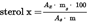- Latest available (Revised)
- Point in Time (01/01/2014)
- Original (As adopted by EU)
Commission Regulation (EEC) No 2568/91Show full title
Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 20/10/20193.52 MB
- Revised 04/12/20163.60 MB
- Revised 11/10/20163.55 MB
- Revised 04/08/20163.60 MB
- Revised 16/10/20153.42 MB
- Revised 01/01/20153.68 MB
- Revised 01/03/20143.69 MB
- Revised 01/01/20142.84 MB
- Revised 09/08/20122.49 MB
- Revised 01/04/20112.46 MB
- Revised 01/10/20081.42 MB
- Revised 01/01/20081.42 MB
- Revised 01/11/20035.95 MB
- Revised 22/05/20020.48 MB
- Revised 01/11/20010.66 MB
- Revised 01/07/20010.66 MB
- Revised 01/06/19990.65 MB
- Revised 01/11/19980.65 MB
- Revised 10/02/19980.65 MB
- Revised 31/10/19950.59 MB
- Revised 28/05/19950.59 MB
- Revised 01/11/19940.55 MB
- Revised 19/02/19940.55 MB
- Revised 05/02/19940.55 MB
- Revised 01/05/19930.55 MB
- Revised 21/03/19930.56 MB
- Revised 20/02/19930.56 MB
- Revised 01/11/19920.55 MB
- Revised 21/07/19920.55 MB
- Revised 03/07/19920.54 MB
- Revised 05/06/19920.54 MB
- Revised 21/12/19910.52 MB
- Revised 06/09/19910.52 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Division 5.
Version Superseded: 01/03/2014
Status:
Point in time view as at 01/01/2014.
Changes to legislation:
There are outstanding changes by UK legislation not yet made to Commission Regulation (EEC) No 2568/91. Any changes that have already been made to the legislation appear in the content and are referenced with annotations.![]()
Changes to Legislation
Changes and effects yet to be applied by the editorial team are only applicable when viewing the latest version or prospective version of legislation. They are therefore not accessible when viewing legislation as at a specific point in time. To view the ‘Changes to Legislation’ information for this provision return to the latest version view using the options provided in the ‘What Version’ box above.
5.PROCEDUREU.K.
5.1.Preparation of the unsaponifiables.U.K.
5.1.1.Using the 500 μl microsyringe [X1introduce, into a 250 ml flask a volume of 0,2 % β-cholestanol solution in chloroform (4.17) containing an amount of cholestanol corresponding to approximately 10 % of the sterol content of the sample aliquot to be taken for the determination.] For example, for 5 g of sample add 500 μl of the 0,2 % α-cholestanol solution in the case of an olive oil and 1 500 μl for[F1 seed oils or] olive-pomaca oil.U.K.Editorial Information
X1
Substituted by Corrigendum to Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis (Official Journal of the European Communities No L 248 of 5 September 1991).
Textual Amendments
Editorial Information
X1 Substituted by Corrigendum to Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis (Official Journal of the European Communities No L 248 of 5 September 1991).
Textual Amendments
Evaporate to [X1dryness in a current] of nitrogen and then weigh accurately 5 g of the dry filtered sample into the same flask.
[F2Oils] containing appreciable quantities of cholesterol may show a peak having a retention time identical to cholestanol. If this occurs the sterol fraction will have to be analyzed in duplicate with and without internal standard[F3or betulinol will have to be used instead of cholestanol].
Textual Amendments
5.1.2.Add 50 ml of [X12 mol/L] ethanolic potassium hydroxide solution, fit the reflux condenser and heat to gentle boiling on a water bath with continuous vigorous stirring until saponification takes place (the solution becomes clear). Continue heating for a further 20 minutes, then add 50 ml of [X1distilled water to the] top of the condenser, detach the condenser and cool the flask to approximately 30 °C.U.K.
5.1.3.Transfer the contents of the flask quantitatively into a 500 ml separating funnel using several rinses of distilled water, amounting in all to about 50 ml. Add approximately 80 ml of [X1diethyl ether], shake vigorously for approximately 30 seconds and allow to settle (Note 1).U.K.
Separate off the lower aqueous phase collecting it in a second separating funnel. Perform two further extractions on the aqueous phase in the same way using 60 to 70 ml of ethyl ether on each occasion.
Note 1.U.K.
Any emulsion can be destroyed by adding small quantities of ethyl or methyl alcohol by means of a spray.U.K.
5.1.4.Pool the ether extracts into a single separating funnel and wash with distilled water (50 ml at a time) until the wash water gives a neutral reaction.U.K.
When the wash water has been removed, dry with anhydrous sodium sulphate and [X1filter through anhydrous] sodium sulphate into a previously weighed 250 ml flask, washing the funnel and filter with small quantities of [X1diethyl ether.]
5.1.5.Distil the ether down to a few ml, then bring to dryness under a slight vacuum or in a current of nitrogen, completing drying in a stove at 100 °C for approximately a quarter of an hour, and then weigh after cooling in a desiccator.U.K.
5.2.Separation of the sterol fraction.U.K.
5.2.1.Preparation of the basic plates. Immerse the silica gel plates (4.4) completely in the [X10,2 mol/L] ethanolic potassium hydroxide solution (4.5) for 10 seconds, then allow to dry in a fume cupboard for two hours and finally place [X1in an oven at] 100 °C for one hour.U.K.
Remove from the stove and keep in a calcium chloride desiccator until required for use (plates treated in this way must be used within 15 days).
Note 2.U.K.
When basic silica gel plates are used to separate the sterol fraction there is no need to treat the unsaponifiables with alumina. In this way all compounds of an acid nature (fatty acids and others) are retained on the spotting line and the sterols band is clearly separated from the aliphatic and triterpene alcohols band.U.K.
5.2.2.Place a 95:5 (v/v) benzene/acetone [X1mixture into the] plate-developing chamber to a depth of approximately 1 cm. As an alternative a 65:35 (v/v) hexane/ethyl ether mixture may be used. Close the chamber with the appropriate cover and leave thus for approximately half an hour so that liquid-vapour equilibrium is established. Strips of filter paper dipping into the eluent may be placed on the internal surfaces of the chamber. This reduces developing time by approximately one-third and brings about more uniform and regular elution of the components.U.K.
Note 3.U.K.
The developing mixture should be replaced for every test in order to achieve perfectly reproducible elution conditions.U.K.
5.2.3.Prepare an approximately 5 % solution of the unsaponifiables (5.1.5) in chloroform and, using the 100 μl microsyringe, streak a chromatographic plate [X1(5.2.1) with 300 μl] approximately 2 cm from one end in a streak which is as thin and as uniform as possible. In line with the streak place 2 to 3 μl of the sterol reference solution (4.11) at one end of the plate so that the sterol band can be identified after developing.U.K.
5.2.4.Place the plate in the developing chamber prepared as specified in 5.2.2. The ambient temperature should be maintained between 15 and 20 °C. Immediately close the chamber with the cover and allow to elute until the solvent front reaches approximately 1 cm from the upper edge of the plate. Remove the plate from the developing chamber and evaporate the solvent in a flow of hot air or by leaving the plate for a short while under a hood.U.K.
5.2.5.Spray the plate lightly and uniformly with the 2,7-dichlorofluoroscein solution. When the plate is observed under ultraviolet light the sterol band can be identified through being aligned with the stain obtained from the reference solution. Mark the limits of the band along the edges of the fluorescence with a black pencil.U.K.
5.2.6.Using a metal spatula scrape off the silica gel in the marked area. Place the finely comminuted material removed into the filter funnel (3.7). Add 10 ml of hot chloroform, mix carefully with the metal spatula and filter under vacuum, collecting the filtrate in the conical flask (3.8) attached to the filter funnel.U.K.
Wash the residue [X1in the funnel three times with diethyl ether] (approximately 10 ml each time) collecting the filtrate in the same flask attached to the funnel. Evaporate the filtrate to a volume of 4 to 5 ml, transfer the residual solution to the previously weighed 10 ml test tube (3.9), evaporate to dryness by mild heating in a gentle flow of nitrogen, make up again using a few drops of acetone, evaporate again to dryness, place in a stove at 105 °C for approximately 10 minutes and then allow to cool in a desiccator and weigh.
The residue contained in the test tube consists of the sterol fraction.
5.3.Preparation of the trimethylsilyl ethers.U.K.
5.3.1.Add the silylation reagent, consisting of a 9:3:1 (v/v/v) mixture of pyridine/hexamethyl disilazane/trimethyl chlorosilane (Note 4) in the ratio of 50 μl for every milligram of sterols to the test tube containing the sterol fraction, avoiding any uptake of moisture (Note 5).U.K.
Note 4.U.K.
Solutions which are ready for use are available commercially. Other silanizing reagents such as, for example, bis-trimethylsilyl, trifluor acetamide + 1 % trimethyl chlorosilane, which has to be diluted with an equal volume of anhydrous pyridine, are also available.U.K.
5.3.2.Stopper the test tube, shake carefully (without overturning) until the sterols are completely dissolved. Stand for at least 15 minutes at ambient temperature and then centrifuge for a few minutes. The clear solution is ready for gas chromatographic analysis.U.K.
Note 5.U.K.
The slight opalescence which may form is normal and does not cause any interference. The formation of a white floc or the appearance of a pink colour are indicative of the presence of moisture or deterioration of the reagent. If these occur the test must be repeated.U.K.
5.4.Gas chromatographic analysis.U.K.
5.4.1.Preliminary operations, column packing.U.K.
5.4.1.1.Fit the column in the gas chromatograph, attaching the inlet end to the evaporator connected to the splitting system and the outlet end to the detector.U.K.
Carry out general checks on the gas chromatograph unit (leaks from the gas circuits, detector efficiency, efficiency of the splitting system and recording system, etc.).
5.4.1.2.If the column is being used for the first time it is recommended that it should be subjected to conditioning. Pass a gentle flow of gas through the column and then switch on the gas chromatography unit and begin gradual heating up to a temperature of at least 20 °C above the operating temperature (Note 6). Hold this temperature for at least two hours, then place the entire unit in operating mode (adjustment of gas flows and splitting, ignition of the flame, connection with the electronic recorder, adjustment of the column chamber, detector and injector temperature, etc.) and then record the signal with a sensitivity at least two times greater than that intended for the analysis. The course of the base line must be linear, without peaks of any kind, and must not drift.U.K.
A negative straight-line drift indicates leakage from the column connections; a positive drift indicates inadequate conditioning of the column.
Note 6.U.K.
The conditioning temperature must always be at least 20 °C less than the maximum temperature specified for the stationary phase used.U.K.
5.4.2.Choice of operating conditions.U.K.
5.4.2.1.The guideline operating conditions are as follows:U.K.
column temperature: 260 ± 5 °C,
evaporator temperature: 280 °C,
detector temperature: 290 °C,
linear velocity of the carrier gas: helium 20 to 35 cm/s, hydrogen 30 to 50 cm/s,
splitting ratio: from 1:50 to 1:100,
instrument sensitivity: from 4 to 16 times the minimum attenuation,
recording sensitivity: 1 to 2 mV f.s.,
paper speed: 30 to 60 cm/hour,
amount of substance injected: 0,5 to 1 μl of TMSE solution.
These conditions may be varied in the light of column and gas-chromatograph characteristics so as to obtain chromatograms which meet the following requirements:
the retention time for β-sitosterol should be 20 ± 5 minutes,
the campesterol peak should be: for olive oil (mean content 3 %) 15 ± 5 % of full scale; for soya oil (mean content 20 %) 80 ± 10 % of full scale,
all the sterols present must be separated. In addition to being separated the peaks must also be completely resolved, i.e. the peak trace should return to the base line before leaving for the next peak. Incomplete resolution is however tolerated provided that the peak at TRR 1,02 can be quantified using the perpendicular.
5.4.3.Analytical procedure.U.K.
5.4.3.1.Using the 10 μl microsyringe take 1 μl of hexane, draw in 0,5 μl of air and then 0,5 to 1 μl of the sample solution. Raise the plunger of the syringe further so the needle is emptied. Push the needle through the membrane of the injection unit and after one to two seconds inject rapidly, then slowly remove the needle after some five seconds.U.K.
5.4.3.2.Continue recording until the TMSE of the sterols present are completely elutedU.K.
The base line must continue to meet the requirements (5.4.1.2).
5.4.4.Peak identification.U.K.
Identify individual peaks on the basis of retention times and by comparison with mixtures of sterol TMSE analysed under the same conditions.
The sterols are eluted in the following order: cholesterol, brassicasterol, 24-methylene cholesterol, campesterol, campestanol, stigmasterol, Δ 7-campesterol, Δ 5,23-stigmastadienol, [X1chlerosterol], β-sistosterol, sitostanol, Δ 5-avenasterol, Δ 5,24-stigmastadienol[X1, Δ 7-stigmasterol,] Δ 7-avenasterol.
The retention times for sitosterol for SE-52 and SE-54 columns are shown in Table 1.
Figures 1 and 2 illustrate typical chromatograms for some oils.
5.4.5.Quantitative evaluation.U.K.
5.4.5.1.Calculate the areas of the [X1β-cholestanol and the sterol peaks using the integrator. Ignore peaks for any compounds which are not included among those listed in Table 1. The response coefficient for β-cholestanol] is to be equal to 1.U.K.
5.4.5.2.Calculate the concentration of each individual sterol in mg/100 g of fatty material as follows:U.K.
where:
=
mass of β-cholestanol] added, im milligrams;
=
mass of the sample used for determination, in grams.
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Whole Annex
PrintThis Division only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources