- Latest available (Revised)
- Original (As adopted by EU)
Commission Directive (EU) 2015/566Show full title
Commission Directive (EU) 2015/566 of 8 April 2015 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells (Text with EEA relevance)
You are here:
- Directives originating from the EU
- 2015 No. 566
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Directive (EU) 2015/566 (Annexes only)
Status:
EU Directives are published on this site to aid cross referencing from UK legislation. Since IP completion day (31 December 2020 11.00 p.m.) no amendments have been applied to this version.
ANNEX IU.K. Minimum requirements concerning the information and documentation to be provided by importing tissue establishment applicants when applying to be accredited, designated, authorised or licensed for the purpose of import activities
When applying for an accreditation, designation, authorisation or licence for the purpose of import activities, the importing tissue establishment applicant shall, unless already provided as part of previous applications for accreditation, designation, authorisation or licensing as a tissue establishment or importing tissue establishment, provide the most up-to-date information and, for part F, documentation on the following:
A. General Information on the Importing Tissue Establishment (ITE) U.K.
Name of the ITE (Company name).
Visiting address of the ITE.
Postal address of the ITE (if different).
Status of the applicant ITE: It should be indicated if this is the first application for accreditation, designation, authorisation or licensing as an ITE or, where applicable, whether this is a renewal application. Where the applicant is already accredited, designated, authorised or licensed as a tissue establishment, the TE compendium code should be provided.
Name of the applying unit (if different from the company name).
Visiting address of the applying unit.
Postal address of the applying unit (if different).
Name of the site of reception of imports (if different from the company name and applying unit).
Visiting address of the site of reception.
Postal address of the site of reception (if different).
B. Contact Details for the Application U.K.
Name of contact person for the application.
Telephone number.
E-mail address.
Name of Responsible Person (if different from contact person).
Telephone number.
E-mail address.
URL of ITE website (if available).
C. Details of Tissues and Cells to be Imported U.K.
A list of the types of tissues and cells to be imported, including one-off imports of specific types of tissues or cells.
The product name (where applicable, in accordance with the EU generic list) of all types of tissues and cells to be imported.
The trade name (if different to the product name) of all types of tissues and cells to be imported.
The name of the third country supplier for each type of tissue and cell to be imported.
D. Location of Activities U.K.
A list specifying which of the activities of donation, procurement, testing, processing, preservation or storage are carried out prior to import by the third country supplier per type of tissue or cell.
A list specifying which of the activities of donation, procurement, testing, processing, preservation or storage are carried out prior to import by sub-contractors of the third country supplier per type of tissue or cell.
A list of all activities carried out by the ITE subsequent to import per type of tissue or cell.
The names of the third countries in which the activities prior to import take place per type of tissue or cell.
E. Details of Third Country Suppliers U.K.
Name of third country supplier(s) (company name).
Name of contact person.
Visiting address.
Postal address (if different).
Telephone number including international dialling code.
Emergency contact number (if different)
E-mail address.
F. Documentation to Accompany the Application U.K.
A copy of the written agreement with the third country supplier(s).
A detailed description of the flow of imported tissues and cells from their procurement to their reception at the importing tissue establishment.
A copy of the third country supplier's export authorisation certificate or, where a specific export authorisation certificate is not issued, certification from the relevant third country competent authority or authorities authorising the third country supplier's activities in the tissue and cells sector including exports. This documentation shall also include the contact details of the third country competent authority or authorities. In third countries where such documentation is not available, alternative forms of documentation shall be provided such as reports of audits of the third country supplier.
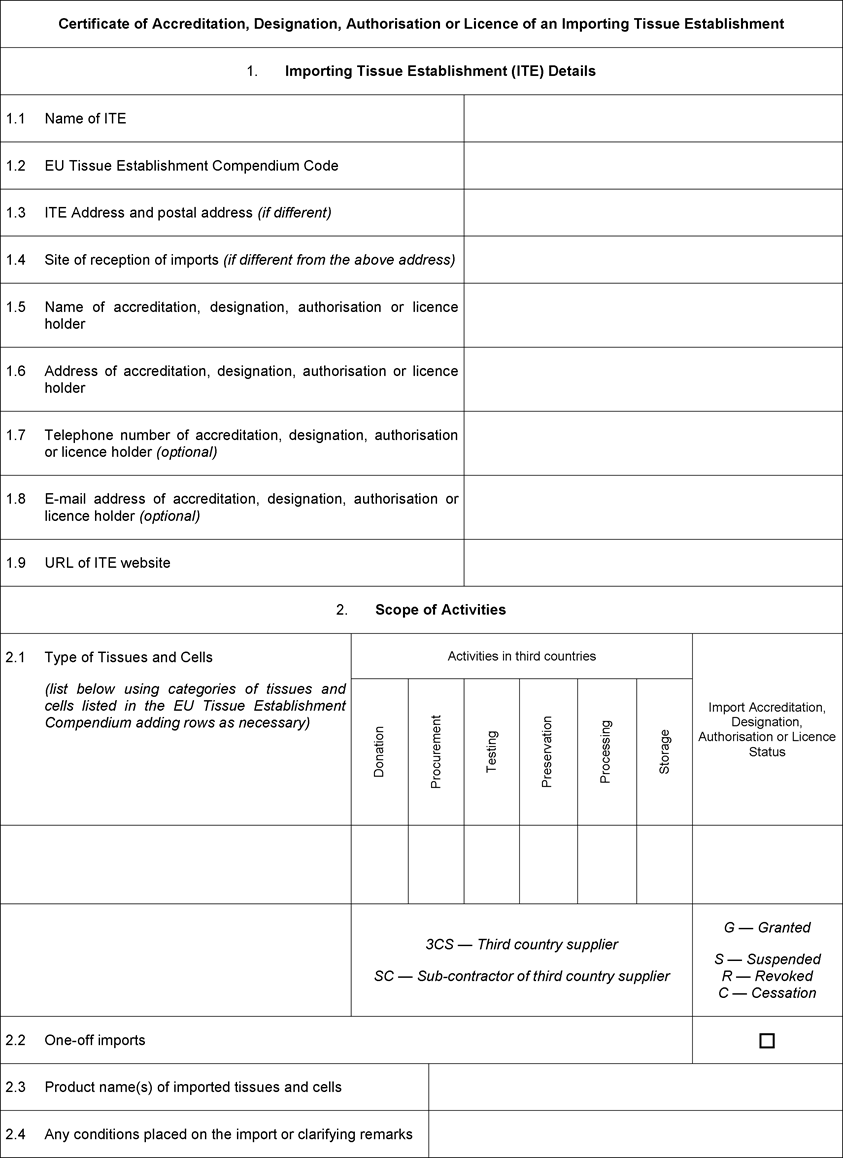
ANNEX IIU.K. Certificate of Accreditation, Designation, Authorisation or Licence to be issued by the competent authority or authorities to importing tissue establishments
ANNEX IIIU.K. Minimum requirements concerning the documentation to be made available to the competent authority or authorities by tissue establishments intending to import tissues and cells from third countries
With the exception of one-off imports as defined in Article 2 of this Directive which have been exempted from these documentation requirements, the applicant importing tissue establishment shall make available and, unless already provided as part of previous applications for accreditation, designation, authorisation or licensing as an importing tissue establishment or tissue establishment, shall provide when requested by the competent authority or authorities the most up-to-date version of the following documents regarding the applicant and its third country supplier(s).
A. Documentation relating to the importing tissue establishment U.K.
A job description of the Responsible Person and details of his/her relevant qualifications and training record as laid down in Directive 2004/23/EC;
A copy of the primary label, repackage label, external package and transport container;
A list of relevant and up-to-date versions of standard operating procedures (SOPs) relating to the establishment's import activities including SOPs on applying the Single European Code, reception and storage of imported tissues and cells at the importing tissue establishment, management of adverse events and reactions, management of recalls and traceability from donor to recipient.
B. Documentation relating to the third country supplier or suppliers U.K.
A detailed description of the criteria used for donor identification and evaluation, information provided to the donor or donor family, how consent is obtained from the donor or donor family and whether the donation was voluntary and unpaid or not;
Detailed information on the testing centre(s) used by third country suppliers and the tests performed by such centres;
Detailed information on the methods used during the processing of the tissues and cells including details of the validation for the critical processing procedure;
A detailed description of the facilities, critical equipment and materials and criteria used for quality control and control of the environment for each activity carried out by the third country supplier;
Detailed information on the conditions for release of tissues and cells by the third country supplier or suppliers;
Details of any sub-contractors used by the third country suppliers including the name, location and activity undertaken;
A summary of the most recent inspection of the third country supplier by the third country competent authority or authorities including the date of the inspection, type of inspection and main conclusions;
A summary of the most recent audit of the third country supplier carried out by, or on behalf of, the importing tissue establishment;
Any relevant national or international accreditation.
ANNEX IV U.K. Minimum requirements concerning the contents of written agreements between importing tissue establishments and their third country suppliers
With the exception of one-off imports as defined in Article 2 of this Directive which have been exempted from these requirements, the written agreement between the importing tissue establishment and the third country supplier shall contain at least the following provisions.
Detailed information on the specifications of the importing tissue establishment aimed at ensuring that the quality and safety standards laid down in Directive 2004/23/EC are met and the mutually agreed roles and responsibilities of both parties in ensuring that imported tissues and cells are of equivalent standards of quality and safety;
A clause ensuring that the third country supplier provides the information set out in Annex III B to this Directive to the importing tissue establishment;
A clause ensuring that the third country supplier informs the importing tissue establishment of any suspected or actual serious adverse events or reactions which may influence the quality and safety of tissues and cells imported or to be imported by the importing tissue establishment;
A clause ensuring that the third country supplier informs the importing tissue establishment of any substantial changes to its activities, including any revocation or suspension, in part or in full, of its authorisation to export tissue and cells or other such decisions of non-compliance by the third country competent authority or authorities, which may influence the quality and safety of tissues and cells imported or to be imported by the importing tissue establishment;
A clause guaranteeing the competent authority or authorities the right to inspect the activities of the third country supplier, including on-site inspections, should it wish to do so as part of its inspection of the importing tissue establishment. The clause should also guarantee the importing tissue establishment the right to regularly audit its third country supplier;
The agreed conditions to be met for the transport of tissues and cells between the third country supplier and importing tissue establishment;
A clause ensuring that donor records relating to imported tissues and cells are kept by the third country supplier or its sub-contractor, in line with EU data protection rules, for 30 years following procurement and that suitable provision is made for their retention should the third country supplier cease to operate;
Provisions for the regular review and, where necessary, revision of the written agreement including in order to reflect any changes in the requirements of the EU quality and safety standards laid out in Directive 2004/23/EC;
A list of all standard operating procedures of the third country supplier relating to the quality and safety of imported tissues and cells and a commitment to provide these on request.
Options/Help
Print Options
PrintThe Whole Directive
PrintThe Annexes only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources