate production units, the competent veterinary authority may derogate from these measures as regards healthy production units on a holding which is infected provided that the authorized veterinarian has confirmed that the structure and size of these production units and the operations carried out there are such that the production units provide completely separate facilities for housing, keeping and feeding, so that the disease in question cannot spread from one production unit to another.
CHAPTER IVU.K.Criteria for suspending or withdrawing approval of an establishment
Approval granted to an establishment must be suspended:
when the conditions laid down in Chapter II are no longer met;
until an investigation appropriate to the disease has been completed:
if avian influenza or Newcastle disease are suspected at the establishment,
if the establishment has received poultry or hatching eggs from an establishment with suspected or actual infection by avain influenza or Newcastle disease,
if contact liable to transmit the infection has occurred between the establishment and the site of an outbreak of avian influenza or Newcastle disease;
until such time as new tests are performed, if the results of surveillance carried out in accordance with the conditions laid down in Chapters II and III for infection by S. pullorum, S. gallinarum, S. arizonae, M. gallisepticum or M. meleagridis give cause to suspect infection;
until completion of the appropriate measures required by the official veterinarian, if the establishment is found not to conform with the requirements of Chapter I, paragraph 1 (a), (b) and (c).
Approval must be withdrawn:
if avian influenza or Newcastle disease occurs at the establishment;
if a second test of an appropriate type confirms the presence of infection by S. pullorum, S. gallinarum, S. arizonae, M. gallisepticum or M. meleagridis;
if, after a second notice served by the official veterinarian, action to bring the establishment into line with the requirements of Chapter I, paragraph 1 (a), (b) and (c), has not been taken.
Conditions for restoring approval:
if approval has been withdrawn because of an occurrence of avian influenza or Newcastle disease, it may be restored 21 days after cleansing and disinfection if sanitary slaughter has been carried out;
if approval has been withdrawn because of infection caused by:
Salmonella pullorum et gallinarum, or Salmonella arizonae, it may be restored after negative results have been recorded in two tests performed with an interval of at least 21 days on the establishment and after disinfection following sanitary slaughter of the infected flock;
Mycoplasma gallisepticum or Mycoplasma meleagridis, it may be restored after negative results have been recorded in two tests performed on the entire flock with an interval of at least 60 days.
[F3ANNEX III U.K. POULTRY VACCINATION CONDITIONS
Textual Amendments
Vaccines used for vaccinating poultry or flocks producing hatching eggs must have a marketing authorization issued by the competent authority of the Member State in which the vaccine is used.
The criteria for using vaccines against Newcastle disease in the context of routine-vaccination programmes may be determined by the Commission.]
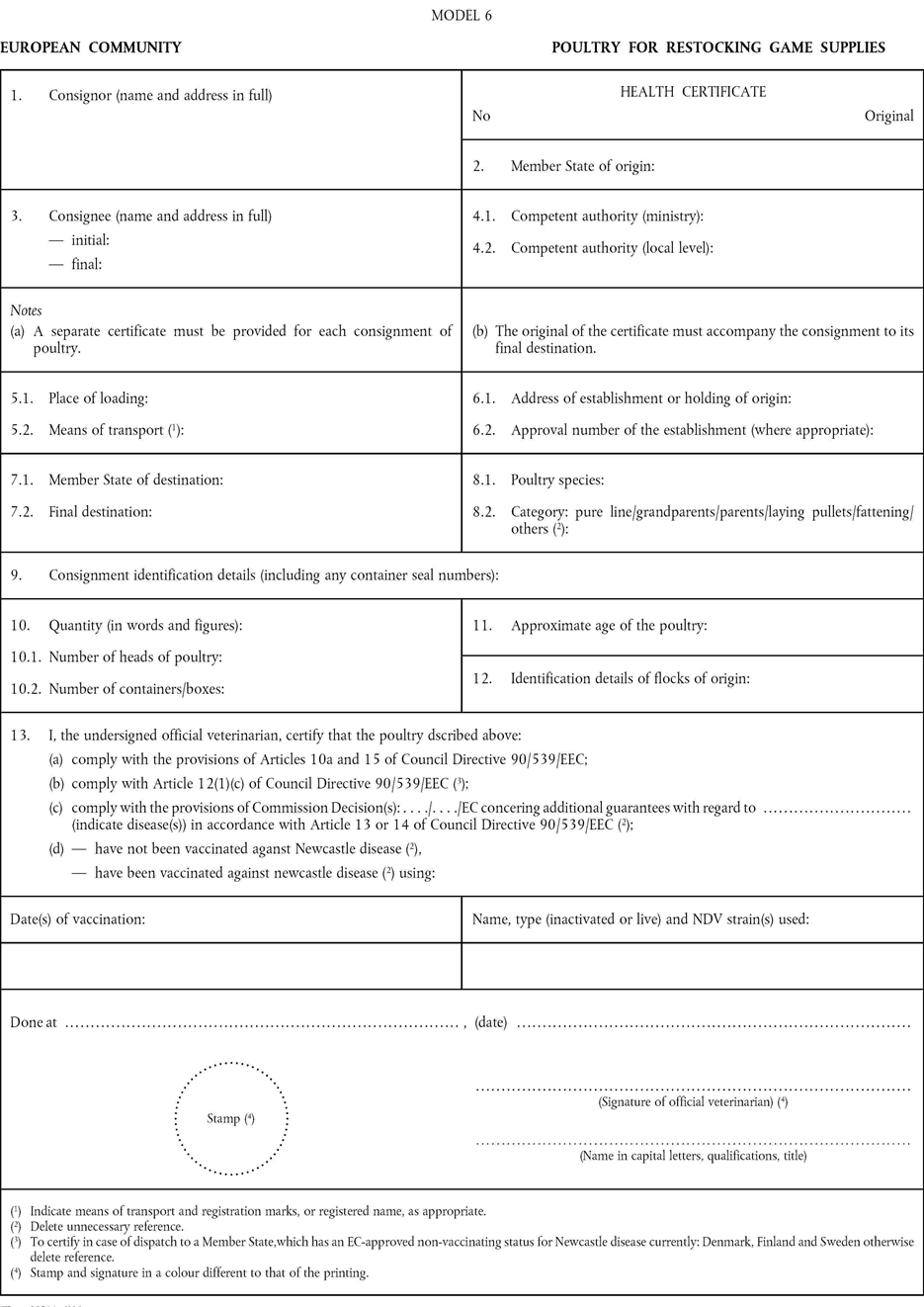
[F4ANNEX IV U.K. HEALTH CERTIFICATES FOR INTRA-COMMUNITY TRADE (Models 1 to 6)]
Textual Amendments
ANNEX VU.K.COMPULSORILY NOTIFIABLE DISEASES
Avian influenza
Newcastle disease
Options/Help
Print Options
PrintThe Whole Directive
PrintThe Annexes only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources