ANNEX IU.K.
Ethylene oxide may not be used for sterilising purposes in food additives.
E 170 (i) CALCIUM CARBONATEU.K.
Purity criteria for this additive are the same as set out for this additive in the Annex to Commission Directive 95/45/EC().
E 200 SORBIC ACIDU.K.
| Definition | |
| Chemical name | Sorbic acid |
| Trans, trans-2,4-hexadienoic acid |
| Einecs | 203-768-7 |
| Chemical formula | C6H8O2 |
| Molecular weight | 112,12 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Colourless needles or white free flowing powder, having a slight characteristic odour and showing no change in colour after heating for 90 minutes at 105 oC |
| Identification | |
A. Melting range | Between 133 oC and 135 oC, after vacuum drying for four hours in a sulphuric acid desiccator |
B. Spectrometry | An isopropanol solution (1 in 4 000 000) shows absorbance maximum at 254 ± 2 nm |
C. Positive test for double bonds | |
D. Sublimation point | 80 oC |
| Purity | |
| Water content | Not more than 0,5 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,2 % |
| Aldehydes | Not more than 0,1 % (as formaldehyde) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 202 POTASSIUM SORBATEU.K.
| Definition | |
| Chemical name | Potassium sorbate |
| Potassium (E, E)-2,4-hexadienoate |
| Potassium salt of trans, trans 2,4-hexadienoic acid |
| Einecs | 246-376-1 |
| Chemical formula | C6H7O2K |
| Molecular weight | 150,22 |
| Assay | Content not less than 99 % on the dried basis |
| Description | White crystalline powder showing no change in colour after heating for 90 minutes at 105 oC |
| Identification | |
A. Melting range of sorbic acid isolated by acidification and not recrystallised 133 oC to 135 oC after vacuum drying in a sulphuric acid desiccator | |
B. Positive tests for potassium and for double bonds | |
| Purity | |
| Loss on drying | Not more than 1,0 % (105 oC, 3h) |
| Acidity or alkalinity | Not more than about 1,0 % (as sorbic acid or K2CO3) |
| Aldehydes | Not more than 0,1 %, calculated as formaldehyde |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 203 CALCIUM SORBATEU.K.
| Definition | |
| Chemical name | Calcium sorbate |
| Calcium salts of trans, trans-2,4-hexadienoic acid |
| Einecs | 231-321-6 |
| Chemical formula | C12H14O4Ca |
| Molecular weight | 262,32 |
| Assay | Content not less than 98 % on the dried basis |
| Description | Fine white crystalline powder not showing any change in colour after heating at 105 oC for 90 minutes |
| Identification | |
A. Melting range of sorbic acid isolated by acidification and not recrystallised 133 oC to 135 oC after vacuum drying in a sulphuric acid desiccator | |
B. Positive tests for calcium and for double bonds | |
| Purity | |
| Loss on drying | Not more than 2,0 %, determined by vacuum drying for four hours in a sulphuric acid desiccator |
| Aldehydes | Not more than 0,1 % (as formaldehyde) |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 210 BENZOIC ACIDU.K.
| |
| |
| |
| |
| Definition | |
| Chemical name | Benzoic acid |
| Benzenecarboxylic acid |
| Phenylcarboxylic acid |
| Einecs | 200-618-2 |
| Chemical formula | C7H6O2 |
| Molecular weight | 122,12 |
| Assay | Content not less than 99,5 % on the anhydrous basis |
| Description | White crystalline powder |
| Identification | |
A. Melting range | 121,5oC to 123,5oC |
B. Positive sublimation test and test for benzoate | |
| Purity | |
| Loss on drying | Not more than 0,5 % after drying for three hours over sulphuric acid |
| pH | About 4 (solution in water) |
| Sulphated ash | Not more than 0,05 % |
| Chlorinated organic compounds | Not more than 0,07 % expressed as chloride corresponding to 0,3 % expressed as monochlorobenzoic acid |
| Readily oxidisable substances | Add 1,5 ml of sulphuric acid to 100 ml of water, heat to boiling point and add 0,1 N KMnO4 in drops, until the pink colour persists for 30 seconds. Dissolve 1 g of the sample, weighed to the nearest mg, in the heated solution, and titrate with 0,1 N KMnO4 to a pink colour that persists for 15 seconds. Not more than 0,5 ml should be required |
| Readily carbonisable substances | A cold solution of 0,5 g of benzoic acid in 5 ml of 94,5 to 95,5 % sulphuric acid must not show a stronger colouring than that of a reference liquid containing 0,2 ml of cobalt chloride TSC, 0,3 ml of ferric chloride TSC, 0,1 ml of copper sulphate TSC and 4,4 ml of water |
| Polycyclic acids | On fractional acidification of a neutralised solution of benzoic acid, the first precipitate must not have a different melting point from that of the benzoic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
|
E 211 SODIUM BENZOATEU.K.
| Definition | |
| Chemical name | Sodium benzoate |
| Sodium salt of benzenecarboxylic acid |
| Sodium salt of phenylcarboxylic acid |
| Einecs | 208-534-8 |
| Chemical formula | C7H5O2Na |
| Molecular weight | 144,11 |
| Assay | Not less than 99 % of C7H5O2Na, after drying at 105 oC for four hours |
| Description | A white, almost odourless, crystalline powder or granules |
| Identification | |
A. Solubility | Freely soluble in water, sparingly soluble in ethanol |
B. Melting range for benzoic acid | Melting range of benzoic acid isolated by acidification and not recrystallised 121,5oC to 123,5oC, after drying in a sulphuric acid desiccator |
C. Positive tests for benzoate and for sodium | |
| Purity | |
| Loss on drying | Not more than 1,5 % after drying at 105 oC for four hours |
| Readily oxidisable substances | Add 1,5 ml of sulphuric acid to 100 ml of water, heat to boiling point and add 0,1 N KMnO4 in drops, until the pink colour persists for 30 seconds. Dissolve 1 g of the sample, weighed to the nearest mg, in the heated solution, and titrate with 0,1 N KMnO4 to a pink colour that persists for 15 seconds. Not more than 0,5 ml should be required |
| Polycyclic acids | On fractional acidification of a (neutralised) solution of sodium benzoate, the first precipitate must not have a different melting range from that of benzoic acid |
| Chlorinated organic compounds | Not more than 0,06 % expressed as chloride, corresponding to 0,25 % expressed as monochlorobenzoic acid |
| Degree of acidity or alkalinity | Neutralisation of 1 g of sodium benzoate, in the presence of phenolphthalein, must not require more than 0,25 ml of 0,1 N NaOH or 0,1 N HCl |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 212 POTASSIUM BENZOATEU.K.
| Definition | |
| Chemical name | Potassium benzoate |
| Potassium salt of benzenecarboxylic acid |
| Potassium salt of phenylcarboxylic acid |
| Einecs | 209-481-3 |
| Chemical formula | C7H5KO2·3H2O |
| Molecular weight | 214,27 |
| Assay | Content not less than 99 % C7H5KO2 after drying at 105 oC to constant weight |
| Description | White crystalline powder |
| Identification | |
A. Melting range of benzoic acid isolated by acidification and not recrystallised 121,5oC to 123,5oC, after vacuum drying in a sulphuric acid desiccator | |
B. Positive tests for benzoate and for potassium | |
| Purity | |
| Loss on drying | Not more than 26,5 %, determined by drying at 105 oC |
| Chlorinated organic compounds | Not more than 0,06 % expressed as chloride, corresponding to 0,25 % expressed as monochlorobenzoic acid |
| Readily oxidisable substances | Add 1,5 ml of sulphuric acid to 100 ml of water, heat to boiling point and add 0,1 N KMnO4 in drops, until the pink colour persists for 30 seconds. Dissolve 1 g of the sample, weighed to the nearest mg, in the heated solution, and titrate with 0,1 N KMnO4 to a pink colour that persists for 15 seconds. Not more than 0,5 ml should be required |
| Readily carbonisable substances | A cold solution of 0,5 g of benzoic acid in 5 ml 94,5 to 95,5 % sulphuric acid must not show a stronger colouring than that of a reference liquid containing 0,2 ml of cobalt chloride TSC, 0,3 ml of ferric chloride TSC, 0,1 ml of copper sulphate TSC and 4,4 ml of water |
| Polycyclic acids | On fractional acidification of a (neutralised) solution of potassium benzoate, the first precipitate must not have a different melting range from that of benzoic acid |
| Degree of acidity or alkalinity | Neutralisation of 1 g of potassium benzoate, in the presence of phenolphthalein, must not require more than 0,25 ml of 0,1 N NaOH or 0,1 N HCl |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 213 CALCIUM BENZOATEU.K.
| Synonyms | Monocalcium benzoate |
| Definition | |
| Chemical name | Calcium benzoate |
| Calcium dibenzoate |
| Einecs | 218-235-4 |
| Chemical formula | Anhydrous: | C14H10O4Ca |
| Monohydrate: | C14H10O4Ca· H2O |
| Trihydrate: | C14H10O4Ca· 3H2O |
| Molecular weight | Anhydrous: | 282,31 |
| Monohydrate: | 300,32 |
| Trihydrate: | 336,36 |
| Assay | Content not less than 99 % after drying at 105 oC |
| Description | White or colourless crystals, or white powder |
| Identification | |
A. Melting range of benzoic acid isolated by acidification and not recrystallised 121,5oC to 123,5oC, after vacuum drying in a sulphuric acid desiccator | |
B. Positive tests for benzoate and for calcium | |
| Purity | |
| Loss on drying | Not more than 17,5 % determined by drying at 105 oC to constant weight |
| Water insoluble matter | Not more than 0,3 % |
| Chlorinated organic compounds | Not more than 0,06 % expressed as chloride, corresponding to 0,25 % expressed as monochlorobenzoic acids |
| Readily oxidisable substances | Add 1,5 ml of sulphuric acid to 100 ml of water, heat to boiling point and add 0,1 N KMnO4 in drops, until the pink colour persists for 30 seconds. Dissolve 1 g of the sample, weighed to the nearest mg, in the heated solution, and titrate with 0,1 N KMnO4 to a pink colour that persists for 15 seconds. Not more than 0,5 ml should be required |
| Readily carbonisable substances | Cold solution of 0,5 g of benzoic acid in 5 ml of 94,5 to 95,5 % sulphuric acid must not show a stronger colouring than that of a reference liquid containing 0,2 ml of cobalt chloride TSC, 0,3 ml of ferric chloride TSC, 0,1 ml of copper sulphate TSC and 4,4 ml of water |
| Polycyclic acids | On fractional acidification of a (neutralised) solution of calcium benzoate, the first precipitate must not be a different melting range from that of benzoic acid |
| Degree of acidity or alkalinity | Neutralisation of 1 g of calcium benzoate, in the presence of phenolphthalein, must not require more than 0,25 ml of 0,1 N NaOH or 0,1 N HCl |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 214 ETHYL p-HYDROXYBENZOATEU.K.
| Synonyms | Ethylparaben |
| Ethyl p-oxybenzoate |
| Definition | |
| Chemical name | Ethyl-p-hydroxybenzoate |
| Ethyl ester of p-hydroxybenzoic acid |
| Einecs | 204-399-4 |
| Chemical formula | C9H10O3 |
| Molecular weight | 166,8 |
| Assay | Content not less than 99,5 % after drying for two hours at 80 oC |
| Description | Almost odourless, small, colourless crystals or a white, crystalline powder |
| Identification | |
A. Melting range | 115 oC to 118 oC |
B. Positive test for p-hydroxybenzoate | Melting range of p-hydroxybenzoic acid isolated by acidification and not recrystallised: 213 oC to 217 oC, after vacuum drying in a sulphuric acid desiccator |
C. Positive test for alcohol | |
| Purity | |
| Loss on drying | Not more than 0,5 % after drying for two hours at 80 oC |
| Sulphated ash | Not more than 0,05 % |
| p-Hydroxybenzoic acid and salicylic acid | Not more than 0,35 % expressed as p-hydroxybenzoic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 215 SODIUM ETHYL p-HYDROXYBENZOATEU.K.
| Definition | |
| Chemical name | Sodium ethyl p-hydroxybenzoate |
| Sodium compound of the ethyl ester of p-hydroxybenzoic acid |
| Einecs | 252-487-6 |
| Chemical formula | C9H9O3Na |
| Molecular weight | 188,8 |
| Assay | Content of ethylester of p-hydroxybenzoic acid not less than 83 % on the anhydrous basis |
| Description | White, crystalline hygroscopic powder |
| Identification | |
A. Melting range | 115 oC to 118 oC, after vacuum drying in a sulphuric acid desiccator |
B. Positive test for p-hydroxybenzoate | Melting range of p-hydroxybenzoic acid derived from the sample is 213 oC to 217 oC |
C. Positive test for sodium | |
D. pH of a 0,1 % aqueous solution must be between 9,9 and 10,3 | |
| Purity | |
| Loss on drying | Not more than 5 %, determined by vacuum drying in a sulphuric acid desiccator |
| Sulphated ash | 37 to 39 % |
| p-Hydroxybenzoic acid and salicylic acid | Not more than 0,35 % expressed as p-hydroxybenzoic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 218 METHYL p-HYDROXYBENZOATEU.K.
| Synonyms | Methylparaben |
| Methyl-p-oxybenzoate |
| Definition | |
| Chemical name | Methyl p-hydroxybenzoate |
| Methyl ester of p-hydroxybenzoic acid |
| Einecs | 243-171-5 |
| Chemical formula | C8H8O3 |
| Molecular weight | 152,15 |
| Assay | Content not less than 99 % after drying for two hours at 80 oC |
| Description | Almost odourless, small colourless crystals or white crystalline powder |
| Identification | |
A. Melting range | 125 oC to 128 oC |
B. Positive test for p-hydroxybenzoate | Melting range of p-hydroxybenzoic acid derived from the sample is 213 oC to 217 oC after drying for two hours at 80 oC |
| Purity | |
| Loss on drying | Not more than 0,5 %, after drying for two hours at 80 oC |
| Sulphated ash | Not more than 0,05 % |
| p-Hydroxybenzoic acid and salicylic acid | Not more than 0,35 % expressed as p-hydroxybenzoic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 219 SODIUM METHYL p-HYDROXYBENZOATEU.K.
| Definition | |
| Chemical name | Sodium methyl p-hydroxybenzoate |
| Sodium compound of the methylester of p-hydroxybenzoic acid |
| Chemical formula | C8H7O3Na |
| Molecular weight | 174,15 |
| Assay | Content not less than 99,5 % on the anhydrous basis |
| Description | White, hygroscopic powder |
| Identification | |
A. The white precipitate formed by acidifying with hydrochloric acid a 10 % (w/v) aqueous solution of the sodium derivative of methyl p-hydroxybenzoate (using litmus paper as indicator) shall, when washed with water and dried at 80 oC for two hours, have a melting range of 125 oC to 128 oC | |
B. Positive test for sodium | |
C. pH of a 0,1 % solution in carbon dioxide free water, not less than 9,7 and not more than 10,3 | |
| Purity | |
| Water content | Not more than 5 % (Karl Fischer method) |
| Sulphated ash | 40 % to 44,5 % on the anhydrous basis |
| p-Hydroxybenzoic acid and salicylic acid | Not more than 0,35 % expressed as p-hydroxybenzoic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 220 SULPHUR DIOXIDEU.K.
| Definition | |
| Chemical name | Sulphur dioxide |
| Sulphurous acid anhydride |
| Einecs | 231-195-2 |
| Chemical formula | SO2 |
| Molecular weight | 64,07 |
| Assay | Content not less than 99 % |
| Description | Colourless, non-flammable gas with strong pungent suffocating odour |
| Identification | |
A. Positive test for sulphurous substances | |
| Purity | |
| Water content | Not more than 0,05 % |
| Non-volatile residue | Not more than 0,01 % |
| Sulphur trioxide | Not more than 0,1 % |
| Selenium | Not more than 10 mg/kg |
| Other gases not normally present in the air | No trace |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 221 SODIUM SULPHITEU.K.
| Definition | |
| Chemical name | Sodium sulphite (anhydrous or heptahydrate) |
| Einecs | 231-821-4 |
| Chemical formula | Anhydrous: | Na2SO3 |
| Heptahydrate: | Na2SO37H2O |
| Molecular weight | Anhydrous: | 126,04 |
| Heptahydrate: | 252,16 |
| Assay | Anhydrous: | Not less than 95 % of Na2SO3 and not less than 48 % of SO2 |
| Heptahydrate: | Not less than 48 % of Na2SO3 and not less than 24 % of SO2 |
| Description | White crystalline powder or colourless crystals |
| Identification | |
A. Positive tests for sulphite and for sodium | |
B. pH of a 10 % solution (anhydrous) or a 20 % solution (heptahydrate) between 8,5 and 11,5 | |
| Purity | |
| Thiosulphate | Not more than 0,1 % based on the SO2 content |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 222 SODIUM BISULPHITEU.K.
| Definition | |
| Chemical name | Sodium bisulphite |
| Sodium hydrogen sulphite |
| Einecs | 231-921-4 |
| Chemical formula | NaHSO3 in aqueous solution |
| Molecular weight | 104,06 |
| Assay | Content not less than 32 % w/w NaHSO3 |
| Description | A clear, colourless to yellow solution |
| Identification | |
A. Positive tests for sulphite and for sodium | |
B. pH of a 10 % aqueous solution between 2,5 and 5,5 | |
| Purity | |
| Iron | Not more than 50 mg/kg of Na2SO3 based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 223 SODIUM METABISULPHITEU.K.
| Synonyms | Pyrosulphite |
| Sodium pyrosulphite |
| Definition | |
| Chemical name | Sodium disulphite |
| Disodium pentaoxodisulphate |
| Einecs | 231-673-0 |
| Chemical formula | Na2S2O5 |
| Molecular weight | 190,11 |
| Assay | Content not less than 95 % Na2S2O5 and not less than 64 % of SO2 |
| Description | White crystals or crystalline powder |
| Identification | |
A. Positive tests for sulphite and for sodium | |
B. pH of a 10 % aqueous solution between 4,0 and 5,5 | |
| Purity | |
| Thiosulphate | Not more than 0,1 % based on the SO2 content |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 224 POTASSIUM METABISULPHITEU.K.
| Synonyms | Potassium pyrosulphite |
| Definition | |
| Chemical name | Potassium disulphite |
| Potassium pentaoxo disulphate |
| Einecs | 240-795-3 |
| Chemical formula | K2S2O5 |
| Molecular weight | 222,33 |
| Assay | Content not less than 90 % of K2S2O5 and not less than 51,8 % of SO2, the remainder being composed almost entirely of potassium sulphate |
| Description | Colourless crystals or white crystalline powder |
| Identification | |
A. Positive tests for sulphite and for potassium | |
| Purity | |
| Thiosulphate | Not more than 0,1 % based on the SO2 content |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 226 CALCIUM SULPHITEU.K.
| Definition | |
| Chemical name | Calcium sulphite |
| Einecs | 218-235-4 |
| Chemical formula | CaSO3·2H2O |
| Molecular weight | 156,17 |
| Assay | Content not less than 95 % of CaSO3·2H2O and not less than 39 % of SO2 |
| Description | White crystals or white crystalline powder |
| Identification | |
A. Positive tests for sulphite and for calcium | |
| Purity | |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 227 CALCIUM BISULPHITEU.K.
| Definition | |
| Chemical name | Calcium bisulphite |
| Calcium hydrogen sulphite |
| Einecs | 237-423-7 |
| Chemical formula | Ca(HSO3)2 |
| Molecular weight | 202,22 |
| Assay | 6 to 8 % (w/v) of sulphur dioxide and 2,5 to 3,5 % (w/v) of calcium dioxide corresponding to 10 to 14 % (w/v) of calcium bisulphite [Ca(HSO3)2] |
| Description | Clear greenish-yellow aqueous solution having a distinct odour of sulphur dioxide |
| Identification | |
A. Positive tests for sulphite and for calcium | |
| Purity | |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 228 POTASSIUM BISULPHITEU.K.
| Definition | |
| Chemical name | Potassium bisulphite |
| Potassium hydrogen sulphite |
| Einecs | 231-870-1 |
| Chemical formula | KHSO3 in aqueous solution |
| Molecular weight | 120,17 |
| Assay | Content not less than 280 g KHSO3 per litre (or 150 g SO2 per litre) |
| Description | Clear colourless aqueous solution |
| Identification | |
A. Positive tests for sulphite and for potassium | |
| Purity | |
| Iron | Not more than 50 mg/kg based on the SO2 content |
| Selenium | Not more than 10 mg/kg based on the SO2 content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 230 BIPHENYLU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
E 231 ORTHOPHENYLPHENOLU.K.
| Synonyms | Orthoxenol |
| Definition | |
| Chemical name | (1,1'-Biphenyl)-2-ol |
| 2-Hydroxydiphenyl |
| o-Hydroxydiphenyl |
| Einecs | 201-993-5 |
| Chemical formula | C12H10O |
| Molecular weight | 170,2 |
| Assay | Content not less than 99 % |
| Description | White or slightly yellowish crystalline powder |
| Identification | |
A. Melting range | 56 oC to 58 oC |
B. Positive test for phenolate | An ethanolic solution (1 g in 10 ml) produces a green colour on addition of 10 % ferric chloride solution |
| Purity | |
| Sulphated ash | Not more than 0,05 % |
| Diphenyl ether | Not more than 0,3 % |
| p-Phenylphenol | Not more than 0,1 % |
| 1-Naphthol | Not more than 0,01 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 232 SODIUM ORTHOPHENYLPHENOLU.K.
| Synonyms | Sodium orthophenylphenate |
| Sodium salt ofo-phenylphenol |
| Definition | |
| Chemical name | Sodium orthophenylphenol |
| Einecs | 205-055-6 |
| Chemical formula | C12H9ONa· 4H2O |
| Molecular weight | 264,26 |
| Assay | Content not less than 97 % of C12H9ONa· 4H2O |
| Description | White or slightly yellowish crystalline powder |
| Identification | |
A. Positive tests for phenolate and for sodium | |
B. Melting range of orthophenylphenol isolated by acidification and not recrystallised derived from the sample 56 oC to 58 oC after drying in a sulphuric acid desiccator | |
C. pH of a 2 % aqueous solution must be between 11,1 and 11,8 | |
| Purity | |
| Diphenylether | Not more than 0,3 % |
| p-phenylphenol | Not more than 0,1 % |
| 1-naphthol | Not more than 0,01 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 233 THIABENDAZOLEU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
[E 234 NISIN U.K.
| Definition | Nisin consists of several closely related polypeptides produced during the fermentation of a milk or sugar medium by certain natural strains of Lactococcus lactis subsp.lactis |
| Einecs | 215-807-5 |
| Chemical formula | C 143 H 230 N 42 O 37 S 7 |
| Molecular weight | 3 354,12 |
| Assay | Nisin concentrate contains not less than 900 units per mg in a mixture of non-fat milk proteins or fermented solids and a minimum sodium chloride content of 50 % |
| Description | White powder |
| Purity |
|---|
| Loss on drying | Not more than 3 % when dried to constant weight at 102 °C to 103 °C |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg] |
E 235 NATAMYCINU.K.
| Synonyms | Pimaricin |
| Definition | Natamycin is a fungicide of the polyene macrolide group, and is produced by natural strains of Streptomyces natalensis or of Streptococcus lactis |
| Einecs | 231-683-5 |
| Chemical formula | C33H47O13N |
| Molecular weight | 665,74 |
| Assay | Content not less than 95 % on the anhydrous basis |
| Description | White to creamy-white crystalline powder |
| Identification | |
A. Colour reactions | On adding a few crystals of natamycin on a spot plate, to a drop of:
concentrated hydrochloric acid, a blue colour develops,
concentrated phosphoric acid, a green colour develops,
which changes into pale red after a few minutes
|
B. Spectrometry | A 0,0005 % w/v solution in 1 % methanolic acetic acid solution has absorption maxima at about 290 nm, 303 nm and 318 nm, a shoulder at about 280 nm and exhibits minima at about 250 nm, 295,5 nm and 311 nm |
C. pH | 5,5 to 7,5 (1 % w/v solution in previously neutralised mixture of 20 parts dimethylformamide and 80 parts of water) |
D. Specific rotation | [α]D 20 = + 250o to + 295o (a 1 % w/v solution in glacial acetic acid, at 20 oC and calculated with reference to the dried material) |
| Purity | |
| Loss on drying | Not more than 8 % (over P2O5, in vacuum at 60 oC to constant weight) |
| Sulphated ash | Not more than 0,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Microbiological criteria: total viable count | Not more than 100/g |
E 239 HEXAMETHYLENE TETRAMINEU.K.
| Synonyms | Hexamine |
| Methenamine |
| Definition | |
| Chemical name | 1,3,5,7-Tetraazatricyclo [3.3.1.13,7]-decane, hexamethylenetetramine |
| Einecs | 202-905-8 |
| Chemical formula | C6H12N4 |
| Molecular weight | 140,19 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Colourless or white crystalline powder |
| Identification | |
A. Positive tests for formaldehyde and for ammonia | |
B. Sublimation point approximately 260 oC | |
| Purity | |
| Loss on drying | Not more than 0,5 % after drying at 105 oC in vacuum over P2O5 for two hours |
| Sulphated ash | Not more than 0,05 % |
| Sulphates | Not more than 0,005 % expressed as SO4 |
| Chlorides | Not more than 0,005 % expressed as Cl |
| Ammonium salts | Not detectable |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 242 DIMETHYL DICARBONATEU.K.
| Synonyms | DMDC |
| Dimethyl pyrocarbonate |
| Definition | |
| Chemical name | Dimethyl dicarbonate |
| Pyrocarbonic acid dimethyl ester |
| Einecs | 224-859-8 |
| Chemical formula | C4H6O5 |
| Molecular weight | 134,09 |
| Assay | Content not less than 99,8 % |
| Description | Colourless liquid, decomposes in aqueous solution. It is corrosive to skin and eyes and toxic by inhalation and ingestion |
| Identification | |
A. Decomposition | After dilution positive tests for CO2 and methanol |
B. Melting point | 17 oC |
| Boiling point | 172 oC with decomposition |
C. Density 20 oC | Approximately 1,25 g/cm3 |
D. Infrared spectrum | Maxima at 1 156 and 1 832 cm- 1 |
| Purity | |
| Dimethyl carbonate | Not more than 0,2 % |
| Chlorine, total | Not more than 3 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 249 POTASSIUM NITRITEU.K.
| |
| Definition | |
| Chemical name | Potassium nitrite |
| Einecs | 231-832-4 |
| Chemical formula | KNO2 |
| Molecular weight | 85,11 |
| Assay | Content not less than 95 % on the anhydrous basis |
| Description | White or slightly yellow, deliquescent granules |
| Identification | |
A. Positive tests for nitrite and for potassium | |
B. pH of a 5 % solution: | Not less than 6,0 and not more than 9,0 |
| Purity | |
| Loss on drying | Not more than 3 % after drying for four hours over silica gel |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 250 SODIUM NITRITEU.K.
| |
| Definition | |
| Chemical name | Sodium nitrite |
| Einecs | 231-555-9 |
| Chemical formula | NaNO2 |
| Molecular weight | 69,0 |
| Assay | Content not less than 97 % on the anhydrous basis |
| Description | White crystalline powder or yellowish lumps |
| Identification | |
A. Positive tests for nitrite and for sodium | |
| Purity | |
| Loss on drying | Not more than 0,25 % after drying over silica gel for four hours |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 251 SODIUM NITRATEU.K.
1.SOLID SODIUM NITRATEU.K.
| Synonyms | Chile saltpetre |
| Cubic or soda nitre |
| Definition | |
| Chemical name | Sodium nitrate |
| Einecs | 231-554-3 |
| Chemical formula | NaNO3 |
| Molecular weight | 85,0 |
| Assay | Content not less than 99 % after drying |
| Description | White crystalline, slightly hygroscopic powder |
| Identification | |
A. Positive tests for nitrate and for sodium | |
B. pH of a 5 % solution | Not less than 5,5 and more than 8,3 |
| Purity | |
| Loss on drying | Not more than 2 % after drying at 105 oC for four hours |
| Nitrites | Not more than 30 mg/kg expressed as NaNO2 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 251 SODIUM NITRATEU.K.
2.LIQUID SODIUM NITRATEU.K.
| Definition | Liquid sodium nitrate is an aqueous solution of sodium nitrate as the direct result of the chemical reaction between sodium hydroxide and nitric acid in stoechiometric amounts, without subsequent crystallisation. Standardised forms prepared from liquid sodium nitrate meeting these specifications may contain nitric acid in excessive amounts, if clearly stated or labelled. |
| Chemical name | Sodium nitrate |
| Einecs | 231-554-3 |
| Chemical formula | NaNO3 |
| Molecular weight | 85,0 |
| Assay | Content between 33,5 % and 40,0 % of NaNO3 |
| Description | Clear colourless liquid |
| Identification | |
A. Positive tests for nitrate and for sodium | |
B. pH | Not less than 1,5 and not more than 3,5 |
| Purity | |
| Free nitric acid | Not more than 0,01 % |
| Nitrites | Not more than 10 mg/kg expressed as NaNO2 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 0,3 mg/kg |
| This specification refers to a 35 % aqueous solution | |
E 252 POTASSIUM NITRATEU.K.
| Synonyms | Chile saltpetre
Cubic or soda nitre
|
| Definition | |
| Chemical name | Potassium nitrate |
| Einecs | 231-818-8 |
| Chemical formula | KNO3 |
| Molecular weight | 101,11 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | White crystalline powder or transparent prisms having a cooling, saline, pungent taste |
| Identification | |
A. Positive tests for nitrate and for potassium | |
B. pH of a 5 % solution | Not less than 4,5 and not more than 8,5 |
| Purity | |
| Loss on drying | Not more than 1 % after drying at 105 oC for four hours |
| Nitrites | Not more than 20 mg/kg expressed as KNO2 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 260 ACETIC ACIDU.K.
| Definition | |
| Chemical name | Acetic acid |
| Ethanoic acid |
| Einecs | 200-580-7 |
| Chemical formula | C2H4O2 |
| Molecular weight | 60,05 |
| Assay | Content not less than 99,8 % |
| Description | Clear, colourless liquid having a pungent, characteristic odour |
| Identification | |
A. Boiling point | 118 oC at 760 mm pressure (of mercury) |
B. Specific gravity | About 1,049 |
C. A one in three solution gives positive tests for acetate | |
D. Solidification point | Not lower than 14,5oC |
| Purity | |
| Non-volatile residue | Not more than 100 mg/kg |
| Formic acid, formates and other oxidisable substances | Not more than 1 000 mg/kg expressed as formic acid |
| Readily oxidisable substances | Dilute 2 ml of the sample in a glass-stoppered container with 10 ml of water and add 0,1 ml of 0,1 N potassium permanganate. The pink colour does not change to brown within 30 minutes |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 261 POTASSIUM ACETATEU.K.
| Definition | |
| Chemical name | Potassium acetate |
| Einecs | 204-822-2 |
| Chemical formula | C2H3O2K |
| Molecular weight | 98,14 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Colourless, deliquescent crystals or a white crystalline powder, odourless or with a faint acetic odour |
| Identification | |
A. pH of a 5 % aqueous solution | Not less than 7,5 and not more than 9,0 |
B. Positive tests for acetate and for potassium | |
| Purity | |
| Loss on drying | Not more than 8 % after drying at 150 oC for two hours |
| Formic acid, formates and other oxidisable substances | Not more than 1 000 mg/kg expressed as formic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 262 (i) SODIUM ACETATEU.K.
| Definition | |
| Chemical name | Sodium acetate |
| Einecs | 204-823-8 |
| Chemical formula | C2H3NaO2·nH2O (n = 0 or 3) |
| Molecular weight | Anhydrous: | 82,03 |
| Trihydrate: | 136,08 |
| Assay | Content (for both of anhydrous and trihydrate form) not less than 98,5 % on the anhydrous basis |
| Description | Anhydrous: | White, odourless, granular, hygroscopic powder |
| Trihydrate: | Colourless, transparent crystals or a granular crystalline powder, odourless or with a faint, acetic odour. Effloresces in warm, dry air |
| Identification | |
A. pH of a 1 % aqueous solution | Not less than 8,0 and not more than 9,5 |
B. Positive tests for acetate and for sodium | |
| Purity | |
| Loss on drying | Anhydrous: | Not more than 2 % (120 oC, 4 hours) |
| Trihydrate: | Between 36 and 42 % (120 oC, 4 hours) |
| Formic acid, formates and other oxidisable substances | Not more than 1 000 mg/kg expressed as formic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 262 (ii) SODIUM DIACETATEU.K.
| Definition | Sodium diacetate is a molecular compound of sodium acetate and acetic acid |
| Chemical name | Sodium hydrogen diacetate |
| Einecs | 204-814-9 |
| Chemical formula | C4H7NaO4·nH2O (n = 0 or 3) |
| Molecular weight | 142,09 (anhydrous) |
| Assay | Content 39 to 41 % of free acetic acid and 58 to 60 % of sodium acetate |
| Description | White, hygroscopic crystalline solid with an acetic odour |
| Identification | |
A. pH of a 10 % aqueous solution | Not less than 4,5 and not more than 5,0 |
B. Positive tests for acetate and for sodium | |
| Purity | |
| Water content | Not more than 2 % (Karl Fischer method) |
| Formic acid, formates and other oxidisable substances | Not more than 1 000 mg/kg expressed as formic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 263 CALCIUM ACETATEU.K.
| Definition | |
| Chemical name | Calcium acetate |
| Einecs | 200-540-9 |
| Chemical formula | Anhydrous: | C4H6O4Ca |
| Monohydrate: | C4H6O4Ca· H2O |
| Molecular weight | Anhydrous: | 158,17 |
| Monohydrate: | 176,18 |
| Assay | Content not less than 98 % on the anhydrous basis |
| Description | Anhydrous calcium acetate is a white, hygroscopic, bulky, crystalline solid with a slightly bitter taste. A slight odour of acetic acid may be present. The monohydrate may be needles, granules or powder |
| Identification | |
A. pH of a 10 % aqueous solution | Not less than 6,0 and not more than 9,0 |
B. Positive tests for acetate and for calcium | |
| Purity | |
| Loss on drying | Not more than 11 % after drying (155 oC to constant weight, for the monohydrate) |
| Water insoluble matter | Not more than 0,3 % |
| Formic acid, formates and other oxidisable substances | Not more than 1 000 mg/kg expressed as formic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 270 LACTIC ACIDU.K.
| Definition | |
| Chemical name | Lactic acid |
| 2-Hydroxypropionic acid |
| 1-Hydroxyethane-1-carboxylic acid |
| Einecs | 200-018-0 |
| Chemical formula | C3H6O3 |
| Molecular weight | 90,08 |
| Assay | Content not less than 76 % and not more than 84 % |
| Description | Colourless or yellowish, nearly odourless, syrupy liquid with an acid taste, consisting of a mixture of lactic acid (C3H6O3) and lactic acid lactate (C6H10O5). It is obtained by the lactic fermentation of sugars or is prepared synthetically |
| Note:
Lactic acid is hygroscopic and when concentrated by boiling, it condenses to form lactic acid lactate, which on dilution and heating hydrolyzes to lactic acid
| |
| Identification | |
A. Positive test for lactate | |
| Purity | |
| Sulphated ash | Not more than 0,1 % |
| Chloride | Not more than 0,2 % |
| Sulphate | Not more than 0,25 % |
| Iron | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Note:
This specification refers to a 80 % aqueous solution; for weaker aqueous solutions, calculate values corresponding to their lactic acid content
| |
E 280 PROPIONIC ACIDU.K.
| Definition | |
| Chemical name | Propionic acid |
| Propanoic acid |
| Einecs | 201-176-3 |
| Chemical formula | C3H6O2 |
| Molecular weight | 74,08 |
| Assay | Content not less than 99,5 % |
| Description | Colourless or slightly yellowish, oily liquid with a slightly pungent odour |
| Indentification | |
A. Melting point | - 22 oC |
B. Distillation range | 138,5oC to 142,5oC |
| Purity | |
| Non-volatile residue | Not more than 0,01 % when dried at 140 oC to constant weight |
| Aldehydes | Not more than 0,1 % expressed as formaldehyde |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 281 SODIUM PROPIONATEU.K.
| Definition | |
| Chemical name | Sodium propionate |
| Sodium propanoate |
| Einecs | 205-290-4 |
| Chemical formula | C3H5O2Na |
| Molecular weight | 96,06 |
| Assay | Content not less than 99 % after drying for two hours at 105 oC |
| Description | White crystalline hygroscopic powder, or a fine white powder |
| Identification | |
A. Positive tests for propionate and for sodium | |
B. pH of a 10 % aqueous solution | Not less than 7,5 and not more than 10,5 |
| Purity | |
| Loss on drying | Not more than 4 % determined by drying for two hours at 105 oC |
| Water insolubles | Not more than 0,1 % |
| Iron | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 282 CALCIUM PROPIONATEU.K.
| Definition | |
| Chemical name | Calcium propionate |
| Einecs | 223-795-8 |
| Chemical formula | C6H10O4Ca |
| Molecular weight | 186,22 |
| Assay | Content not less than 99 %, after drying for two hours at 105 oC |
| Description | White crystalline powder |
| Identification | |
A. Positive tests for propionate and for calcium | |
B. pH of a 10 % aqueous solution | Between 6,0 and 9,0 |
| Purity | |
| Loss on drying | Not more than 4 %, determined by drying for two hours at 105 oC |
| Water insolubles | Not more than 0,3 % |
| Iron | Not more than 50 mg/kg |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 283 POTASSIUM PROPIONATEU.K.
| Definition | |
| Chemical name | Potassium propionate |
| Potassium propanoate |
| Einecs | 206-323-5 |
| Chemical formula | C3H5KO2 |
| Molecular weight | 112,17 |
| Assay | Content not less than 99 % after drying for two hours at 105 oC |
| Description | White crystalline powder |
| Identification | |
A. Positive tests for propionate and for potassium | |
| Purity | |
| Loss on drying | Not more than 4 %, determined by drying for two hours at 105 oC |
| Water-insoluble substances | Not more than 0,3 % |
| Iron | Not more than 30 mg/kg |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 284 BORIC ACIDU.K.
| Synonyms | Boracic acid |
| Orthoboric acid |
| Borofax |
| Definition | |
| Einecs | 233-139-2 |
| Chemical formula | H3BO3 |
| Molecular weight | 61,84 |
| Assay | Content not less than 99,5 % |
| Description | Colourless, odourless, transparent crystals or white granules or powder; slightly unctuous to the touch; occurs in nature as the mineral sassolite |
| Identification | |
A. Melting point | At approximately 171 oC |
B. Burns with a nice green flame | |
C. pH of a 3,3 % aqueous solution | Between 3,8 and 4,8 |
| Purity | |
| Peroxides | No colour develops with added KI-solution |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 285 SODIUM TETRABORATE (BORAX)U.K.
| Synonyms | Sodium borate |
| Definition | |
| Chemical name | Sodium tetraborate |
| Sodium biborate |
| Sodium pyroborate |
| Anhydrous tetraborate |
| Einecs | 215-540-4 |
| Chemical formula | Na2B4O7 |
| Na2B4O7·10H2O |
| Molecular weight | 201,27 |
| Description | Powder or glass-like plates becoming opaque on exposure to air; slowly soluble in water |
| Identification | |
A. Melting range | Between 171 oC and 175 oC with decomposition |
| Purity | |
| Peroxides | No colour develops with added KI-solution |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 290 CARBON DIOXIDEU.K.
| Synonyms | Carbonic acid gas |
| Dry ice (solid form) |
| Carbonic anhydride |
| Definition | |
| Chemical name | Carbon dioxide |
| Einecs | 204-696-9 |
| Chemical formula | CO2 |
| Molecular weight | 44,01 |
| Assay | Content not less than 99 % v/v on the gaseous basis |
| Description | A colourless gas under normal environmental conditions with a slight pungent odour. Commercial carbon dioxide is shipped and handled as a liquid in pressurised cylinders or bulk storage systems, or in compressed solid blocks of ‘dry ice’. Solid (dry ice) forms usually contain added substances, such as propylene glycol or mineral oil, as binders |
| Identification | |
A. Precipitation (Precipitate formation) | When a stream of the sample is passed through a solution of barium hydroxide, a white precipitate is produced which dissolves with effervescence in dilute acetic acid |
| Purity | |
| Acidity | 915 ml of gas bubbled through 50 ml of freshly boiled water must not render the latter more acid to methylorange than is 50 ml freshly boiled water to which has been added 1 ml of hydrochloric acid (0,01 N) |
| Reducing substances, hydrogen phosphide and sulphide | 915 ml of gas bubbled through 25 ml of ammoniacal silver nitrate reagent to which has been added 3 ml of ammonia must not cause clouding or blackening of this solution |
| Carbon monoxide | Not more than 10 μl/l |
| [Oil content | Not more than 5 mg/kg] |
E 296 MALIC ACIDU.K.
| Synonyms | DL-Malic acid, pomalous acid |
| Definition | |
| Chemical name | DL-Malic acid, hydroxybutanedioic acid, hydroxysuccinic acid |
| Einecs | 230-022-8 |
| Chemical formula | C4H6O5 |
| Molecular weight | 134,09 |
| Assay | Content not less than 99,0 % |
| Description | White or nearly white crystalline powder or granules |
| Identification | |
A. Melting range between 127 oC and 132 oC | |
B. Positive test for malate | |
C. Solutions of this substance are optically inactive in all concentrations | |
| Purity | |
| Sulphated ash | Not more than 0,1 % |
| Fumaric acid | Not more than 1,0 % |
| Maleic acid | Not more than 0,05 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 297 FUMARIC ACIDU.K.
| Definition | |
| Chemical name | Trans-butenedioic acid, trans-1,2-ethylene-dicarboxylic acid |
| Einecs | 203-743-0 |
| Chemical formula | C4H4O4 |
| Molecular weight | 116,07 |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | White crystalline powder or granules |
| Identification | |
A. Melting range | 286 oC-302 oC (closed capillary, rapid heating) |
B. Positive tests for double bonds and for 1,2-dicarboxylic acid | |
C. pH of a 0,05 % solution at 25 oC | 3,0-3,2 |
| Purity | |
| Loss on drying | Not more than 0,5 % (120 oC, 4h) |
| Sulphated ash | Not more than 0,1 % |
| Maleic acid | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 300 ASCORBIC ACIDU.K.
| Definition | |
| Chemical name | L-ascorbic acid |
| Ascorbic acid |
| 2,3-Didehydro-L-threo-hexono-1,4-lactone |
| 3-Keto-L-gulofuranolactone |
| Einecs | 200-066-2 |
| Chemical formula | C6H8O6 |
| Molecular weight | 176,13 |
| Assay | Ascorbic acid, after drying in a vacuum desiccator over sulphuric acid for 24 hours, contains not less than 99 % of C6H8O6 |
| Description | White to pale yellow, odourless crystalline solid |
| Identification | |
A. Melting range | Between 189 oC and 193 oC with decomposition |
B. Positive tests for ascorbic acid | |
| Purity | |
| Loss on drying | Not more than 0,4 % after drying in a vacuum desiccator over sulphuric acid for 24 hours |
| Sulphated ash | Not more than 0,1 % |
| Specific rotation | [α]D 20 between + 20,5o and + 21,5o (10 % w/v aqueous solution) |
| pH of a 2 % aqueous solution | Between 2,4 and 2,8 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 301 SODIUM ASCORBATEU.K.
| Definition | |
| Chemical name | Sodium ascorbate |
| Sodium L-ascorbate |
| 2,3-Didehydro-L-threo-hexono-1,4-lactone sodium enolate |
| 3-Keto-L-gulofurano-lactone sodium enolate |
| Einecs | 205-126-1 |
| Chemical formula | C6H7O6Na |
| Molecular weight | 198,11 |
| Assay | Sodium ascorbate, after drying in a vacuum desiccator over sulphuric acid for 24 hours, contains not less than 99 % of C6H7O6Na |
| Description | White or almost white, odourless crystalline solid which darkens on exposure to light |
| Identification | |
A. Positive tests for ascorbate and for sodium | |
| Purity | |
| Loss on drying | Not more than 0,25 % after drying in a vacuum desiccator over sulphuric acid for 24 hours |
| Specific rotation | [α]D 20 between + 103o and + 106o (10 % w/v aqueous solution) |
| pH of 10 % aqueous solution | Between 6,5 and 8,0 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 302 CALCIUM ASCORBATEU.K.
| Definition | |
| Chemical name | Calcium ascorbate dihydrate |
| Calcium salt of 2,3-didehydro-L-threo-hexono-1,4-lactone dihydrate |
| Einecs | 227-261-5 |
| Chemical formula | C12H14O12Ca· 2H2O |
| Molecular weight | 426,35 |
| Assay | Content not less than 98 % on a volatile matter-free basis |
| Description | White to slightly pale greyish-yellow odourless crystalline powder |
| Identification | |
A. Positive tests for ascorbate and for calcium | |
| Purity | |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Specific rotation | [α]D 20 between + 95o and + 97o (5 % w/v aqueous solution) |
| pH of 10 % aqueous solution | Between 6,0 and 7,5 |
| Volatile matter | Not more than 0,3 % determined by drying at room temperature for 24 hours in a desiccator containing sulphuric acid or phosphorus pentoxide |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 304 (i) ASCORBYL PALMITATEU.K.
| Definition | |
| Chemical name | Ascorbyl palmitate |
| L-ascorbyl palmitate |
| 2,3-didehydro-L-threo-hexono-1,4-lactone-6-palmitate |
| 6-palmitoyl-3-keto-L-gulofuranolactone |
| Einecs | 205-305-4 |
| Chemical formula | C22H38O7 |
| Molecular weight | 414,55 |
| Assay | Content not less than 98 % on the dried basis |
| Description | White or yellowish-white solid with a citrus-like odour |
| Identification | |
A. Melting range | Between 107 oC and 117 oC |
| Purity | |
| Loss on drying | Not more than 2,0 % after drying in a vacuum oven at 56 oC and 60 oC for one hour |
| Sulphated ash | Not more than 0,1 % |
| Specific rotation | [α]D 20 between + 21o and + 24o (5 % w/v in methanol solution) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 304 (ii) ASCORBYL STEARATEU.K.
| Definition | |
| Chemical name | Ascorbyl stearate |
| L-ascorbyl stearate |
| 2,3-didehydro-L-threo-hexono-1,4-lactone-6-stearate |
| 6-stearoyl-3-keto-L-gulofuranolactone |
| Einecs | 246-944-9 |
| Chemical formula | C24H42O7 |
| Molecular weight | 442,6 |
| Assay | Content not less than 98 % |
| Description | White or yellowish, white solid with a citrus-like odour |
| Identification | |
A. Melting point | About 116 oC |
| Purity | |
| Loss on drying | Not more than 2,0 % after drying in a vacuum oven at 56 oC to 60 oC for one hour |
| Sulphated ash | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 306 TOCOPHEROL-RICH EXTRACTU.K.
| Definition | Product obtained by the vacuum steam distillation of edible vegetable oil products, comprising concentrated tocopherols and tocotrienols |
| Contains tocopherols such as d-α-, d-β-, d-γ- and d-ς-tocopherols |
| Molecular weight | 430,71 (d-α-tocopherol) |
| Assay | Content not less than 34 % of total tocopherols |
| Description | Brownish red to red, clear, viscous oil having a mild, characteristic odour and taste. May show a slight separation of wax-like constituents in microcrystalline form |
| Identification | |
A. By suitable gas liquid chromatographic method | |
B. Solubility tests | Insoluble in water. Soluble in ethanol. Miscible in ether |
| Purity | |
| Sulphated ash | Not more than 0,1 % |
| Specific rotation | [α]D 20 not less than + 20o |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 307 ALPHA-TOCOPHEROLU.K.
| Synonyms | DL-α-Tocopherol |
| Definition | |
| Chemical name | DL-5,7,8-Trimethyltocol |
| DL-2,5,7,8-tetramethyl-2-(4′,8′,12′-trimethyltridecyl)-6-chromanol |
| Einecs | 233-466-0 |
| Chemical formula | C29H50O2 |
| Molecular weight | 430,71 |
| Assay | Content not less than 96 % |
| Description | Slightly yellow to amber, nearly odourless, clear, viscous oil which oxidises and darkens on exposure to air or light |
| Identification | |
A. Solubility tests | Insoluble in water, freely soluble in ethanol, miscible in ether |
B. Spectro-photometry | In absolute ethanol the maximum absorption is about 292 nm |
| Purity | |
| Refractive index | n D 201,503-1,507 |
Specific absorption  in ethanol in ethanol | 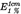 (292 nm) 72-76 (292 nm) 72-76
(0,01 g in 200 ml of absolute ethanol)
|
| Sulphated ash | Not more than 0,1 % |
| Specific rotation | [α]D 25 0o ± 0,05o (1 in 10 solution in chloroform) |
| Lead | Not more than 2 mg/kg |
E 308 GAMMA-TOCOPHEROLU.K.
| Synonyms | dl-γ-Tocopherol |
| Definition | |
| Chemical name | 2,7,8-trimethyl-2-(4′,8′,12′-trimethyltridecyl)-6-chromanol |
| Einecs | 231-523-4 |
| Chemical formula | C28H48O2 |
| Molecular weight | 416,69 |
| Assay | Content not less than 97 % |
| Description | Clear, viscous, pale yellow oil which oxidises and darkens on exposure to air or light |
| Identification | |
A. Spectrometry | Maximum absorptions in absolute ethanol at about 298 nm and 257 nm |
| Purity | |
Specific absorption  in ethanol in ethanol |  (298 nm) between 91 and 97 (298 nm) between 91 and 97 |
 (257 nm) between 5,0 and 8,0 (257 nm) between 5,0 and 8,0 |
| Refractive index |  1,503-1,507 1,503-1,507 |
| Sulphated ash | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 309 DELTA-TOCOPHEROLU.K.
| Definition | |
| Chemical name | 2,8-dimethyl-2-(4′,8′,12′-trimethyltridecyl)-6-chromanol |
| Einecs | 204-299-0 |
| Chemical formula | C27H46O2 |
| Molecular weight | 402,7 |
| Assay | Content not less than 97 % |
| Description | Clear, viscous, pale yellowish or orange oil which oxidises and darkens on exposure to air or light |
| Identification | |
A. Spectrometry | Maximum absorptions in absolute ethanol at about 298 nm and 257 nm |
| Purity | |
Specific absorption 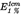 in ethanol in ethanol |  (298 nm) between 89 and 95 (298 nm) between 89 and 95 |
 (257 nm) between 3,0 and 6,0 (257 nm) between 3,0 and 6,0 |
| Refractive index |  1,5-1,504 1,5-1,504 |
| Sulphated ash | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 310 PROPYL GALLATEU.K.
| Definition | |
| Chemical name | Propyl gallate |
| Propyl ester of gallic acid |
| n-propyl ester of 3,4,5-trihydroxybenzoic acid |
| Einecs | 204-498-2 |
| Chemical formula | C10H12O5 |
| Molecular weight | 212,2 |
| Assay | Content not less than 98 % on the anhydrous basis |
| Description | White to creamy-white, crystalline, odourless solid |
| Identification | |
A. Solubility tests | Slightly soluble in water, freely soluble in ethanol, ether and propane-1,2-diol |
B. Melting range | Between 146 oC and 150 oC after drying at 110 oC for four hours |
| Purity | |
| Loss on drying | Not more than 1,0 % (110 oC, four hours) |
| Sulphated ash | Not more than 0,1 % |
| Free acid | Not more than 0,5 % (as gallic acid) |
| Chlorinated organic compound | Not more than 100 mg/kg (as C1) |
Specific absorption  in ethanol in ethanol |  (275 nm) not less than 485 and not more than 520 (275 nm) not less than 485 and not more than 520 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 311 OCTYL GALLATEU.K.
| Definition | |
| Chemical name | Octyl gallate |
| Octyl ester of gallic acid |
| n-octyl ester of 3,4,5-trihydroxybenzoic acid |
| Einecs | 213-853-0 |
| Chemical formula | C15H22O5 |
| Molecular weight | 282,34 |
| Assay | Content not less than 98 % after drying at 90 oC for six hours |
| Description | White to creamy-white odourless solid |
| Identification | |
A. Solubility tests | Insoluble in water, freely soluble in ethanol, ether and propane-1,2-diol |
B. Melting range | Between 99 oC and 102 oC after drying at 90 oC for six hours |
| Purity | |
| Loss on drying | Not more than 0,5 % (90 oC, six hours) |
| Sulphated ash | Not more than 0,05 % |
| Free acid | Not more than 0,5 % (as gallic acid) |
| Chlorinated organic compound | Not more than 100 mg/kg (as C1) |
Specific absorption  in ethanol in ethanol |  (275 nm) not less than 375 and not more than 390 (275 nm) not less than 375 and not more than 390 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 312 DODECYL GALLATEU.K.
| Synonyms | Lauryl gallate |
| Definition | |
| Chemical name | Dodecyl gallate |
| n-dodecyl (or lauryl) ester of 3,4,5-trihydroxybenzoic acid |
| Dodecyl ester of gallic acid |
| Einecs | 214-620-6 |
| Chemical formula | C19H30O5 |
| Molecular weight | 338,45 |
| Assay | Content not less than 98 % after drying at 90 oC for six hours |
| Description | White or creamy-white odourless solid |
| Identification | |
A. Solubility tests | Insoluble in water, freely soluble in ethanol and ether |
B. Melting range | Between 95 oC and 98 oC after drying at 90 oC for six hours |
| Purity | |
| Loss on drying | Not more than 0,5 % (90 oC, six hours) |
| Sulphated ash | Not more than 0,05 % |
| Free acid | Not more than 0,5 % (as gallic acid) |
| Chlorinated organic compound | Not more than 100 mg/kg (as Cl) |
Specific absorption  in ethanol in ethanol |  (275 nm) not less than 300 and not more than 325 (275 nm) not less than 300 and not more than 325 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 30 mg/kg |
E 315 ERYTHORBIC ACIDU.K.
| Synonyms | Isoascorbic acid |
| D-Araboascorbic acid |
| Definition | |
| Chemical name | D-Erythro-hex-2-enoic acid γ-lactone |
| Isoascorbic acid |
| D-Isoascorbic acid |
| Einecs | 201-928-0 |
| Chemical formula | C6H8O6 |
| Molecular weight | 176,13 |
| Assay | Content not less than 98 % on the anhydrous basis |
| Description | White to slightly yellow crystalline solid which darkens gradually on exposure to light |
| Identification | |
A. Melting range | About 164 oC to 172 oC with decomposition |
B. Positive test for ascorbic acid/colour reaction | |
| Purity | |
| Loss on drying | Not more than 0,4 % after drying under reduced pressure on silica gel for 3 hours |
| Sulphated ash | Not more than 0,3 % |
| Specific rotation | [α]10 % (w/v) aqueous solution between - 16,5o to - 18,0o |
| Oxalate | To a solution of 1 g in 10 ml of water add 2 drops of glacial acetic acid and 5 ml of 10 % calcium acetate solution. The solution should remain clear |
| Lead | Not more than 2 mg/kg |
E 316 SODIUM ERYTHORBATEU.K.
| Synonyms | Sodium isoascorbate |
| Definition | |
| Chemical name | Sodium isoascorbate |
| Sodium D-isoascorbic acid |
| Sodium salt of 2,3-didehydro-D-erythro-hexono-1,4-lactone |
| 3-keto-D-gulofurano-lactone sodium enolate monohydrate |
| Einecs | 228-973-9 |
| Chemical formula | C6H7O6Na· H2O |
| Molecular weight | 216,13 |
| Assay | Content not less than 98 % after drying in a vacuum desiccator over sulphuric acid for 24 hours expressed on the monohydrate basis |
| Description | White crystalline solid |
| Identification | |
A. Solubility tests | Freely soluble in water, very slightly soluble in ethanol |
B. Positive test for ascorbic acid/colour reaction | |
C. Positive test for sodium | |
| Purity | |
| Loss on drying | Not more than 0,25 % after drying in a vacuum desiccator over sulphuric acid for 24 hours |
| Specific rotation | [α]10 % (w/v) aqueous solution between + 95o and + 98o |
| pH of a 10 % aqueous solution | 5,5 to 8,0 |
| Oxalate | To a solution of 1 g in 10 ml of water add 2 drops of glacial acetic acid and 5 ml of 10 % calcium acetate solution. The solution should remain clear |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 319 TERTIARY-BUTYLHYDROQUINONE (TBHQ)U.K.
| Synonyms | TBHQ |
| Definition | |
| Chemical names | Tert-butyl-1,4-benzenediol |
| 2-(1,1-Dimethylethyl)-1,4-benzenediol |
| Einecs | 217-752-2 |
| Chemical formula | C10H14O2 |
| Molecular weight | 166,22 |
| Assay | Content not less than 99 % of C10H14O2 |
| Description | White crystalline solid having a characteristic odour |
| Identification | |
A. Solubility | Practically insoluble in water; soluble in ethanol |
B. Melting point | Not less than 126,5oC |
C. Phenolics | Dissolve about 5 mg of the sample in 10 ml of methanol and add 10,5 ml of dimethylamine solution (1 in 4). A red to pink colour is produced |
| Purity | |
| Tertiary-Butyl-p-benzoquinone | Not more than 0,2 % |
| 2,5-Di-tertiary-butyl hydroquinone | Not more than 0,2 % |
| Hydroxyquinone | Not more than 0,1 % |
| Toluene | Not more than 25 mg/kg |
| Lead | Not more than 2 mg/kg |
E 320 BUTYLATED HYDROXYANISOLE (BHA)U.K.
| Synonyms | BHA |
| Definition | |
| Chemical names | 3-Tertiary-butyl-4-hydroxyanisole |
| A mixture of 2-tertiary-butyl-4-hydroxyanisole and 3-tertiary-butyl-4-hydroxyanisole |
| Einecs | 246-563-8 |
| Chemical formula | C11H16O2 |
| Formula weight | 180,25 |
| Assay | Content not less than 98,5 % of C11H16O2 and not less than 85 % of 3-tertiary-butyl-4-hydroxyanisole isomer |
| Description | White or slightly yellow crystals or waxy solid with a slight aromatic smell |
| Identification | |
A. Solubility | Insoluble in water, freely soluble in ethanol |
B. Melting range | Between 48 °C and 63 °C |
C. Colour reaction | Passes test for phenol groups |
| Purity | |
| Sulphated ash | Not more than 0,05 % after calcination at 800 ± 25 °C |
| Phenolic impurities | Not more than 0,5 % |
Specific absorption  | 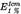 (290 nm) not less than 190 and not more than 210 (290 nm) not less than 190 and not more than 210 |
Specific absorption  |  (228 nm) not less than 326 and not more than 345 (228 nm) not less than 326 and not more than 345 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 321 BUTYLATED HYDROXYTOLUENE (BHT)U.K.
| Synonyms | BHT |
| Definition | |
| Chemical name | 2,6-Ditertiary-butyl-p-cresol |
| 4-Methyl-2,6-ditertiarybutylphenol |
| Einecs | 204-881-4 |
| Chemical formula | C15H24O |
| Molecular weight | 220,36 |
| Assay | Content not less than 99 % |
| Description | White, crystalline or flaked solid, odourless or having a characteristic faint aromatic odour |
| Identification | |
A. Solubility tests | Insoluble in water and propane- 1,2-diol |
| Freely soluble in ethanol |
B. Melting point | At 70 oC |
C. Absorbance maximum | The absorption in the range 230 to 320 nm of a 2 cm layer of a 1 in 100 000 solution in dehydrated ethanol exhibits a maximum only at 278 nm |
| Purity | |
| Sulphated ash | Not more than 0,005 % |
| Phenolic impurities | Not more than 0,5 % |
Specific absorption  in ethanol in ethanol |  (278 nm) not less than 81 and not more than 88 (278 nm) not less than 81 and not more than 88 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 322 LECITHINSU.K.
| Synonyms | Phosphatides |
| Phospholipids |
| Definition | Lecithins are mixtures or fractions of phosphatides obtained by physical procedures from animal or vegetable foodstuffs; they also include hydrolysed products obtained through the use of harmless and appropriate enzymes. The final product must not show any signs of residual enzyme activity The lecithins may be slightly bleached in aqueous medium by means of hydrogen peroxide. This oxidation must not chemically modify the lecithin phosphatides |
| Einecs | 232-307-2 |
| Assay | |
| |
| Description | |
| |
| Identification | |
A. Positive tests for choline, for phosphorus and fatty acids | |
B. Test for hydrolysed lecithin | To a 800 ml beaker add 500 ml of water (30 oC-35 oC). Then slowly add 50 ml of the sample with constant stirring. Hydrolysed lecithin will form a homogeneous emulsion. Non-hydrolysed lecithin will form a distinct mass of about 50 g |
| Purity | |
| Loss on drying | Not more than 2,0 % determined by drying at 105 oC for one hour |
| Toluene-insoluble matter | Not more than 0,3 % |
| Acid value | |
| |
| Peroxide value | Equal to or less than 10 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 325 SODIUM LACTATEU.K.
| Definition | |
| Chemical name | Sodium lactate |
| Sodium 2-hydroxypropanoate |
| Einecs | 200-772-0 |
| Chemical formula | C3H5NaO3 |
| Molecular weight | 112,06 (anhydrous) |
| Assay | Content not less than 57 % and not more than 66 % |
| Description | Colourless, transparent, liquid. Odourless, or with a slight, characteristic odour |
| Identification | |
A. Positive test for lactate | |
B. Positive test for sodium | |
| Purity | |
| Acidity | Not more than 0,5 % after drying expressed as lactic acid |
| pH of a 20 % aqueous solution | 6,5 to 7,5 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Reducing substances | No reduction of Fehling's solution |
| Note:
This specification refers to a 60 % aqueous solution
| |
E 326 POTASSIUM LACTATEU.K.
| Definition | |
| Cheminal name | Potassium lactate |
| Potassium 2-hydroxypropanoate |
| Einecs | 213-631-3 |
| Chemical formula | C3H5O3K |
| Molecular weight | 128,17 (anhydrous) |
| Assay | Content not less than 57 % and not more than 66 % |
| Description | Slightly viscous, almost odourless clear liquid. Odourless, or with a slight, characteristic odour |
| Identification | |
A. Ignition | Ignite potassium lactate solution to an ash. The ash is alkaline, and an effervescence occurs when acid is added |
B. Colour reaction | Overlay 2 ml of potassium lactate solution on 5 ml of a 1 in 100 solution of catechol in sulphuric acid. A deep red colour is produced at the zone of contact |
C. Positive tests for potassium and for lactate | |
| Purity | |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Acidity | Dissolve 1 g of potassium lactate solution in 20 ml of water, add 3 drops of phenolphthalein TS and titrate with 0,1 N sodium hydroxide. Not more than 0,2 ml should be required |
| Reducing substances | Potassium lactate solution shall not cause any reduction of Fehling's solution |
| Note:
This specification refers to a 60 % aqueous solution
| |
E 327 CALCIUM LACTATEU.K.
| Definition | |
| Chemical name | Calcium dilactate |
| Calcium dilactate hydrate |
| 2-Hydroxypropanoic acid calcium salt |
| Einecs | 212-406-7 |
| Chemical formula | (C3H5O2)2 Ca· nH2O (n = 0-5) |
| Molecular weight | 218,22 (anhydrous) |
| Assay | Content not less than 98 % on the anhydrous basis |
| Description | Almost odourless, white crystalline powder or granules |
| Identification | |
A. Positive tests for lactate and for calcium | |
B. Solubility tests | Soluble in water and practically insoluble in ethanol |
| Purity | |
| Loss on drying | Determined by drying at 120 oC for four hours: |
| |
| |
| |
| |
| Acidity | Not more than 0,5 % of the dry matter expressed as lactic acid |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| pH of a 5 % solution | Between 6,0 and 8,0 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Reducing substances | No reduction of Fehling's solution |
E 330 CITRIC ACIDU.K.
| Definition | |
| Chemical name | Citric acid |
| 2-Hydroxy-1,2,3-propanetricarboxylic acid |
| β-Hydroxytricarballytic acid |
| Einecs | 201-069-1 |
| Chemical formula | |
(b) C6H8O7·H2O (monohydrate)
|
| Molecular weight | |
| |
| Assay | Citric acid may be anhydrous or it may contain 1 molecule of water. Citric acid contains not less than 99,5 % of C6H8O7, calculated on the anhydrous basis |
| Description | Citric acid is a white or colourless, odourless, crystalline solid, having a strongly acid taste. The monohydrate effloresces in dry air |
| Identification | |
A. Solubility tests | Very soluble in water; freely soluble in ethanol; soluble in ether |
| Purity | |
| Water content | Anhydrous citric acid contains not more than 0,5 % water; citric acid monohydrate contains not more than 8,8 % water (Karl Fischer method) |
| Sulphated ash | Not more than 0,05 % after calcination at 800 ± 25 oC |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
| Oxalates | Not more than 100 mg/kg, expressed as oxalic acid, after drying |
| Readily carbonisable substances | Heat 1 g of powdered sample with 10 ml of 98 % minimum sulphuric acid in a water bath at 90 oC in the dark for one hour. Not more than a pale brown colour should be produced (Matching Fluid K) |
E 331 (i) MONOSODIUM CITRATEU.K.
| Synonyms | Monosodium citrate
Monobasic sodium citrate
|
| Definition | |
| Chemical name | Monosodium citrate |
| Monosodium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Chemical formula | |
(b) C6H7O7Na· H2O (monohydrate)
|
| Molecular weight | |
| |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Crystalline white powder or colourless crystals |
| Identification | |
A. Positive tests for citrate and for sodium | |
| Purity | |
| Loss on drying | Determined by drying at 180 oC for four hours:
|
| |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 1 % aqueous solution | Between 3,5 and 3,8 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 331 (ii) DISODIUM CITRATEU.K.
| Synonyms | Disodium citrate
Dibasic sodium citrate
|
| Definition | |
| Chemical name | Disodium citrate |
| Disodium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Disodium salt of citric acid with 1,5 molecules of water |
| Einecs | 205-623-3 |
| Chemical formula | C6H6O7Na2·1,5H2O |
| Molecular weight | 263,11 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Crystalline white powder or colourless crystals |
| Identification | |
A. Positive tests for citrate and for sodium | |
| Purity | |
| Loss on drying | Not more than 13,0 % by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 1 % aqueous solution | Between 4,9 and 5,2 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 331 (iii) TRISODIUM CITRATEU.K.
| Synonyms | Trisodium citrate
Tribasic sodium citrate
|
| Definition | |
| Chemical name | Trisodium citrate |
| Trisodium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Trisodium salt of citric acid, in anhydrous, dihydrate or pentahydrate form |
| Einecs | 200-675-3 |
| Chemical formula | Anhydrous: | C6H5O7Na3 |
| Hydrated: | C6H5O7Na3·nH2O (n = 2 or 5) |
| Molecular weight | 258,07 (anhydrous) |
| Assay | Not less than 99 % on the anhydrous basis |
| Description | Crystalline white powder or colourless crystals |
| Identification | |
A. Positive tests for citrate and for sodium | |
| Purity | |
| Loss on drying | Determined by drying at 180 oC for four hours: |
| | not more than 1,0 % |
| | not more than 13,5 % |
| | not more than 30,3 % |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 5 % aqueous solution | Between 7,5 and 9,0 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 332 (i) MONOPOTASSIUM CITRATEU.K.
| Synonyms | Monopotassium citrate
Monobasic potassium citrate
|
| Definition | |
| Chemical name | Monopotassium citrate |
| Monopotassium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Anhydrous monopotassium salt of citric acid |
| Einecs | 212-753-4 |
| Chemical formula | C6H7O7K |
| Molecular weight | 230,21 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | White, hygroscopic, granular powder or transparent crystals |
| Identification | |
A. Positive tests for citrate and for potassium | |
| Purity | |
| Loss on drying | Not more than 1,0 % determined by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 1 % aqueous solution | Between 3,5 and 3,8 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 332 (ii) TRIPOTASSIUM CITRATEU.K.
| Synonyms | Tripotassium citrate
Tribasic potassium citrate
|
| Definition | |
| Chemical name | Tripotassium citrate |
| Tripotassium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Monohydrated tripotassium salt of citric acid |
| Einecs | 212-755-5 |
| Chemical formula | C6H5O7K3·H2O |
| Molecular weight | 324,42 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | White, hygroscopic, granular powder or transparent crystals |
| Identification | |
A. Positive tests for citrate and for potassium | |
| Purity | |
| Loss on drying | Not more than 6,0 % determined by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 5 % aqueous solution | Between 7,5 and 9,0 |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 333 (i) MONOCALCIUM CITRATEU.K.
| Synonyms | Monocalcium citrate
Monobasic calcium citrate
|
| Definition | |
| Chemical name | Monocalcium citrate |
| Monocalcium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Monohydrate monocalcium salt of citric acid |
| Chemical formula | (C6H7O7)2Ca· H2O |
| Molecular weight | 440,32 |
| Assay | Content not less than 97,5 % on the anhydrous basis |
| Description | Fine white powder |
| Identification | |
A. Positive tests for citrate and for calcium | |
| Purity | |
| Loss on drying | Not more than 7,0 % determined by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 1 % aqueous solution | Between 3,2 and 3,5 |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
| Carbonates | Dissolving 1 g of calcium citrate in 10 ml 2 N hydrochloric acid must not liberate more than a few isolated bubbles |
E 333 (ii) DICALCIUM CITRATEU.K.
| Synonyms | Dicalcium citrate
Dibasic calcium citrate
|
| Definition | |
| Chemical name | Dicalcium citrate |
| Dicalcium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Trihydrated dicalcium salt of citric acid |
| Chemical formula | (C6H7O7)2Ca2·3H2O |
| Molecular weight | 530,42 |
| Assay | Not less than 97,5 % on the anhydrous basis |
| Description | Fine white powder |
| Identification | |
A. Positive tests for citrate and for calcium | |
| Purity | |
| Loss on drying | Not more than 20,0 % determined by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
| Carbonates | Dissolving 1 g of calcium citrate in 10 ml 2 N hydrochloric acid must not liberate more than a few isolated bubbles |
E 333 (iii) TRICALCIUM CITRATEU.K.
| Synonyms | Tricalcium citrate
Tribasic calcium citrate
|
| Definition | |
| Chemical name | Tricalcium citrate |
| Tricalcium salt of 2-hydroxy-1,2,3-propanetricarboxylic acid |
| Tetrahydrated tricalcium salt of citric acid |
| Einecs | 212-391-7 |
| Chemical formula | (C6H6O7)2Ca3·4H2O |
| Molecular weight | 570,51 |
| Assay | Not less than 97,5 % on the anhydrous basis |
| Description | Fine white powder |
| Identification | |
A. Positive tests for citrate and for calcium | |
| Purity | |
| Loss on drying | Not more than 14,0 % determined by drying at 180 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
| Carbonates | Dissolving 1 g of calcium citrate in 10 ml 2 N hydrochloric acid must not liberate more than a few isolated bubbles |
E 334 L(+)-TARTARIC ACIDU.K.
| Definition | |
| Chemical name | L-tartaric acid |
| L-2,3-dihydroxybutanedioic acid |
| d-α, β-dihydroxysuccinic acid |
| Einecs | 201-766-0 |
| Chemical formula | C4H6O6 |
| Molecular weight | 150,09 |
| Assay | Content not less than 99,5 % on the anhydrous basis |
| Description | Colourless or translucent crystalline solid or white crystalline powder |
| Identification | |
A. Melting range | Between 168 oC and 170 oC |
B. Positive test for tartrate | |
| Purity | |
| Loss on drying | Not more than 0,5 % (over P2O5, three hours) |
| Sulphated ash | Not more than 1 000 mg/kg after calcination at 800 ± 25 oC |
| Specific optical rotation of a 20 % w/v aqueous solution | [α] 20 D between + 11,5o and + 13,5o |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
E 335 (i) MONOSODIUM TARTRATEU.K.
| Synonyms | Monosodium salt of L-(+)-tartaric acid |
| Definition | |
| Chemical name | Monosodium salt of L-2,3-dihydroxybutanedioic acid |
| Monohydrated monosodium salt of L-(+)-tartaric acid |
| Chemical formula | C4H5O6Na· H2O |
| Molecular weight | 194,05 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Transparent colourless crystals |
| Identification | |
A. Positive tests for tartrate and for sodium | |
| Purity | |
| Loss on drying | Not more than 10,0 % determined by drying at 105 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 335 (ii) DISODIUM TARTRATEU.K.
| Definition | |
| Chemical name | Disodium L-tartrate |
| Disodium (+)-tartrate |
| Disodium (+)-2,3-dihydroxybutanedioic acid |
| Dihydrated disodium salt of L-(+)-tartaric acid |
| Einecs | 212-773-3 |
| Chemical formula | C4H4O6Na2·2H2O |
| Molecular weight | 230,8 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Transparent, colourless crystals |
| Identification | |
A. Positive tests for tartrate and for sodium | |
B. Solubility tests | 1 gram is insoluble in 3 ml of water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 17,0 % determined by drying at 150 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of a 1 % aqueous solution | Between 7,0 and 7,5 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 336 (i) MONOPOTASSIUM TARTRATEU.K.
| Synonyms | Monobasic potassium tartrate |
| Definition | |
| Chemical name | Anhydrous monopotassium salt of L-(+)-tartaric acid |
| Monopotassium salt of L-2,3-dihydroxybutanedioic acid |
| Chemical formula | C4H5O6K |
| Molecular weight | 188,16 |
| Assay | Content not less than 98 % on the anhydrous basis |
| Description | White crystalline or granulated powder |
| Identification | |
A. Positive tests for tartrate and for potassium | |
B. Melting point | 230 oC |
| Purity | |
| pH of a 1 % aqueous solution | 3,4 |
| Loss on drying | Not more than 1,0 % determined by drying at 105 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 336 (ii) DIPOTASSIUM TARTRATEU.K.
| Synonyms | Dibasic potassium tartrate |
| Definition | |
| Chemical name | Dipotassium salt of L-2,3-dihydroxybutanedioic acid |
| Dipotassium salt with half a molecule of water of L-(+)-tartaric acid |
| Einecs | 213-067-8 |
| Chemical formula | C4H4O6K2·1/2H2O |
| Molecular weight | 235,2 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | White crystalline or granulated powder |
| Identification | |
A. Positive tests for tartrate and for potassium | |
| Purity | |
| pH of a 1 % aqueous solution | Between 7,0 and 9,0 |
| Loss on drying | Not more than 4,0 % determined by drying at 150 oC for four hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 337 POTASSIUM SODIUM TARTRATEU.K.
| Synonyms | Potassium sodium L-(+)-tartrate |
| Rochelle salt |
| Seignette salt |
| Definition | |
| Chemical name | Potassium sodium salt of L-2,3-dihydroxybutanedioic acid |
| Potassium sodium L-(+)-tartrate |
| Einecs | 206-156-8 |
| Chemical formula | C4H4O6KNa· 4H2O |
| Molecular weight | 282,23 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Colourless crystals or white crystalline powder |
| Identification | |
A. Positive tests for tartrate, for potassium and for sodium | |
B. Solubility tests | 1 gram is soluble in 1 ml of water, insoluble in ethanol |
C. Melting range | Between 70 and 80 oC |
| Purity | |
| Loss on drying | Not more than 26,0 % and not less than 21,0 % determined by drying at 150 oC for three hours |
| Oxalates | Not more than 100 mg/kg expressed as oxalic acid, after drying |
| pH of 1 % aqueous solution | Between 6,5 and 8,5 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 338 PHOSPHORIC ACIDU.K.
| Synonyms | Orthophosphoric acid |
| Monophosphoric acid |
| Definition | |
| Chemical name | Phosphoric acid |
| Einecs | 231-633-2 |
| Chemical formula | H3PO4 |
| Molecular weight | 98,0 |
| Assay | Phosphoric acid is commercially available as an aqueous solution at variable concentrations. Content not less than 67,0 % and not more than 85,7 %. |
| Description | Clear, colourless, viscous liquid |
| Identification | |
A. Positive tests for acid and for phosphate | |
| Purity | |
| Volatile acids | Not more than 10 mg/kg (as acetic acid) |
| Chlorides | Not more than 200 mg/kg (expressed as chlorine) |
| Nitrates | Not more than 5 mg/kg (as NaNO3) |
| Sulphates | Not more than 1 500 mg/kg (as CaSO4) |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Note:
This specification refers to a 75 % aqueous solution
| |
E 339 (i) MONOSODIUM PHOSPHATEU.K.
| Synonyms | Monosodium monophosphate |
| Acid monosodium monophosphate |
| Monosodium orthophosphate |
| Monobasic sodium phosphate |
| Sodium dihydrogen monophosphate |
| Definition | |
| Chemical name | Sodium dihydrogen monophosphate |
| Einecs | 231-449-2 |
| Chemical formula | Anhydrous: NaH2PO4 |
| Monohydrate: NaH2PO4 · H2O |
| Dihydrate: NaH2PO4 · 2H2O |
| Molecular weight | Anhydrous: 119,98 |
| Monohydrate: 138,0 |
| Dihydrate: 156,01 |
| Assay | After drying at 60 oC for one hour and then at 105 oC for four hours, contains not less than 97 % of NaH2PO4 |
| P2O5 content | Between 58,0 % and 60,0 % on the anhydrous basis |
| Description | A white odourless, slightly deliquescent powder, crystals or granules |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol or ether |
C. pH of a 1 % solution | Between 4,1 and 5,0 |
| Purity | |
| Loss on drying | The anhydrous salt loses not more than 2,0 %, the monohydrate not more than 15,0 %, and the dihydrate not more than 25 % when dried first at 60 oC for one hour, then at 105 oC for four hours |
| Water-insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 339 (ii) DISODIUM PHOSPHATEU.K.
| Synonyms | Disodium monophosphate |
| Secondary sodium phosphate |
| Disodium orthophosphate |
| Acid disodium phosphate |
| Definition | |
| Chemical name | Disodium hydrogen monophosphate |
| Disodium hydrogen orthophosphate |
| Einecs | 231-448-7 |
| Chemical formula | Anhydrous: Na2HPO4 |
| Hydrat: Na2HPO4 · nH2O (n = 2,7 or 12) |
| Molecular weight | 141,98 (anhydrous) |
| Assay | After drying at 40 oC for three hours and subsequently at 105 oC for five hours, contains not less than 98 % of Na2HPO4 |
| P2O5 content | Between 49 % and 51 % on the anhydrous basis |
| Description | Anhydrous disodium hydrogen phosphate is a white, hygroscopic, odourless powder. Hydrated forms available include the dihydrate: a white crystalline, odourless solid; the heptahydrate: white, odourless, efflorescent crystals or granular powder; and the dodecahydrate: white, efflorescent, odourless powder or crystals |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 8,4 and 9,6 |
| Purity | |
| Loss on drying | When dried at 40 oC for three hours and then at 105 oC for five hours, the losses in weight are as follows: anhydrous not more than 5,0 %, dihydrate not more than 22,0 %, heptahydrate not more than 50,0 %, dodecahydrate not more than 61,0 % |
| Water-insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 339 (iii) TRISODIUM PHOSPHATEU.K.
| Synonyms | Sodium phosphate |
| Tribasic sodium phosphate |
| Trisodium orthophosphate |
| Definition | Trisodium phosphate is obtained from aqueous solutions and crystallises in the anhydrous form and with 1/2, 1, 6, 8 or 12 H2O. The dodecahydrate always crystallises from aqueous solutions with an excess of sodium hydroxide. It contains 1/4 molecule of NaOH |
| Chemical name | Trisodium monophosphate |
| Trisodium phosphate |
| Trisodium orthophosphate |
| Einecs | 231-509-8 |
| Chemical formula | Anhydrous: Na3PO4 |
| Hydrated: Na3PO4 · nH2O (n = 1/2, 1, 6, 8, or 12) |
| Molecular weight | 163,94 (anhydrous) |
| Assay | Sodium phosphate anhydrous and the hydrated forms, with the exception of the dodecahydrate, contain not less than 97,0 % of Na3PO4 calculated on the dried basis. Sodium phosphate dodecahydrate contains not less than 92,0 % of Na3PO4 calculated on the ignited basis |
| P2O5 content | Between 40,5 % and 43,5 % on the anhydrous basis |
| Description | White odourless crystals, granules or crystalline powder |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 11,5 and 12,5 |
| Purity | |
| Loss on ignition | When dried at 120 oC for two hours and then ignited at about 800 oC for 30 minutes, the losses in weight are as follows: anhydrous not more than 2,0 %, monohydrate not more than 11,0 %, dodecahydrate: between 45,0 % and 58,0 % |
| Water insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 340 (i) MONOPOTASSIUM PHOSPHATEU.K.
| Synonyms | Monobasic potassium phosphate |
| Monopotassium monophosphate |
| Potassium orthophosphate |
| Definition | |
| Chemical name | Potassium dihydrogen phosphate |
| Monopotassium dihydrogen orthophosphate |
| Monopotassium dihydrogen monophosphate |
| Einecs | 231-913-4 |
| Chemical formula | KH2PO4 |
| Molecular weight | 136,09 |
| Assay | Content not less than 98,0 % after drying at 105 oC for four hours |
| P2O5 content | Between 51,0 % and 53,0 % on the anhydrous basis |
| Description | Odourless, colourless crystals or white granular or crystalline powder, hygroscopic |
| Identification | |
A. Positive tests for potassium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 4,2 and 4,8 |
| Purity | |
| Loss on drying | Not more than 2,0 % determined by drying at 105 oC for four hours |
| Water-insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 340 (ii) DIPOTASSIUM PHOSPHATEU.K.
| Synonyms | Dipotassium monophosphate |
| Secondary potassium phosphate |
| Dipotassium acid phosphate |
| Dipotassium orthophosphate |
| Dibasic potassium phosphate |
| Definition | |
| Chemical name | Dipotassium hydrogen monophosphate |
| Dipotassium hydrogen phosphate |
| Dipotassium hydrogen orthophosphate |
| Einecs | 231-834-5 |
| Chemical formula | K2HPO4 |
| Molecular weight | 174,18 |
| Assay | Content not less than 98 % after drying at 105 oC for four hours |
| P2O5 content | Between 40,3 % and 41,5 % on the anhydrous basis |
| Description | Colourless or white granular powder, crystals or masses; deliquescent substance |
| Identification | |
A. Positive tests for potassium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 8,7 and 9,4 |
| Purity | |
| Loss on drying | Not more than 2,0 % determined by drying at 105 oC for four hours |
| Water-insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 340 (iii) TRIPOTASSIUM PHOSPHATEU.K.
| Synonyms | Potassium phosphate |
| Tribasic potassium phosphate |
| Tripotassium orthophosphate |
| Definition | |
| Chemical name | Tripotassium monophosphate |
| Tripotassium phosphate |
| Tripotassium orthophosphate |
| Einecs | 231-907-1 |
| Chemical formula | Anhydrous: K3PO4 |
| Hydrated: K3PO4 · nH2O (n = 1 or 3) |
| Molecular weight | 212,27 (anhydrous) |
| Assay | Content not less than 97 % calculated on the ignited basis |
| P2O5 content | Between 30,5 % and 33,0 % on the ignited basis |
| Description | Colourless or white, odourless hygroscopic crystals or granules. Hydrated forms available include the monohydrate and trihydrate |
| Identification | |
A. Positive tests for potassium and for phosphate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 11,5 and 12,3 |
| Purity | |
| Loss on ignition | Anhydrous: not more than 3,0 %; hydrated: not more than 23,0 %. Determined by drying at 105 oC for one hour and then ignite at about 800 oC ± 25 oC for 30 minutes |
| Water insoluble substances | Not more than 0,2 % on the anhydrous basis |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 341 (i) MONOCALCIUM PHOSPHATEU.K.
| Synonyms | Monobasic calcium phosphate |
| Monocalcium orthophosphate |
| Definition | |
| Chemical name | Calcium dihydrogen phosphate |
| Einecs | 231-837-1 |
| Chemical formula | Anhydrous: Ca(H2PO4)2 |
| Monohydrate: Ca(H2PO4)2 · H2O |
| Molecular weight | 234,05 (anhydrous) |
| 252,08 (monohydrate) |
| Assay | Content not less than 95 % on the dried basis |
| P2O5 content | Between 55,5 % and 61,1 % on the anhydrous basis |
| Description | Granular powder or white, deliquescent crystals or granules |
| Identification | |
A. Positive tests for calcium and for phosphate | |
B. CaO content | Between 23,0 % and 27,5 % (anhydrous) |
| Between 19,0 % and 24,8 % (monohydrate) |
| Purity | |
| Loss on drying | Not more than 14 % determined by drying at 105 oC for four hours (anhydrous) |
| Not more than 17,5 % determined by drying at 60 oC for one hour, then at 105 oC for four hours (monohydrate) |
| Loss on ignition | Not more than 17,5 % after ignition at 800 oC ± 25 oC for 30 minutes (anhydrous) |
| Not more than 25,0 % determined by drying at 105 oC for one hour, then ignite at 800 oC ± 25 oC for 30 minutes (monohydrate) |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 341 (ii) DICALCIUM PHOSPHATEU.K.
| Synonyms | Dibasic calcium phosphate |
| Dicalcium orthophosphate |
| Definition | |
| Chemical name | Calcium monohydrogen phosphate |
| Calcium hydrogen orthophosphate |
| Secondary calcium phosphate |
| Einecs | 231-826-1 |
| Chemical formula | Anhydrous: CaHPO4 |
| Dihydrate: CaHPO4 · 2H2O |
| Molecular weight | 136,06 (anhydrous) |
| 172,09 (dihydrate) |
| Assay | Dicalcium phosphate, after drying at 200 oC for three hours, contains not less than 98 % and not more than the equivalent of 102 % of CaHPO4 |
| P2O5 content | Between 50,0 % and 52,5 % on the anhydrous basis |
| Description | White crystals or granules, granular powder or powder |
| Identification | |
A. Positive tests for calcium and for phosphate | |
B. Solubility tests | Sparingly soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on ignition | Not more than 8,5 % (anhydrous), or 26,5 % (dihydrate) after ignition at 800 oC ± 25 oC for 30 minutes |
| Fluoride | Not more than 50 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 341 (iii) TRICALCIUM PHOSPHATEU.K.
| Synonyms | Calcium phosphate, tribasic |
| Calcium orthophosphate |
| Pentacalcium hydroxy monophosphate |
| Calcium hydroxyapatite |
| Definition | Tricalcium phosphate consists of a variable mixture of calcium phosphates obtained from neutralisation of phosphoric acid with calcium hydroxide and having the approximate composition of 10CaO · 3P2O5 · H2O |
| Chemical name | Pentacalcium hydroxy monophosphate |
| Tricalcium monophosphate |
| Einecs | 235-330-6 (Pentacalcium hydroxy monophosphate) |
| 231-840-8 (Calcium orthophosphate) |
| Chemical formula | Ca5(PO4)3· OH or Ca3(PO4)2 |
| Molecular weight | 502 or 310 |
| Assay | Content not less than 90 % calculated on the ignited basis |
| P2O5 content | Between 38,5 % and 48,0 % on the anhydrous basis |
| Description | A white, odourless powder which is stable in air |
| Identification | |
A. Positive tests for calcium and for phosphate | |
B. Solubility | Practically insoluble in water; insoluble in ethanol soluble in dilute hydrochloric and nitric acid |
| Purity | |
| Loss on ignition | Not more than 8 % after ignition at 800 oC ± 25 oC, to constant weight |
| Fluoride | Not more than 50 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 343(i) MONOMAGNESIUM PHOSPHATEU.K.
| Synonyms | Magnesiumdihydrogenphosphate |
| Magnesiumphosphate, monobasic |
| Monomagnesium orthophosphate |
| Definition | |
| Chemical name | Monomagnesiumdihydrogenmonophosphate |
| Einecs | 236-004-6 |
| Chemical formula | Mg(H2PO4)2 · nH2O (where n = 0 to 4) |
| Molecular weight | 218,3 (anhydrous) |
| Assay | Not less than 51,0 % after ignition |
| Description | White, odourless, crystalline powder, slightly soluble in water |
| Identification | |
A. Positive test for magnesium and for phosphate | |
B. MgO content | Not less than 21,5 % after ignition |
| Purity | |
| Fluoride | Not more than 10 mg/kg (as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 4 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 343(ii) DIMAGNESIUM PHOSPHATEU.K.
| Synonyms | Magnesiumhydrogenphosphate |
| Magnesiumphosphate, dibasic |
| Dimagnesium orthophosphate |
| Secondary magnesiumphosphate |
| Definition | |
| Chemical name | Dimagnesiummonohydrogenmonophosphate |
| Einecs | 231-823-5 |
| Chemical formula | MgHPO4 · nH2O (where n = 0-3) |
| Molecular weight | 120,3 (anhydrous) |
| Assay | Not less than 96 % after ignition |
| Description | White, odourless, crystalline powder, slightly soluble in water |
| Identification | |
A. Positive test for magnesium and for phosphate | |
B. MgO content: | Not less than 33,0 % calculated on an anhydrous basis |
| Purity | |
| Fluoride | Not more than 10 mg/kg (as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 4 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 350 (i) SODIUM MALATEU.K.
| Synonyms | Sodium salt of malic acid |
| Definition | |
| Chemical name | Disodium DL-malate, disodium salt of hydroxybutanedioic acid |
| Chemical formula | Hemihydrate: C4H4Na2O5 · 1/2 H2O |
| Trihydrate: C4H4Na2O5 · 3H2O |
| Molecular weight | Hemihydrate: 187,05 |
| Trihydrate: 232,1 |
| Assay | Content not less than 98,0 % on the anhydrous basis |
| Description | White crystalline powder or lumps |
| Identification | |
A. Positive tests for 1,2-dicarboxylic acid and for sodium | |
B. Azo dye formation | Positive |
C. Solubility | Freely soluble in water |
| Purity | |
| Loss on drying | Not more than 7,0 % (130 oC, 4h) for the hemihydrate, or 20,5 %-23,5 % (130 oC, 4h) for the trihydrate |
| Alkalinity | Not more than 0,2 % as Na2CO3 |
| Fumaric acid | Not more than 1,0 % |
| Maleic acid | Not more than 0,05 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 350 (ii) SODIUM HYDROGEN MALATEU.K.
| Synonyms | Monosodium salt of DL-malic acid |
| Definition | |
| Chemical name | Monosodium DL-malate, monosodium 2-DL-hydroxy succinate |
| Chemical formula | C4H5NaO5 |
| Molecular weight | 156,07 |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | White powder |
| Identification | |
A. Positive tests for 1,2-dicarboxylic acid and for sodium | |
B. Azo dye formation | Positive |
| Purity | |
| Loss on drying | Not more than 2,0 % (110 oC, 3h) |
| Maleic acid | Not more than 0,05 % |
| Fumaric acid | Not more than 1,0 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 351 POTASSIUM MALATEU.K.
| Synonyms | Potassium salt of malic acid |
| Definition | |
| Chemical name | Dipotassium DL-malate, dipotassium salt of hydroxybutanedioic acid |
| Chemical formula | C4H4K2O5 |
| Molecular weight | 210,27 |
| Assay | Content not less than 59,5 % |
| Description | Colourless or almost colourless aqueous solution |
| Identification | |
A. Positive tests for 1,2-dicarboxylic acid and for potassium | |
B. Azo dye formation | Positive |
| Purity | |
| Alkalinity | Not more than 0,2 % as K2CO3 |
| Fumaric acid | Not more than 1,0 % |
| Maleic acid | Not more than 0,05 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 352 (i) CALCIUM MALATEU.K.
| Synonyms | Calcium salt of malic acid |
| Definition | |
| Chemical name | Calcium DL-malate, calcium-α-hydroxysuccinate, calcium salt of hydroxybutanedioic acid |
| Chemical formula | C4H5CaO5 |
| Molecular weight | 172,14 |
| Assay | Content not less than 97,5 % on the anhydrous basis |
| Description | White powder |
| Identification | |
A. Positive tests for malate, 1,2-dicarboxylic acid and for calcium | |
B. Azo dye formation | Positive |
C. Solubility | Slightly soluble in water |
| Purity | |
| Loss on drying | Not more than 2 % (100 oC, 3h) |
| Alkalinity | Not more than 0,2 % as CaCO3 |
| Maleic acid | Not more than 0,05 % |
| Fumaric acid | Not more than 1,0 % |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 352 (ii) CALCIUM HYDROGEN MALATEU.K.
| Synonyms | Monocalcium salt of DL-malic acid |
| Definition | |
| Chemical name | Monocalcium DL-malate, monocalcium 2-DL-hydroxysuccinate |
| Chemical formula | (C4H5O5)2Ca |
| Assay | Content not less than 97,5 % on the anhydrous basis |
| Description | White powder |
| Identification | |
A. Positive tests for 1,2-dicarboxylic acid and for calcium | |
B. Azo dye formation | Positive |
| Purity | |
| Loss on drying | Not more than 2,0 % (110 oC, 3h) |
| Maleic acid | Not more than 0,05 % |
| Fumaric acid | Not more than 1,0 % |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 353 METATARTARIC ACIDU.K.
| Synonyms | Ditartaric acid |
| Definition | |
| Chemical name | Metatartaric acid |
| Chemical formula | C4H6O6 |
| Assay | Not less than 99,5 % |
| Description | Crystalline or powder form with a white or yellowish colour. Very deliquescent with a faint odour of caramel |
| Identification | |
| A. | Very soluble in water and ethanol |
| B. | Place a sample of 1 to 10 mg of this substance in a test tube with 2 ml of concentrated sulfuric acid and 2 drops of sulpho-resorcinol reagent. When heated to 150 oC, an intense violet coloration appears |
| Purity | |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 354 CALCIUM TARTRATEU.K.
| Synonyms | L-Calcium tartrate |
| Definition | |
| Chemical name | Calcium L(+)-2,3-dihydroxybutanedioate di-hydrate |
| Chemical formula | C4H4CaO6 · 2H2O |
| Molecular weight | 224,18 |
| Assay | Not less than 98,0 % |
| Description | Fine crystalline powder with a white or off-white colour |
| Identification | |
A. Slightly soluble in water. Solubility approximately 0,01 g/100 ml water (20 oC). Sparingly soluble in ethanol. Slightly soluble in diethyl ether. Soluble in acids | |
B. Specific rotation [α]20 D | + 7,0o to + 7,4o (0,1 % in a 1N de HCl solution) |
C. pH of a 5 % slurry | Between 6,0 and 9,0 |
| Purity | |
| Sulphates (as H2SO4) | Not more than 1 g/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 355 ADIPIC ACIDU.K.
| Definition | |
| Chemical name | Hexanedioic acid, 1,4-butanedicarboxylic acid |
| Einecs | 204-673-3 |
| Chemical formula | C6H10O4 |
| Molecular weight | 146,14 |
| Assay | Content not less than 99,6 % |
| Description | White odourless crystals or crystalline powder |
| Identification | |
A. Melting range | 151,5oC-154,0oC |
B. Solubility | Slightly soluble in water. Freely soluble in ethanol |
| Purity | |
| Water | Not more than 0,2 % (Karl Fischer method) |
| Sulphated ash | Not more than 20 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 356 SODIUM ADIPATEU.K.
| Definition | |
| Chemical name | Sodium adipate |
| Einecs | 231-293-5 |
| Chemical formula | C6H8Na2O4 |
| Molecular weight | 190,11 |
| Assay | Content not less than 99,0 % (on anhydrous basis) |
| Description | White odourless crystals or crystalline powder |
| Identification | |
A. Melting range | 151 oC-152 oC (for adipic acid) |
B. Solubility | Approximately 50 g/100 ml water (20 oC) |
C. Positive test for sodium | |
| Purity | |
| Water | Not more than 3 % (Karl Fischer) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 357 POTASSIUM ADIPATEU.K.
| Definition | |
| Chemical name | Potassium adipate |
| Einecs | 242-838-1 |
| Chemical formula | C6H8K2O4 |
| Molecular weight | 222,32 |
| Assay | Content not less than 99,0 % (on anhydrous basis) |
| Description | White odourless crystals or crystalline powder |
| Identification | |
A. Melting range | 151 oC-152 oC (for adipic acid) |
B. Solubility | Approximately 60 g/100 ml water (20 oC) |
C. Positive test for potassium | |
| Purity | |
| Water | Not more than 3 % (Karl Fischer) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 363 SUCCINIC ACIDU.K.
| Definition | |
| Chemical name | Butanedioic acid |
| Einecs | 203-740-4 |
| Chemical formula | C4H6O4 |
| Molecular weight | 118,09 |
| Assay | Content no less than 99,0 % |
| Description | Colourless or white, odourless crystals |
| Identification | |
A. Melting range | Between 185,0oC and 190,0oC |
| Purity | |
| Residue on ignition | Not more than 0,025 % (800 oC, 15 min) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 380 TRIAMMONIUM CITRATEU.K.
| Synonyms | Tribasic ammonium citrate |
| Definition | |
| Chemical name | Triammonium salt of 2-hydroxypropan-1,2,3-tricarboxylic acid |
| Einecs | 222-394-5 |
| Chemical formula | C6H17N3O7 |
| Molecular weight | 243,22 |
| Assay | Content not less than 97,0 % |
| Description | White to off-white crystals or powder |
| Identification | |
A. Positive tests for ammonium and for citrate | |
B. Solubility | Freely soluble in water |
| Purity | |
| Oxalate | Not more than 0,04 % (as oxalic acid) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 385 CALCIUM DISODIUM ETHYLENEDIAMINETETRAACETATEU.K.
| Synonyms | Calcium disodium EDTA |
| Calcium disodium edetate |
| Definition | |
| Chemical name | N, N′-1,2-Ethanediylbis [N-(carboxymethyl)-glycinate] [(4-)-O,O′,ON,ON]calciate(2)-disodium |
| Calcium disodium ethylenediaminetetra acetate Calcium disodium (ethylenedinitrilo)tetra acetate |
| Einecs | 200-529-9 |
| Chemical formula | C10H12O8CaN2Na2·2H2O |
| Molecular weight | 410,31 |
| Assay | Content not less than 97 % on the anhydrous basis |
| Description | White, odourless crystalline granules or white to nearly white powder, slightly hygroscopic |
| Identification | |
A. Positive tests for sodium and for calcium | |
B. Chelating activity to metal ions positive | |
C. pH of a 1 % solution between 6,5 and 7,5 | |
| Purity | |
| Water content | 5 to 13 % (Karl Fischer method) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
[E 392 EXTRACTS OF ROSEMARY
| GENERAL SPECIFICATION |
| Synonym | Extract of rosemary leaf (antioxidant) |
| Definition | Extracts of rosemary contain several components, which have been proven to exert antioxidative functions. These components belong mainly to the classes of phenolic acids, flavonoids, diterpenoids. Besides the antioxidant compounds, the extracts can also contain triterpenes and organic solvent extractable material specifically defined in the following specification |
| EINECS | 283-291-9 |
| Chemical name | Rosemary extract ( Rosmarinus officinalis ) |
| Description | Rosemary leaf extract antioxidant is prepared by extraction of the leaves of Rosmarinus officinalis using a food approved solvent system. Extracts may then be deodorised and decolourised. Extracts may be standardised |
| Identification |
|---|
| Reference antioxidative compounds: phenolic diterpenes | Carnosic acid (C 20 H 28 O 4 ) and Carnosol (C 20 H 26 O 4 ) (which comprise not less than 90 % of the total phenolic diterpenes) |
| Reference key volatiles | Borneol, Bornyl Acetate, Camphor, 1,8-Cineol, Verbenone |
| Density | > 0,25 g/ml |
| Solubility | Insoluble in water |
| Purity |
|---|
| Loss on Drying | < 5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| 1. Extracts of rosemary produced from dried rosemary leaves by acetone extraction |
| Description | Extracts of rosemary are produced from dried rosemary leaves by acetone extraction, filtration, purification and solvent evaporation, followed by drying and sieving to obtain a fine powder or a liquid |
| Identification |
|---|
| Content of reference antioxidative compounds | ≥ 10 % w/w, expressed as the total of carnosic acid and carnosol |
| Antioxidant/Volatiles — Ratio | (Total % w/w of carnosic acid and carnosol) ≥ 15
(% w/w of reference key volatiles)*
(* as a percentage of total volatiles in the extract, measured by Gas Chromatography — Mass Spectrometry Detection, ‘ GC-MSD ’ )
|
| Residual Solvents | Acetone: not more than 500 mg/kg |
| 2. Extracts of rosemary prepared by extraction of dried rosemary leaves by means of supercritical carbon dioxide |
| Extracts of rosemary produced from dried rosemary leaves extracted by means of supercritical carbon dioxide with a small amount of ethanol as entrainer.
|
| Identification |
|---|
| Content of reference antioxidative compounds | ≥ 13 % w/w, expressed as the total of carnosic acid and carnosol |
| Antioxidant/Volatiles — Ratio | (Total % w/w of carnosic acid and carnosol) ≥ 15
(% w/w of reference key volatiles)*
(* as a percentage of total volatiles in the extract, measured by Gas Chromatography — Mass Spectrometry Detection, ‘ GC-MSD ’ )
|
| Residual Solvents | Ethanol: not more than 2 % |
| 3. Extracts of rosemary prepared from a deodorised ethanolic extract of rosemary |
| Extracts of rosemary which are prepared from a deodorised ethanolic extract of rosemary. The extracts may be further purified, for example by treatment with active carbon and/or molecular distillation. The extracts may be suspended in suitable and approved carriers or spray dried.
|
| Identification |
|---|
| Content of reference antioxidative compounds | ≥ 5 % w/w, expressed as the total of carnosic acid and carnosol |
| Antioxidant/Volatiles — Ratio | (Total % w/w of carnosic acid and carnosol) ≥ 15
(% w/w of reference key volatiles)*
(* as a percentage of total volatiles in the extract, measured by Gas Chromatography — Mass Spectrometry Detection, ‘ GC-MSD ’ )
|
| Residual Solvents | Ethanol: not more than 500 mg/kg |
| 4. Extracts of rosemary decolourised and deodorised, obtained by a two-step extraction using hexane and ethanol |
| Extracts of rosemary which are prepared from a deodorised ethanolic extract of rosemary, undergone a hexane extraction. The extract may be further purified, for example by treatment with active carbon and/or molecular distillation. They may be suspended in suitable and approved carriers or spray dried.
|
| Identification |
|---|
| Content of reference antioxidative compounds | ≥ 5 % w/w, expressed as the total of carnosic acid and carnosol |
| Antioxidant/Volatiles — Ratio | (Total % w/w of carnosic acid and carnosol) ≥ 15
(% w/w of reference key volatiles)*
(* as a percentage of total volatiles in the extract, measured by Gas Chromatography — Mass Spectrometry Detection, ‘ GC-MSD ’ )
|
| Residual solvents | Hexane: not more than 25 mg/kg
Ethanol: not more than 500 mg/kg]
|
[E 400 ALGINIC ACID U.K.
| Definition | Linear glycuronoglycan consisting mainly of β-(1-4) linked D-mannuronic and α-(1-4) linked L-guluronic acid units in pyranose ring form. Hydrophilic colloidal carbohydrate extracted by the use of dilute alkali from natural strains of various species of brown seaweeds ( Phaeophyceae ) |
| Einecs | 232-680-1 |
| Chemical formula | (C 6 H 8 O 6 ) n |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Alginic acid yields, on the anhydrous basis, not less than 20 % and not more than 23 % of carbon dioxide (CO 2 ), equivalent to not less than 91 % and not more than 104,5 % of alginic acid (C 6 H 8 O 6 ) n (calculated on equivalent weight basis of 200) |
| Description | Alginic acid occurs in filamentous, grainy, granular and powdered forms. It is a white to yellowish brown and nearly odourless |
| Identification |
|---|
A. Solubility | Insoluble in water and organic solvents, slowly soluble in solutions of sodium carbonate, sodium hydroxide and trisodium phosphate |
B. Calcium chloride precipitation test | To a 0,5 % solution of the sample in 1 M sodium hydroxide solution, add one fifth of its volume of a 2,5 % solution of calcium chloride. A voluminous, gelatinous precipitate is formed. This test distinguishes alginic acid from acacia gum, sodium carboxymethyl cellulose, carboxymethyl starch, carrageenan, gelatin, gum ghatti, karaya gum, locust bean gum, methyl cellulose and tragacanth gum |
C. Ammonium sulphate precipitation test | To a 0,5 % solution of the sample in 1 M sodium hydroxide solution, add one half of its volume of a saturated solution of ammonium sulphate. No precipitate is formed. This test distinguishes alginic acid from agar, sodium carboxymethyl cellulose, carrageenan, de-esterified pectin, gelatin, locust bean gum, methyl cellulose and starch |
D. Colour reaction | Dissolve as completely as possible 0,01 g of the sample by shaking with 0,15 ml of 0,1 N sodium hydroxide and add 1 ml of acid ferric sulphate solution. Within 5 minutes, a cherry-red colour develops that finally becomes deep purple |
| Purity |
|---|
| pH of a 3 % suspension | Between 2,0 and 3,5 |
| Loss on drying | Not more than 15 % (105 °C, 4 hours) |
| Sulphated ash | Not more than 8 % on the anhydrous basis |
| Sodium hydroxide (1 M solution) | Not more than 2 % on the anhydrous basis insoluble matter |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 401 SODIUM ALGINATE U.K.
| Definition |
|---|
| Chemical name | Sodium salt of alginic acid |
| Chemical formula | (C 6 H 7 NaO 6 ) n |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Yields, on the anhydrous basis, not less than 18 % and not more than 21 % of carbon dioxide corresponding to not less than 90,8 % and not more than 106,0 % of sodium alginate (calculated on equivalent weight basis of 222) |
| Description | Nearly odourless, white to yellowish fibrous or granular powder |
| Identification |
|---|
| Positive test for sodium and alginic acid | |
| Purity |
|---|
| Loss on drying | Not more than 15 % (105 °C, 4 hours) |
| Water-insoluble matter | Not more than 2 % on the anhydrous basis |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 402 POTASSIUM ALGINATE U.K.
| Definition |
|---|
| Chemical name | Potassium salt of alginic acid |
| Chemical formula | (C 6 H 7 KO 6 ) n |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Yields, on the anhydrous basis, not less than 16,5 % and not more than 19,5 % of carbon dioxide corresponding to not less than 89,2 % and not more than 105,5 % of potassium alginate (calculated on an equivalent weight basis of 238) |
| Description | Nearly odourless, white to yellowish fibrous or granular powder |
| Identification |
|---|
| Positive test for potassium and for alginic acid | |
| Purity |
|---|
| Loss on drying | Not more than 15 % (105 °C, 4 hours) |
| Water-insoluble matter | Not more than 2 % on the anhydrous basis |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 403 AMMONIUM ALGINATE U.K.
| Definition |
|---|
| Chemical name | Ammonium salt of alginic acid |
| Chemical formula | (C 6 H 11 NO 6 ) n |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Yields, on the anhydrous basis, not less than 18 % and not more than 21 % of carbon dioxide corresponding to not less than 88,7 % and not more than 103,6 % ammonium alginate (calculated on an equivalent weight basis of 217) |
| Description | White to yellowish fibrous or granular powder |
| Identification |
|---|
| Positive test for ammonium and alginic acid | |
| Purity |
|---|
| Loss on drying | Not more than 15 % (105 °C, 4 hours) |
| Sulphated ash | Not more than 7 % on the dried basis |
| Water-insoluble matter | Not more than 2 % on the anhydrous basis |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 404 CALCIUM ALGINATE U.K.
| Synonyms | Calcium salt of alginate |
| Definition |
|---|
| Chemical name | Calcium salt of alginic acid |
| Chemical formula | (C 6 H 7 Ca 1/2 O 6 ) n |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Yields, on the anhydrous basis, not less than 18 % and not more than 21 % carbon dioxide corresponding to not less than 89,6 % and not more than 104,5 % of calcium alginate (calculated on an equivalent weight basis of 219) |
| Description | Nearly odourless, white to yellowish fibrous or granular powder |
| Identification |
|---|
| Positive test for calcium and alginic acid | |
| Purity |
|---|
| Loss on drying | Not more than 15,0 % (105 °C, 4 hours) |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 405 PROPANE-1,2-DIOL ALGINATE U.K.
| Synonyms | Hydroxypropyl alginate
1,2-propanediol ester of alginic acid
Propylene glycol alginate
|
| Definition |
|---|
| Chemical name | Propane-1,2-diol ester of alginic acid; varies in composition according to its degree of esterification and the percentage of free and neutralised carboxyl groups in the molecule |
| Chemical formula | (C 9 H 14 O 7 ) n (esterified) |
| Molecular weight | 10 000 - 600 000 (typical average) |
| Assay | Yields, on the anhydrous basis, not less than 16 % and not more than 20 % of CO 2 of carbon dioxide |
| Description | Nearly odourless, white to yellowish brown fibrous or granular powder |
| Identification |
|---|
| Positive test for 1,2-propanediol and alginic acid after hydrolysis | |
| Purity |
|---|
| Loss on drying | Not more than 20 % (105 °C, 4 hours) |
| Total propane-1,2-diol content | Not less than 15 % and not more than 45 % |
| Free propane-1,2-diol content | Not more than 15 % |
| Water-insoluble matter | Not more than 2 % on the anhydrous basis |
| Formaldehyde | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 500 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
E 406 AGARU.K.
| Synonyms | Gelose |
| Japan agar |
| Bengal, Ceylon, Chinese or Japanese isinglass |
| Layor Carang |
| Definition | |
| Chemical name | Agar is a hydrophilic colloidal polysaccharide consisting mainly of D-galactose units. On about every tenth D-galactopyranose unit one of the hydroxyl groups is esterified with sulphuric acid which is neutralised by calcium, magnesium, potassium or sodium. It is extracted from certain natural strains of marine algae of the families Gelidiaceae and Sphaerococcaceae and related red algae of the class Rhodophyceae |
| Einecs | 232-658-1 |
| Assay | The threshold gel concentration should not be higher than 0,25 % |
| Description | Agar is odourless or has a slight characteristic odour. Unground agar usually occurs in bundles consisting of thin, membranous, agglutinated strips, or in cut, flaked or granulated forms. It may be light yellowish-orange, yellowish-grey to pale yellow, or colourless. It is tough when damp, brittle when dry. Powdered agar is white to yellowish-white or pale yellow. When examined in water under a microscope, the agar appears granular and somewhat filamentous. A few fragments of the spicules of sponges and a few frustules of diatoms may be present. In chloral hydrate solution, the powdered agar appears more transparent than in water, more or less granular, striated, angular and occasionally contains frustules of diatoms. Gel strength may be standardised by the addition of dextrose and maltodextrines or sucrose |
| Identification | |
A. Solubility | Insoluble in cold water; soluble in boiling water |
| Purity | |
| Loss on drying | Not more than 22 % (105 oC, 5 hours) |
| Ash | Not more than 6,5 % on the anhydrous basis determined at 550 oC |
| Acid-insoluble ash (insoluble in approximately 3N Hydrochloric acid) | Not more than 0,5 % determined at 550 oC on the anhydrous basis |
| Insoluble matter (in hot water) | Not more than 1,0 % |
| Starch | Not detectable by the following method: to a 1 in 10 solution of the sample add a few drops of iodine solution. No blue colour is produced |
| Gelatin and other proteins | Dissolve about 1 g of agar in 100 ml of boiling water and allow to cool of about 50 oC. To 5 ml of the solution add 5 ml of trinitrophenol solution (1 g of anhydrous trinitrophenol/100 ml of hot water). No turbidity appears within 10 minutes |
| Water absorption | Place 5 g to agar in a 100 ml graduated cylinder, fill to the mark with water, mix and allow to stand at about 25 oC for 24 hours. Pour the contents of the cylinder through moistened glass wool, allowing the water to drain into a second 100 ml graduated cylinder. Not more than 75 ml of water is obtained |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
[E 407 CARRAGEENAN U.K.
| Synonyms | Products of commerce are sold under different names such as:
Irish moss gelose
Eucheuman (from Eucheuma spp.)
Iridophycan (from Iridaea spp.)
Hypnean (from Hypnea spp.)
Furcellaran or Danish agar (from Furcellaria fastigiata )
Carrageenan (from Chondrus and Gigartina spp.)
|
| Definition | Carrageenan is obtained by aqueous extraction of natural strains of seaweeds of Gigartinaceae, Solieriaceae, Hypneaeceae and Furcellariaceae , families of the class Rhodophyceae (red seaweeds). No organic precipitant shall be used other than methanol, ethanol and propane-2-ol. Carrageenan consists chiefly of the potassium, sodium, magnesium and calcium salts of polysaccharide sulphate esters which, on hydrolysis, yield galactose and 3,6-anhydrogalactose. Carrageenan shall not be hydrolysed or otherwise chemically degraded. Formaldehyde may be present as an adventitious impurity up to a maximum level of 5 mg/kg |
| Einecs | 232-524-2 |
| Description | Yellowish to colourless, coarse to fine powder which is practically odourless |
| Identification |
|---|
| Positive tests for galactose, for anhydrogalactose and for sulphate | |
| Purity |
|---|
| Methanol, ethanol, propane-2-ol content | Not more than 0,1 % singly or in combination |
| Viscosity of a 1,5 % solution at 75 °C | Not less than 5 mPa.s |
| Loss on drying | Not more than 12 % (105 °C, four hours) |
| Sulphate | Not less than 15 % and not more than 40 % on the dried basis (as SO 4 ) |
| Ash | Not less than 15 % and not more than 40 % determined on the dried basis at 550 °C |
| Acid-insoluble ash | Not more than 1 % on the dried basis (insoluble in 10 % hydrochloric acid) |
| Acid-insoluble matter | Not more than 2 % on the dried basis (insoluble in 1 % v/v sulphuric acid) |
| Low molecular weight carrageenan
(Molecular weight fraction below 50 kDa)
| Not more than 5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 2 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 300 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
[E 407a PROCESSED EUCHEUMA SEAWEED U.K.
| Synonyms | PES (acronym for processed eucheuma seaweed) |
| Definition | Processed eucheuma seaweed is obtained by aqueous alkaline (KOH) treatment of the natural strains of seaweeds Eucheuma cottonii and Eucheuma spinosum , of the class Rhodophyceae (red seaweeds) to remove impurities and by fresh water washing and drying to obtain the product. Further purification may be achieved by washing with methanol, ethanol or propane-2-ol and drying. The product consist chiefly of the potassium salt of polysaccharide sulphate esters which, on hydrolysis, yield galactose and 3,6-anhydrogalactose. Sodium, calcium and magnesium salts of the polysaccharide sulphate esters are present in lesser amounts. Up to 15 % algal cellulose is also present in the product. The carrageenan in processed eucheuma seaweed shall not be hydrolysed or otherwise chemically degraded. Formaldehyde may be present as an adventitious impurity up to a maximum level of 5 mg/kg. |
| Description | Tan to yellowish, coarse to fine powder which is practically odourless |
| Identification |
|---|
A. Positive tests for galactose, for anhydrogalactose and for sulphate | |
B. Solubility | Forms cloudy viscous suspensions in water. Insoluble in ethanol |
| Purity |
|---|
| Methanol, ethanol, propane-2-ol content | Not more than 0,1 % singly or in combination |
| Viscosity of a 1,5 % solution at 75 °C | Not less than 5 mPa.s |
| Loss on drying | Not more than 12 % (105 °C, four hours) |
| Sulphate | Not less than 15 % and not more than 40 % on the dried basis (as SO 4 ) |
| Ash | Not less than 15 % and not more than 40 % determined on the dried basis at 550 °C |
| Acid-insoluble ash | Not more than 1 % on the dried basis (insoluble in 10 % hydrochloric acid) |
| Acid-insoluble matter | Not less than 8 % and not more than 15 % on the dried basis (insoluble in 1 % v/v sulphuric acid) |
| Low molecular weight carrageenan
(Molecular weight fraction below 50 kDa)
| Not more than 5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 2 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and moulds | Not more than 300 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g] |
E 410 LOCUST BEAN GUMU.K.
| Synonyms | Carob bean gum |
| Algaroba gum |
| Definition | Locust bean gum is the ground endosperm of the seeds of the natural strains of carob tree, Cerationia siliqua (L.) Taub. (family Leguminosae). Consists mainly of a high molecular weight hydrocolloidal polysaccharide, composed of galactopyranose and mannopyranose units combined through glycosidic linkages, which may be described chemically as galactomannan |
| Molecular weight | 50 000-3 000 000 |
| Einecs | 232-541-5 |
| Assay | Galactomannan content not less than 75 % |
| Description | White to yellowish-white, nearly odourless powder |
| Identification | |
A. Positive tests for galactose mannose | |
B. Microscopic examination | Place some ground sample in an aqueous solution containing 0,5 % iodine and 1 % potassium iodide on a glass slide and examine under microscope. Locust bean gum contains long stretched tubiform cells, separated or slightly interspaced. Their brown contents are much less regularly formed in guar gum. Guar gum shows close groups of round to pear shaped cells. Their contents are yellow to brown |
C. Solubility | Soluble in hot water, insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 15 % (105 oC, 5 hours) |
| Ash | Not more than 1,2 % determined at 800 oC |
| Protein (N × 6,25) | Not more than 7 % |
| Acid-insoluble matter | Not more than 4 % |
| Starch | Not detectable by the following method: to a 1 in 10 solution of the sample add a few drops of iodine solution. No blue colour is produced |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Ethanol and propane-2-ol | Not more than 1 %, single or in combination |
[E 412 GUAR GUM U.K.
| Synonyms | Gum cyamopsis
Guar flour
|
| Definition | Guar gum is the ground endosperm of the seeds of natural strains of the guar plant, Cyamopsis tetragonolobur (L.) Taub. (family Leguminosae ). Consists mainly of a high molecular weight hydrocolloidal polysaccharide composed of galactopyranose and mannopyranose units combined through glycosidic linkages, which may be described chemically as a galactomannan. The gum may be partially hydrolysed by either heat treatment, mild acid or alkaline oxidative treatment for viscosity adjustment. |
| Einecs | 232-536-0 |
| Molecular weight | Consists mainly of a high molecular weight hydrocolloidal polysaccharide ( 50 000 - 8 000 000 ) |
| Assay | Galactomannan content not less than 75 % |
| Description | A white to yellowish-white, nearly odourless powder |
| Identification |
|---|
A. Positive tests for galactose and for mannose | |
B. Solubility | Soluble in cold water |
| Purity |
|---|
| Loss on drying | Not more than 15 % (105 °C, 5 hours) |
| Ash | Not more than 5,5 % determined at 800 °C |
| Acid-insoluble matter | Not more than 7 % |
| Protein (N × 6,25) | Not more than 10 % |
| Starch | Not detectable by the following method: to a 1 in 10 solution of the sample add a few drops of iodine solution (no blue colour is produced) |
| Organic peroxides | Not more than 0,7 meq active oxygen/kg sample |
| Furfural | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg] |
E 413 TRAGACANTHU.K.
| Synonyms | Tragacanth gum |
| Tragant |
| Definition | Tragacanth is a dried exudation obtained from the stems and branches of natural strains of Astragalus gummifer Labillardiere and other Asiatic species of Astragalus (family Leguminosae). It consists mainly of high molecular weight polysaccharides (galactoarabans and acidic polysaccharides) which, on hydrolysis, yield galacturonic acid, galactose, arabinose, xylose and fucose. Small amounts of rhamnose and of glucose (derived from traces of starch and/or cellulose) may also be present |
| Molecular weight | Approximately 800 000 |
| Einecs | 232-252-5 |
| Description | Unground Tragacanth gum occurs as flattened, lamellated, straight or curved fragments or as spirally twisted pieces 0,5-2,5 mm thick and up to 3 cm in length. It is white to pale yellow in colour but some pieces may have a red tinge. The pieces are horny in texture, with a short fracture. It is odourless and solutions have an insipid mucilaginous taste. Powdered tragacanth is white to pale yellow or pinkish brown (pale tan) in colour |
| Identification | |
A. Solubility | 1 g of the sample in 50 ml of water swells to form a smooth, stiff, opalescent mucilage; insoluble in ethanol and does not swell in 60 % (w/v) aqueous ethanol |
| Purity | |
| Negative test for Karaya gum | Boil 1 g with 20 ml of water until a mucilage is formed. Add 5 ml of hydrochloric acid and again boil the mixture for five minutes. No permanent pink or red colour develops |
| Loss on drying | Not more than 16 % (105 oC, 5 hours) |
| Total ash | Not more than 4 % |
| Acid insoluble ash | Not more than 0,5 % |
| Acid insoluble matter | Not more than 2 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Salmonella spp. | Negative in 10 g |
| E. coli | Negative in 5 g |
E 414 ACACIA GUMU.K.
| Synonyms | Gum arabic |
| Definition | Acacia gum is a dried exudation obtained from the stems and branches of natural strains of Acacia senegal (L) Willdenow or closely related species of Acacia (family Leguminosae). It consists mainly of high molecular weight polysaccharides and their calcium, magnesium and potassium salts, which on hydrolysis yield arabinose, galactose, rhamnose and glucuronic acid |
| Molecular weight | Approximately 350 000 |
| Einecs | 232-519-5 |
| Description | Unground acacia gum occurs as white or yellowish-white spheroidal tears of varying sizes or as angular fragments and is sometimes mixed with darker fragments. It is also available in the form of white to yellowish-white flakes, granules, powder or spray-dried material. |
| Identification | |
A. Solubility | 1 g dissolves in 2 ml of cold water forming a solution which flows readily and is acid to litmus, insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 17 % (105 oC, 5 hours) for granular and not more than 10 % (105 oC, 4 hours) for spray-dried material |
| Total ash | Not more than 4 % |
| Acid insoluble ash | Not more than 0,5 % |
| Acid insoluble matter | Not more than 1 % |
| Starch or dextrin | Boil a 1 in 50 solution of the gum and cool. To 5 ml add 1 drop of iodine solution. No bluish or reddish colours are produced |
| Tannin | To 10 ml of a 1 in 50 solution add about 0,1 ml of ferric chloride solution (9 g FeCl3.6H2O made up to 100 ml with water). No blackish colouration or blackish precipitate is formed |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Hydrolysis products | Mannose, xylose and galacturonic acid are absent (determined by chromatography) |
| Salmonella spp. | Negative in 10 g |
| E. coli | Negative in 5 g |
E 415 XANTHAN GUMU.K.
| Definition | Xanthan gum is a high molecular weight polysaccharide gum produced by a pure-culture fermentation of a carbohydrate with natural strains of Xanthomonas campestris, purified by recovery with ethanol or propane-2-ol, dried and milled. It contains D-glucose and D-mannose as the dominant hexose units, along with D-glucuronic acid and pyruvic acid, and is prepared as the sodium, potassium or calcium salt. Its solutions are neutral |
| Molecular weight | Approximately 1 000 000 |
| Einecs | 234-394-2 |
| Assay | Yields, on dried basis, not less than 4,2 % and not more than 5 % of CO2 corresponding to between 91 % and 108 % of xanthan gum |
| Description | Cream-coloured powder |
| Identification | |
A. Solubility | Soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 15 % (105 oC, 21/2 hours) |
| Total ash | Not more than 16 % on the anhydrous basis determined at 650 oC after drying at 105 oC for four hours |
| Pyruvic acid | Not less than 1,5 % |
| Nitrogen | Not more than 1,5 % |
| Ethanol and propan-2-ol | Not more than 500 mg/kg singly or in combination |
| Lead | Not more than 2 mg/kg |
| Total plate count | Not more than 5 000 colonies per gram |
| Yeast and mould | Not more than 300 colonies per gram |
| E. coli | Absent in 5 g |
| Salmonella spp. | Absent in 10 g |
| Xanthomonas campestris | Viable cells absent in 1 g |
E 416 KARAYA-GUMU.K.
| Synonyms | Katilo |
| Kadaya |
| Gum sterculia |
| Sterculia |
| Karaya, gum karaya |
| Kullo |
| Kuterra |
| Definition | Karaya gum is a dried exudation from the stems and branches of natural strains of: Sterculia urens Roxburgh and other species of Sterculia (family Sterculiaceae) or from Cochlospermum gossypium A.P. De Candolle or other species of Cochlospermum (family Bixaceae). It consists mainly of high molecular weight acetylated polysaccharides, which on hydrolysis yield galactose, rhamnose, and galacturonic acid, together with minor amounts of glucuronic acid |
| Einecs | 232-539-4 |
| Description | Karaya gum occurs in tears of variable size and in broken irregular pieces having a characteristic semi-crystalline appearance. It is pale yellow to pinkish brown in colour, translucent and horny. Powdered karaya gum is a pale grey to pinkish brown. The gum has a distinctive odour of acetic acid |
| Identification | |
A. Solubility | Insoluble in ethanol |
B. Swelling in ethanol solution | Karaya gum swells in 60 % ethanol distinguishing it from other gums |
| Purity | |
| Loss on drying | Not more than 20 % (105 oC, 5 hours) |
| Total ash | Not more than 8 % |
| Acid insoluble ash | Not more than 1 % |
| Acid insoluble matter | Not more than 3 % |
| Volatile acid | Not less than 10 % (as acetic acid) |
| Starch | Not detectable |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Salmonella spp. | Negative in 10 g |
| E. coli | Negative in 5 g |
E 417 TARA GUMU.K.
| Definition | Tara gum is obtained by grinding the endosperm of the seeds of natural strains of Caesalpinia spinosa (family Leguminosae). It consists chiefly of polysaccharides of high molecular weight composed mainly of galactomannans. The principal component consists of a linear chain of (1-4)-β-D-mannopyranose units with α-D-galactopyranose units attached by (1-6) linkages. The ratio of mannose to galactose in tara gum is 3:1. (In locust bean gum this ratio is 4:1 and in guar gum 2:1) |
| Einecs | 254-409-6 |
| Description | A white to white-yellow odourless powder |
| Identification | |
A. Solubility | Soluble in water |
| Insoluble in ethanol |
B. Gel formation | To an aqueous solution of the sample add small amounts of sodium borate. A gel is formed |
| Purity | |
| Loss on drying | Not more than 15 % |
| Ash | Not more than 1,5 % |
| Acid insoluble matter | Not more than 2 % |
| Protein | Not more than 3,5 % (factor N × 5,7) |
| Starch | Not detectable |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 418 GELLAN GUMU.K.
| Definition | Gellan gum is a high molecular weight polysaccharide gum produced by a pure culture fermentation of a carbohydrate by natural strains of Pseudomonas elodea, purified by recovery with isopropyl alcohol, dried, and milled. The high molecular weight polysaccharide is principally composed of a tetrasaccharide repeating unit of one rhamnose, one glucuronic acid, and two glucoses, and substituted with acyl (glyceryl and acetyl) groups as the O-glycosidically linked esters. The glucuronic acid is neutralised to a mixed potassium, sodium, calcium, and magnesium salt |
| Einecs | 275-117-5 |
| Molecular weight | Approximately 500 000 |
| Assay | Yields, on the dried basis, not less than 3,3 % and not more than 6,8 % of CO2 |
| Description | An off-white powder |
| Identification | |
A. Solubility | Soluble in water, forming a viscous solution. |
| Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 15 % after drying (105 oC, 21/2 hours) |
| Nitrogen | Not more than 3 % |
| Propane-2-ol | Not more than 750 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Total plate count | Not more than 10 000 colonies per gram |
| Yeast and mould | Not more than 400 colonies per gram |
| E. coli | Negative in 5 g |
| Salmonella spp. | Negative in 10 g |
E 420(i) SORBITOLU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Commission Directive 2008/60/EC().
E 420(ii) SORBITOL SYRUPU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 421 MANNITOLU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 422 GLYCEROLU.K.
| Synonyms | Glycerin |
| Glycerine |
| Definition | |
| Chemical names | 1,2,3-propanetriol |
| Glycerol |
| Trihydroxypropane |
| Einecs | 200-289-5 |
| Chemical formula | C3H8O3 |
| Molecular weight | 92,1 |
| Assay | Content not less than 98 % of glycerol on the anhydrous basis |
| Description | Clear, colourless hygroscopic syrupy liquid with not more than a slight characteristic odour, which is neither harsh nor disagreeable |
| Identification | |
A. Acrolein formation on heating | Heat a few drops of the sample in a test tube with about 0,5 g of potassium bisulphate. The characteristic pungent vapours of acrolein are evolved |
B. Specific gravity (25/25 oC) | Not less than 1,257 |
C. Refractive index [n]D20 | Between 1,471 and 1,474 |
| Purity | |
| Water | Not more than 5 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,01 % determined at 800 ± 25 oC |
| Butanetriols | Not more than 0,2 % |
| Acrolein, glucose and ammonium compounds | Heat a mixture of 5 ml of glycerol and 5 ml of potassium hydroxide solution (1 in 10) at 60 oC for five minutes. It neither becomes yellow nor emits an odour of ammonia |
| Fatty acids and esters | Not more than 0,1 % calculated as butyric acid |
| Chlorinated compounds | Not more than 30 mg/kg (as chlorine) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 425(i) KONJAC GUMU.K.
| Definition | Konjac gum is a water-soluble hydrocolloid obtained from the Konjac flour by aqueous extraction. Konjac flour is the unpurified raw product from the root of the perennial plant Amorphophallus konjac. The main component of Konjac gum is the water-soluble high-molecular-weight polysaccharide glucomannan, which consists of D-mannose and D-glucose units at a molar ratio of 1,6:1,0, connected by β(1-4)-glycosidic bonds. Shorter side chains are attached through β(1-3)-glycosidic bonds, and acetyl groups occur at random at a ratio of about 1 group per 9 to 19 sugar units |
| Molecular weight | The main component, glucomannan, has an average molecular weight of 200 000 to 2 000 000 |
| Assay | Not less than 75 % carbohydrate |
| Description | A white to cream to light tan powder |
| Identification | |
A. Solubility | Dispersible in hot or cold water forming a highly viscous solution with a pH between 4,0 and 7,0 |
B. Gel formation | Add 5 ml of a 4 % sodium borate solution to a 1 % solution of the sample in a test tube, and shake vigorously. A gel forms |
C. Formation of heat-stable gel | Prepare a 2 % solution of the sample by heating it in a boiling water bath for 30 min, with continuous agitation and then cooling the solution to room temperature. For each g of the sample used to prepare 30 g of the 2 % solution, add 1 ml of 10 % potassium carbonate solution to the fully hydrated sample at ambient temperature. Heat the mixture in a water bath to 85 oC, and maintain for 2 h without agitation. Under these conditions a thermally stable gel is formed |
D. Viscosity (1 % solution) | Not less than 3 kgm-1s-1 at 25 oC |
| Purity | |
| Loss on drying | Not more than 12 % (105 oC, 5 h) |
| Starch | Not more than 3 % |
| Protein | Not more than 3 % (N × 5,7) |
| Determine nitrogen by Kjeldahl method. The percentage of nitrogen in the sample multiplied by 5,7 gives the percent of protein in the sample |
| Ether-soluble material | Not more than 0,1 % |
| Total ash | Not more than 5,0 % (800 oC, 3 to 4h) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Salmonella spp. | Absent in 12,5 g |
| E. coli | Absent in 5 g |
E 425(ii) KONJAC GLUCOMANNANU.K.
| Definition | Konjac glucomannan is a water-soluble hydrocolloid obtained from Konjac flour by washing with water-containing ethanol. Konjac flour is the unpurified raw product from the tuber of the perennial plant Amorphophallus konjac. The main component is the water-soluble high-molecular-weight polysaccharide glucomannan, which consists of D-mannose and D-glucose units at a molar ratio of 1,6:1,0, connected by β(1-4)-glycosidic bonds with a branch at about each 50th or 60th unit. About each 19th sugar residue is acetylated |
| Molecular weight | 500 000 to 2 000 000 |
| Assay | Total dietary fibre: not less than 95 % on a dry weight basis |
| Description | White to slightly brownish fine particle size, free flowing and odourless powder |
| Identification | |
A. Solubility | Dispersible in hot or cold water forming a highly viscous solution with a pH between 5,0 and 7,0. Solubility is increased by heat and mechanical agitation |
B. Formation of heat-stable gel | Prepare a 2 % solution of the sample by heating it in a boiling water bath for 30 min, with continuous agitation and then cooling the solution to room temperature. For each g of the sample used to prepare 30 g of the 2 % solution, add 1 ml of 10 % potassium carbonate solution to the fully hydrated sample at ambient temperature. Heat the mixture in a water bath to 85 oC, and maintain for 2 h without agitation. Under these conditions a thermally stable gel is formed |
C. Viscosity (1 % solution) | Not less than 20 kgm-1s-1 at 25 oC |
| Purity | |
| Loss on drying | Not more than 8 % (105 oC, 3h) |
| Starch | Not more than 1 % |
| Protein | Not more than 1,5 % (N × 5,7) |
| Determine nitrogen by Kjeldahl method. The percentage of nitrogen in the sample multiplied by 5,7 gives the percent of protein in the sample |
| Ether-soluble material | Not more than 0,5 % |
| Sulphite (as SO2) | Not more than 4 mg/kg |
| Chloride | Not more than 0,02 % |
| 50 % Alcohol-soluble | Not more than 2,0 % material |
| Total ash | Not more than 2,0 % (800 oC, 3 to 4h) |
| Lead | Not more than 1 mg/kg |
| Salmonella spp. | Absent in 12,5 g |
| E. coli | Absent in 5 g |
E 426 SOYBEAN HEMICELLULOSEU.K.
| [Definition | Soybean Hemicellulose is a refined water-soluble polysaccharide obtained from natural strain soybean fibre by hot water extraction. No organic precipitant shall be used other than ethanol] |
| Chemical names | Water soluble soybean polysaccharides |
| Water soluble soybean fibre |
| Assay | Not less than 74 % carbohydrate |
| [Description | Free flowing white or yellowish white powder] |
| Identification | |
A. Solubility pH of 1 % solution | Soluble in hot and cold water without gel formation |
| 5,5 ± 1,5 |
B. Viscosity of 10 % solution | Not more than 200 mPa.s |
| Purity | |
| Loss on drying | Not more than 7 % (105 oC, 4h) |
| Protein | Not more than 14 % |
| Total ash | Not more than 9,5 % (600 oC, 4h) |
| Arsenic | Not more than 2 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Standard plate count | Not more than 3 000 colonies per gram |
| Yeast and mould | Not more than 100 colonies per gram |
| E. coli | Negative in 10 g |
| [Ethanol | Not more than 2 %] |
[E 427 CASSIA GUM U.K.
| Synonyms | |
| Definition | Cassia gum is the ground purified endosperm of the seeds of Cassia tora and Cassia obtusifoli ( Leguminosae ) containing less than 0,05 % of Cassia occidentalis . It consists mainly of high molecular weight polysaccharides composed primarily of a linear chain of 1,4-β-D-mannopyranose units linked with 1,6-α-D-galactopyranose units. The ratio of mannose to galactose is about 5:1
In the manufacture the seeds are dehusked and degermed by thermal mechanical treatment followed by milling and screening of the endosperm. The ground endosperm is further purified by extraction with isopropanol
|
| Assay | Not less than 75 % of Galactomannan |
| Description | Pale yellow to off-white, odourless powder |
| Identification |
|---|
| Solubility | Insoluble in ethanol. Disperses well in cold water forming a colloidal solution |
| Gel formation with borate | To an aqueous dispersion of the sample add sufficient sodium borate test solution (TS) to raise the pH to above 9; a gel is formed |
| Gel formation with xanthan gum | Weigh 1,5 g of the sample and 1,5 g of xanthan gum and blend them. Add this blend (with rapid stirring) into 300 ml water at 80 °C in a 400 ml beaker. Stir until the mixture is dissolved and continue stirring for an extra 30 min after dissolution (maintain the temperature above 60 °C during the stirring process). Discontinue stirring and allow the mixture to cool at room temperature for at least 2 h
A firm, viscoelastic gel forms after the temperature drops below 40 °C, but no such gel forms in a 1 % control solution of cassia gum or xanthan gum alone prepared in a similar manner
|
| Viscosity | Less than 500 mPa.s (25 °C, 2h, 1 % solution) corresponding to an average molecular weight of 200 000 - 300 000 D |
| Purity |
|---|
| Acid insoluble matter | Not more than 2,0 % |
| pH | 5,5-8 (1 % aqueous solution) |
| Crude fat | Not more than 1 % |
| Proteins | Not more than 7 % |
| Total ash | Not more than 1,2 % |
| Loss on drying | Not more than 12 % (5 h, 105 °C) |
| Total Anthraquinones | Not more than 0,5 mg/kg (detection limit) |
| Solvent residues | Not more than 750 mg/kg Isopropyl alcohol |
| Lead | Not more than 1 mg/kg |
| Microbiological criteria |
|---|
| Total plate count | Not more than 5 000 colony forming units per gram |
| Yeast and mould | Not more than 100 colony forming units per gram |
| Salmonella spp. | Absent in 25 g |
| E. Coli | Absent in 1 g] |
E 431 POLYOXYETHYLENE (40) STEARATEU.K.
| Synonyms | Polyoxyl (40) stearate |
| polyoxyethylene (40) monostearate |
| Definition | A mixture of the mono- and diesters of edible commercial stearic acid and mixed polyoxyethylene diols (having an average polymer length of about 40 oxyethylene units) together with free polyol |
| Assay | Content not less than 97,5 % on the anhydrous basis |
| Description | Cream-coloured flakes or waxy solid at 25 oC with a faint odour |
| Identification | |
A. Solubility | Soluble in water, ethanol, methanol and ethyl acetate. Insoluble in mineral oil |
B. Congealing range | 39 oC-44 oC |
C. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 1 |
| Saponification value | Not less than 25 and not more than 35 |
| Hydroxyl value | Not less than 27 and not more than 40 |
| 1,4-Dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 432 POLYOXYETHYLENE SORBITAN MONOLAURATE (POLYSORBATE 20)U.K.
| Synonyms | Polysorbate 20 |
| Polyoxyethylene (20) sorbitan monolaurate |
| Definition | A mixture of the partial esters of sorbitol and its mono- and dianhydrides with edible commercial lauric acid and condensed with approximately 20 moles of ethylene oxide per mole of sorbitol and its anhydrides |
| Assay | Content not less than 70 % of oxyethylene groups, equivalent to not less than 97,3 % of polyoxyethylene (20) sorbitan monolaurate on the anhydrous basis |
| Description | A lemon to amber-coloured oily liquid at 25 oC with a faint characteristic odour |
| Identification | |
A. Solubility | Soluble in water, ethanol, methanol, ethyl acetate and dioxane. Insoluble in mineral oil and petroleum ether |
B. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 2 |
| Saponification value | Not less than 40 and not more than 50 |
| Hydroxyl value | Not less than 96 and not more than 108 |
| 1,4-dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 433 POLYOXYETHYLENE SORBITAN MONOOLEATE (POLYSORBATE 80)U.K.
| Synonyms | Polysorbate 80 |
| Polyoxyethylene (20) sorbitan monooleate |
| Definition | A mixture of the partial esters of sorbitol and its mono- and dianhydrides with edible commercial oleic acid and condensed with approximately 20 moles of ethylene oxide per mole of sorbitol and its anhydrides |
| Assay | Content not less than 65 % of oxyethylene groups, equivalent to not less than 96,5 % of polyoxyethylene (20) sorbitan monooleate on the anhydrous basis |
| Description | A lemon to amber-coloured oily liquid at 25 oC with a faint characteristic odour |
| Identification | |
A. Solubility | Soluble in water, ethanol, methanol, ethyl acetate and toluene. Insoluble in mineral oil and petroleum ether |
B. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 2 |
| Saponification value | Not less than 45 and not more than 55 |
| Hydroxyl value | Not less than 65 and not more than 80 |
| 1,4-dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 434 POLYOXYETHYLENE SORBITAN MONOPALMITATE (POLYSORBATE 40)U.K.
| Synonyms | Polysorbate 40 |
| Polyoxyethylene (20) sorbitan monopalmitate |
| Definition | A mixture of the partial esters of sorbitol and its mono- and dianhydrides with edible commercial palmitic acid and condensed with approximately 20 moles of ethylene oxide per mole of sorbitol and its anhydrides |
| Assay | Content not less than 66 % of oxyethylene groups, equivalent to not less than 97 % of polyoxyethylene (20) sorbitan monopalmitate on the anhydrous basis |
| Description | A lemon to orange-coloured oily liquid or semi-gel at 25 oC with a faint characteristic odour |
| Identification | |
A. Solubility | Soluble in water, ethanol, methanol, ethyl acetate and acetone. Insoluble in mineral oil |
B. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 2 |
| Saponification value | Not less than 41 and not more than 52 |
| Hydroxyl value | Not less than 90 and not more than 107 |
| 1,4-dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 435 POLYOXYETHYLENE SORBITAN MONOSTEARATE (POLYSORBATE 60)U.K.
| Synonyms | Polysorbate 60 |
| Polyoxyethylene (20) sorbitan monostearate |
| Definition | A mixture of the partial esters of sorbitol and its mono- and dianhydrides with edible commercial stearic acid and condensed with approximately 20 moles of ethylene oxide per mole of sorbitol and its anhydrides |
| Assay | Content not less than 65 % of oxyethylene groups, equivalent to not less than 97 % of polyoxyethylene (20) sorbitan monostearate on the anhydrous basis |
| Description | A lemon to orange-coloured oily liquid or semi-gel at 25 oC with a faint characteristic odour |
| Identification | |
A. Solubility | Soluble in water, ethyl acetate and toluene. Insoluble in mineral oil and vegetable oils |
B. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 2 |
| Saponification value | Not less than 45 and not more than 55 |
| Hydroxyl value | Not less than 81 and not more than 96 |
| 1,4-dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 436 POLYOXYETHYLENE SORBITAN TRISTEARATE (POLYSORBATE 65)U.K.
| Synonyms | Polysorbate 65 |
| Polyoxyethylene (20) sorbitan tristearate |
| Definition | A mixture of the partial esters of sorbitol and its mono- and dianhydrides with edible commercial stearic acid and condensed with approximately 20 moles of ethylene oxide per mole of sorbitol and its anhydrides |
| Assay | Content not less than 46 % of oxyethylene groups, equivalent to not less than 96 % of polyoxyethylene (20) sorbitan tristearate on the anhydrous basis |
| Description | A tan-coloured, waxy solid at 25 oC with a faint characteristic odour |
| Identification | |
A. Solubility | Dispersible in water. Soluble in mineral oil, vegetal oils, petroleum ether, acetone, ether, dioxane, ethanol and methanol |
B. Congealing range | 29-33 oC |
C. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyoxyethylated polyol |
| Purity | |
| Water | Not more than 3 % (Karl Fischer method) |
| Acid value | Not more than 2 |
| Saponification value | Not less than 88 and not more than 98 |
| Hydroxyl value | Not less than 40 and not more than 60 |
| 1,4-dioxane | Not more than 5 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycols (mono- and di-) | Not more than 0,25 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 440 (i) PECTINU.K.
| Definition | Pectin consists mainly of the partial methyl esters of polygalacturonic acid and their ammonium, sodium, potassium and calcium salts. It is obtained by extraction in an aqueous medium of natural strains of appropriate edible plant material, usually citrus fruits or apples. No organic precipitant shall be used other than methanol, ethanol and propane-2-ol |
| Einecs | 232-553-0 |
| Assay | Content not less than 65 % of galacturonic acid on the ash-free and anhydrous basis after washing with acid and alcohol |
| Description | White, light yellow, light grey or light brown powder |
| Identification | |
A. Solubility | Soluble in water forming a colloidal, opalescent solution. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 12 % (105 oC, 2 hours) |
| Acid insoluble ash | Not more than 1 % (insoluble in approximately 3N hydrochloric acid) |
| Sulphur dioxide | Not more than 50 mg/kg on the anhydrous basis |
| Nitrogen content | Not more than 1,0 % after washing with acid and ethanol |
| Free methanol, ethanol and propane-2-ol | Not more than 1 %, singly or in combination, on the anhydrous basis |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 440 (ii) AMIDATED PECTINU.K.
| Definition | Amidated pectin consists mainly of the partial methyl esters and amides of polygalacturonic acid and their ammonium, sodium, potassium and calcium salts. It is obtained by extraction in an aqueous medium of appropriate natural strains of edible plant material, usually citrus fruits or apples and treatment with ammonia under alkaline conditions. No organic precipitant shall be used other than methanol, ethanol and propane-2-ol |
| Assay | Content not less than 65 % of galacturonic acid on the ash-free and anhydrous basis after washing with acid and alcohol |
| Description | White, light yellow, light greyish or light brownish powder |
| Identification | |
A. Solubility | Soluble in water forming a colloidal, opalescent solution. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 12 % (105 oC, 2 hours) |
| Acid-insoluble ash | Not more than 1 % (insoluble in approximately 3N hydrochloric acid) |
| Degree of amidation | Not more than 25 % of total carboxyl groups |
| Sulphur dioxide residue | Not more than 50 mg/kg on the anhydrous basis |
| Nitrogen content | Not more than 2,5 % after washing with acid and ethanol |
| Free methanol, ethanol and propane-2-ol | Not more than 1 % single or in combination, on a volatile matter-free basis |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 442 AMMONIUM PHOSPHATIDESU.K.
| Synonyms | Ammonium salts of phosphatidic acid, mixed ammonium salts of phoshorylated glycerides |
| Definition | A mixture of the ammonium compounds of phosphatidic acids derived from edible fat and oil (usually partially hardened rapeseed oil). One or two or three glyceride moieties may be attached to phosphorus. Moreover, two phosphorus esters may be linked together as phosphatidyl phosphatides |
| Assay | The phosphorus content is not less than 3 % and not more than 3,4 % by weight; the ammonium content is not less than 1,2 % and not more than 1,5 % (calculated as N) |
| Description | Unctuous semi-solid |
| Identification | |
A. Solubility | Soluble in fats. Insoluble in water. Partially soluble in ethanol and in acetone |
B. Positive tests for glycerol, for fatty acid and for phosphate | |
| Purity | |
| Petroleum ether insoluble matter | Not more than 2,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 444 SUCROSE ACETATE ISOBUTYRATEU.K.
| Synonyms | SAIB |
| Definition | Sucrose acetate isobutyrate is a mixture of the reaction products formed by the esterification of food grade sucrose with acetic acid anhydride and isobutyric anhydride, followed by distillation. The mixture contains all possible combinations of esters in which the molar ratio of acetate to butyrate is about 2:6 |
| Einecs | 204-771-6 |
| Chemical name | Sucrose diacetate hexaisobutyrate |
| Chemical formulae | C40H62O19 |
| Molecular weight | 832-856 (approximate), C40H62O19: 846,9 |
| Assay | Content not less than 98,8 % and not more than 101,9 % of C40H62O19 |
| Description | A pale straw-coloured liquid, clear and free of sediment and having a bland odour |
| Identification | |
A. Solubility | Insoluble in water. Soluble in most organic solvents |
B. Refractive index | [n]40 D: 1,4492-1,4504 |
C. Specific gravity | [d]25 D: 1,141-1,151 |
| Purity | |
| Triacetin | Not more than 0,1 % |
| Acid value | Not more than 0,2 |
| Saponification value | Not less than 524 and not more than 540 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 3 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 5 mg/kg |
E 445 GLYCEROL ESTERS OF WOOD ROSINU.K.
| Synonyms | Ester gum |
| Definition | A complex mixture of tri- and diglycerol esters of resin acids from wood rosin. The rosin is obtained by the solvent extraction of aged pine stumps followed by a liquid-liquid solvent refining process. Excluded from these specifications are substances derived from gum rosin, and exudate of living pine trees, and substances derived from tall oil rosin, a by-product of kraft (paper) pulp processing. The final product is composed of approximately 90 % resin acids and 10 % neutrals (non-acidic compounds). The resin acid fraction is a complex mixture of isomeric diterpenoid monocarboxylic acids having the empirical molecular formula of C20H30O2, chiefly abietic acid. The substance is purified by steam stripping or by countercurrent steam distillation |
| Description | Hard, yellow to pale amber-coloured solid |
| Identification | |
A. Solubility | Insoluble in water, soluble in acetone |
B. Infrared absorption spectrum | Characteristic of the compound |
| Purity | |
| Specific gravity of solution | [d]20 25 not less than 0,935 when determined in a 50 % solution in d-limonene (97 %, boilding point 175,5-176 oC, d20 4: 0,84) |
| Ring and ball softening range | Between 82 oC and 90 oC |
| Acid value | Not less than 3 and not more than 9 |
| Hydroxyl value | Not less than 15 and not more than 45 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Test for absence of tall oil rosin (sulphur test) | When sulphur-containing organic compounds are heated in the presence of sodium formate, the sulphur is converted to hydrogen sulphide which can readily be detected by the use of lead acetate paper. A positive test indicates the use of tall oil rosin instead of wood rosin |
E 450 (i) DISODIUM DIPHOSPHATEU.K.
| Synonyms | Disodium dihydrogen diphosphate |
| Disodium dihydrogen pyrophosphate |
| Sodium acid pyrophosphate |
| Disodium pyrophosphate |
| Definition | |
| Chemical name | Disodium dihydrogen diphosphate |
| Einecs | 231-835-0 |
| Chemical formula | Na2H2P2O7 |
| Molecular weight | 221,94 |
| Assay | Content not less than 95 % of disodium diphosphate |
| P2O5 Content | Not less than 63,0 % and not more than 64,5 % |
| Description | White powder or grains |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Soluble in water |
C. pH of a 1 % solution | Between 3,7 and 5,0 |
| Purity | |
| Loss on drying | Not more than 0,5 % (105 oC, four hours) |
| Water-insoluble matter | Not more than 1 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 450 (ii) TRISODIUM DIPHOSPHATEU.K.
| Synonyms | Acid trisodium pyrophosphate |
| Trisodium monohydrogen diphosphate |
| Definition | |
| Einecs | 238-735-6 |
| Chemical formula | Monohydrate: Na3HP2O7 · H2O |
| Anhydrous: Na3HP2O7 |
| Molecular weight | Monohydrate: 261,95 |
| Anhydrous: 243,93 |
| Assay | Content not less than 95 % on the anhydrous basis |
| P2O5 content | Not less than 57 % and not more than 59 % |
| Description | White powder or grains, occurs anhydrous or as a monohydrate |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Soluble in water |
C. pH of a 1 % solution | Between 6,7 and 7,5 |
| Purity | |
| Loss on ignition | Not more than 4,5 % on the anhydrous compound |
| Not more than 11,5 % on the monohydrous basis |
| Loss on drying | Not more than 0,5 % (105 oC, four hours) |
| Water-insoluble matter | Not more than 0,2 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 450 (iii) TETRASODIUM DIPHOSPHATEU.K.
| Synonyms | Tetrasodium pyrophosphate |
| Sodium pyrophosphate |
| Definition | |
| Chemical name | Tetrasodium diphosphate |
| Einecs | 231-767-1 |
| Chemical formula | Anhydrous: Na4P2O7 |
| Decahydrate: Na4P2O7 · 10H2O |
| Molecular weight | Anhydrous: 265,94 |
| Decahydrate: 446,09 |
| Assay | Content not less than 95 % of Na4P2O7 on the ignited basis |
| P2O5 content | Not less than 52,5 % and not more than 54,0 % |
| Description | Colourless or white crystals, or a white crystalline or granular powder. The decahydrate effloresces slightly in dry air |
| Identification | |
A. Positive tests for sodium and for phosphate | |
B. Solubility | Soluble in water. Insoluble in ethanol |
C. pH of a 1 % solution | Between 9,8 and 10,8 |
| Purity | |
| Loss on ignition | Not more than 0,5 % for the anhydrous salt, not less than 38 % and not more than 42 % for the decahydrate, in both cases determined after drying at 105 oC for four hours, followed by ignition at 550 oC for 30 minutes |
| Water-insoluble matter | Not more than 0,2 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 450 (v) TETRAPOTASSIUM DIPHOSPHATEU.K.
| Synonyms | Potassium pyrophosphate |
| Tetrapotassium pyrophosphate |
| Definition | |
| Chemical name | Tetrapotassium diphosphate |
| Einecs | 230-785-7 |
| Chemical formula | K4P2O7 |
| Molecular weight | 330,34 (anhydrous) |
| Assay | Content not less than 95 % on the ignited basis |
| P2O5 content | Not less than 42,0 % and not more than 43,7 % on the anhydrous basis |
| Description | Colourless crystals or white, very hygroscopic powder |
| Identification | |
A. Positive tests for potassium and for phosphate | |
B. Solubility | Soluble in water, insoluble in ethanol |
C. pH of a 1 % solution | Between 10,0 and 10,8 |
| Purity | |
| Loss on ignition | Not more than 2 % after drying at 105 oC for four hours and then ignition at 550 oC for 30 minutes |
| Water-insoluble substances | Not more than 0,2 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 450 (vi) DICALCIUM DIPHOSPHATEU.K.
| Synonyms | Calcium pyrophosphate |
| Definition | |
| Chemical name | Dicalcium diphosphate |
| Dicalcium pyrophosphate |
| Einecs | 232-221-5 |
| Chemical formula | Ca2P2O7 |
| Molecular weight | 254,12 |
| Assay | Content not less than 96 % |
| P2O5 content | Not less than 55 % and not more than 56 % |
| Description | A fine, white, odourless powder |
| Identification | |
A. Positive tests for calcium and for phosphate | |
B. Solubility | Insoluble in water. Soluble in dilute hydrochloric and nitric acids |
C. pH of a 10 % suspension in water | Between 5,5 and 7,0 |
| Purity | |
| Loss on ignition | Not more than 1,5 % at 800 oC ± 25 oC for 30 minutes |
| Fluoride | Not more than 50 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 450 (vii) CALCIUM DIHYDROGEN DIPHOSPHATEU.K.
| Synonyms | Acid calcium pyrophosphate |
| Monocalcium dihydrogen pyrophosphate |
| Definition | |
| Chemical name | Calcium dihydrogen diphosphate |
| Einecs | 238-933-2 |
| Chemical formula | CaH2P2O7 |
| Molecular weight | 215,97 |
| Assay | Content not less than 90 % on the anhydrous basis |
| P2O5 content | Not less than 61 % and not more than 64 % |
| Description | White crystals or powder |
| Identification | |
A. Positive tests for calcium and for phosphate | |
| Purity | |
| Acid-insoluble matter | Not more than 0,4 % |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 451 (i) PENTASODIUM TRIPHOSPHATEU.K.
| Synonyms | Pentasodium tripolyphosphate |
| Sodium tripolyphosphate |
| Definition | |
| Chemical name | Pentasodium triphosphate |
| Einecs | 231-838-7 |
| Chemical formula | Na5O10P3 · nH2O (n = 0 or 6) |
| Molecular weight | 367,86 |
| Assay | Content not less than 85,0 % (anhydrous) or 65,0 % (hexahydrate) |
| P2O5 content | Not less than 56 % and not more than 59 % (anhydrous) or not less than 43 % and not more than 45 % (hexahydrate) |
| Description | White, slightly hygroscopic granules or powder |
| Identification | |
A. Solubility | Freely soluble in water. Insoluble in ethanol |
B. Positive tests for sodium and for phosphate | |
C. pH of a 1 % solution | Between 9,1 and 10,2 |
| Purity | |
| Loss on drying | Anhydrous: Not more than 0,7 % (105 oC, one hour) |
| Hexahydrate: Not more than 23,5 % (60 oC, one hour, followed by drying at 105 oC, four hours) |
| Water-insoluble substances | Not more than 0,1 % |
| Higher polyphosphates | Not more than 1 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 451 (ii) PENTAPOTASSIUM TRIPHOSPHATEU.K.
| Synonyms | Pentapotassium tripolyphosphate |
| Potassium triphosphate |
| Potassium tripolyphosphate |
| Definition | |
| Chemical name | Pentapotassium triphosphate |
| Pentapotassium tripolyphosphate |
| Einecs | 237-574-9 |
| Chemical formula | K5O10P3 |
| Molecular weight | 448,42 |
| Assay | Content not less than 85 % on the anhydrous basis |
| P2O5 content | Not less than 46,5 % and not more than 48 % |
| Description | White, very hygroscopic powder or granules |
| Identification | |
A. Solubility | Very soluble in water |
B. Positive tests for potassium and for phosphate | |
C. pH of a 1 % solution | Between 9,2 and 10,5 |
| Purity | |
| Loss on ignition | Not more than 0,4 % (after drying at 105 oC, four hours, followed by ignition at 550 oC, 30 minutes) |
| Water-insoluble matter | Not more than 2 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 452 (i) SODIUM POLYPHOSPHATEU.K.
1.SOLUBLE POLYPHOSPHATEU.K.
| Synonyms | Sodium hexametaphosphate |
| Sodium tetrapolyphosphate |
| Graham's salt |
| Sodium polyphosphates, glassy |
| Sodium polymetaphosphate |
| Sodium metaphosphate |
| Definition | Soluble sodium polyphosphates are obtained by fusion and subsequent chilling of sodium orthophosphates. These compounds are a class consisting of several amorphous, water-soluble polyphosphates composed of linear chains of metaphosphate units, (NaPO3)x where x ≥ 2, terminated by Na2PO4 groups. These substances are usually identified by their Na2O/P2O5 ratio or their P2O5 content. The Na2O/P2O5 ratios vary from about 1,3 for sodium tetrapolyphosphate, where x = approximately 4; to about 1,1 for Graham's salt, commonly called sodium hexametaphosphate, where x = 13 to 18; and to about 1,0 for the higher molecular weight sodium polyphosphates, where x = 20 to 100 or more. The pH of their solutions varies from 3,0 to 9,0 |
| Chemical name | Sodium polyphosphate |
| Einecs | 272-808-3 |
| Chemical formula | Heterogenous mixtures of sodium salts of linear condensed polyphosphoric acids of general formula H(n + 2)PnO(3n + 1) where ‘n’ is not less than 2 |
| Molecular weight | (102)n |
| Assay P2O5 content | Not less than 60 % and not more than 71 % on the ignited basis |
| Description | Colourless or white, transparent platelets, granules, or powders |
| Identification | |
A. Solubility | Very soluble in water |
B. Positive tests for sodium and for phosphate | |
C. pH of a 1 % solution | Between 3,0 and 9,0 |
| Purity | |
| Loss on ignition | Not more than 1 % |
| Water-insoluble matter | Not more than 0,1 % |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
2.INSOLUBLE POLYPHOSPHATEU.K.
| Synonyms | Insoluble sodium metaphosphate |
| Maddrell's salt |
| Insoluble sodium polyphosphate, IMP |
| Definition | Insoluble sodium metaphosphate is a high molecular weight sodium polyphosphate composed of two long metaphosphate chains (NaPO3)x that spiral in opposite directions about a common axis. The Na2O/P2O5 ratio is about 1,0. The pH of 1 in 3 suspension in water is about 6,5 |
| Chemical name | Sodium polyphosphate |
| Einecs | 272-808-3 |
| Chemical formula | Heterogenous mixtures of sodium salts of linear condensed polyphosphoric acids of general formula H(n + 2)PnO(3n + 1) where ‘n’ is not less than 2 |
| Molecular weight | (102)n |
| P2O5 content | Not less than 68,7 % and not more than 70,0 % |
| Description | White crystalline powder |
| Identification | |
A. Solubility | Insoluble in water, soluble in mineral acids and in solutions of potassium and ammonium (but not sodium) chlorides |
B. Positive tests for sodium and for phosphate | |
C. pH of 1 in 3 suspension in water | About 6,5 |
| Purity | |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 452 (ii) POTASSIUM POLYPHOSPHATEU.K.
| Synonyms | Potassium metaphosphate |
| Potassium polymetaphosphate |
| Kurrol salt |
| Definition | |
| Chemical name | Potassium polyphosphate |
| Einecs | 232-212-6 |
| Chemical formula | (KPO3)n |
| Heterogenous mixtures of potassium salts of linear condensed polyphosphoric acids of general formula H(n + 2)PnO(3n + 1) where ‘n’ is not less than 2 |
| Molecular weight | (118)n |
| P2O5 content | Not less than 53,5 % and not more than 61,5 % on the ignited basis |
| Description | Fine white powder or crystals or colourless glassy platelets |
| Identification | |
A. Solubility | 1 g dissolves in 100 ml of a 1 in 25 solution of sodium acetate |
B. Positive tests for potassium and for phosphate | |
C. pH of a 1 % suspension | Not more than 7,8 |
| Purity | |
| Loss on ignition | Not more than 2 % (105 oC, four hours followed by ignition at 550 oC, 30 minutes) |
| Cyclic phosphate | Not more than 8 % on P2O5 content |
| Fluoride | Not more than 10 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 452(iii) SODIUM CALCIUM POLYPHOSPHATEU.K.
| Synonym | Sodium calcium polyphosphate, glassy |
| Definition | |
| Chemical name | Sodium calcium polyphosphate |
| Einecs | 233-782-9 |
| Chemical formula | (NaPO3)n CaO where n is typically 5 |
| Assay | Not less than 61 % and not more than 69 % as P2O5 |
| Description | White glassy crystals, spheres |
| Identification | |
A. pH of a 1 % m/m slurry | Approximately 5 to 7 |
B. CaO content | 7 %-15 % m/m |
| Purity | |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 4 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 452 (iv) CALCIUM POLYPHOSPHATEU.K.
| Synonyms | Calcium metaphosphate |
| Calcium polymetaphosphate |
| Definition | |
| Chemical name | Calcium polyphosphate |
| Einecs | 236-769-6 |
| Chemical formula | (CaP2O6)n |
| Heterogenous mixtures of calcium salts of condensed polyphosphoric acids of general formula H(n + 2)PnO(n + 1) where ‘n’ is not less than 2 |
| Molecular weight | (198)n |
| P2O5 content | Not less than 71 % and not more than 73 % on the ignited basis |
| Description | Odourless, colourless crystals or white powder |
| Identification | |
A. Solubility | Usually sparingly soluble in water. Soluble in acid medium |
B. Positive tests for calcium and for phosphate | |
C. CaO content | 27 to 29,5 % |
| Purity | |
| Loss on ignition | Not more than 2 % (105 oC, four hours followed by ignition at 550 oC, 30 minutes) |
| Cyclic phosphate | Not more than 8 % on P2O5 content |
| Fluoride | Not more than 30 mg/kg (expressed as fluorine) |
| Arsenic | Not more than 3 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Lead | Not more than 4 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 459 BETA-CYCLODEXTRINU.K.
| Definition | Beta-cyclodextrin is a non-reducing cyclic saccharide consisting of seven α-1,4-linked D-glucopyranosyl units. The product is manufactured by the action of the enzyme cycloglycosyltransferase (CGTase) obtained from Bacillus circulans, Paenibacillus macerans or recombinant Bacillus licheniformis strain SJ1608 on partially hydrolysed starch |
| Chemical name | Cycloheptaamylose |
| Einecs | 231-493-2 |
| Chemical formula | (C6H10O5)7 |
| Molecular weight | 1 135 |
| Assay | Content not less than 98,0 % of (C6H10O5)7 on an anhydrous basis |
| Description | Virtually odourless white or almost white crystalline solid |
| Identification | |
A. Solubility | Sparingly soluble in water; freely soluble in hot water; slightly soluble in ethanol |
B. Specific rotation | [α]25 D: + 160o to + 164o (1 % solution) |
| Purity | |
| Water | Not more than 14 % (Karl Fischer method) |
| Other cyclodextrins | Not more than 2 % on an anhydrous basis |
| Residual solvents (toluene and trichloroethylene) | Not more than 1 mg/kg for each solvent |
| Sulphated ash | Not more than 0,1 % |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
E 460 (i) MICROCRISTALLINE CELLULOSEU.K.
| Synonyms | Cellulose gel |
| Definition | Microcrystalline cellulose is purified, partally depolymerised cellulose prepared by treating alpha-cellulose, obtained as a pulp from natural strains of fibrous plant material, with mineral acids. The degree of polymerisation is typically less than 400 |
| Chemical name | Cellulose |
| Einecs | 232-674-9 |
| Chemical formula | (C6H10O5)n |
| Molecular weight | About 36 000 |
| Assay | Not less than 97 % calculated as cellulose on the anhydrous basis |
| Description | A fine white or almost white odourless powder |
| Identification | |
A. Solubility | Insoluble in water, ethanol, ether and dilute mineral acids. Slightly soluble in sodium hydroxide solution |
B. Colour reaction | To 1 mg of the sample, add 1 ml of phosphoric acid and heat on a water bath for 30 minutes. Add 4 ml of a 1 in 4 solution of pyrocatechol in phosphoric acid and heat for 30 minutes. A red colour is produced |
C. To be identified by IR spectroscopy | |
D. Suspension test | Mix 30 g of the sample with 270 ml of water in a high-speed (12 000 rpm) power blender for 5 minutes. The resultant mixture will be either a free-following suspension or a heavy, lumpy suspension which flows poorly, if at all, settles only slightly and contains many trapped air bubbles. If a free-flowing suspension is obtained, transfer 100 ml into a 100-ml graduated cylinder and allow to stand for 1 hour. The solids settles and a supernatant liquid appears |
| Purity | |
| Loss on drying | Not more than 7 % (105 oC, 3 hours) |
| Water-soluble matter | Not more than 0,24 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| pH of a 10 % suspension in water | The pH of the supernatant liquid is between 5,0 and 7,5 |
| Starch | Not detectable |
| To 20 ml of the dispersion obtained in identification, test D, add a few drops of iodine solution and mix. No purplish to blue or blue colour should be produced |
| Particle size | Not less than 5 μm (not more than 10 % of particles of less than 5 μm) |
| Carboxyl groups | Not more than 1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 460 (ii) POWDERED CELLULOSEU.K.
| Definition | Purified, mechanically disintegrated celluslose prepared by processing alpha-cellulose obtained as a pulp from natural strains of fibrous plant materials |
| Chemical name | Cellulose |
| Linear polymer of 1:4 linked glucose residues |
| Einecs | 232-674-9 |
| Chemical formula | (C6H10O5)n |
| Molecular weight | (162)n (n is predominantly 1 000 and greater) |
| Assay | Content not less than 92 % |
| Description | A white, odourless powder |
| Identification | |
A. Solubility | Insoluble in water, ethanol, ether and dilute mineral acids. Slightly soluble in sodium hydroxide solution |
B. Suspension test | Mix 30 g of the sample with 270 ml of water in a high-speed (12 000 rpm) power blender for 5 minutes. The resultant mixture will be either a free-flowing suspension or a heavy, lumpy suspension which flows poorly, if at all, settles only slightly and contains many trapped air bubbles. If a free-flowing suspension is obtained, transfer 100 ml into a 100-ml graduated cylinder and allow to stand for 1 hour. The solids settle and a supernatant liquid appears |
| Purity | |
| Loss on drying | Not more than 7 % (105 oC, 3 hours) |
| Water-soluble matter | Not more than 1,0 % |
| Sulphated ash | Not more than 0,3 % determined at 800 ± 25 oC |
| pH of a 10 % suspension in water | The pH of the supernatant liquid is between 5,0 and 7,5 |
| Starch | Not detectable |
| To 20 ml of the dispersion obtained in identification, test B, add a few drops of iodine solution and mix. No purplish to blue or blue colour should be produced |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Particle size | Not less than 5 μm (not more than 10 % of particles of less than 5 μm) |
E 461 METHYL CELLULOSEU.K.
| Synonyms | Cellulose methyl ether |
| Definition | Methyl cellulose is cellulose obtained directly from natural strains of fibrous plant material and partially etherified with methyl groups |
| Chemical name | Methyl ether of cellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2)(OR3) where R1, R2, R3 each may be one of the following:
|
| Molecular weight | From about 20 000 to 380 000 |
| Assay | Content not less than 25 % and not more than 33 % of methoxyl groups (-OCH3) and not more than 5 % of hydroxyethoxyl groups (-OCH2CH2OH) |
| Description | Slightly hygroscopic white or slightly yellowish or greyish odourless and tasteless, granular or fibrous powder |
| Identification | |
A. Solubility | Swelling in water, producing a clear to opalescent, viscous, colloidal solution. |
| Insoluble in ethanol, ether and chloroform. |
| Soluble in glacial acetic acid |
| Purity | |
| Loss on drying | Not more than 10 % (105 oC, 3 hours) |
| Sulphated ash | Not more than 1,5 % determined at 800 ± 25 oC |
| pH of a 1 % colloidal solution | Not less than 5,0 and not more than 8,0 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 462 ETHYL CELLULOSEU.K.
| Synonyms | Cellulose ethyl ether |
| Definition | Ethyl cellulose is cellulose obtained directly from fibrous plant material and partially etherified with ethyl groups |
| Chemical name | Ethyl ether of cellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2) where R1 and R2 may be any of the following:
|
| Assay | Content not less than 44 % and not more than 50 % of ethoxyl groups (-OC2H5) on the dried basis (equivalent to not more than 2,6 ethoxyl groups per anhydroglucose unit) |
| Description | Slightly hygroscopic white to off-white, odourless and tasteless powder |
| Identification | |
A. Solubility | Practically insoluble in water, in glycerol and in propane-1,2-diol but soluble in varying proportions in certain organic solvents depending upon the ethoxyl content. Ethyl cellulose containing less than 46 to 48 % of ethoxyl groups is freely soluble in tetrahydrofuran, in methyl acetate, in chloroform and in aromatic hydrocarbon ethanol mixtures. Ethyl cellulose containing 46 to 48 % or more of ethoxyl groups is freely soluble in ethanol, in methanol, in toluene, in chloroform and in ethyl acetate |
B. Film forming test | Dissolve 5 g of the sample in 95 g of an 80:20 (w/w) mixture of toluene ethanol. A clear, stable, slightly yellow solution is formed. Pour a few ml of the solution onto a glass plate and allow the solvent to evaporate. A thick, tough, continuous, clear film remains. The film is flammable |
| Purity | |
| Loss on drying | Not more than 3 % (105 oC, 2 hours) |
| Sulphated ash | Not more than 0,4 % |
| pH of a 1 % colloidal solution | Neutral to litmus |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 463 HYDROXYPROPYL CELLULOSEU.K.
| Synonyms | Cellulose hydroxypropyl ether |
| Definition | Hydroxypropylcellulose is cellulose obtained directly from natural strains of fibrous plant material and partially etherified with hydroxypropyl groups |
| Chemical name | Hydroxypropyl ether of cellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2)(OR3), where R1, R2, R3 each may be one of the following:
|
| Molecular weight | From about 30 000 to 1 000 000 |
| [Assay | Content not more than 80,5 % of hydroxypropoxyl groups (-OCH 2 CHOHCH 3 ) equivalent to not more than 4,6 hydroxypropyl groups per anhydroglucose unit on the anhydrous basis] |
| Description | Slightly hygroscopic white or slightly yellowish or greyish odourless and tasteless, granular or fibrous powder |
| Identification | |
A. Solubility | Swelling in water, producing a clear to opalescent, viscous, colloidal solution. Soluble in ethanol. Insoluble in ether |
B. Gas chromatography | Determine the substituents by gas chromotography |
| Purity | |
| Loss on drying | Not more than 10 % (105 oC, 3 hours) |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| pH of a 1 % colloidal solution | Not less than 5,0 and not more than 8,0 |
| Propylene chlorohydrins | Not more than 0,1 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 464 HYDROXYPROPYL METHYL CELLULOSEU.K.
| Definition | Hydroxypropyl methyl cellulose is cellulose obtained directly from natural strains of fibrous plant material and partially etherified with methyl groups and containing a small degree of hydroxypropyl substitution |
| Chemical name | 2-Hydroxypropyl ether of methylcellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2)(OR3), where R1, R2 R3 each may be one of the following:
|
| Molecular weight | From about 13 000 to 200 000 |
| Assay | Content not less than 19 % and not more than 30 % methoxyl groups (-OCH3) and not less than 3 % and not more than 12 % hydroxypropoxyl groups (-OCH2CHOHCH3), on the anhydrous basis |
| Description | Slightly hygroscopic white or slightly yellowish or greyish odourless and tasteless, granular or fibrous powder |
| Identification | |
A. Solubility | Swelling in water, producing a clear to opalescent, viscous, colloidal solution. Insoluble in ethanol |
B. Gas chromatography | Determine the substituents by gas chromatography |
| Purity | |
| Loss on drying | Not more than 10 % (105 oC, 3 hours) |
| Sulphated ash | Not more than 1,5 % for products with viscosities of 50 mPa.s or above |
| Not more than 3 % for products with viscosities below 50 mPa.s |
| pH of a 1 % colloidal solution | Not less than 5,0 and not more than 8,0 |
| Propylene chlorohydrins | Not more than 0,1 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 465 ETHYL METHYL CELLULOSEU.K.
| Synonyms | Methylethylcellulose |
| Definition | Ethyl methyl cellulose is cellulose obtained directly from natural strains of fibrous plant material and partially etherified with methyl and ethyl groups |
| Chemical name | Ethyl methyl ether of cellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2)(OR3), where R1, R2 R3 each may be one of the following:
|
| Molecular weight | From about 30 000 to 40 000 |
| Assay | Content on the anhydrous basis not less than 3,5 % and not more than 6,5 % of methoxyl groups (-OCH3) and not less than 14,5 % and not more than 19 % of ethoxyl groups (-OCH2CH3), and not less than 13,2 % and not more than 19,6 % of total alkoxyl groups, calculated as methoxyl |
| Description | Slightly hygroscopic white or slightly yellowish or greyish odourless and tasteless, granular or fibrous powder |
| Identification | |
A. Solubility | Swelling in water, producing a clear to opalescent, viscous, colloidal solution. Soluble in ethanol. Insoluble in ether |
| Purity | |
| Loss on drying | Not more than 15 % for the fibrous form, and not more than 10 % for the powdered form (105 oC to constant weight) |
| Sulphated ash | Not more than 0,6 % |
| pH of a 1 % colloidal solution | Not less than 5,0 and not more than 8,0 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
E 466 SODIUM CARBOXY METHYL CELLULOSEU.K.
| Synonyms | Carboxy methyl cellulose |
| CMC |
| NaCMC |
| Sodium CMC |
| Cellulose gum |
| Definition | Carboxy methyl cellulose is the partial sodium salt of a carboxymethyl ether of cellulose, the cellulose being obtained directly from natural strains of fibrous plant material |
| Chemical name | Sodium salt of the carboxymethyl ether of cellulose |
| Chemical formula | The polymers contain substituted anhydroglucose units with the following general formula: |
| C6H7O2(OR1)(OR2)(OR3), where R1, R2 R3 each may be one of the following:
|
| Molecular weight | Higher than approximately 17 000 (degree of polymerisation approximately 100) |
| Assay | Content on the anhydrous basis not less than 99,5 % |
| Description | Slightly hygroscopic white or slightly yellowish or greyish odourless and tasteless, granular or fibrous powder |
| Identification | |
A. Solubility | Yields a viscous colloidal solution with water. Insoluble in ethanol |
B. Foam test | A 0,1 % solution of the sample is shaken vigorously. No layer of foam appears. (This test permits the distinction of sodium carboxymethyl cellulose from other cellulose ethers) |
C. Precipitate formation | To 5 ml of a 0,5 % solution of the sample, add 5 ml of 5 % solution of copper sulphate or of aluminium sulphate. A precipitate appears. (This test permits the distinction of sodium carboxymethyl cellulose from other cellulose ethers and from gelatine, locust bean gum and tragacanth) |
D. Colour reaction | Add 0,5 g powdered carboxy methyl cellulose sodium to 50 ml of water, while stirring to produce an uniform dispersion. Continue the stirring until a clear solution is produced, and use the solution for the following test:
To 1 mg of the sample, diluted with an equal volume of water, in a small test tube, add 5 drops of 1-naphthol solution. Incline the test tube, and carefully introduce down the side of the tube 2 ml of sulphuric acid so that it forms a lower layer. A red-purple colour develops at the interface
|
| Purity | |
| Degree of substitution | Not less than 0,2 and not more than 1,5 carboxymethyl groups (-CH2COOH) per anhydroglucose unit |
| Loss on drying | Not more than 12 % (105 oC to constant weight) |
| pH of a 1 % colloidal solution | Not less than 5,0 and not more than 8,5 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 20 mg/kg |
| Total glycolate | Not more than 0,4 %, calculated as sodium glycolate on the anhydrous basis |
| Sodium | Not more than 12,4 % on the anhydrous basis |
E 468 CROSS-LINKED SODIUM CARBOXYMETHYLCELLULOSEU.K.
| Synonyms | Cross-linked carboxymethyl cellulose |
| Cross-linked CMC |
| Cross-linked sodium CMC |
| Cross-linked cellulose gum |
| Definition | Cross-linked sodium carboxymethyl cellulose is the sodium salt of thermally cross-linked partly O-carboxymethylated cellulose |
| Chemical name | Sodium salt of the cross-linked carboxymethyl ether cellulose |
| Chemical formula | The polymers containing substituted anhydroglucose units with the general formula: |
| C6H7O2(OR1)(OR2)(OR3) |
| where R1, R2 and R3 may be any of the following:
|
| Description | Slightly hygroscopic, white to off white, odourless powder |
| Identification | |
| A. | Shake 1 g with 100 ml of a solution containing 4 mg/kg methylene blue and allow to settle. The substance to be examined absorbs the methylene blue and settles as a blue, fibrous mass |
| B. | Shake 1 g with 50 ml of water. Transfer 1 ml of the mixture to a test tube, add 1 ml water and 0,05 ml of freshly prepared 40 g/l solution of alpha-naphthol in methanol. Incline the test tube and add carefully 2 ml of sulphuric acid down the side so that it forms a lower layer. A reddish-violet colour develops at the interface |
| C. | It gives the reaction of sodium |
| Purity | |
| Loss on drying | Not more than 6 % (105 oC, 3h) |
| Water solubles | Not more than 10 % |
| Degree of substitution | Not less than 0,2 and not more than 1,5 carboxymethyl groups per anhydroglucose unit |
| pH of 1 % | Not less than 5,0 and not more than 7,0 |
| Sodium content | Not more than 12,4 % on anhydrous basis |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 469 ENZYMATICALLY HYDROLYSED CARBOXYMETHYLCELLULOSEU.K.
| Synonyms | Sodium carboxymethyl cellulose, enzymatically hydrolysed |
| Definition | Enzymatically hydrolysed carboxymethylcellulose is obtained from carboxymethylcellulose by enzymatic digestion with a cellulase produced by Trichoderma longibrachiatum (formerly T. reesei) |
| Chemical name | Carboxymethyl cellulose, sodium, partially enzymatically hydrolysed |
| Chemical formula | Sodium salts of polymers containing substituted anhydroglucose units with the general formula: |
| [C6H7O2(OH)x(OCH2COONa)y]n |
| where n is the degree of polymerisation |
| x = 1,5 to 2,8 |
| y = 0,2 to 1,5 |
| x + y = 3,0 |
| (y = degree of substitution) |
| Formula weight | 178,14 where y = 0,2 |
| 282,18 where y = 1,5 |
| Macromolecules: Not less than 800 (n about 4) |
| Assay | Not less than 99,5 %, including mono- and disaccharides, on the dried basis |
| Description | White or slightly yellowish or greyish, odourless, slightly hygroscopic granular or fibrous powder |
| Identification | |
A. Solubility | Soluble in water, insoluble in ethanol |
B. Foam test | Vigorously shake a 0,1 % solution of the sample. No layer of foam appears. This test distinguishes sodium carboxymethyl cellulose, whether hydrolysed or not, from other cellulose ethers and from alginates and natural gums |
C. Precipitate formation | To 5 ml of a 0,5 % solution of the sample add 5 ml of a 5 % solution of copper or aluminium sulphate. A precipitate appears. This test distinguishes sodium carboxymethyl cellulose, whether hydrolysed or not, from other cellulose ethers and from gelatine, carob bean gum and tragacanth gum |
D. Colour reaction | Add 0,5 g of the powdered sample to 50 ml of water, while stirring to produce a uniform dispersion. Continue the stirring until a clear solution is produced. Dilute 1 ml of the solution with 1 ml of water in a small test tube. Add 5 drops of 1-naphthol TS. Incline the tube, and carefully introduce down the side of the tube 2 ml of sulphuric acid so that it forms a lower layer. A red-purple colour develops at the interface |
E. Viscosity (60 % solids) | Not less than 2,5 kgm-1s-1 at 25 oC corresponding to an average molecule weight of 5 000 D |
| Purity | |
| Loss on drying | Not more than 12 % (105 oC to constant weight) |
| Degree of substitution | Not less than 0,2 and not more than 1,5 carboxymethyl groups per anhydroglucose unit on the dried basis |
| pH of a 1 % colloidal solution | Not less than 6,0 and not more than 8,5 |
| Sodium chloride and sodium glycolate | Not more than 0,5 % singly or in combination |
| Residual enzyme activity | Passes test. No change in viscosity of test solution occurs, which indicates hydrolysis of the sodium carboxymethyl cellulose |
| Lead | Not more than 3 mg/kg |
E 470a SODIUM, POTASSIUM AND CALCIUM SALTS OF FATTY ACIDSU.K.
| Definition | Sodium, potassium and calcium salts of fatty acids occurring in food oils and fats, these salts being obtained either from edible fats and oils or from distilled food fatty acids |
| Assay | Content on the anhydrous basis not less than 95 % |
| Description | White or creamy white light powders, flakes or semi-solids |
| Identification | |
A. Solubility | Sodium and potassium salts: soluble in water and ethanol calcium salts: |
| insoluble in water, ethanol and ether |
B. Positive tests for cations and for fatty acids | |
| Purity | |
| Sodium | Not less than 9 % and not more than 14 % expressed as Na2O |
| Potassium | Not less than 13 % and not more than 21,5 % expressed as K2O |
| Calcium | Not less than 8,5 % and not more than 13 % expressed as CaO |
| Unsaponifiable matter | Not more than 2 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Free alkali | Not more than 0,1 % expressed as NaOH |
| Matter insoluble in alcohol | Not more than 0,2 % (sodium and potassium salts only) |
E 470b MAGNESIUM SALTS OF FATTY ACIDSU.K.
| Definition | Magnesium salts of fatty acids occurring in foods oils and fats, these salts being obtained either from edible fats and oils or from distilled food fatty acids |
| Assay | Content on the anhydrous basis not less than 95 % |
| Description | White or creamy-white light powders, flakes or semi-solids |
| Identification | |
A. Solubility | Insoluble in water, partially soluble in ethanol and ether |
B. Positive tests for magnesium and for fatty acids | |
| Purity | |
| Magnesium | Not less than 6,5 % and not more than 11 % expressed as MgO |
| Free alkali | Not more than 0,1 % expressed as MgO |
| Unsaponifiable matter | Not more than 2 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 471 MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Glyceryl monostearate |
| Glyceryl monopalmitate |
| Glyceryl monooleate, etc. |
| Monostearin, monopalmitin, monoolein, etc. |
| GMS (for glyceryl monostearate) |
| Definition | Mono- and diglycerides of fatty acids consist of mixtures of glycerol mono-, di- and triesters of fatty acids occurring in food oils and fats. They may contain small amounts of free fatty acids and glycerol |
| Assay | Content of mono- and diesters: not less than 70 % |
| Description | The product varies from a pale yellow to pale brown oily liquid to a white or slightly off-white hard waxy solid. The solids may be in the form of flakes, powders or small beads |
| Identification | |
A. Infrared spectrum | Characteristic of a partial fatty acid ester of a polyol |
B. Positive tests for glycerol and for fatty acids | |
C. Solubility | Insoluble in water, soluble in ethanol and toluene |
| Purity | |
| Water content | Not more than 2 % (Karl Fischer method) |
| Acid value | Not more than 6 |
| Free glycerol | Not more than 7 % |
| Polyglycerols | Not more than 4 % diglycerol and not more than 1 % higher polyglycerols both based on total glycerol content |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total glycerol | Not less than 16 % and not more than 33 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
E 472 a ACETIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Acetic acid esters of mono- and diglycerides |
| Acetoglycerides |
| Acetylated mono- and diglycerides |
| Acetic and fatty acid esters of glycerol |
| Definition | Esters of glycerol with acetic and fatty acids occurring in food fats and oils. They may contain small amounts of free glycerol, free fatty acids, free acetic acid and free glycerides |
| Description | Clear, mobile liquids to solids, from white to pale yellow in colour |
| Identification | |
A. Positive tests for glycerol, for fatty acids and for acetic acid | |
B. Solubility | Insoluble in water. Soluble in ethanol |
| Purity | |
| Acids other than acetic and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total acetic acid | Not less than 9 % and not more than 32 % |
| Free fatty acids (and acetic acid) | Not more than 3 % estimated as oleic acid |
| Total glycerol | Not less than 14 % and not more than 31 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
E 472 b LACTIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Lactic acid esters of mono- and diglycerides |
| Lactoglycerides |
| Mono- and diglycerides of fatty acids esterified with lactic acid |
| Definition | Esters of glycerol with lactic acid and fatty acids occurring in food fats and oils. They may contain small amounts of free glycerol, free fatty acids, free lactic acid and free glycerides |
| Description | Clear, mobile liquids to waxy solids of variable consistency, from white to pale yellow in colour |
| Identification | |
A. Positive tests for glycerol, for fatty acids and for lactic acid | |
B. Solubility | Insoluble in cold water but dispersible in hot water |
| Purity | |
| Acids other than lactic and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total lactic acid | Not less than 13 % and not more than 45 % |
| Free fatty acids (and lactic acid) | Not more than 3 % estimated as oleic acid |
| Total glycerol | Not less than 13 % and not more than 30 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
E 472 c CITRIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however, these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Citrem |
| Citric acid esters of mono- and diglycerides |
| Citroglycerides |
| Mono- and diglycerides of fatty acids esterified with citric acid |
| Definition | Esters of glycerol with citric acid and fatty acids occurring in food oils and fats. They may contain small amounts of free glycerol, free fatty acids, free citric acid and free glycerides. They may be partially or wholly neutralised with sodium hydroxide or with potassium hydroxide |
| Description | Yellowish or light brown liquids to waxy solids or semi-solids |
| Identification | |
A. Positive tests for glycerol, for fatty acids and for citric acid | |
B. Solubility | Insoluble in cold water |
| Dispersible in hot water |
| Soluble in oils and fats |
| Insoluble in cold ethanol |
| Purity | |
| Acids other than citric and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Total glycerol | Not less than 8 % and not more than 33 % |
| Total citric acid | Not less than 13 % and not more than 50 % |
| Sulphated ash (determined at 800 ± 25 oC) | Non-neutralised products: not more than 0,5 % |
| Partially or wholly neutralised products: not more than 10 % |
| Lead | Not more than 2 mg/kg |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
E 472 d TARTARIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Tartaric acid esters of mono- and diglycerides |
| Mono- and diglycerides of fatty acids esterified with tartaric acid |
| Definition | Esters of glycerol with tartaric acid and fatty acids occurring in food fats and oils. They may contain small amounts of free glycerol, free fatty acids, free tartaric acid and free glycerides |
| Description | Sticky viscous yellowish liquids to hard yellow waxes |
| Identification | |
A. Positive tests for glycerol, for fatty acids and for tartaric acid | |
| Purity | |
| Acids other than tartaric and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Total glycerol | Not less than 12 % and not more than 29 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total tartaric acid | Not less than 15 % and not more than 50 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
E 472 e MONO- AND DIACETYLTARTARIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Diacetyltartaric acid esters of mono- and diglycerides |
| Mono-and diglycerides of fatty acids esterified with mono- and diacetyltartaric acid |
| Diacetyltartaric and fatty acid esters of glycerol |
| Definition | Mixted esters of glycerol with mono- and diacetyltartaric acids (obtained from tartaric acid) and fatty acids occurring in food fats and oils. They may contain small amounts of free glycerol, free fatty acids, free tartaric and acetic acids and their combinations, and free glycerides. Contains also tartaric and acetic esters of fatty acids |
| Description | Sticky viscous liquids through a fat-like consistency to yellow waxes which hydrolyse in moist air to liberate acetic acid |
| Identification | |
A. Positive tests for glycerol, for fatty acids, for tartaric acid and for acetic acid | |
| Purity | |
| Acids other than acetic, tartaric and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Total glycerol | Not less than 11 % and not more than 28 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total tartaric acid | Not less than 10 % and not more than 40 % |
| Total acetic acid | Not less than 8 % and not more than 32 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
E 472 f MIXED ACETIC AND TARTARIC ACID ESTERS OF MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Mono- and diglycerides of fatty acids esterified with acetic acid and tartaric acid |
| Definition | Esters of glycerol with acetic and tartaric acids and fatty acids occurring in food fats and oils. They may contain small amounts of free glycerol, free fatty acids, free tartaric and ecetic acids, and free glycerides. May contain mono- and diacetyltartaric esters of mono- and diglycerides of fatty acids |
| Description | Sticky liquids to solids, from white to pale-yellow in colour |
| Identification | |
A. Positive tests for glycerol, for fatty acids, for tartaric acid and for acetic acid | |
| Purity | |
| Acids other than acetic, tartaric and fatty acids | Not detectable |
| Free glycerol | Not more than 2 % |
| Total glycerol | Not less than 12 % and not more than 27 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Total acetic acid | Not less than 10 % and not more than 20 % |
| Total tartaric acid | Not less than 20 % and not more than 40 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
E 473 SUCROSE ESTERS OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Sucroesters |
| Sugar esters |
| Definition | Essentially the mono-, di- and triesters of sucrose with fatty acids occurring in food fats and oils. They may be prepared from sucrose and the methyl and ethyl esters of food fatty acids or by extraction from sucroglycerides. No organic solvent other than dimethylsulphoxide, dimethylformamide, ethyl acetate, propane-2-ol, 2-methyl-1-propanol, propylene glycol and methyl ethyl ketone may be used for their preparation |
| Assay | Content not less than 80 % |
| Description | Stiff gels, soft solids or white to slightly greyish-white powders |
| Identification | |
A. Positive tests for sugar for fatty acids | |
B. Solubility | Sparingly soluble in water |
| Soluble in ethanol |
| Purity | |
| Sulphated ash | Not more than 2 % determined at 800 ± 25 oC |
| Free sugar | Not more than 5 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Methanol | Not more than 10 mg/kg |
| Dimethylsulphoxide | Not more than 2 mg/kg |
| Dimethylformamide | Not more than 1 mg/kg |
| 2-methyl-1-propanol | Not more than 10 mg/kg |
| Ethylacetate | | Not more than 350 mg/kg, singly or in combination |
| Propane-2-ol |
| Prolyleneglycol |
| Methyl ethyl ketone | Not more than 10 mg/kg |
E 474 SUCROGLYCERIDESU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Sugar glycerides |
| Definition | Sucroglycerides are produced by reacting sucrose with an edible fat or oil to produce a mixture of essentially mono-, di- and triesters of sucrose and fatty acids together with residual mono-, di- and triglycerides from fat or oil. No organic solvents shall be used in their preparation other than cyclohexane, dimethylformamide, ethyl acetate, 2-methyl-1-propanol and propane-2-ol |
| Assay | Content not less than 40 % and not more than 60 % of sucrose fatty acid esters |
| Description | Soft solid masses, stiff gels or white to off-white powders |
| Identification | |
A. Positive tests for sugar and for fatty acids | |
B. Solubility | Insoluble in cold water |
| Soluble in ethanol |
| Purity | |
| Sulphated ash | Not more than 2 % determined at 800 ± 25 oC |
| Free sugar | Not more than 5 % |
| Free fatty acids | Not more than 3 % estimated as oleic acid |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Methanol | Not more than 10 mg/kg |
| Dimethylformamide | Not more than 1 mg/kg |
| 2-methyl-1-propanol | | Not more than 10 mg/kg, single or in combination |
| Cyclohexane |
| Ethylacetate | | Not more than 350 mg/kg, single or in combination |
| Propane-2-ol |
E 475 POLYGLYCEROL ESTERS OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Polyglycerol fatty acid esters |
| Polyglycerin esters of fatty acid esters |
| Definition | Polyglycerol esters of fatty acids are produced by the esterification of polyglycerol with food fats and oils or with fatty acids occurring in foods fats and oils. The polyglycerol moiety is predominantly di-, tri- and tetraglycerol and contains not more than 10 % of polyglycerols equal to or higher than heptaglycerol |
| Assay | Content of total fatty acid ester not less than 90 % |
| Description | Light yellow to amber, oily to very viscous liquids; light tan to medium brown, plastic or soft solids; and light tan to brown, hard, waxy solids |
| Identification | |
A. Positive tests for glycerol, for polyglycerols and for fatty acids | |
B. Solubility | The esters range from very hydrophilic to very lipophilic, but as a class tend to be dispersible in water and soluble in organic solvents and oils |
| Purity | |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| Acids other than fatty acids | Not detectable |
| Free fatty acids | Not more than 6 % estimated as oleic acid |
| Total glycerol and polyglycerol | Not less than 18 % and not more than 60 % |
| Free glycerol and polyglycerol | Not more than 7 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 476 POLYGLYCEROL POLYRICINOLEATEU.K.
| Synonyms | Glycerol esters of condensed castor oil fatty acids |
| Polyglycerol esters of polycondensed fatty acids from castor oil |
| Polyglycerol esters of interesterified ricinoleic acid |
| PGPR |
| Definition | Polyglycerol polyricinoleate is prepared by the esterification of polyglycerol with condensed castor oil fatty acids |
| Description | Clear, highly viscous liquid |
| Identification | |
A. Solubility | Insoluble in water and in ethanol. |
| Soluble in ether, hydrocarbons and halogenated hydrocarbons |
B. Positive tests for glycerol, polyglycerol and for ricinoleic acid | |
C. Refractive index [n]65 | Between 1,463 and 1,4665 |
| Purity | |
| Polyglycerols | The polyglycerol moiety shall be composed of not less than 75 % of di-, tri- and tetraglycerols and shall contain not more than 10 % of polyglycerols equal to or higher than heptaglycerol |
| Hydroxyl value | Not less than 80 and not more than 100 |
| Acid value | Not more than 6 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 477 PROPANE-1,2-DIOL ESTERS OF FATTY ACIDSU.K.
| Note: Purity criteria apply to the additive free of sodium, potassium and calcium salts of fatty acids, however these substances may be present up to a maximum level of 6 % (expressed as sodium oleate). |
| Synonyms | Propylene glycol esters of fatty acids |
| Definition | Consists of mixtures of propane-1,2-diol mono- and diesters of fatty acids occurring in food fats and oils. The alcohol moiety is exclusively propane-1,2-diol together with dimer and traces of trimer. Organic acids other than food fatty acids are absent |
| Assay | Content of total fatty acid ester not less than 85 % |
| Description | Clear liquids or waxy white flakes, beads or solids having a bland odour |
| Identification | |
A. Positive tests for propylene glycol and for fatty acids | |
| Purity | |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| Acids other than fatty acids | Not detectable |
| Free fatty acids | Not more than 6 % estimated as oleic acid |
| Total propane-1,2-diol | Not less than 11 % and not more than 31 % |
| Free propane-1,2-diol | Not more than 5 % |
| Dimer and trimer of propylene glycol | Not more than 0,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 479 b THERMALLY OXIDISED SOYA BEAN OIL INTERACTED WITH MONO- AND DIGLYCERIDES OF FATTY ACIDSU.K.
| Synonyms | TOSOM |
| Definition | Thermally oxidised soya bean oil interacted with mono- and diglycerides of fatty acids is a complex mixture of esters of glycerol and fatty acids found in edible fat and fatty acids from thermally oxidised soya bean oil. It is produced by interaction and desodorisation under vacuum at 130 oC of 10 % of thermally oxidised soya bean oil and 90 % mono- and diglycerides of food fatty acids. Soya bean oil is exclusively made from natural strains of soya beans |
| Description | Pale yellow to light brown a waxy or solid consistency |
| Identification | |
A. Solubility | Insoluble in water. Soluble in hot oil or fat |
| Purity | |
| Melting range | 55-65 oC |
| Free fatty acids | Not more than 1,5 % estimated as oleic acid |
| Free glycerol | Not more than 2 % |
| Total fatty acids | 83-90 % |
| Total glycerol | 16-22 % |
| Fatty acid methyl esters, not forming adduct with urea | Not more than 9 % of total fatty acid methyl esters |
| Fatty acids, insoluble in petroleum ether | Not more than 2 % of total fatty acids |
| Peroxide value | Not more than 3 |
| Epoxides | Not more than 0,03 % oxirane oxygen |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 481 SODIUM STEAROYL-2-LACTYLATEU.K.
| Synonyms | Sodium stearoyl lactylate |
| Sodium stearoyl lactate |
| Definition | A mixture of the sodium salts of stearoyl lactylic acids and its polymers and minor amounts of sodium salts of other related acids, manufactured by the reaction of stearic acid and lactic acid. Other food fatty acids may also be present, free or esterified, due to their presence in the stearic acid used |
| Chemical names | Sodium di-2-stearoyl lactate |
| Sodium di(2-stearoyloxy)propionate |
| Einecs | 246-929-7 |
| Chemical formula (major components) | C21H39O4Na |
| C19H35O4Na |
| Description | White or slightly yellowish powder or brittle solid with a characteristic odour |
| Identification | |
A. Positive tests for sodium, for fatty acids and for lactic acid | |
B. Solubility | Insoluble in water. Soluble in ethanol |
| Purity | |
| Sodium | Not less than 2,5 % and not more than 5 % |
| Ester value | Not less than 90 and not more than 190 |
| Acid value | Not less than 60 and not more than 130 |
| Total lactic acid | Not less than 15 % and not more than 40 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 482 CALCIUM STEAROYL-2-LACTYLATEU.K.
| Synonyms | Calcium stearoyl lactate |
| Definition | A mixture of the calcium salts of stearoyl lactylic acids and its polymers and minor amounts of calcium salts of other related acids, manufactured by the reaction of stearic acid and lactic acid. Other food fatty acids may also be present, free or esterified, due to their presence in the stearic acid used |
| Chemical name | Calcium di-2-stearoyl lactate |
| Calcium di(2-stearoyloxy)propionate |
| Einecs | 227-335-7 |
| Chemical formula | C42H78O8Ca |
| C38H70O8Ca |
| Description | White or slightly yellowish powder or brittle solid with a characteristic odour |
| Identification | |
A. Positive tests for calcium, for fatty acids and for lactid acid | |
B. Solubility | Slightly soluble in hot water |
| Purity | |
| Calcium | Not less than 1 % and not more than 5,2 % |
| Ester value | Not less than 125 and not more than 190 |
| Total lactic acid | Not less than 15 % and not more than 40 % |
| Acid value | Not less than 50 and not more than 130 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 483 STEARYL TARTRATEU.K.
| Synonyms | Stearyl palmityl tartrate |
| Definition | Product of the esterification of tartaric acid with commercial stearyl alcohol, which consists essentially of stearyl and palmityl alcohols. It consists mainly of diester, with minor amounts of monoester and of unchanged starting materials |
| Chemical name | Distearyl tartrate |
| Dipalmityl tartrate |
| Chemical formula | C38H74O6 to C40H78O6 |
| Molecular weight | 627 to 655 |
| Assay | Content of total ester not less than 90 % corresponding to an ester value of not less than 163 and not more than 180 |
| Description | Cream-coloured unctuous solid (at 25 oC) |
| Identification | |
A. Positive tests for tartare | |
B. Melting range | Between 67 oC and 77 oC. After saponification the saturated long chain fatty alcohols have a melting range of 49 oC to 55 oC |
| Purity | |
| Hydroxyl value | Not less than 200 and not more than 220 |
| Acid value | Not more than 5,6 |
| Total tartaric acid content | Not less than 18 % and not more than 35 % |
| Sulphated ash | Not more than 0,5 % determined at 800 ± 25 oC |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Unsaponifiable matter | Not less than 77 % and not more than 83 % |
| Iodine value | Not more than 4 (Wijs method) |
E 491 SORBITAN MONOSTEARATEU.K.
| Definition | A mixture of the partial esters of sorbitol and its anhydrides with edible, commercial stearic acid |
| Einecs | 215-664-9 |
| Assay | Content not less than 95 % of a mixture of sorbitol, sorbitan, and isosorbide esters |
| Description | Light, cream- to tan-coloured beads or flakes or a hard, waxy solid with a slight characteristic odour |
| Identification | |
A. Solubility | Soluble at temperatures above its melting point in toluene, dioxane, carbon tetrachloride, ether, methanol, ethanol and aniline; insoluble in petroleum ether and acetone; insoluble in cold water but dispersible in warm water; soluble with haze at temperatures above 50 oC in mineral oil and ethyl acetate |
B. Congealing range | 50-52 oC |
C. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyol |
| Purity | |
| Water | Not more than 2 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,5 % |
| Acid value | Not more than 10 |
| Saponification value | Not less than 147 and not more than 157 |
| Hydroxyl value | Not less than 235 and not more than 260 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 492 SORBITAN TRISTEARATEU.K.
| Definition | A mixture of the partial esters of sorbitol and its anhydrides with edible, commercial stearic acid |
| Einecs | 247-891-4 |
| Assay | Content not less than 95 % of a mixture of sorbitol, sorbitan, and isosorbide esters |
| Description | Light, cream- to tan-coloured beads or flakes or hard, waxy solid with a slight odour |
| Identification | |
A. Solubility | Slightly soluble in toluene, ether, carbon tetrachloride and ethyl acetate; dispersible in petroleum ether, mineral oil, vegetable oils, acetone and dioxane; insoluble in water, methanol and ethanol |
B. Congealing range | 47-50 oC |
C. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyol |
| Purity | |
| Water | Not more than 2 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,5 % |
| Acid value | Not more than 15 |
| Saponification value | Not less than 176 and not more than 188 |
| Hydroxyl value | Not less than 66 and not more than 80 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 493 SORBITAN MONOLAURATEU.K.
| Definition | A mixture of the partial esters of sorbitol and its anhydrides with edible, commercial lauric acid |
| Einecs | 215-663-3 |
| Assay | Content not less than 95 % of a mixture of sorbitol, sorbitan, and isosorbide esters |
| Description | Amber-coloured oily viscous liquid, light cream to tan-coloured beads or flakes or a hard, waxy solid with a slight odour |
| Identification | |
A. Solubility | Dispersible in hot and cold water |
B. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of a polyol |
| Purity | |
| Water | Not more than 2 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,5 % |
| Acid value | Not more than 7 |
| Saponification value | Not less than 155 and not more than 170 |
| Hydroxyl value | Not less than 330 and not more than 358 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 494 SORBITAN MONOOLEATEU.K.
| Definition | A mixture of the partial esters of sorbitol and its anhydrides with edible, commercial oleic acid. Major constituent is 1,4-sorbitan monooleate. Other constituents include isosorbide monooleate, sorbitan dioleate and sorbitan trioleate |
| Einecs | 215-665-4 |
| Assay | Content not less than 95 % of a mixture of sorbitol, sorbitan and isosorbide esters |
| Description | Amber-coloured viscous liquid, light cream to tan-coloured beads or flakes or a hard, waxy solid with a slight characteristic odour |
| Identification | |
A. Solubility | Soluble at temperatures above its melting point in ethanol, ether, ethyl acetate, aniline, toluene, dioxane, petroleum ether and carbon tetrachloride. Insoluble in cold water, dispersible in warm water |
B. Iodine value | The residue of oleic acid, obtained from the saponification of the sorbitan monoleate in assay, has a iodine value between 80 and 100 |
| Purity | |
| Water | Not more than 2 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,5 % |
| Acid value | Not more than 8 |
| Saponification value | Not less than 145 and not more than 160 |
| Hydroxyl value | Not less than 193 and not more than 210 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 495 SORBITAN MONOPALMITATEU.K.
| Synonyms | Sorbitan palmitate |
| Definition | A mixture of the partial esters of sorbitol and its anhydrides with edible, commercial palmitic acid |
| Einecs | 247-568-8 |
| Assay | Content not less than 95 % of a mixture of sorbitol, sorbitan, and isosorbide esters |
| Description | Light cream to tan-coloured beads or flakes or a hard, waxy solid with a slight characteristic odour |
| Identification | |
A. Solubility | Soluble at temperatures above its melting point in ethanol, methanol, ether, ethyl acetate, aniline, toluene, dioxane, petroleum ether and carbon tetrachloride. Insoluble in cold water but dispersible in warm water |
B. Congealing range | 45-47 oC |
C. Infrared absorption spectrum | Characteristic of a partial fatty acid ester of polyol |
| Purity | |
| Water | Not more than 2 % (Karl Fischer method) |
| Sulphate ash | Not more than 0,5 % |
| Acid value | Not more than 7,5 |
| Saponification value | Not less than 140 and not more than 150 |
| Hydroxyl value | Not less than 270 and not more than 305 |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 500(i) SODIUM CARBONATEU.K.
| Synonyms | Soda ash |
| Definition | |
| Chemical name | Sodium carbonate |
| Einecs | 207-838-8 |
| Chemical formula | Na2CO3 · nH2O (n = 0, 1 or 10) |
| Molecular weight | 106,0 (anhydrous) |
| Assay | Content not less than 99 % of Na2CO3 on the anhydrous basis |
| Description | Colourless crystals or white, granular or crystalline powder |
| The anhydrous form is hygroscopic, the decahydrate efflorescent |
| Identification | |
A. Positive tests for sodium and for carbonate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 2 % (anhydrous), 15 % (monohydrate) or 55 %-65 % (decahydrate) (70 oC raising gradually to 300 oC, to constant weight) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 500(ii) SODIUM HYDROGEN CARBONATEU.K.
| Synonyms | Sodium bicarbonate, sodium acid carbonate, bicarbonate of soda, baking soda |
| Definition | |
| Chemical name | Sodium hydrogen carbonate |
| Einecs | 205-633-8 |
| Chemical formula | NaHCO3 |
| Molecular weight | 84,01 |
| Assay | Content not less than 99 % on the anhydrous basis |
| Description | Colourless or white crystalline masses or crystalline powder |
| Identification | |
A. Positive tests for sodium and for carbonate | |
B. pH of a 1 % solution | Between 8,0 and 8,6 |
C. Solubility | Soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 0,25 % (over silica gel, 4h) |
| Ammonium salts | No odour of ammonia detectable after heating |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 500(iii) SODIUM SESQUICARBONATEU.K.
| Definition | |
| Chemical name | Sodium monohydrogen dicarbonate |
| Einecs | 208-580-9 |
| Chemical formula | Na2(CO)3 · NaHCO3 · 2H2O |
| Molecular weight | 226,03 |
| Assay | Content between 35,0 % and 38,6 % of NaHCO3 and between 46,4 % and 50,0 % of Na2CO3 |
| Description | White flakes, crystals or crystalline powder |
| Identification | |
A. Positive tests for sodium and for carbonate | |
B. Solubility | Freely soluble in water |
| Purity | |
| Sodium chloride | Not more than 0,5 % |
| Iron | Not more than 20 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 501(i) POTASSIUM CARBONATEU.K.
| Definition | |
| Chemical name | Potassium carbonate |
| Einecs | 209-529-3 |
| Chemical formula | K2CO3 · nH2O (n = 0 or 1,5) |
| Molecular weight | 138,21 (anhydrous) |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | White, very deliquescent powder. |
| The hydrate occurs as small, white, translucent crystals or granules |
| Identification | |
A. Positive tests for potassium and for carbonate | |
B. Solubility | Very soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 5 % (anhydrous) or 18 % (hydrate) (180 oC, 4h) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 501(ii) POTASSIUM HYDROGEN CARBONATEU.K.
| Synonyms | Potassium bicarbonate, acid potassium carbonate |
| Definition | |
| Chemical name | Potassium hydrogen carbonate |
| Einecs | 206-059-0 |
| Chemical formula | KHCO3 |
| Molecular weight | 100,11 |
| Assay | Content not less than 99,0 % and not more than 101,0 % KHCO3 on the anhydrous basis |
| Description | Colourless crystals or white powder or granules |
| Identification | |
A. Positive tests for potassium and for carbonate | |
B. Solubility | Freely soluble in water. Insoluble in ethanol |
| Purity | |
| Loss on drying | Not more than 0,25 % (over silica gel, 4h) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 503(i) AMMONIUM CARBONATEU.K.
| Definition | Ammonium carbonate consists of ammonium carbamate, ammonium carbonate and ammonium hydrogen carbonate in varying proportions |
| Chemical name | Ammonium carbonate |
| Einecs | 233-786-0 |
| Chemical formula | CH6N2O2, CH8N2O3 and CH5NO3 |
| Molecular weight | Ammonium carbamate 78,06; ammonium carbonate 98,73; ammonium hydrogen carbonate 79,06 |
| Assay | Content not less than 30,0 % and not more than 34,0 % of NH3 |
| Description | White powder or hard, white or translucent masses or crystals. Becomes opaque on exposure to air and is finally converted into white porous lumps or powder (of ammonium bicarbonate) due to loss of ammonia and carbon dioxide |
| Identification | |
A. Positive tests for ammonium and for carbonate | |
B. pH of a 5 % solution | about 8,6 |
C. Solubility | Soluble in water |
| Purity | |
| Non-volatile matter | Not more than 500 mg/kg |
| Chlorides | Not more than 30 mg/kg |
| Sulphate | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 503(ii) AMMONIUM HYDROGEN CARBONATEU.K.
| Synonyms | Ammonium bicarbonate |
| Definition | |
| Chemical name | Ammonium hydrogen carbonate |
| Einecs | 213-911-5 |
| Chemical formula | CH5NO3 |
| Molecular weight | 79,06 |
| Assay | Content not less than 99,0 % |
| Description | White crystals or crystalline powder |
| Identification | |
A. Positive tests for ammonium and for carbonate | |
B. pH of a 5 % solution | about 8,0 |
C. Solubility | Freely soluble in water. Insoluble in ethanol |
| Purity | |
| Non-volatile matter | Not more than 500 mg/kg |
| Chlorides | Not more than 30 mg/kg |
| Sulphate | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
[E 504(i) MAGNESIUM CARBONATE U.K.
| Synonyms | Hydromagnesite |
| Definition | Magnesium carbonate is a basic hydrated or a monohydrated magnesium carbonate or a mixture of the two |
| Chemical name | Magnesium carbonate |
| Chemical formula | MgCO 3 .nH 2 O |
| Einecs | 208-915-9 |
| Assay | Not less than 24 % and not more than 26,4 % of Mg |
| Description | Odourless, light, white friable masses or as a bulky white powder |
| Identification |
|---|
A. Solubility | Practically insoluble both in water or ethanol |
B. Positive tests for magnesium and for carbonate | |
| Purity |
|---|
| Acid insoluble matter | Not more than 0,05 % |
| Water soluble matter | Not more than 1 % |
| Calcium | Not more than 0,4 % |
| Arsenic | Not more than 4 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg] |
E 504(ii) MAGNESIUM HYDROXIDE CARBONATEU.K.
| Synonyms | Magnesium hydrogen carbonate, magnesium subcarbonate (light or heavy), hydrated basic magnesium carbonate, magnesium carbonate hydroxide |
| Definition | |
| Chemical name | Magnesium carbonate hydroxide hydrated |
| Einecs | 235-192-7 |
| Chemical formula | 4MgCO3Mg(OH)25H2O |
| Molecular weight | 485 |
| Assay | Mg content not less than 40,0 % and not more than 45,0 % calculated as MgO |
| Description | Light, white friable mass or bulky white powder |
| Identification | |
A. Positive tests for magnesium and for carbonate | |
B. Solubility | Practically insoluble in water. Insoluble in ethanol |
| Purity | |
| Acid insoluble matter | Not more than 0,05 % |
| Water soluble matter | Not more than 1,0 % |
| Calcium | Not more than 1,0 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 507 HYDROCHLORIC ACIDU.K.
| Synonyms | Hydrogen chloride, muriatic acid |
| Definition | |
| Chemical name | Hydrochloric acid |
| Einecs | 231-595-7 |
| Chemical formula | HCl |
| Molecular weight | 36,46 |
| Assay | Hydrochloric acid is commercially available in varying concentrations. Concentrated hydrochloric acid contains not less than 35,0 % HCl |
| Description | Clear, colourless or slightly yellowish, corrosive liquid having a pungent odour |
| Identification | |
A. Positive tests for acid and for chloride | |
B. Solubility | Soluble in water and in ethanol |
| Purity | |
| Total organic compounds | Total organic compounds (non-fluorine containing): not more than 5 mg/kg |
| Benzene: not more than 0,05 mg/kg |
| Fluorinated compounds (total): not more than 25 mg/kg |
| Non-volatile matter | Not more than 0,5 % |
| Reducing substances | Not more than 70 mg/kg (as SO2) |
| Oxidising substances | Not more than 30 mg/kg (as Cl2) |
| Sulphate | Not more than 0,5 % |
| Iron | Not more than 5 mg/kg |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 508 POTASSIUM CHLORIDEU.K.
| Synonyms | Sylvine |
| Sylvite |
| Definition | |
| Chemical name | Potassium chloride |
| Einecs | 231-211-8 |
| Chemical formulae | KCl |
| Molecular weight | 74,56 |
| Assay | Content not less than 99 % on the dried basis |
| Description | Colourless, elongated, prismatic or cubital crystals or white granular powder. Odourless |
| Identification | |
A. Solubility | Freely soluble in water. Insoluble in ethanol |
B. Positive tests for potassium and for chloride | |
| Purity | |
| Loss on drying | Not more than 1 % (105 oC, 2 hours) |
| Sodium | Negative test |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
E 509 CALCIUM CHLORIDEU.K.
| Definition | |
| Chemical name | Calcium chloride |
| Einecs | 233-140-8 |
| Chemical formula | CaCl2 · nH2O (n = 0,2 or 6) |
| Molecular weight | 110,99 (anhydrous), 147,02 (dihydrate), 219,08 (hexahydrate) |
| Assay | Content not less than 93,0 % on the anhydrous basis |
| Description | White, odourless, hygroscopic powder or deliquescent crystals |
| Identification | |
A. Positive tests for calcium and for chloride | |
B. Solubility | Anhydrous calcium chloride: freely soluble in water and ethanol |
| Dihydrate: freely soluble in water, soluble in ethanol |
| Hexahydrate: very soluble in water and ethanol |
| Purity | |
| Magnesium and alkali salts | Not more than 5 % on the anhydrous basis |
| Fluoride | Not more than 40 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 511 MAGNESIUM CHLORIDEU.K.
| Definition | |
| Chemical name | Magnesium chloride |
| Einecs | 232-094-6 |
| Chemical formula | MgCl2 · 6H2O |
| Molecular weight | 203,3 |
| Assay | Content not less than 99,0 % |
| Description | Colourless, odourless, very deliquescent flakes or crystals |
| Identification | |
A. Positive tests for magnesium and for chloride | |
B. Solubility | Very soluble in water, freely soluble in ethanol |
| Purity | |
| Ammonium | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 512 STANNOUS CHLORIDEU.K.
| Synonyms | Tin chloride, tin dichloride |
| Definition | |
| Chemical name | Stannous chloride dihydrate |
| Einecs | 231-868-0 |
| Chemical formula | SnCl2 · 2H2O |
| Molecular weight | 225,63 |
| Assay | Content not less than 98,0 % |
| Description | Colourless or white crystals |
| May have a slight odour of hydrochloric acid |
| Identification | |
A. Positive tests for tin (II) and for chloride | |
B. Solubility | Water: soluble in less than its own weight of water, but it forms an insoluble basic salt with excess water |
| Ethanol: soluble |
| Purity | |
| Sulphate | Not more than 30 mg/kg |
| Arsenic | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
E 513 SULPHURIC ACIDU.K.
| Synonyms | Oil of vitriol, dihydrogen sulphate |
| Definition | |
| Chemical name | Sulphuric acid |
| Einecs | 231-639-5 |
| Chemical formula | H2SO4 |
| Molecular weight | 98,07 |
| Assay | Sulphuric acid is commercially available in varying concentrations. The concentrated form contains not less than 96,0 % |
| Description | Clear, colourless or slightly brown, very corrosive oily liquid |
| Identification | |
A. Positive tests for acid and for sulphate | |
B. Solubility | Miscible with water, with generation of much heat, also with ethanol |
| Purity | |
| Ash | Not more than 0,02 % |
| Reducing matter | Not more than 40 mg/kg (as SO2) |
| Nitrate | Not more than 10 mg/kg (on H2SO4 basis) |
| Chloride | Not more than 50 mg/kg |
| Iron | Not more than 20 mg/kg |
| Selenium | Not more than 20 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 514(i) SODIUM SULPHATEU.K.
| Definition | |
| Chemical name | Sodium sulphate |
| Chemical formula | Na2SO4 · nH2O (n = 0 or 10) |
| Molecular weight | 142,04 (anhydrous) |
| 322,04 (decahydrate) |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | Colourless crystals or a fine, white, crystalline powder |
| The decahydrate is efflorescent |
| Identification | |
A. Positive tests for sodium and for sulphate | |
B. Acidity of a 5 % solution: neutral or slightly alkaline to litmus paper | |
| Purity | |
| Loss on drying | Not more than 1,0 % (anhydrous) or not more than 57 % (decahydrate) at 130 °C |
| Selenium | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 514(ii) SODIUM HYDROGEN SULPHATEU.K.
| Synonyms | Acid sodium sulphate, sodium bisulphate, nitre cake |
| Definition | |
| Chemical name | Sodium hydrogen sulphate |
| Chemical formula | NaHSO4 |
| Molecular weight | 120,06 |
| Assay | Content not less than 95,2 % |
| Description | White, odourless crystals or granules |
| Identification | |
A. Positive tests for sodium and for sulphate | |
B. Solutions are strongly acidic | |
| Purity | |
| Loss on drying | Not more than 0,8 % |
| Water insoluble | Not more than 0,05 % |
| Selenium | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 515(i) POTASSIUM SULPHATEU.K.
| Definition | |
| Chemical name | Potassium sulphate |
| Chemical formula | K2SO4 |
| Molecular weight | 174,25 |
| Assay | Content not less than 99,0 % |
| Description | Colourless or white crystals or crystalline powder |
| Identification | |
A. Positive tests for potassium and for sulphate | |
B. pH of a 5 % solution | Between 5,5 and 8,5 |
C. Solubility | Freely soluble in water, insoluble in ethanol |
| Purity | |
| Selenium | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 515(ii) POTASSIUM HYDROGEN SULPHATEU.K.
| Definition | |
| Synonyms | Potassium bisulphate, potassium acid sulphate |
| Chemical name | Potassium hydrogen sulphate |
| Chemical formula | KHSO4 |
| Molecular weight | 136,17 |
| Assay | Content not less than 99 % |
| Melting point | 197 oC |
| Description | White deliquescent crystals, pieces or granules |
| Identification | |
A. Positive test for potassium | |
B. Solubility | Freely soluble in water, insoluble in ethanol |
| Purity | |
| Selenium | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 516 CALCIUM SULPHATEU.K.
| Synonyms | Gypsum, selenite, anhydrite |
| Definition | |
| Chemical name | Calcium sulphate |
| Einecs | 231-900-3 |
| Chemical formula | CaSO4 · nH2O (n = 0 or 2) |
| Molecular weight | 136,14 (anhydrous), 172,18 (dihydrate) |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | Fine, white to slightly yellowish-white odourless powder |
| Identification | |
A. Positive tests for calcium and for sulphate | |
B. Solubility | Slightly soluble in water, insoluble in ethanol |
| Purity | |
| Loss on drying | Anhydrous: not more than 1,5 % (250 oC, constant weight) |
| Dihydrate: not more than 23 % (ibid.) |
| Fluoride | Not more than 30 mg/kg |
| Selenium | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 517 AMMONIUM SULPHATEU.K.
| Definition | |
| Chemical name | Ammonium sulphate |
| Einecs | 231-984-1 |
| Chemical formula | (NH4)2SO4 |
| Molecular weight | 132,14 |
| Assay | Content not less than 99,0 % and not more than 100,5 % |
| Description | White powder, shining plates or crystalline fragments |
| Identification | |
A. Positive tests for ammonium and for sulphate | |
B. Solubility | Freely soluble in water, insoluble in ethanol |
| Purity | |
| Loss on ignition | Not more than 0,25 % |
| Selenium | Not more than 30 mg/kg |
| Lead | Not more than 5 mg/kg |
E 520 ALUMINIUM SULPHATEU.K.
| Synonyms | Alum |
| Definition | |
| Chemical name | Aluminium sulphate |
| Einecs | 233-135-0 |
| Chemical formula | Al2(SO4)3 |
| Molecular weight | 342,13 |
| Assay | Content not less than 99,5 % on the ignited basis |
| Description | White powder, shining plates or crystalline fragments |
| Identification | |
A. Positive tests for aluminium and for sulphate B. pH of a 5 % solution 2,9 or above | |
C. Solubility | Freely soluble in water, insoluble in ethanol |
| Purity | |
| Loss on ignition | Not more than 5 % (500 oC, 3h) |
| Alkalies and alkaline earths | Not more than 0,4 % |
| Selenium | Not more than 30 mg/kg |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 521 ALUMINIUM SODIUM SULPHATEU.K.
| Synonyms | Soda alum, sodium alum |
| Definition | |
| Chemical name | Aluminium sodium sulphate |
| Einecs | 233-277-3 |
| Chemical formula | AlNa(SO4)2 · nH2O (n = 0 or 12) |
| Molecular weight | 242,09 (anhydrous) |
| Assay | Content on the anhydrous basis not less than 96,5 % (anhydrous) and 99,5 % (dodecahydrate) |
| Description | Transparent crystals or white crystalline powder |
| Identification | |
A. Positive tests for aluminium, for sodium and for sulphate | |
B. Solubility | Dodecahydrate is freely soluble in water. The anhydrous form is slowly soluble in water. Both forms are insoluble in ethanol |
| Purity | |
| Loss on drying | Anhydrous form: not more than 10,0 % (220 oC, 16h) |
| Dodecahydrate: not more than 47,2 % (50 oC-55 oC, 1h then 200 oC, 16h) |
| Ammonium salts | No odour of ammonia detectable after heating |
| Selenium | Not more than 30 mg/kg |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 522 ALUMINIUM POTASSIUM SULPHATEU.K.
| Synonyms | Potassium alum, potash alum |
| Definition | |
| Chemical name | Aluminium potassium sulphate dodecahydrate |
| Einecs | 233-141-3 |
| Chemical formula | AlK(SO4)2 · 12 H2O |
| Molecular weight | 474,38 |
| Assay | Content not less than 99,5 % |
| Description | Large, transparent crystals or white crystalline powder |
| Identification | |
A. Positive tests for aluminium, for potassium and for sulphate | |
B. pH of a 10 % solution between 3,0 and 4,0 | |
C. Solubility | Freely soluble in water, insoluble in ethanol |
| Purity | |
| Ammonium salts | No odour of ammonia detectable after heating |
| Selenium | Not more than 30 mg/kg |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 523 ALUMINIUM AMMONIUM SULPHATEU.K.
| Synonyms | Ammonium alum |
| Definition | |
| Chemical name | Aluminium ammonium sulphate |
| Einecs | 232-055-3 |
| Chemical formula | AlNH4(SO4)2 · 12 H2O |
| Molecular weight | 453,32 |
| Assay | Content not less than 99,5 % |
| Description | Large, colourless crystals or white powder |
| Identification | |
A. Positive tests for aluminium, for ammonium and for sulphate | |
B. Solubility | Freely soluble in water, soluble in ethanol |
| Purity | |
| Alkali metals and alkaline earths | Not more than 0,5 % |
| Selenium | Not more than 30 mg/kg |
| Fluoride | Not more than 30 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 524 SODIUM HYDROXIDEU.K.
| Synonyms | Caustic soda, lye |
| Definition | |
| Chemical name | Sodium hydroxide |
| Einecs | 215-185-5 |
| Chemical formula | NaOH |
| Molecular weight | 40,0 |
| Assay | Content of solid forms not less than 98,0 % of total alkali (as NaOH). Content of solutions accordingly, based on the stated or labelled percentage of NaOH |
| Description | White or nearly white pellets, flakes, sticks, fused masses or other forms. Solutions are clear or slightly turbid, colourless or slightly coloured, strongly caustic and hygroscopic and when exposed to the air they absorb carbon dioxide, forming sodium carbonate |
| Identification | |
A. Positive tests for sodium | |
B. A 1 % solution is strongly alkaline | |
C. Solubility | Very soluble in water. Freely soluble in ethanol |
| Purity | |
| Water insoluble and organic matter | A 5 % solution is completely clear and colourless to slightly coloured |
| Carbonate | Not more than 0,5 % (as Na2CO3) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 0,5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 525 POTASSIUM HYDROXIDEU.K.
| Synonyms | Caustic potash |
| Definition | |
| Chemical name | Potassium hydroxide |
| Einecs | 215-181-3 |
| Chemical formula | KOH |
| Molecular weight | 56,11 |
| Assay | Content not less than 85,0 % of alkali calculated as KOH |
| Description | White or nearly white pellets, flakes, sticks, fused masses or other forms |
| Identification | |
A. Positive tests for potassium | |
B. A 1 % solution is strongly alkaline | |
C. Solubility | Very soluble in water. Freely soluble in ethanol |
| Purity | |
| Water insoluble matter | A 5 % solution is completely clear and colourless |
| Carbonate | Not more than 3,5 % (as K2CO3) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
[E 526 CALCIUM HYDROXIDE U.K.
| Synonyms | Slaked lime, hydrated lime |
| Definition |
|---|
| Chemical name | Calcium hydroxide |
| Einecs | 215-137-3 |
| Chemical formula | Ca(OH) 2 |
| Molecular weight | 74,09 |
| Assay | Content not less than 92 % |
| Description | White powder |
| Identification |
|---|
A. Positive tests for alkali and for calcium | |
B. Solubility | Slightly soluble in water. Insoluble in ethanol. Soluble in glycerol |
| Purity |
|---|
| Acid insoluble ash | Not more than 1,0 % |
| Magnesium and alkali salts | Not more than 2,7 % |
| Barium | Not more than 300 mg/kg |
| Fluoride | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 6 mg/kg] |
E 527 AMMONIUM HYDROXIDEU.K.
| Synonyms | Aqua ammonia, strong ammonia solution |
| Definition | |
| Chemical name | Ammonium hydroxide |
| Chemical formula | NH4OH |
| Molecular weight | 35,05 |
| Assay | Content not less than 27 % of NH3 |
| Description | Clear, colourless solution, having an exceedingly pungent, characteristic odour |
| Identification | |
A. Positive tests for ammonia | |
| Purity | |
| Non-volatile matter | Not more than 0,02 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
E 528 MAGNESIUM HYDROXIDEU.K.
| Definition | |
| Chemical name | Magnesium hydroxide |
| Einecs | 215-170-3 |
| Chemical formula | Mg(OH)2 |
| Molecular weight | 58,32 |
| Assay | Content not less than 95,0 % on the anhydrous basis |
| Description | Odourless, white bulky powder |
| Identification | |
A. Positive test for magnesium and for alkali | |
B. Solubility | Practically insoluble in water and in ethanol |
| Purity | |
| Loss on drying | Not more than 2,0 % (105 oC, 2h) |
| Loss on ignition | Not more than 33 % (800 oC to constant weight) |
| Calcium oxide | Not more than 1,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
[E 529 CALCIUM OXIDE U.K.
| Synonyms | Burnt lime |
| Definition | |
| Chemical name | Calcium oxide |
| Einecs | 215-138-9 |
| Chemical formula | CaO |
| Molecular weight | 56,08 |
| Assay | Content not less than 95 % on the ignited basis |
| Description | Odourless, hard, white or greyish white masses of granules, or white to greyish powder |
| Identification |
|---|
A. Positive test for alkali and for calcium | |
B. Heat is generated on moistening the sample in water | |
C. Solubility | Slightly soluble in water. Insoluble in ethanol. Soluble in glycerol |
| Purity |
|---|
| Loss on ignition | Not more than 10 % (ca. 800 °C to constant weight) |
| Acid insoluble matter | Not more than 1 % |
| Barium | Not more than 300 mg/kg |
| Magnesium and alkali salts | Not more than 3,6 % |
| Fluoride | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 7 mg/kg] |
E 530 MAGNESIUM OXIDEU.K.
| Definition | |
| Chemical name | Magnesium oxide |
| Einecs | 215-171-9 |
| Chemical formula | MgO |
| Molecular weight | 40,31 |
| Assay | Content not less than 98,0 % on the ignited basis |
| Description | A very bulky, white powder known as light magnesium oxide or a relative dense, white powder known as heavy magnesium oxide. 5 g of light magnesium oxide occupy a volume of 40 to 50 ml, while 5 g of heavy magnesium oxide occupy a volume of 10 to 20 ml |
| Identification | |
A. Positive test for alkali and for magnesium | |
B. Solubility | Practically insoluble in water. Insoluble in ethanol |
| Purity | |
| Loss on ignition | Not more than 5,0 % (ca 800 oC to constant weight) |
| Calcium oxide | Not more than 1,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
E 535 SODIUM FERROCYANIDEU.K.
| Synonyms | Yellow prussiate of soda, sodium hexacyanoferrate |
| Definition | |
| Chemical name | Sodium ferrocyanide |
| Einecs | 237-081-9 |
| Chemical formula | Na4Fe(CN)6 · 10 H2O |
| Molecular weight | 484,1 |
| Assay | Content not less than 99,0 % |
| Description | Yellow crystals or crystalline powder |
| Identification | |
A. Positive test for sodium and for ferrocyanide | |
| Purity | |
| Free moisture | Not more than 1,0 % |
| Water insoluble matter | Not more than 0,03 % |
| Chloride | Not more than 0,2 % |
| Sulphate | Not more than 0,1 % |
| Free cyanide | Not detectable |
| Ferricyanide | Not detectable |
| Lead | Not more than 5 mg/kg |
E 536 POTASSIUM FERROCYANIDEU.K.
| Synonyms | Yellow prussiate of potash, potassium hexacyanoferrate |
| Definition | |
| Chemical name | Potassium ferrocyanide |
| Einecs | 237-722-2 |
| Chemical formula | K4Fe(CN)6· 3 H2O |
| Molecular weight | 422,4 |
| Assay | Content not less than 99,0 % |
| Description | Lemon yellow crystals |
| Identification | |
A. Positive test for potassium and for ferrocyanide | |
| Purity | |
| Free moisture | Not more than 1,0 % |
| Water insoluble matter | Not more than 0,03 % |
| Chloride | Not more than 0,2 % |
| Sulphate | Not more than 0,1 % |
| Free cyanide | Not detectable |
| Ferricyanide | Not detectable |
| Lead | Not more than 5 mg/kg |
E 538 CALCIUM FERROCYANIDEU.K.
| Synonyms | Yellow prussiate of lime, calcium hexacyanoferrate |
| Definition | |
| Chemical name | Calcium ferrocyanide |
| Einecs | 215-476-7 |
| Chemical formula | Ca2Fe(CN)6 · 12H2O |
| Molecular weight | 508,3 |
| Assay | Content not less than 99,0 % |
| Description | Yellow crystals or crystalline powder |
| Identification | |
A. Positive test for calcium and for ferrocyanide | |
| Purity | |
| Free moisture | Not more than 1,0 % |
| Water insoluble matter | Not more than 0,03 % |
| Chloride | Not more than 0,2 % |
| Sulphate | Not more than 0,1 % |
| Free cyanide | Not detectable |
| Ferricyanide | Not detectable |
| Lead | Not more than 5 mg/kg |
E 541 SODIUM ALUMINIUM PHOSPHATE, ACIDICU.K.
| Synonyms | SALP |
| Definition | |
| Chemical name | Sodium trialuminium tetradecahydrogen octaphosphate tetrahydrate (A) or |
| Trisodium dialuminium pentadecahydrogen octaphosphate (B) |
| Einecs | 232-090-4 |
| Chemical formula | NaAl3H14(PO4)8 · 4H2O (A) |
| Na3Al2H15(PO4)8 (B) |
| Molecular weight | 949,88 (A) |
| 897,82 (B) |
| Assay | Content not less than 95,0 % (both forms) |
| Description | White odourless powder |
| Identification | |
A. Positive test for sodium, for aluminium and for phosphate | |
B. pH | Acid to litmus |
C. Solubility | Insoluble in water. Soluble in hydrochloric acid |
| Purity | |
| Loss on ignition | 19,5 %-21,0 % (A) } (750 oC-800 oC, 2h) |
| 15 %-16 % (B) } (750 oC-800 oC, 2h) |
| Fluoride | Not more than 25 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 4 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 551 SILICON DIOXIDEU.K.
| Synonyms | Silica, silicium dioxide |
| Definition | Silicon dioxide is an amorphous substance, which is produced synthetically by either a vapour-phase hydrolysis process, yielding fumed silica, or by a wet process, yielding precipitated silica, silica gel, or hydrous silica. Fumed silica is produced in essentially an anhydrous state, whereas the wet-process products are obtained as hydrates or contain surface absorbed water |
| Chemical name | Silicon dioxide |
| Einecs | 231-545-4 |
| Chemical formula | (SiO2)n |
| Molecular weight | 60,08 (SiO2) |
| Assay | Content after ignition not less than 99,0 % (fumed silica) or 94,0 % (hydrated forms) |
| Description | White, fluffy powder or granules |
| Hygroscopic |
| Identification | |
A. Positive test for silica | |
| Purity | |
| Loss on drying | Not more than 2,5 % (fumed silica, 105 oC, 2h) |
| Not more than 8,0 % (precipitated silica and silica gel, 105 oC, 2h) |
| Not more than 70 % (hydrous silica, 105 oC, 2h) |
| Loss on ignition | Not more than 2,5 % after drying (1 000oC, fumed silica) |
| Not more than 8,5 % after drying (1 000oC, hydrated forms) |
| Soluble ionisable salts | Not more than 5,0 % (as Na2SO4) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 552 CALCIUM SILICATEU.K.
| Definition | Calcium silicate is a hydrous or anhydrous silicate with varying proportions of CaO and SiO2 |
| Chemical name | Calcium silicate |
| Einecs | 215-710-8 |
| Assay | Content on the anhydrous basis:
|
| Description | White to off-white free-flowing powder that remains so after absorbing relatively large amounts of water or other liquids |
| Identification | |
A. Positive test for silicate and for calcium | |
B. Forms a gel with mineral acids | |
| Purity | |
| Loss on drying | Not more than 10 % (105 oC, 2h) |
| Loss on ignition | Not less than 5 % and not more than 14 % (1 000oC, constant weight) |
| Sodium | Not more than 3 % |
| Fluoride | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 553a(i) MAGNESIUM SILICATEU.K.
| Definition | Magnesium silicate is a synthetic compound of which the molar ratio of magnesium oxide to silicon dioxide is approximately 2:5 |
| Assay | Content not less than 15 % of MgO and not less than 67 % of SiO2 on the ignited basis |
| Description | Very fine, white, odourless powder, free from grittiness |
| Identification | |
A. Positive test for magnesium and for silicate | |
B. pH of a 10 % slurry | Between 7,0 and 10,8 |
| Purity | |
| Loss on drying | Not more than 15 % (105 oC, 2h) |
| Loss on ignition | Not more than 15 % after drying (1 000oC, 20 min) |
| Water soluble salts | Not more than 3 % |
| Free alkali | Not more than 1 % (as NaOH) |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 553a(ii) MAGNESIUM TRISILICATEU.K.
| Definition | |
| Chemical name | Magnesium trisilicate |
| Chemical formula | Mg2Si3O8 · xH2O (approximate composition) |
| Einecs | 239-076-7 |
| Assay | Content not less than 29,0 % of MgO and not less than 65,0 % of SiO2 both on the ignited basis |
| Description | Fine, white powder, free from grittiness |
| Identification | |
A. Positive test for magnesium and for silicate | |
B. pH of a 5 % slurry | Between 6,3 and 9,5 |
| Purity | |
| Loss on ignition | Not less than 17 % and not more than 34 % (1 000oC) |
| Water soluble salts | Not more than 2 % |
| Free alkali | Not more than 1 % (as NaOH) |
| Fluoride | Not more than 10 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 553b TALCU.K.
| Synonyms | Talcum |
| Definition | Naturally occurring form of hydrous magnesium silicate containing varying proportions of such associated minerals as alpha-quartz, calcite, chlorite, dolomite, magnesite, and phlogopite |
| Chemical name | Magnesium hydrogen metasilicate |
| Einecs | 238-877-9 |
| Chemical formula | Mg3(Si4O10)(OH)2 |
| Molecular weight | 379,22 |
| Description | Light, homogeneous, white or almost white powder, greasy to the touch |
| Identification | |
A. IR absorption | Characteristic peaks at 3 677, 1 018 and 669 cm-1 |
B. X-ray diffraction | Peaks at 9,34/4,66/3,12 Å |
C. Solubility | Insoluble in water and ethanol |
| Purity | |
| Loss on drying | Not more than 0,5 % (105 oC, 1h) |
| Acid-soluble matter | Not more than 6 % |
| Water-soluble matter | Not more than 0,2 % |
| Acid-soluble iron | Not detectable |
| Arsenic | Not more than 10 mg/kg |
| Lead | Not more than 5 mg/kg |
E 554 SODIUM ALUMINIUM SILICATEU.K.
| Synonyms | Sodium silicoaluminate, sodium aluminosilicate, aluminium sodium silicate |
| Definition | |
| Chemical name | Sodium aluminium silicate |
| Assay | Content on the anhydrous basis:
as SiO2 not less than 66,0 % and not more than 88,0 %
as Al2O3 not less than 5,0 % and not more than 15,0 %
|
| Description | Fine white amorphous powder or beads |
| Identification | |
A. Positive tests for sodium, for aluminium and for silicate | |
B. pH of a 5 % slurry | Between 6,5 and 11,5 |
| Purity | |
| Loss on drying | Not more than 8,0 % (105 oC, 2h) |
| Loss on ignition | Not less than 5,0 % and not more than 11,0 % on the anhydrous basis (1 000oC, constant weight) |
| Sodium | Not less than 5 % and not more than 8,5 % (as Na2O) on the anhydrous basis |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 555 POTASSIUM ALUMINIUM SILICATEU.K.
| Synonyms | Mica |
| Definition | Natural mica consists of mainly potassium aluminium silicate (muscovite) |
| Einecs | 310-127-6 |
| Chemical name | Potassium aluminium silicate |
| Chemical formulae | KAl2[AlSi3O10](OH)2 |
| Molecular weight | 398 |
| Assay | Content not less than 98 % |
| Description | Light grey to white crystalline platelets or powder |
| Identification | |
A. Solubility | Insoluble in water, diluted acids and alkali and organic solvents |
| Purity | |
| Loss on drying | Not more than 0,5 % (105 oC, 2h) |
| Antimony | Not more than 20 mg/kg |
| Zinc | Not more than 25 mg/kg |
| Barium | Not more than 25 mg/kg |
| Chromium | Not more than 100 mg/kg |
| Copper | Not more than 25 mg/kg |
| Nickel | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 2 mg/kg |
| Lead | Not more than 10 mg/kg |
E 556 CALCIUM ALUMINIUM SILICATEU.K.
| Synonyms | Calcium aluminosilicate, calcium silicoaluminate, aluminium calcium silicate |
| Definition | |
| Chemical name | Calcium aluminium silicate |
| Assay | Content on the anhydrous basis:
as SiO2 not less than 44,0 % and not more than 50,0 %
as Al2O3 not less than 3,0 % and not more than 5,0 %
as CaO not less than 32,0 % and not more than 38,0 %
|
| Description | Fine white, free-flowing powder |
| Identification | |
A. Positive tests for calcium, for aluminium and for silicate | |
| Purity | |
| Loss on drying | Not more than 10,0 % (105 oC, 2h) |
| Loss on ignition | Not less than 14,0 % and not more than 18,0 on the anhydrous basis (1 000oC, constant weight) |
| Fluoride | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 10 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 558 BENTONITEU.K.
| Definition | Bentonite is a natural clay containing a high proportion of montmorillonite, a native hydrated aluminium silicate in which some aluminium and silicon atoms were naturally replaced by other atoms such as magnesium and iron. Calcium and sodium ions are trapped between the mineral layers. There are four common types of bentonite: natural sodium bentonite, natural calcium bentonite, sodium-activated bentonite and acid-activated bentonite |
| Einecs | 215-108-5 |
| Chemical formula | (Al, Mg)8(Si4O10) 4(OH)8 · 12H2O |
| Molecular weight | 819 |
| Assay | Montmorillonite content not less than 80 % |
| Description | Very fine, yellowish or greyish white powder or granules. The structure of bentonite allows it to absorb water in its structure and on its external surface (swelling properties) |
| Identification | |
A. Methylene blue test | |
B. X-Ray diffraction | Characteristic peaks at 12,5/15 A |
C. IR absorption | Peaks at 428/470/530/1 110-1 020/3 750 — 3 400 cm-1 |
| Purity | |
| Loss on drying | Not more than 15,0 % (105 oC, 2h) |
| Arsenic | Not more than 2 mg/kg |
| Lead | Not more than 20 mg/kg |
E 559 ALUMINIUM SILICATE (KAOLIN)U.K.
| Synonyms | Kaolin, light or heavy |
| Definition | Aluminium silicate hydrous (kaolin) is a purified white plastic clay composed of kaolinite, potassium aluminium silicate, feldspar and quartz. Processing should not include calcination. The raw kaolinitic clay used in the production of aluminium silicate shall have a level of dioxin which does not make it injurious to health or unfit for human consumption |
| Einecs | 215-286-4 (kaolinite) |
| Chemical formula | Al2Si2O5(OH)4 (kaolinite) |
| Molecular weight | 264 |
| Assay | Content not less than 90 % (sum of silica and alumina, after ignition) |
| Silica (SiO2) | Between 45 % and 55 % |
| Alumina (Al2O3) | Between 30 % and 39 % |
| Description | Fine, white or greyish white, unctuous powder. Kaolin is made up of loose aggregations of randomly oriented stacks of kaolinite flakes or of individual hexagonal flakes |
| Identification | |
A. Positive tests for alumina and for silicate | |
B. X-ray diffraction: | Characteristic peaks at 7,18/3,58/2,38/1,78 Å |
C. IR absorption: | Peaks at 3 700 and 3 620 cm-1 |
| Purity | |
| Loss on ignition | Between 10 and 14 % (1 000oC, constant weight) |
| Water soluble matter | Not more than 0,3 % |
| Acid soluble matter | Not more than 2 % |
| Iron | Not more than 5 % |
| Potassium oxide (K2O) | Not more than 5 % |
| Carbon | Not more than 0,5 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 570 FATTY ACIDSU.K.
| Definition | Linear fatty acids, caprylic acid (C8), capric acid (C10), laurinc acid (C12), myristic acid (C14), palmitic acid (C16), stearic acid (C18), oleic acid (C18:1) |
| Chemical name | octanoic acid (C8), decanoic acid (C10), dodecanoic acid (C12), tetradecanoic acid (C14), hexadecanoic acid (C16), octadecanoic acid (C18), 9-octadecenoic acid (C18:1) |
| Assay | Not less than 98 % by chromatography |
| Description | A colourless liquid or white solid obtained from oils and fats |
| Identification | |
A. Individual fatty acids can be identified by acid value, iodine value, gas chromatog-raphy and molecular weight | |
| Purity | |
| Residue on ignition | Not more than 0,1 % |
| Unsaponifiable matter | Not more than 1,5 % |
| Water | Not more than 0,2 % (Karl Fischer method) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 1 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 574 GLUCONIC ACIDU.K.
| Synonyms | D-gluconic acid, dextronic acid |
| Definition | Gluconic acid is an aqueous solution of gluconic acid and glucono-delta-lactone |
| Chemical name | Gluconic acid |
| Chemical formula | C6H12O7 (gluconic acid) |
| Molecular weight | 196,2 |
| Assay | Content not less than 50,0 % (as gluconic acid) |
| Description | Colourless to light yellow, clear syrupy liquid |
| Identification | |
A. Formation of phenylhydrazine derivative positive | Compound formed melts between 196 oC and 202 oC with decomposition |
| Purity | |
| Residue on ignition | Not more than 1,0 % |
| Reducing matter | Not more than 0,75 % (as D-glucose) |
| Chloride | Not more than 350 mg/kg |
| Sulphate | Not more than 240 mg/kg |
| Sulphite | Not more than 20 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 575 GLUCONO-DELTA-LACTONEU.K.
| Synonyms | Gluconolactone, GDL, D-gluconic acid delta-lactone, delta-gluconolactone |
| Definition | Glucono-delta-lactone is the cyclic 1,5-intramolecular ester of D-gluconic acid. In aqueous media it is hydrolysed to an equilibrium mixture of D-gluconic acid (55 %-66 %) and the delta- and gamma-lactones |
| Chemical name | D-Glucono-1,5-lactone |
| Einecs | 202-016-5 |
| Chemical formula | C6H10O6 |
| Molecular weight | 178,14 |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | Fine, white, nearly odourless, crystalline powder |
| Identification | |
A. Formation of phenylhydrazine derivative of gluconic acid positive | Compound formed melts between 196 oC and 202 oC with decomposition |
B. Solubility | Freely soluble in water. Sparingly soluble in ethanol |
C. Melting point | 152 oC ± 2 oC |
| Purity | |
| Water | Not more than 1,0 % (Karl Fischer method) |
| Reducing substances | Not more than 0,75 % (as D-glucose) |
| Lead | Not more than 2 mg/kg |
E 576 SODIUM GLUCONATEU.K.
| Synonyms | Sodium salt of D-gluconic acid |
| Definition | |
| Chemical name | Sodium D-gluconate |
| Einecs | 208-407-7 |
| Chemical formula | C6H11NaO7 (anhydrous) |
| Molecular weight | 218,14 |
| Assay | Content not less than 98,0 % |
| Description | White to tan, granular to fine, crystalline powder |
| Identification | |
A. Positive test for sodium and for gluconate | |
B. Solubility | Very soluble in water. Sparingly soluble in ethanol |
C. pH of a 10 % solution | Between 6,5 and 7,5 |
| Purity | |
| Reducing matter | Not more than 1,0 % (as D-glucose) |
| Lead | Not more than 2 mg/kg |
E 577 POTASSIUM GLUCONATEU.K.
| Synonyms | Potassium salt of D-gluconic acid |
| Definition | |
| Chemical name | Potassium D-gluconate |
| Einecs | 206-074-2 |
| Chemical formula | C6H11KO7 (anhydrous) |
| C6H11KO7 · H2O (monohydrate) |
| Molecular weight | 234,25 (anhydrous) |
| 252,26 (monohydrate) |
| Assay | Content not less than 97,0 % and not more than 103,0 % on dried basis |
| Description | Odourless, free flowing white to yellowish white, crystalline powder or granules |
| Identification | |
A. Positive test for potassium and for gluconate | |
B. pH of a 10 % solution | Between 7,0 and 8,3 |
| Purity | |
| Loss on drying | Anhydrous: not more than 3,0 % (105 oC, 4h, vacuum)
Monohydrate: not less than 6 % and not more than 7,5 % (105 oC, 4h, vacuum)
|
| Reducing substances | Not more than 1,0 % (as D-glucose) |
| Lead | Not more than 2 mg/kg |
E 578 CALCIUM GLUCONATEU.K.
| Synonyms | Calcium salt of D-gluconic acid |
| Definition | |
| Chemical name | Calcium di-D-gluconate |
| Einecs | 206-075-8 |
| Chemical formula | C12H22CaO14 (anhydrous) |
| C12H22CaO14 · H2O (monohydrate) |
| Molecular weight | 430,38 (anhydrous form) |
| 448,39 (monohydrate) |
| Assay | Content not less than 98,0 % and not more than 102 % on the anhydrous and monohydrate basis |
| Description | Odourless, white crystalline granules or powder, stable in air |
| Identification | |
A. Positive test for calcium and for gluconate | |
B. Solubility | Soluble in water, insoluble in ethanol |
C. pH of a 5 % solution | Between 6,0 and 8,0 |
| Purity | |
| Loss on drying | Not more than 3,0 % (105 oC, 16h) (anhydrous) |
| Not more than 2,0 % (105 oC, 16h) (monohydrate) |
| Reducing substances | Not more than 1,0 % (as D-glucose) |
| Lead | Not more than 2 mg/kg |
E 579 FERROUS GLUCONATEU.K.
| Definition | |
| Chemical name | Ferrous di-D-gluconate dihydrate |
| Iron(II) di-gluconate dihydrate |
| Einecs | 206-076-3 |
| Chemical formulae | C12H22FeO14·2H2O |
| Molecular weight | 482,17 |
| Assay | Content not less than 95 % on the dried basis |
| Description | Pale greenish-yellow to yellowish-grey powder or granules, which may have a faint odour of burnt sugar |
| Identification | |
A. Solubility | Soluble with slight heating in water. Practically insoluble in ethanol |
B. Positive test for ferrous ion | |
C. Formation of phenylhy-drazine derivative of gluconic acid positive | |
D. pH of a 10 % solution | Between 4 and 5,5 |
| Purity | |
| Loss on drying | Not more than 10 % (105 oC, 16 hours) |
| Oxalic acid | Not detectable |
| Iron (Fe III) | Not more than 2 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
| Reducing substances | Not more than 0,5 % expressed as glucose |
E 585 FERROUS LACTATEU.K.
| Synonyms | Iron(II) lactate |
| Iron(II) 2-hydroxy propanoate |
| Propanoic acid, 2-hydroxy-iron(2 +) salt (2:1) |
| Definition | |
| Chemical name | Ferrous 2-hydroxy propanoate |
| Einecs | 227-608-0 |
| Chemical formulae | C6H10FeO6·xH2O (x = 2 or 3) |
| Molecular weight | 270,02 (dihydrate) |
| 288,03 (trihydrate) |
| Assay | Content not less than 96 % on the dried basis |
| Description | Greenish-white crystals or light green powder having a characteristic smell |
| Identification | |
A. Solubility | Soluble in water. Practically insoluble in ethanol |
B. Positive test for ferrous ion and for lactate | |
C. pH of a 2 % solution | Between 4 and 6 |
| Purity | |
| Loss on drying | Not more than 18 % (100 oC, under vacuum, approximately 700 mm Hg) |
| Iron (Fe III) | Not more than 0,6 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Cadmium | Not more than 1 mg/kg |
E 586 4-HEXYLRESORCINOLU.K.
| Synonyms | 4-Hexyl-1,3-benzenediol |
| Hexylresorcinol |
| Definition | |
| Chemical name | 4-Hexylresorcinol |
| Einecs | 205-257-4 |
| Chemical formula | C12H18O2 |
| Molecular weight | 197,24 |
| Assay | Not less than 98 % on the dried basis |
| Description | White powder |
| Identification | |
A. Solubility | Freely soluble in ether and acetone; very slightly soluble in water |
B. Nitric acid test | To 1 ml of a saturated solution of the sample, add 1 ml of nitric acid. A light red colour appears |
C. Bromine test | To 1 ml of saturated solution of the sample, add 1 ml of bromine TS. A yellow, flocculent precipitate dissolves producing a yellow solution |
D. Melting range | 62 to 67 oC |
| Purity | |
| Acidity | Not more than 0,05 % |
| Sulphated ash | Not more than 0,1 % |
| Resorcinol and other phenols | Shake about 1 g of the sample with 50 ml of water for a few minutes, filter, and to the filtrate add 3 drops of ferric chloride TS. No red or blue colour is produced |
| Nickel | Not more than 2 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 3 mg/kg |
E 620 GLUTAMIC ACIDU.K.
| Synonyms | L-Glutamic acid, L-α-aminoglutaric acid |
| Definition | |
| Chemical name | L-Glutamic acid, L-2-amino-pentanedioic acid |
| Einecs | 200-293-7 |
| Chemical formula | C5H9NO4 |
| Molecular weight | 147,13 |
| Assay | Content not less than 99,0 % and not more than 101,0 % on the anhydrous basis |
| Description | White crystals or crystalline powder |
| Identification | |
A. Positive test for glutamic acid by thin layer chromatography | |
B. Specific rotation [α]D20 | Between + 31,5o and + 32,2o |
| (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
C. pH of a saturated solution | Between 3,0 and 3,5 |
| Purity | |
| Loss on drying | Not more than 0,2 % (80 oC, 3h) |
| Sulphated ash | Not more than 0,2 % |
| Chloride | Not more than 0,2 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 621 MONOSODIUM GLUTAMATEU.K.
| Synonyms | Sodium glutamate, MSG |
| Definition | |
| Chemical name | Monosodium L-glutamate monohydrate |
| Einecs | 205-538-1 |
| Chemical formula | C5H8NaNO4 · H2O |
| Molecular weight | 187,13 |
| Assay | Content not less than 99,0 % and not more than 101,0 % on the anhydrous basis |
| Description | White, practically odourless crystals or crystalline powder |
| Identification | |
A. Positive test for sodium | |
B. Positive test for glutamic acid by thin-layer chromatography | |
C. Specific rotation [α]D 20 | Between + 24,8o and + 25,3o |
| (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
D. pH of a 5 % solution | Between 6,7 and 7,2 |
| Purity | |
| Loss on drying | Not more than 0,5 % (98 oC, 5h) |
| Chloride | Not more than 0,2 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 622 MONOPOTASSIUM GLUTAMATEU.K.
| Synonyms | Potassium glutamate, MPG |
| Definition | |
| Chemical name | Monopotassium L-glutamate monohydrate |
| Einecs | 243-094-0 |
| Chemical formula | C5H8KNO4 · H2O |
| Molecular weight | 203,24 |
| Assay | Content not less than 99,0 % and not more than 101,0 % on the anhydrous basis |
| Description | White, practically odourless crystals or crystalline powder |
| Identification | |
A. Positive test for potassium | |
B. Positive test for glutamic acid by thin-layer chromatog-raphy | |
C. Specific rotation [α]D 20 | Between + 22,5o and + 24,0o |
| (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
D. pH of a 2 % solution | Between 6,7 and 7,3 |
| Purity | |
| Loss on drying | Not more than 0,2 % (80 oC, 5h) |
| Chloride | Not more than 0,2 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 623 CALCIUM DIGLUTAMATEU.K.
| Synonyms | Calcium glutamate |
| Definition | |
| Chemical name | Monocalcium di-L-glutamate |
| Einecs | 242-905-5 |
| Chemical formula | C10H16CaN2O8 · x H2O (x = 0, 1, 2 or 4) |
| Molecular weight | 332,32 (anhydrous) |
| Assay | Content not less than 98,0 % and not more than 102,0 % on the anhydrous basis |
| Description | White, practically odourless crystals or crystalline powder |
| Identification | |
A. Positive test for calcium | |
B. Positive test for glutamic acid by thin-layer chromatog-raphy | |
C. Specific rotation [α]D 20 | Between + 27,4 and + 29,2 (for calcium diglutamate with x = 4) (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
| Purity | |
| Water | Not more than 19,0 % (for calcium diglutamate with x = 4) (Karl Fischer) |
| Chloride | Not more than 0,2 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 624 MONOAMMONIUM GLUTAMATEU.K.
| Synonyms | Ammonium glutamate |
| Definition | |
| Chemical name | Monoammonium L-glutamate monohydrate |
| Einecs | 231-447-1 |
| Chemical formula | C5H12N2O4 · H2O |
| Molecular weight | 182,18 |
| Assay | Content not less than 99,0 % and not more 101,0 % on the anhydrous basis |
| Description | White, practically odourless crystals or crystalline powder |
| Identification | |
A. Positive test for ammonium | |
B. Positive test for glutamic acid by thin-layer chromatog-raphy | |
C. Specific rotation [α]D 20 | Between + 25,4o and + 26,4o |
| (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
D. pH of a 5 % solution | Between 6,0 and 7,0 |
| Purity | |
| Loss on drying | Not more than 0,5 % (50 oC, 4h) |
| Sulphated ash | Not more than 0,1 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 625 MAGNESIUM DIGLUTAMATEU.K.
| Synonyms | Magnesium glutamate |
| Definition | |
| Chemical name | Monomagnesium di-L-glutamate tetrahydrate |
| Einecs | 242-413-0 |
| Chemical formula | C10H16MgN2O8 · 4H2O |
| Molecular weight | 388,62 |
| Assay | Content not less than 95,0 % and not more than 105,0 % on the anhydrous basis |
| Description | Odourless, white or off-white crystals or powder |
| Identification | |
A. Positive test for magnesium | |
B. Positive test for glutamic acid by thin-layer chromatog-raphy | |
C. Specific rotation [α]D 20 | Between + 23,8o and + 24,4o |
| (10 % solution (anhydrous basis) in 2N HCl, 200 mm tube) |
D. pH of a 10 % solution | Between 6,4 and 7,5 |
| Purity | |
| Water | Not more than 24 % (Karl Fischer) |
| Chloride | Not more than 0,2 % |
| Pyrrolidone carboxylic acid | Not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
E 626 GUANYLIC ACIDU.K.
| Synonyms | Guanylic acid |
| Definition | |
| Chemical name | Guanosine-5'-monophosphoric acid |
| Einecs | 201-598-8 |
| Chemical formula | C10H14N5O8P |
| Molecular weight | 363,22 |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or white crystalline powder |
| Identification | |
A. Positive test for ribose and for organic phosphate | |
B. pH of a 0,25 % solution | Between 1,5 and 2,5 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 256 nm |
| Purity | |
| Loss on drying | Not more than 1,5 % (120 oC, 4h) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 627 DISODIUM GUANYLATEU.K.
| Synonyms | Sodium guanylate, sodium 5'-guanylate |
| Definition | |
| Chemical name | Disodium guanosine-5'-monophosphate |
| Einecs | 221-849-5 |
| Chemical formula | C10H12N5Na2O8P· x H2O (x = ca. 7) |
| Molecular weight | 407,19 (anhydrous) |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or white crystalline powder |
| Identification | |
A. Positive test for ribose, for organic phosphate, and for sodium | |
B. pH of a 5 % solution | Between 7,0 and 8,5 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 256 nm |
| Purity | |
| Loss on drying | Not more than 25 % (120 oC, 4h) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 628 DIPOTASSIUM GUANYLATEU.K.
| Synonyms | Potassium guanylate, potassium 5'-guanylate |
| Definition | |
| Chemical name | Dipotassium guanosine-5'-monophosphate |
| Einecs | 226-914-1 |
| Chemical formula | C10H12K2N5O8P |
| Molecular weight | 439,4 |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or white crystalline powder |
| Identification | |
A. Positive test for ribose, for organic phosphate, and for potassium | |
B. pH of a 5 % solution | Between 7,0 and 8,5 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 256 nm |
| Purity | |
| Loss on drying | Not more than 5 % (120 oC, 4h) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 629 CALCIUM GUANYLATEU.K.
| Synonyms | Calcium 5'-guanylate |
| Definition | |
| Chemical name | Calcium guanosine-5'-monophosphate |
| Chemical formula | C10H12CaN5O8P· nH2O |
| Molecular weight | 401,2 (anhydrous) |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, white or off-white crystals or powder |
| Identification | |
A. Positive test for ribose, for organic phosphate, and for calcium | |
B. pH of a 0,05 % solution | Between 7,0 and 8,0 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 256 nm |
| Purity | |
| Loss on drying | Not more than 23,0 % (120 oC, 4h) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 630 INOSINIC ACIDU.K.
| Synonyms | 5'-Inosinic acid |
| Definition | |
| Chemical name | Inosine-5'-monophosphoric acid |
| Einecs | 205-045-1 |
| Chemical formula | C10H13N4O8P |
| Molecular weight | 348,21 |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate | |
B. pH of a 5 % solution | Between 1,0 and 2,0 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 250 nm |
| Purity | |
| Loss on drying | Not more than 3,0 % (120 oC, 4h) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 631 DISODIUM INOSINATEU.K.
| Synonyms | Sodium inosinate, sodium 5'-inosinate |
| Definition | |
| Chemical name | Disodium inosine-5'-monophosphate |
| Einecs | 225-146-4 |
| Chemical formula | C10H11N4Na2O8P· H2O |
| Molecular weight | 392,17 (anhydrous) |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate and for sodium | |
B. pH of a 5 % solution | Between 7,0 and 8,5 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 250 nm |
| Purity | |
| Water | Not more than 28,5 % (Karl Fischer) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 632 DIPOTASSIUM INOSINATEU.K.
| Synonyms | Potassium inosinate, potassium 5'-inosinate |
| Definition | |
| Chemical name | Dipotassium inosine-5'-monophosphate |
| Einecs | 243-652-3 |
| Chemical formula | C10H11K2N4O8P |
| Molecular weight | 424,39 |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate and for potassium | |
B. pH of a 5 % solution | Between 7,0 and 8,5 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 250 nm |
| Purity | |
| Water | Not more than 10,0 % (Karl Fischer) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 633 CALCIUM INOSINATEU.K.
| Synonyms | Calcium 5'-inosinate |
| Definition | |
| Chemical name | Calcium inosine-5'-monophosphate |
| Chemical formula | C10H11CaN4O8P· nH2O |
| Molecular weight | 386,19 (anhydrous) |
| Assay | Content not less than 97,0 % on the anhydrous basis |
| Description | Odourless, colourless or white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate and for calcium | |
B. pH of a 0,05 % solution | Between 7,0 and 8,0 |
C. Spectrometry: | maximum absorption of a 20 mg/l solution in 0,01N HCl at 250 nm |
| Purity | |
| Water | Not more than 23,0 % (Karl Fischer) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 634 CALCIUM 5'-RIBONUCLEOTIDEU.K.
| Definition | |
| Chemical name | Calcium 5'-ribonucleotide is essentially a mixture of calcium inosine-5'-monophosphate and calcium guanosine-5'-monophosphate |
| Chemical formula | C10H11N4CaO8P· nH2O y |
| C10H12N5CaO8P· nH2O |
| Assay | Content of both major components not less than 97,0 %, and of each component not less than 47,0 % and not more than 53 %, in every case on the anhydrous basis |
| Description | Odourless, white or nearly white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate and for calcium | |
B. pH of a 0,05 % solution | Between 7,0 and 8,0 |
| Purity | |
| Water | Not more than 23,0 % (Karl Fischer) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 635 DISODIUM 5'-RIBONUCLEOTIDEU.K.
| Synonyms | Sodium 5'-ribonucleotide |
| Definition | |
| Chemical name | Disodium 5'-ribonucleotide is essentially a mixture of disodium inosine-5'-monophosphate and disodium guanosine-5'-monophosphate |
| Chemical formula | C10H11N4Na2O8P · nH2O and |
| C10H12N5Na2O8P· nH2O |
| Assay | Content of both major components not less than 97,0 %, and of each component not less than 47,0 % and not more than 53 %, in every case on the anhydrous basis |
| Description | Odourless, white or nearly white crystals or powder |
| Identification | |
A. Positive test for ribose, and for organic phosphate and for sodium | |
B. pH of a 5 % solution | Between 7,0 and 8,5 |
| Purity | |
| Water | Not more than 26,0 % (Karl Fischer) |
| Other nucleotides | Not detectable by thin-layer chromatography |
| Lead | Not more than 2 mg/kg |
E 640 GLYCINE AND ITS SODIUM SALTU.K.
| Synonyms (gly) | Aminoacetic acid, glycocoll |
| (Na salt) | Sodium glycinate |
| Definition | |
| Chemical name (gly) | Aminoacetic acid |
| (Na salt) | Sodium glycinate |
| Chemical formula (gly) | C2H5NO2 |
| (Na salt) | C2H5NO2 Na |
| Einecs (gly) | 200-272-2 |
| (Na salt) | 227-842-3 |
| Molecular weight (gly) | 75,07 |
| (Na salt) | 98 |
| Assay | Content not less than 98,5 % on the anhydrous basis |
| Description | White crystals or crystalline powder |
| Identification | |
A. Positive test for amino acid (gly and Na salt) | |
B. Positive test for sodium (Na salt) | |
| Purity | |
| Loss on drying (gly) | Not more than 0,2 % (105 oC, 3h) |
| (Na salt) | Not more than 0,2 % (105 oC, 3h) |
| Residue on ignition (gly) | Not more than 0,1 % |
| (Na salt) | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 650 ZINC ACETATEU.K.
| Synonyms | Acetic acid, zinc salt, dihydrate |
| Definition | |
| Chemical name | Zinc acetate dihydrate |
| Chemical formula | C4H6O4 Zn· 2H2O |
| Molecular weight | 219,51 |
| Assay | Content not less than 98 % and not more than 102 % of C4H6O4 Zn · 2H2O |
| Description | Colourless crystals or fine, off-white powder |
| Identification | |
A. Positive tests for acetate and for zinc | |
B. pH of a 5 % solution | Between 6,0 and 8,0 |
| Purity | |
| Insoluble matter | Not more than 0,005 % |
| Chlorides | Not more than 50 mg/kg |
| Sulphates | Not more than 100 mg/kg |
| Alkalines and alkaline earths | Not more than 0,2 % |
| Organic volatile impurities | Passes test |
| Iron | Not more than 50 mg/kg |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 20 mg/kg |
| Cadmium | Not more than 5 mg/kg |
E 900 DIMETHYL POLYSILOXANEU.K.
| Synonyms | Polydimethyl siloxane, silicone fluid, silicone oil, dimethyl silicone |
| Definition | Dimethylpolysiloxane is a mixture of fully methylated linear siloxane polymers containing repeating units of the formula (CH3)2 SiO and stablised with trimethylsiloxy end-blocking units of the formula (CH3)3 SiO |
| Chemical name | Siloxanes and silicones, di-methyl |
| Chemical formula | (CH3)3-Si-[O-Si(CH3)2]n-O-Si(CH3)3 |
| Assay | Content of total silicon not less than 37,3 % and not more than 38,5 % |
| Description | Clear, colourless, viscous liquid |
| Identification | |
A. Specific gravity (25o/25 oC) | Between 0,964 and 0,977 |
B. Refractive index [n]D 25 | Between 1,4 and 1,405 |
C. Infrared spectrum characteristic of the compound | |
| Purity | |
| Loss on drying | Not more than 0,5 % (150 oC, 4h) |
| Viscosity | Not less than 1,0 · 10-4 m2s-1 at 25 oC |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
[E 901 BEESWAX U.K.
| Synonyms | White wax, yellow wax |
| Definition | Yellow beeswax is the wax obtained by melting the walls of the honeycomb made by the honey bee, Apis mellifera L ., with hot water and removing foreign matter
White beeswax is obtained by bleaching yellow beeswax
|
| Einecs | 232-383-7 (beeswax) |
| Description | Yellowish white (white form) or yellowish to greyish brown (yellow form) pieces or plates with a fine-grained and non-crystalline fracture, having an agreeable, honey-like odour |
| Identification |
|---|
A. Melting range | Between 62 °C and 65 °C |
B. Specific gravity | About 0,96 |
C. Solubility | Insoluble in water
Sparingly soluble in alcohol
Very soluble in chloroform and ether
|
| Purity |
|---|
| Acid value | Not less than 17 and not more than 24 |
| Saponification value | 87-104 |
| Peroxide value | Not more than 5 |
| Glycerol and other polyols | Not more than 0,5 % (as glycerol) |
| Ceresin, paraffins and certain other waxes | Absent |
| Fats, Japan wax, rosin and soaps | Absent |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 1 mg/kg] |
E 902 CANDELILLA WAXU.K.
| Definition | Candelilla wax is a purified wax obtained from the leaves of the candelilla plant, Euphorbia antisyphilitica |
| Einecs | 232-347-0 |
| Description | Hard, yellowish brown, opaque to translucent wax |
| Identification | |
A. Specific gravity | About 0,983 |
B. Melting range | Between 68,5oC and 72,5oC |
C. Solubility | Insoluble in water |
| Soluble in chloroform and toluene |
| Purity | |
| Acid value | Not less than 12 and not more than 22 |
| Saponification value | Not less than 43 and not more than 65 |
| Glycerol and other polyols | Not more than 0,5 % (as glycerol) |
| Ceresin, paraffins and certain other waxes | Absent |
| Fats, Japan wax, rosin and soaps | Absent |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 903 CARNAUBA WAXU.K.
| Definition | Carnauba wax is a purified wax obtained from the leaf buds and leaves of the Brazilian Mart wax palm, Copernicia cerifera |
| Einecs | 232-399-4 |
| Description | Light brown to pale yellow powder or flakes or hard and brittle solid with a resinous fracture |
| Identification | |
A. Specific gravity | About 0,997 |
B. Melting range | Between 82 oC and 86 oC |
C. Solubility | Insoluble in water |
| Partly soluble in boiling ethanol |
| Soluble in chloroform and diethyl ether |
| Purity | |
| Sulphated ash | Not more than 0,25 % |
| Acid value | Not less than 2 and not more than 7 |
| Ester value | Not less than 71 and not more than 88 |
| Unsaponifiable matter | Not less than 50 % and not more than 55 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 904 SHELLACU.K.
| Synonyms | Bleached shellac, white shellac |
| Definition | Shellac is the purified and bleached lac, the resinous secretion of the insect Laccifer (Tachardia) lacca Kerr (Fam. Coccidae) |
| Einecs | 232-549-9 |
| Description | Bleached shellac — off-white, amorphous, granular resin |
| Wax-free bleached shellac — light yellow, amorphous, granular resin |
| Identification | |
A. Solubility | Insoluble in water; freely (though very slowly) soluble in alcohol; slightly soluble in acetone |
B. Acid value | Between 60 and 89 |
| Purity | |
| Loss on drying | Not more than 6,0 % (40 oC, over silica gel, 15h) |
| Rosin | Absent |
| Wax | Bleached shellac: not more than 5,5 % |
| Wax-free bleached shellac: not more than 0,2 % |
| Lead | Not more than 2 mg/kg |
[E 905 MICROCRYSTALLINE WAX U.K.
| Synonyms | Petroleum wax, hydrocarbon wax, Fischer-Tropsch wax, synthetic wax, synthetic paraffin |
| Definition | Refined mixtures of solid, saturated hydrocarbons, obtained from petroleum or synthetic feedstocks |
| Description | White to amber, odourless wax |
| Identification |
|---|
A. Solubility | Insoluble in water, very slightly soluble in ethanol |
B. Refractive Index | n D 100 1,434-1,448
Alternative: n D 120 1,426-1,440
|
| Purity |
|---|
| Molecular weight | Average not less than 500 |
| Viscosity | Not less than 1,1 × 10 -5 m 2 s -1 at 100 °C
Alternative: Not less than 0,8 × 10 -5 m 2 s -1 at 120 °C, if solid at 100 °C
|
| Residue on ignition | Not more than 0,1 wt % |
| Carbon number at 5 % distillation point | Not more than 5 % of molecules with carbon number less than 25 |
| Colour | Passes test |
| Sulphur | Not more than 0,4 wt % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 3 mg/kg |
| Polycyclic aromatic compounds | The polycyclic aromatic hydrocarbons, obtained by extraction with dimethyl sulfoxide, shall meet the following ultraviolet absorbency limits:
| Nm | Maximum absorbance per cm path length |
|---|
| 280-289 | 0,15 | | 290-299 | 0,12 | | 300-359 | 0,08 | | 360-400 | 0,02 |
Alternative, if solid at 100 °C
PAC method as per 21 CFR& 175.250;
Absorbency at 290 nm in decahydronaphthalene at 88 °C: Not exceeding 0,01]
|
E 907 HYDROGENATED POLY-1-DECENEU.K.
| Synonyms | Hydrogenated polydec-1-ene |
| Hydrogenated poly-alpha-olefin |
| Definition | |
| Chemical formula | C10nH20n+2 where n = 3-6 |
| Molecular weight | 560 (average) |
| Assay | Not less than 98,5 % of hydrogenated poly-1-decene, having the following oligomer distribution: |
| C30: 13-37 % |
| C40: 35-70 % |
| C50: 9-25 % |
| C60: 1-7 % |
| Description | |
| Identification | |
A. Solubility | Insoluble in water; slightly soluble in ethanol; soluble in toluene |
B. Burning | Burns with a bright flame and a paraffin-like characteristic smell |
| Purity | |
| Viscosity | Between 5,7 × 10-6 and 6,1 × 10-6 m2s-1 at 100 oC |
| Compounds with carbon number less than 30 | Not more than 1,5 % |
| Readily carbonisable substances | After 10 minutes shaking in a boiling water bath, a tube of sulphuric acid with a 5 g sample of hydrogenated poly-1-decene is not darker than a very slight straw colour |
| Nickel | Not more than 1 mg/kg |
| Lead | Not more than 1 mg/kg |
E 912 MONTAN ACID ESTERSU.K.
| Definition | Montan acids and/or esters with ethylene glycol and/or 1,3-butanediol and/or glycerol |
| Chemical name | Montan acid esters |
| Description | Almost white to yellowish flakes, powder, granules or pellets |
| Identification | |
A. Density (20 oC) | Between 0,98 and 1,05 |
B. Drop point | Greater than 77 oC |
| Purity | |
| Acid value | Not more than 40 |
| Glycerol | Not more than 1 % (by gas chromatography) |
| Other polyols | Not more than 1 % (by gas chromatography) |
| Other wax types | Not detectable (by differential scanning calorimetry and/or infrared spectroscopy) |
| Arsenic | Not more than 2 mg/kg |
| Chromium | Not more than 3 mg/kg |
| Lead | Not more than 2 mg/kg |
E 914 OXIDISED POLYETHYLENE WAXU.K.
| Definition | Polar reaction products from mild oxidation of polyethylene |
| Chemical name | Oxidised polyethylene |
| Description | Almost white flakes, powder, granules or pellets |
| Identification | |
A. Density (20 oC) | Between 0,92 and 1,05 |
B. Drop point | Greater than 95 oC |
| Purity | |
| Acid value | Not more than 70 |
| Viscosity at 120 oC | Not less than 8,1 · 10-5 m2s-1 |
| Other wax types | Not detectable (by differential scanning calorimetry and/or infrared spectroscopy) |
| Oxygen | Not more than 9,5 % |
| Chromium | Not more than 5 mg/kg |
| Lead | Not more than 2 mg/kg |
E 920 L-CYSTEINEU.K.
| Definition | L-cysteine hydrochloride or hydrochloride monohydrate. Human hair may not be used as a source for this substance |
| Einecs | 200-157-7 (anhydrous) |
| Chemical formula | C3H7NO2S· HCl· n H20 (where n = 0 or 1) |
| Molecular weight | 157,62 (anhydrous) |
| Assay | Content not less than 98,0 % and not more than 101,5 % on the anhydrous basis |
| Description | White powder or colourless crystals |
| Identification | |
A. Solubility | Freely soluble in water and in ethanol |
B. Melting range | Anhydrous form melts at about 175 oC |
C. Specific rotation | [α]20 D: between + 5,0o and + 8,0o or |
| [α]25 D: between + 4,9o and 7,9o |
| Purity | |
| Loss on drying | Between 8,0 % and 12,0 % |
| Not more than 2,0 % (anhydrous form) |
| Residue on ignition | Not more than 0,1 % |
| Ammonium-ion | Not more than 200 mg/kg |
| Arsenic | Not more than 1,5 mg/kg |
| Lead | Not more than 5 mg/kg |
E 927b CARBAMIDEU.K.
| Synonyms | Urea |
| Definition | |
| Einecs | 200-315-5 |
| Chemical formula | CH4N2O |
| Molecular weight | 60,06 |
| Assay | Content not less than 99,0 % on the anhydrous basis |
| Description | Colourless to white, prismatic, crystalline powder or small, white pellets |
| Identification | |
A. Solubility | Very soluble in water |
| Soluble in ethanol |
B. Precipitation with nitric acid | To pass the test a white, crystalline precipitate is formed |
C. Colour reaction | To pass the test a reddish-violet colour is produced |
D. Melting range | 132 oC to 135 oC |
| Purity | |
| Loss on drying | Not more than 1,0 % (105 oC, 1h) |
| Sulphated ash | Not more than 0,1 % |
| Ethanol-insoluble matter | Not more than 0,04 % |
| Alkalinity | Passes test |
| Ammonium-ion | Not more than 500 mg/kg |
| Biuret | Not more than 0,1 % |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
E 938 ARGONU.K.
| Definition | |
| Chemical name | Argon |
| Einecs | 231-147-0 |
| Chemical formula | Ar |
| Molecular weight | 40 |
| Assay | Not less than 99 % |
| Description | Colourless, odourless, non-flammable gas |
| Purity | |
| Water | Not more than 0,05 % |
| Methane and other hydrocarbons calculated as methane | Not more than 100 μl/l |
E 939 HELIUMU.K.
| Definition | |
| Chemical name | Helium |
| Einecs | 231-168-5 |
| Chemical formula | He |
| Molecular weight | 4 |
| Assay | Not less than 99 % |
| Description | Colourless, odourless, non-flammable gas |
| Purity | |
| Water | Not more than 0,05 % |
| Methane and other hydrocarbons calculated as methane | Not more than 100 μl/l |
E 941 NITROGENU.K.
| Definition | |
| Chemical name | Nitrogen |
| Einecs | 231-783-9 |
| Chemical formula | N2 |
| Molecular weight | 28 |
| Assay | Not less than 99 % |
| Description | Colourless, odourless, non-flammable gas |
| Purity | |
| Water | Not more than 0,05 % |
| Carbon monoxide | Not more than 10 μl/l |
| Methane and other hydrocarbons calculated as methane | Not more than 100 μl/l |
| Nitrogen dioxide and nitrogen oxide | Not more than 10 μl/l |
| Oxygen | Not more than 1 % |
E 942 NITROUS OXIDEU.K.
| Definition | |
| Chemical name | Nitrous oxide |
| Einecs | 233-032-0 |
| Chemical formula | N2O |
| Molecular weight | 44 |
| Assay | Not less than 99 % |
| Description | Colourless, non-flammable gas, sweetish odour |
| Purity | |
| Water | Not more than 0,05 % |
| Carbon monoxide | Not more than 30 μl/l |
| Nitrogen dioxide and nitrogen oxide | Not more than 10 μl/l |
E 943a BUTANEU.K.
| Synonyms | n-Butane |
| Definition | |
| Chemical name | Butane |
| Chemical formula | CH3CH2CH2CH3 |
| Molecular weight | 58,12 |
| Assay | Content not less than 96 % |
| Description | Colourless gas or liquid with mild, characteristic odour |
| Identification | |
A. Vapour pressure | 108,935 kPa at 20 oC |
| Purity | |
| Methane | Not more than 0,15 % v/v |
| Ethane | Not more than 0,5 % v/v |
| Propane | Not more than 1,5 % v/v |
| Isobutane | Not more than 3,0 % v/v |
| 1,3-butadiene | Not more than 0,1 % v/v |
| Moisture | Not more than 0,005 % |
E 943b ISOBUTANEU.K.
| Synonyms | 2-methyl propane |
| Definition | |
| Chemical name | 2-methyl propane |
| Chemical formula | (CH3)2CH CH3 |
| Molecular weight | 58,12 |
| Assay | Content not less than 94 % |
| Description | Colourless gas or liquid with mild, characteristic odour |
| Identification | |
A. Vapour pressure | 205,465 kPa at 20 oC |
| Purity | |
| Methane | Not more than 0,15 % v/v |
| Ethane | Not more than 0,5 % v/v |
| Propane | Not more than 2,0 % v/v |
| n-Butane | Not more than 4,0 % v/v |
| 1,3-butadiene | Not more than 0,1 % v/v |
| Moisture | Not more than 0,005 % |
E 944 PROPANEU.K.
| Definition | |
| Chemical name | Propane |
| Chemical formula | CH3CH2CH3 |
| Molecular weight | 44,09 |
| Assay | Content not less than 95 % |
| Description | Colourless gas or liquid with mild, characteristic odour |
| Identification | |
A. Vapour pressure | 732,91 kPa at 20 oC |
| Purity | |
| Methane | Not more than 0,15 % v/v |
| Ethane | Not more than 1,5 % v/v |
| Isobutane | Not more than 2,0 % v/v |
| n-Butane | Not more than 1,0 % v/v |
| 1,3-butadiene | Not more than 0,1 % v/v |
| Moisture | Not more than 0,005 % |
E 948 OXYGENU.K.
| Definition | |
| Chemical name | Oxygen |
| Einecs | 231-956-9 |
| Chemical formula | O2 |
| Molecular weight | 32 |
| Assay | Not less than 99 % |
| Description | Colourless, odourless, non-flammable gas |
| Purity | |
| Water | Not more than 0,05 % |
| Methane and other hydrocarbons calculated as methane | Not more than 100 μl/l |
E 949 HYDROGENU.K.
| Definition | |
| Chemical name | Hydrogen |
| Einecs | 215-605-7 |
| Chemical formula | H2 |
| Molecular weight | 2 |
| Assay | Content not less than 99,9 % |
| Description | Colourless, odourless, highly flammable gas |
| Purity | |
| Water | Not more than 0,005 % v/v |
| Oxygen | Not more than 0,001 % v/v |
| [Nitrogen | Not more than 0,07 % v/v] |
E 950 ACESULFAME KU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 951 ASPARTAMEU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 953 ISOMALTU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 957 THAUMATINU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 959 NEOHESPERIDINE DIHYDROCHALCONEU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 965(i) MALTITOLU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 965(ii) MALTITOL SYRUPU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 966 LACTITOLU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 967 XYLITOLU.K.
Purity criteria for this additive are the same as set out for this additive in Annex I to Directive 2008/60/EC.
E 999 QUILLAIA EXTRACTU.K.
| Synonyms | Soapbark extract, Quillay bark extract, Panama bark extract, Quillai extract, Murillo bark extract, China bark extract |
| Definition | Quillaia extract is obtained by aqueous extraction of Quillaia saponaria Molina, or other Quillaia species, trees of the family Rosaceae. It contains a number of triterpenoid saponins consisting of glycosides of quillaic acid. Some sugars including glucose, galactose, arabinose, xylose, and rhamnose are also present, along with tannin, calcium oxalate and other minor components |
| Description | Quillaia extract in the powder form is light brown with a pink tinge. It is also available as an aqueous solution |
| Identification | |
A. pH of a 2,5 % solution | Between 4,5 and 5,5 |
| Purity | |
| Water | Not more than 6,0 % (Karl Fischer method) (powder form only) |
| Arsenic | Not more than 2 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
E 1103 INVERTASEU.K.
| Definition | Invertase is produced from Saccharomyces cerevisiae |
| Systematic name | β-D-Fructofuranoside fructohydrolase |
| Enzyme Commission No | EC 3.2.1.26 |
| Einecs | 232-615-7 |
| Purity | |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
| Cadmium | Not more than 0,5 mg/kg |
| Total bacterial count | Not more than 50 000/g |
| Salmonella spp. | Absent by test in 25 g |
| Coliforms | Not more than 30/g |
| E. coli | Absent by test in 25 g |
E 1105 LYSOZYMEU.K.
| Synonyms | Lysozyme hydrochloride |
| Muramidase |
| Definition | Lysozyme is a linear polypeptide obtained from hens' egg whites consisting of 129 amino acids. It possesses enzymatic activity in its ability to hydrolyse the β(1-4) linkages between N-acetylmuramic acid and N-acetylglucosamine in the outer membranes of bacterial species, in particular gram-positive organisms. Is usually obtained as the hydrochloride |
| Chemical name | Enzyme Commission (EC) No: 3.2.1.17 |
| Einecs | 232-620-4 |
| Molecular weight | About 14 000 |
| Assay | Content not less than 950 mg/g on the anhydrous basis |
| Description | White, odourless powder having a slightly sweet taste |
| Identification | |
A. Isoelectric point 10,7 | |
B. pH of a 2 % aqueous solution between 3,0 and 3,6 | |
C. Absorption maximum of an aqueous solution (25 mg/100 ml) at 281 nm, a minimum at 252 nm | |
| Purity | |
| Water content | Not more than 6,0 % (Karl Fischer method) (powder form only) |
| Residue on ignition | Not more than 1,5 % |
| Nitrogen | Not less than 16,8 % and not more than 17,8 % |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
| Mercury | Not more than 1 mg/kg |
| Heavy metals (as Pb) | Not more than 10 mg/kg |
| Microbiological criteria | |
| Total bacterial count | Not more than 5 × 104 col/g |
| Salmonellae | Absent in 25 g |
| Staphylococcus aureus | Absent in 1 g |
| Escherichia coli | Absent in 1 g |
E 1200 POLYDEXTROSEU.K.
| Synonyms | Modified polydextroses |
| Definition | Randomly bonded glucose polymers with some sorbitol end-groups, and with citric acid or phosphoric acid residues attached to the polymers by mono or diester bonds. They are obtained by melting and condensation of the ingredients and consist of approximately 90 parts D-glucose, 10 parts sorbitol and 1 part citric acid or 0,1 part phosphoric acid. The 1,6-glucosidic linkage predominates in the polymers but other linkages are present. The products contain small quantities of free glucose, sorbitol, levoglucosan (1,6-anhydro-D-glucose) and citric acid and may be neutralised with any food grade base and/or decolorised and deionised for further purification. The products may also be partially hydrogenated with Raney nickel catalyst to reduce residual glucose. Polydextrose-N is neutralised polydextrose |
| Assay | Content not less than 90 % of polymer on the ash free and anhydrous basis |
| Description | White to light tan-coloured solid. Polydextroses dissolve in water to give a clear, colourless to straw coloured solution |
| Identification | |
A. Positive tests for sugar and for reducing sugar | |
B. pH of a 10 % solution | Between 2,5 and 7,0 for polydextrose |
| Between 5,0 and 6,0 for polydextrose-N |
| Purity | |
| Water | Not more than 4,0 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,3 % (polydextrose) |
| Not more than 2,0 % (polydextrose N) |
| Nickel | Not more than 2 mg/kg for hydrogenated polydextroses |
| 1,6-Anhydro-D-glucose | Not more than 4,0 % on the ash-free and the dried basis |
| Glucose and sorbitol | Not more than 6,0 % combined on the ash-free and the dried basis; glucose and sorbitol are determined separately |
| Molecular weight limit | Negative test for polymers of molecular weight greater than 22 000 |
| 5-Hydroxy-methylfurfural | Not more than 0,1 % (polydextrose) |
| Not more than 0,05 % (polydextrose-N) |
| Lead | Not more than 0,5 mg/kg |
E 1201 POLYVINYLPYRROLIDONEU.K.
| Synonyms | Povidone |
| PVP |
| Soluble polyvinylpyrrolidone |
| Definition | |
| Chemical name | Polyvinylpyrrolidone, poly-[1-(2-oxo-1-pyrrolidinyl)-ethylene] |
| Chemical formula | (C6H9NO)n |
| Molecular weight | Not less than 25 000 |
| Assay | Content not less than 11,5 % and not more than 12,8 % of nitrogen (N) on the anhydrous basis |
| Description | White or nearly white powder |
| Identification | |
A. Solubility | Soluble in water and in ethanol. Insoluble in ether |
B. pH of a 5 % solution | Between 3,0 and 7,0 |
| Purity | |
| Water | Not more than 5 % (Karl Fischer) |
| Total ash | Not more than 0,1 % |
| Aldehyde | Not more than 500 mg/kg (as acetaldehyde) |
| Free-N-vinylpyrrolidone | Not more than 10 mg/kg |
| Hydrazine | Not more than 1 mg/kg |
| Lead | Not more than 5 mg/kg |
[E 1203 POLYVINYL ALCOHOL U.K.
| Synonyms | Vinyl alcohol polymer, PVOH |
| Definition | Polyvinyl alcohol is a synthetic resin prepared by the polymerisation of vinyl acetate, followed by partial hydrolysis of the ester in the presence of an alkaline catalyst. The physical characteristics of the product depend on the degree of polymerisation and the degree of hydrolysis |
| Chemical name | Ethenol homopolymer |
| Chemical formula | (C 2 H 3 OR) n where R = H or COCH 3 |
| Description | Odourless, tasteless, translucent, white or cream-coloured granular powder |
| Identification |
|---|
| Solubility | Soluble in water; sparingly soluble in ethanol |
| Precipitation reaction | Dissolve 0,25 g of the sample in 5 ml of water with warming and let the solution cool to room temperature. The addition of 10 ml of ethanol to this solution leads to a white, turbid or flocculent precipitate |
| Colour reaction | Dissolve 0,01 g of the sample in 100 ml of water with warming and let the solution cool to room temperature. A blue colour is produced when adding (to 5 ml solution) one drop of iodine test solution (TS) and a few drops of boric acid solution
Dissolve 0,5 g of the sample in 10 ml of water with warming and let the solution cool to room temperature. A dark red to blue colour is produced after adding one drop of iodine TS to 5 ml of solution
|
| Viscosity | 4,8 to 5,8 mPa.s (4 % solution at 20 °C) corresponding to an average molecular weight of 26 000 - 30 000 D |
| Purity |
|---|
| Water insoluble matter | Not more than 0,1 % |
| Ester value | Between 125 and 153 mg KOH/g |
| Degree of hydrolysis | 86,5 to 89,0 % |
| Acid value | Not more than 3,0 |
| Solvent residues | Not more than 1,0 % Methanol, 1,0 % Methyl acetate |
| pH | 5,0 to 6,5 (4 % solution) |
| Loss on drying | Not more than 5,0 % (105 °C, 3 H) |
| Residue in ignition | Not more than 1,0 % |
| Lead | Not more than 2,0 mg/kg] |
E 1202 POLYVINYLPOLYPYRROLIDONEU.K.
| Synonyms | Crospovidone |
| Cross linked polyvidone |
| Insoluble polyvinylpyrrolidone |
| Definition | Polyvinylpolypyrrolidone is a poly-[1-(2-oxo-1-pyrrolidinyl)-ethylene], cross linked in a random fashion. It is produced by the polymerisation of N-vinyl-2-pyrrolidone in the presence of either caustic catalyst or N, N'-divinyl-imidazolidone. Due to its insolubility in all common solvents the molecular weight range is not amenable to analytical determination |
| Chemical name | Polyvinylpyrrolidone, poly-[1-(2-oxo-1-pyrrolidinyl)-ethylene] |
| Chemical formula | (C6H9NO)n |
| Assay | Content not less than 11 % and not more than 12,8 % nitrogen (N) on the anhydrous basis |
| Description | A white hygroscopic powder with a faint, non-objectionable odour |
| Identification | |
A. Solubility | Insoluble in water, ethanol and ether |
B. pH of a 1 % suspension in water | Between 5,0 and 8,0 |
| Purity | |
| Water | Not more than 6 % (Karl Fischer) |
| Sulphated ash | Not more than 0,4 % |
| Water-soluble matter | Not more than 1 % |
| Free-N-vinylpyrrolidone | Not more than 10 mg/kg |
| Free-N, N'-divinyl-imidazolidone | Not more than 2 mg/kg |
| Lead | Not more than 5 mg/kg |
E 1204 PULLULANU.K.
| Definition | Linear, neutral glucan consisting mainly of maltotriose units connected by - 1,6 glycosidic bonds. It is produced by fermentation from a food-grade hydrolysed starch using a non-toxin-producing strain of Aureobasidium pullulans. After completion of the fermentation, the fungal cells are removed by microfiltration, the filtrate is heat-sterilised and pigments and other impurities are removed by adsorption and ion exchange chromatography |
| Einecs | 232-945-1 |
| Chemical formula | (C6H10O5)x |
| Assay | Not less than 90 % of glucan on the dried basis |
| Description | White to off-white odourless powder |
| Identification | |
A. Solubility | Soluble in water, practically insoluble in ethanol |
B. pH of 10 % solution | 5,0 to 7,0 |
C. Precipitation with polyethylene glycol 600 | Add 2 ml of polyethylene glycol 600 to 10 ml of a 2 % aqueous solution of pullulan. A white precipitate is formed |
D. Depoly-merisation with pullulanase | Prepare two test tubes each with 10 ml of a 10 % pullulan solution. Add 0,1 ml pullulanase solution having activity 10 units/g to one test tube, and 0,1 ml water to the other. After incubation at about 25 oC for 20 minutes, the viscosity of the pullulanase-treated solution is visibly lower than that of the untreated solution |
| Purity | |
| Loss on drying | Not more than 6 % (90 oC, pressure not more than 50 mm Hg, 6 h) |
| Mono-, di- and oligosaccharides | Not more than 10 % expressed as glucose |
| Viscosity | 100 to 180 mm2/s (10 % w/w aqueous solution at 30 oC) |
| Lead | Not more than 1 mg/kg |
| Yeast and moulds | Not more than 100 colonies per gram |
| Coliforms | Absent in 25 g |
| Salmonella | Absent in 25 g |
E 1404 OXIDISED STARCHU.K.
| Definition | Oxidised starch is starch treated with sodium hypochlorite |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Carboxyl groups | Not more than 1,1 % |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1410 MONOSTARCH PHOSPHATEU.K.
| Definition | Monostarch phosphate is starch esterified with ortho-phosphoric acid, or sodium or potassium ortho-phosphate or sodium tripolyphosphate |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Residual phosphate | Not more than 0,5 % (as P) for wheat or potato starch |
| Not more than 0,4 % (as P) for other starches |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1412 DISTARCH PHOSPHATEU.K.
| Definition | Distarch phosphate is starch cross-linked with sodium trimetaphosphate or phosphorus oxychloride |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Residual phosphate | Not more than 0,5 % (as P) for wheat or potato starch |
| Not more than 0,4 % (as P) for other starches |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1413 PHOSPHATED DISTARCH PHOSPHATEU.K.
| Definition | Phosphated distarch phosphate is starch having undergone a combination of treatments as described for monostarch phosphate and for distarch phosphate |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Residual phosphate | Not more than 0,5 % (as P) for wheat or potato starch |
| Not more than 0,4 % (as P) for other starches |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1414 ACETYLATED DISTARCH PHOSPHATEU.K.
| Definition | Acetylated distarch phosphate is starch cross-linked with sodium trimetaphosphate or phosphorus oxychloride and esterified by acetic anhydride or vinyl acetate |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Acetyl groups | Not more than 2,5 % |
| Residual phosphate | Not more than 0,14 % (as P) for wheat or potato starch |
| Not more than 0,04 % (as P) for other starches |
| Vinyl acetate | Not more than 0,1 mg/kg |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1420 ACETYLATED STARCHU.K.
| Synonyms | Starch acetate |
| Definition | Acetylated starch is starch esterified with acetic anhydride or vinyl acetate |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Acetyl groups | Not more than 2,5 % |
| Vinyl acetate | Not more than 0,1 mg/kg |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1422 ACETYLATED DISTARCH ADIPATEU.K.
| Definition | Acetylated distarch adipate is starch cross-linked with adipic anhydride and esterified with acetic anhydride |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Acetyl groups | Not more than 2,5 % |
| Adipate groups | Not more than 0,135 % |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1440 HYDROXYPROPYL STARCHU.K.
| Definition | Hydroxypropyl starch is starch etherified with propylene oxide |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Hydroxypropyl groups | Not more than 7,0 % |
| Propylene chlorohydrin | Not more than 1 mg/kg |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1442 HYDROXYPROPYL DISTARCH PHOSPHATEU.K.
| Definition | Hydroxypropyl distarch phosphate is starch cross-linked with sodium trimetaphosphate or phosphorus oxychloride and etherified with propylene oxide |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Hydroxypropyl groups | Not more than 7,0 % |
| Residual phosphate | Not more than 0,14 % (as P) for wheat or potato starch |
| Not more than 0,04 (as P) for other starches |
| Propylene chlorohydrin | Not more than 1 mg/kg |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1450 STARCH SODIUM OCTENYL SUCCINATEU.K.
| Synonyms | SSOS |
| Definition | Starch sodium octenyl succinate is starch esterified with octenylsuccinic anhydride |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Octenylsuccinyl groups | Not more than 3 % |
| Octenylsuccinic acid residue | Not more than 0,3 % |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1451 ACETYLATED OXIDISED STARCHU.K.
| Definition | Acetylated oxidised starch is starch treated with sodium hypochlorite followed by esterification with acetic anhydride |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 15,0 % for cereal starch |
| Not more than 21,0 % for potato starch |
| Not more than 18,0 % for other starches |
| Carboxyl groups | Not more than 1,3 % |
| Acetyl groups | Not more than 2,5 % |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
E 1452 STARCH ALUMINIUM OCTENYL SUCCINATEU.K.
| Synonyms | SAOS |
| Definition | Starch aluminium octenyl succinate is starch esterified with octenylsuccinic anhydride and treated with aluminium sulphate |
| Description | White or nearly white powder or granules or (if pregelatinised) flakes, amorphous powder or coarse particles |
| Identification | |
A. If not pregelatinised: by microscopic observation | |
B. Iodine staining positive (dark blue to light red colour) | |
| Purity (all values expressed on an anhydrous basis except for loss on drying) | |
| Loss on drying | Not more than 21,0 % |
| Octenylsuccinyl groups | Not more than 3 % |
| Octenylsuccinic acid residue | Not more than 0,3 % |
| Sulphur dioxide | Not more than 50 mg/kg for modified cereal starches |
| Not more than 10 mg/kg for the other modified starches, unless otherwise specified |
| Arsenic | Not more than 1 mg/kg |
| Lead | Not more than 2 mg/kg |
| Mercury | Not more than 0,1 mg/kg |
| Aluminium | Not more than 0,3 % |
E 1505 TRIETHYL CITRATEU.K.
| Synonyms | Ethyl citrate |
| Definition | |
| Chemical name | Triethyl-2-hydroxypropan-1,2,3-tricarboxylate |
| Einecs | 201-070-7 |
| Chemical formula | C12H20O7 |
| Molecular weight | 276,29 |
| Assay | Content not less than 99,0 % |
| Description | Odourless, practically colourless, oily liquid |
| Identification | |
A. Specific gravity | d25 25: 1,135-1,139 |
B. Refractive index | [n]D 20: 1,439-1,441 |
| Purity | |
| Water | Not more than 0,25 % (Karl Fischer method) |
| Acidity | Not more than 0,02 % (as citric acid) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
E 1517 GLYCERYL DIACETATEU.K.
| Synonyms | Diacetin |
| Definition | Glyceryl diacetate consist predominantly of a mixture of the 1,2- and 1,3-diacetates of glycerol, with minor amounts of the mono- and tri-esters |
| Chemical names | Glyceryl diacetate |
| 1, 2, 3-propanetriol diacetate |
| Chemical formula | C7H12O5 |
| Molecular weight | 176,17 |
| Assay | Not less than 94,0 % |
| Description | Clear, colourless, hygroscopic, somewhat oily liquid with a slight, fatty odour |
| Identification | |
A. Solubility | Soluble in water. Miscible with ethanol |
B. Positive tests for glycerol and acetate | |
C. Specific gravity | d20 20: 1,175-1,195 |
D. Boiling range | Between 259 and 261 oC |
| Purity | |
| Total ash | Not more than 0,02 % |
| Acidity | Not more than 0,4 % (as ascetic acid) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
E 1518 GLYCERYL TRIACETATEU.K.
| Synonyms | Triacetin |
| Definition | |
| Chemical name | Glyceryl triacetate |
| Einecs | 203-051-9 |
| Chemical formula | C9H14O6 |
| Molecular weight | 218,21 |
| Assay | Content not less than 98,0 % |
| Description | Colourless, somewhat oily liquid having a slightly fatty odour |
| Identification | |
A. Positive tests for acetate and for glycerol | |
B. Refractive index | Between 1,429 and 1,431 at 25 oC |
C. Specific gravity (25 oC/25 oC) | Between 1,154 and 1,158 |
D. Boiling range | Between 258 and 270 oC |
| Purity | |
| Water | Not more than 0,2 % (Karl Fischer method) |
| Sulphated ash | Not more than 0,02 % (as citric acid) |
| Arsenic | Not more than 3 mg/kg |
| Lead | Not more than 5 mg/kg |
E 1519 BENZYL ALCOHOLU.K.
| Synonyms | Phenylcarbinol |
| Phenylmethyl alcohol |
| Benzenemethanol |
| Alpha-hydroxytoluene |
| Definition | |
| Chemical names | Benzyl alcohol |
| Phenylmethanol |
| Chemical formula | C7H8O |
| Molecular weight | 108,14 |
| Assay | Not less than 98,0 % |
| Description | Colourless, clear liquid with a faint, aromatic odour |
| Identification | |
A. Solubility | Soluble in water, ethanol and ether |
B. Refractive index | [n]D 20: 1,538-1,541 |
C. Specific gravity | d25 25: 1,042-1,047 |
D. Positive test for peroxides | |
| Purity | |
| Distillation range | Not less than 95 % v/v distils between 202 and 208 oC |
| Acid value | Not more than 0,5 |
| Aldehydes | Not more than 0,2 % v/v (as bezaldehyde) |
| Lead | Not more than 5 mg/kg |
E 1520 PROPANE-1,2-DIOLU.K.
| Synonyms | Propylene glycol |
| Definition | |
| Chemical names | 1,2-dihydroxypropane |
| Einecs | 200-338-0 |
| Chemical formula | C3H8O2 |
| Molecular weight | 76,1 |
| Assay | Content not less than 99,5 % on the anhydrous basis |
| Description | Clear, colourless, hygroscopic, viscous liquid |
| Identification | |
A. Solubility | Soluble in water, ethanol and acetone |
B. Specific gravity | d20 20: 1,035-1,04 |
C. Refractive index | [n]20 D: 1,431-1,433 |
| Purity | |
| Distillation range | 99 % v/v distils between 185 oC-189 oC |
| Sulphated ash | Not more than 0,07 % |
| Water | Not more than 1,0 % (Karl Fischer method) |
| Lead | Not more than 5 mg/kg |
[E 1521 POLYETHYLENE GLYCOLS U.K.
| Synonyms | PEG, Macrogol, Polyethylene oxide |
| Definition | Addition polymers of ethylene oxide and water usually designated by a number roughly corresponding to the molecular weight |
| Chemical name | alpha-Hydro-omega-hydroxypoly (oxy-1,2-ethanediol) |
| Chemical formula | HOCH 2 - (CH 2 - O - CH 2 ) n - CH 2 OH |
| Average molecular weight | 380 to 9 000 D |
| Assay | PEG 400: Not less than 95 % and not more than 105 %
PEG 3000: Not less than 90 % and not more than 110 %
PEG 3350: Not less than 90 % and not more than 110 %
PEG 4000: Not less than 90 % and not more than 110 %
PEG 6000: Not less than 90 % and not more than 110 %
PEG 8000: Not less than 87,5 % and not more than 112,5 %
|
| Description | PEG 400 is a clear, viscous, colourless or almost colourless hygroscopic liquid
PEG 3000, PEG 3350, PEG 4000, PEG 6000 and PEG 8000 are white or almost white solids with a waxy or paraffin-like appearance
|
| Identification |
|---|
| Melting point | PEG 400: 4-8 °C
PEG 3000: 50-56 °C
PEG 3350: 53-57 °C
PEG 4000: 53-59 °C
PEG 6000: 55-61 °C
PEG 8000: 55-62 °C
|
| Viscosity | PEG 400: 105 to 130 mPa.s at 20 °C
PEG 3000: 75 to 100 mPa.s at 20 °C
PEG 3350: 83 to 120 mPa.s at 20 °C
PEG 4000: 110 to 170 mPa.s at 20 °C
PEG 6000: 200 to 270 mPa.s at 20 °C
PEG 8000: 260 to 510 mPa.s at 20 °C
For polyethylene glycols having a average molecular weight greater than 400, the viscosity is determined on a 50 per cent m/m solution of the candidate substance in water
|
| Solubility | PEG 400 is miscible with water, very soluble in acetone, in alcohol and in methylene chloride, practically insoluble in fatty oils and in mineral oils
PEG 3000 and PEG 3350: very soluble in water and in methylene chloride, very slightly soluble in alcohol, practically insoluble in fatty oils and in mineral oils
PEG 4000, PEG 6000 and PEG 8000: very soluble in water and in methylene chloride, practically insoluble in alcohol and in fatty oils and in mineral oils
|
| Purity |
|---|
| Acidity or alkalinity | Dissolve 5,0 g in 50 ml of carbon dioxide-free water and add 0,15 ml of bromothymol blue solution . The solution is yellow or green. Not more than 0,1 ml of 0,1 M sodium hydroxide is required to change the colour of the indicator to blue |
| Hydroxyl value | PEG 400: 264-300
PEG 3000: 34-42
PEG 3350: 30-38
PEG 4000: 25-32
PEG 6000: 16-22
PEG 8000: 12-16
|
| Sulphated ash | Not more than 0,2 % |
| 1,4-Dioxane | Not more than 10 mg/kg |
| Ethylene oxide | Not more than 0,2 mg/kg |
| Ethylene glycol and diethylene glycol | Total not more than 0,25 % w/w individually or in combination |
| Lead | Not more than 1 mg/kg] |
ANNEX IIU.K.
PART AU.K.Repealed Directive with list of its successive amendments(referred to in Article 2)
| Commission Directive 96/77/EC
Commission Directive 98/86/EC
Commission Directive 2000/63/EC
Commission Directive 2001/30/EC
Commission Directive 2002/82/EC
Commission Directive 2003/95/EC
Commission Directive 2004/45/EC
Commission Directive 2006/129/EC
| (OJ L 339, 30.12.1996, p. 1)
(OJ L 334, 9.12.1998, p. 1)
(OJ L 277, 30.10.2000, p. 1)
(OJ L 146, 31.5.2001, p. 1)
(OJ L 292, 28.10.2002, p. 1)
(OJ L 283, 31.10.2003, p. 71)
(OJ L 113, 20.4.2004, p. 19)
(OJ L 346, 9.12.2006, p. 15)
|
PART BU.K.List of time-limits for transposition into national law(referred to in Article 2)
| |
| |
| |
| |
| |
| |
| Directive | Time-limit for transposition |
|---|
| 96/77/EC | 1 July 1997 |
| 98/86/EC | 1 July 1999 |
| 2000/63/EC | 31 March 2001 |
| 2001/30/EC | 1 June 2002 |
| 2002/82/EC | 31 August 2003 |
| 2003/95/EC | 1 November 2004 |
| 2004/45/EC | 1 April 2005 |
| 2006/129/EC | 15 February 2008 |
ANNEX IIIU.K.
Correlation table
| Directive 96/77/EC | This Directive |
|---|
| Article 1 | Article 1 |
| Article 2 | — |
| Article 3 | — |
| — | Article 2 |
| Article 4 | Article 3 |
| Article 5 | Article 4 |
| Annex | Annex I |
| — | Annex II |
| — | Annex III |