- Latest available (Revised)
- Point in Time (28/07/2010)
- Original (As adopted by EU)
Regulation (EC) No 1774/2002 of the European Parliament and of the Council (repealed)Show full title
Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption (repealed)
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 04/03/20110.44 MB
- Revised 28/09/201032.86 MB
- Revised 28/07/201033.23 MB
- Revised 07/08/200932.95 MB
- Revised 25/08/20089.02 MB
- Revised 01/07/200832.94 MB
- Revised 24/07/20073.76 MB
- Revised 01/01/20073.77 MB
- Revised 01/04/20064.90 MB
- Revised 15/03/20053.81 MB
- Revised 01/01/20053.81 MB
- Revised 01/05/20043.64 MB
- Revised 01/05/20031.69 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: ANNEX X
Version Superseded: 28/09/2010
Alternative versions:
Status:
Point in time view as at 28/07/2010.
Changes to legislation:
There are currently no known outstanding effects for the Regulation (EC) No 1774/2002 of the European Parliament and of the Council (repealed),
ANNEX X
.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
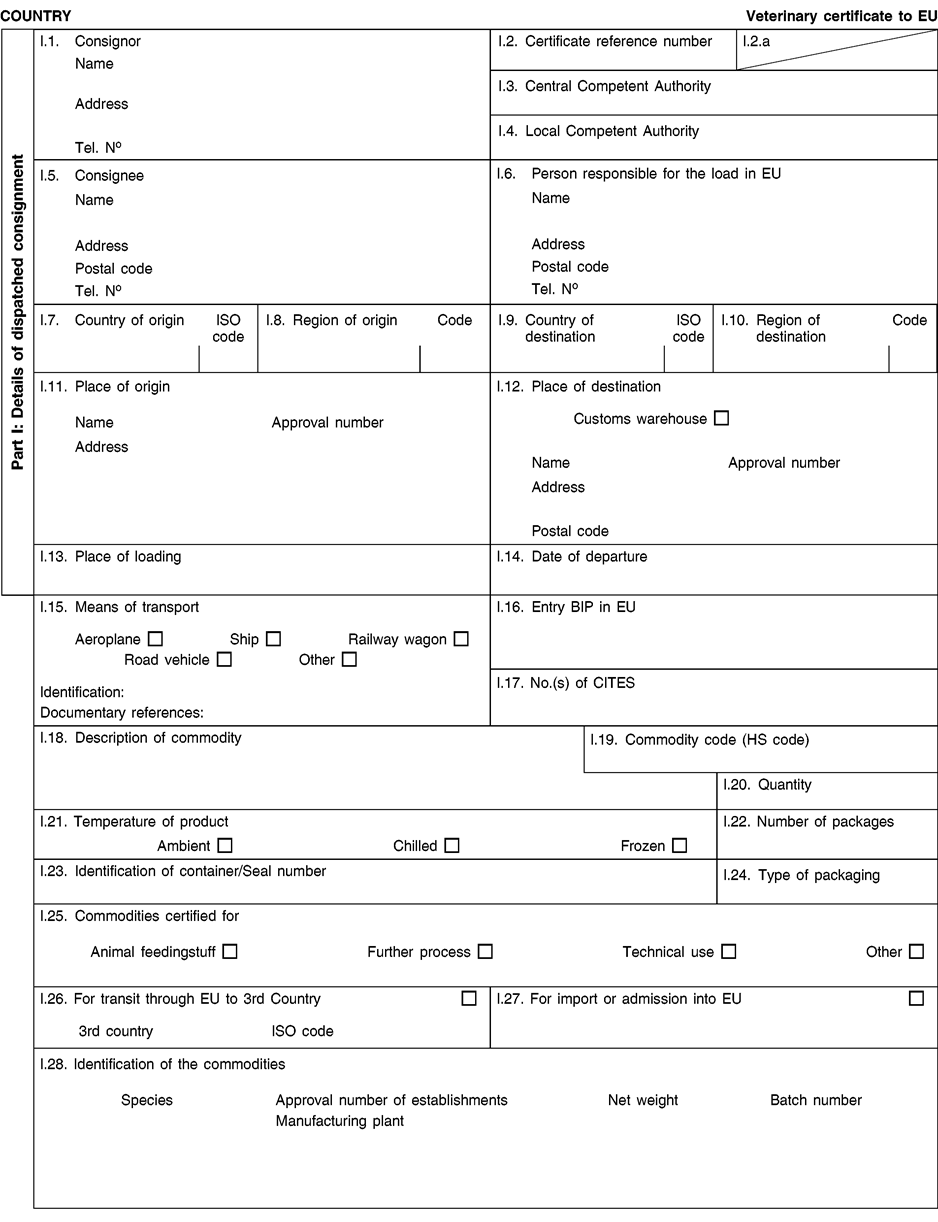
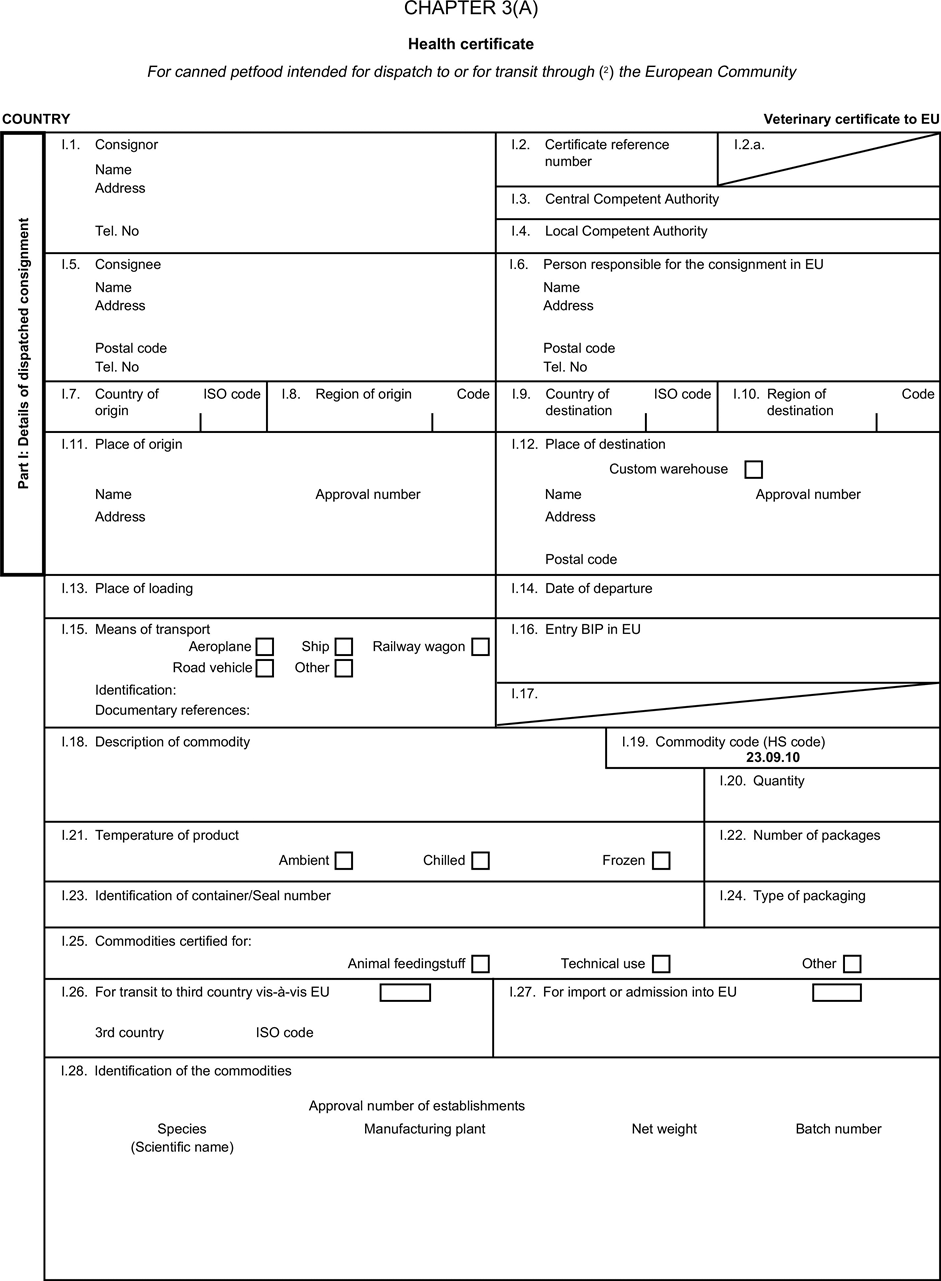
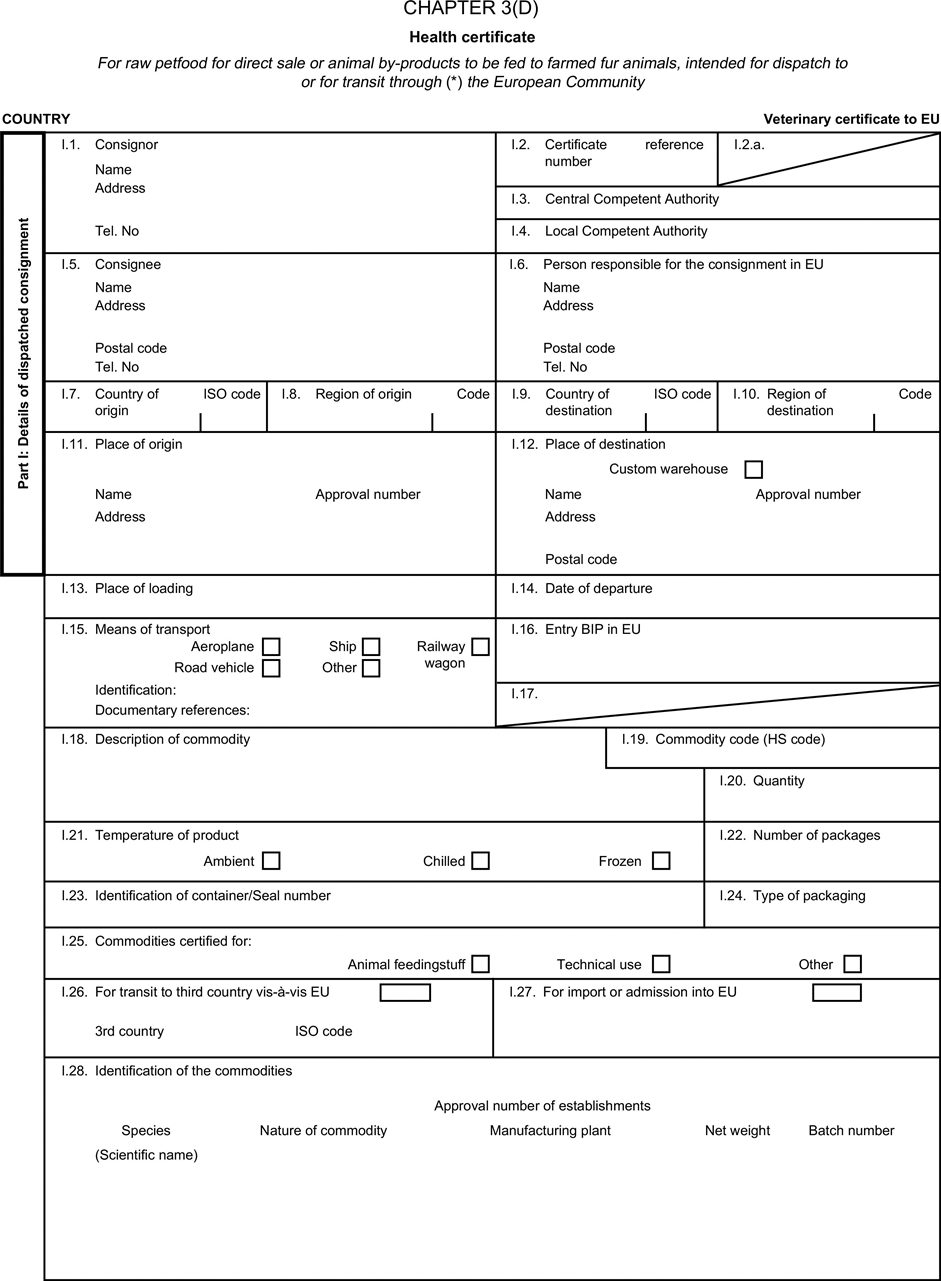
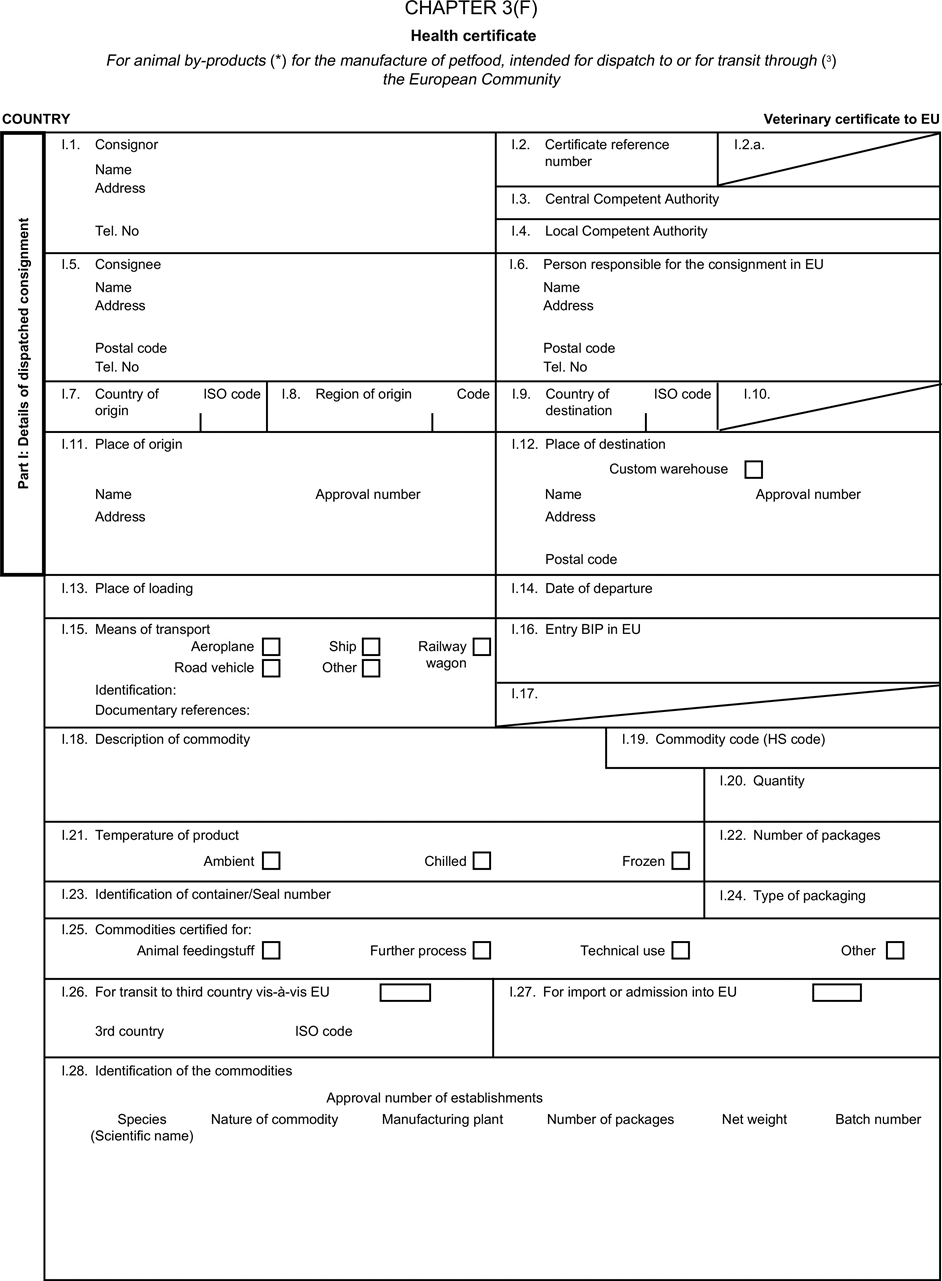
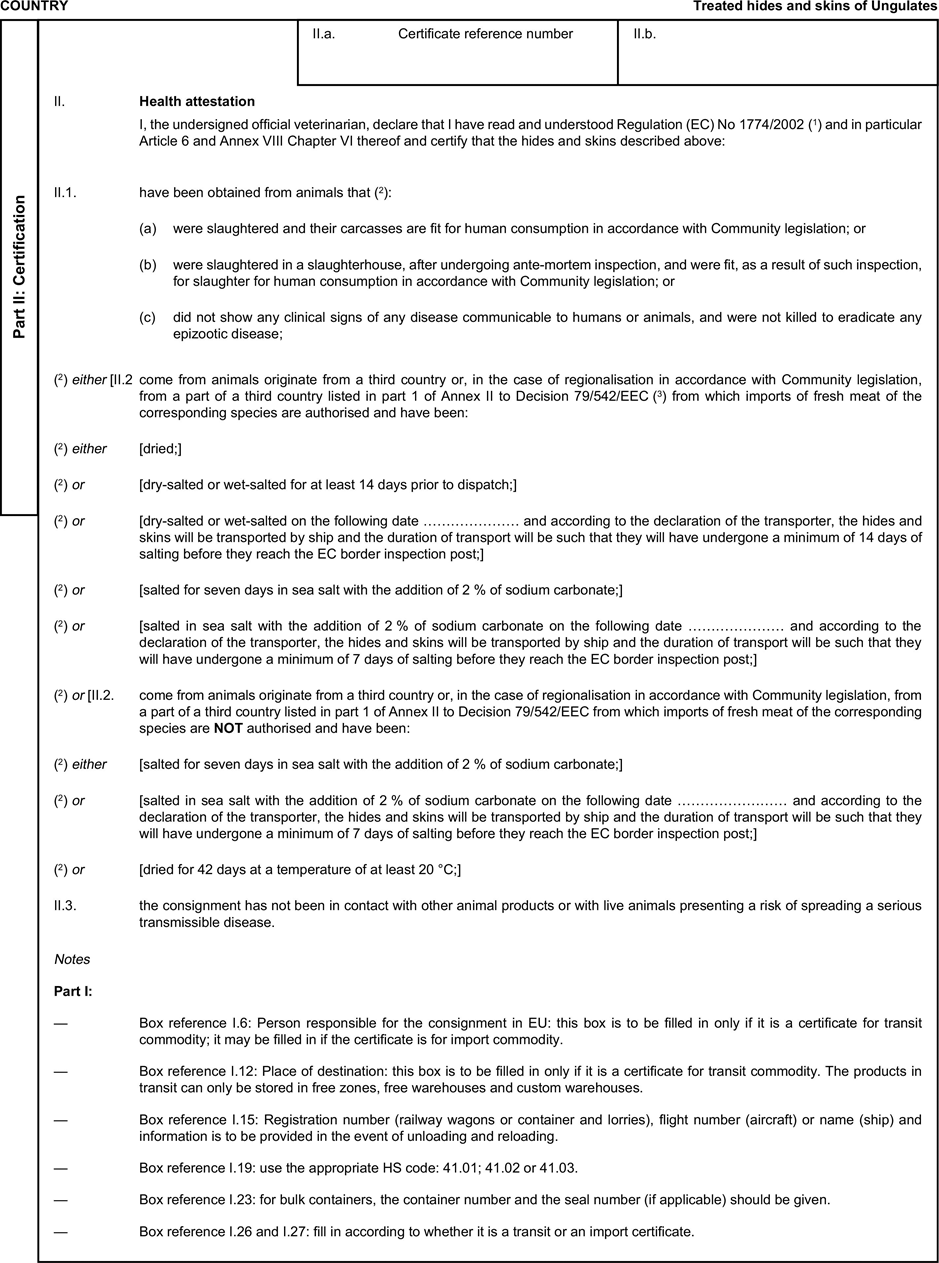
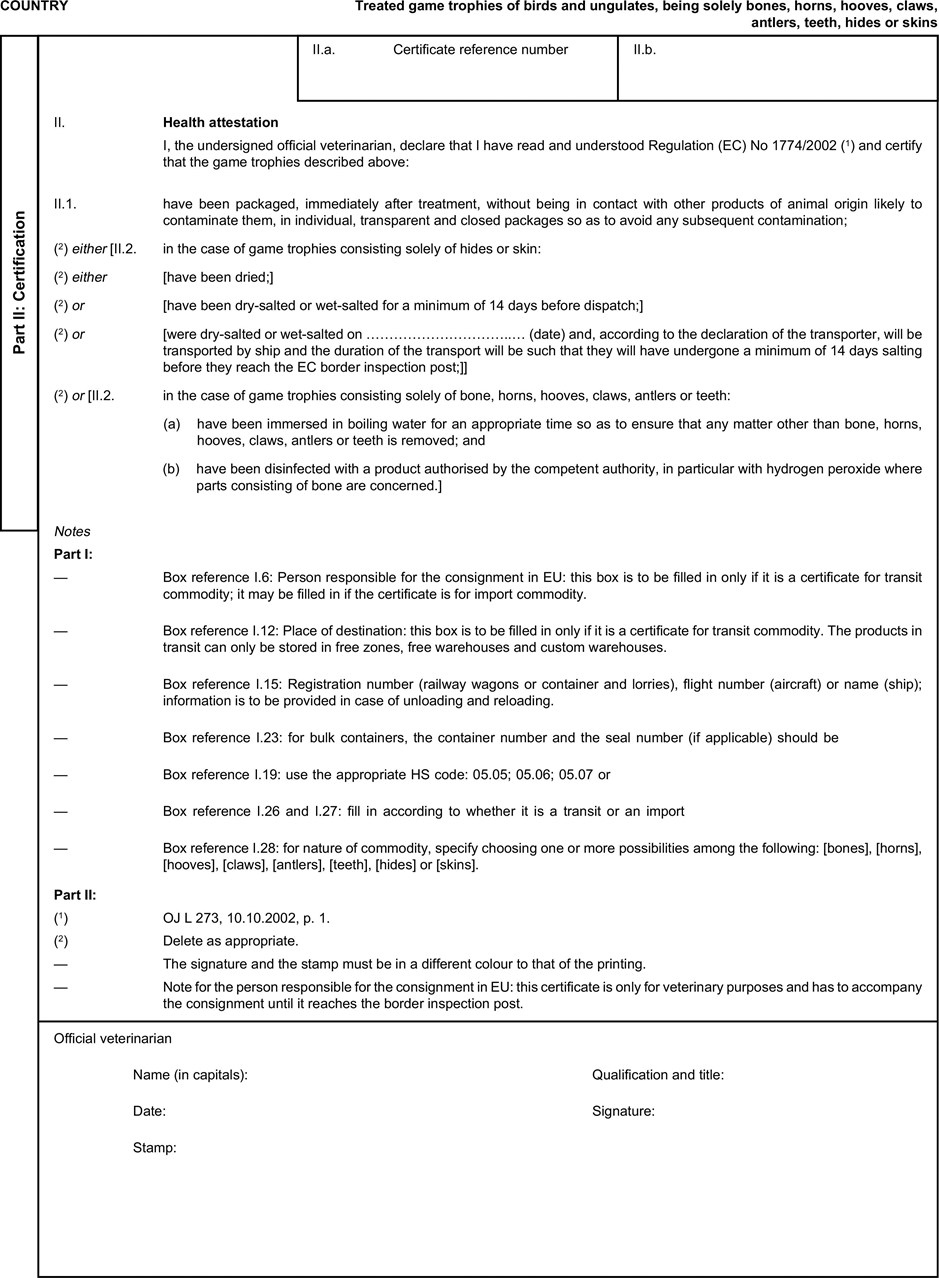
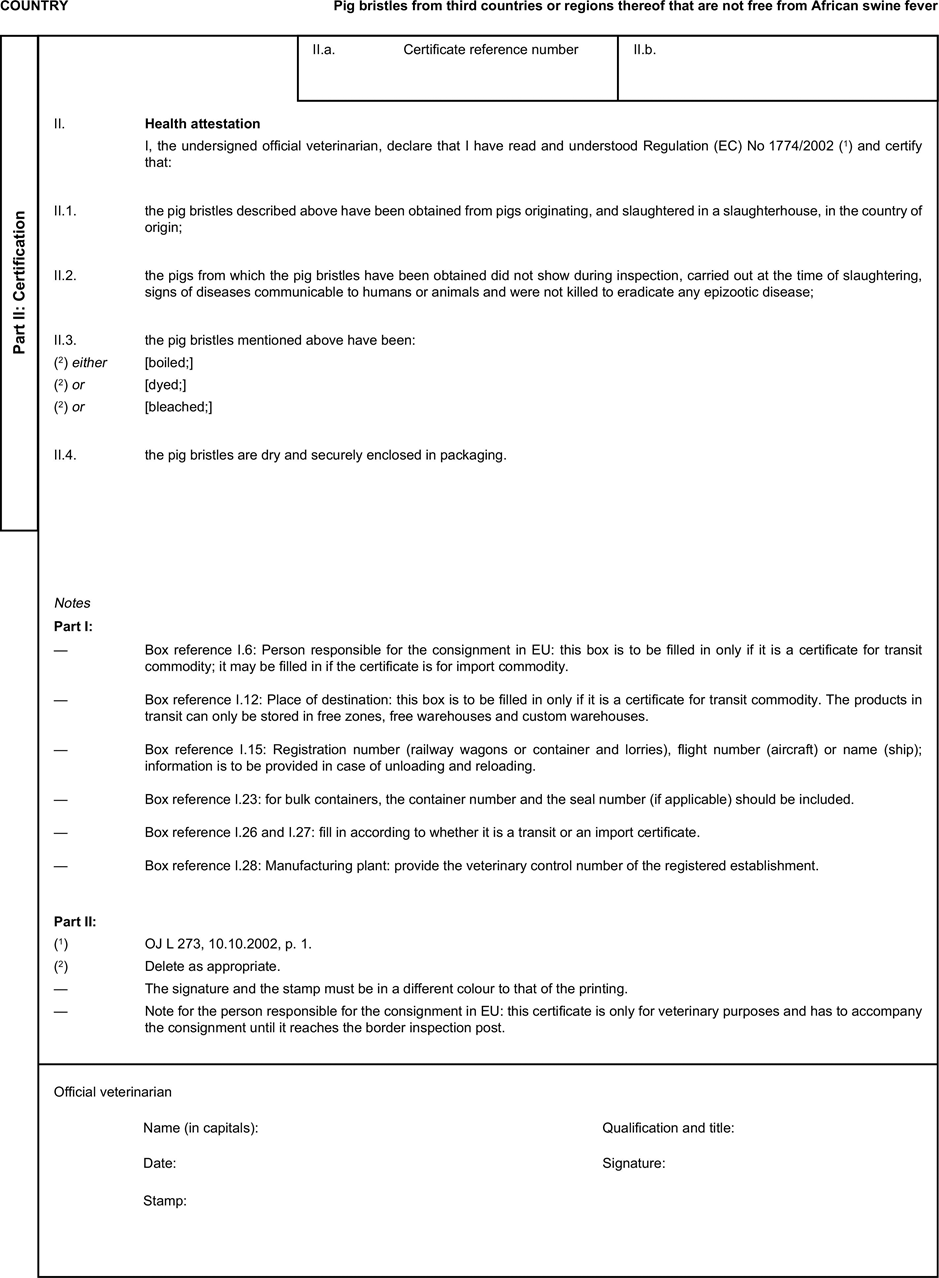
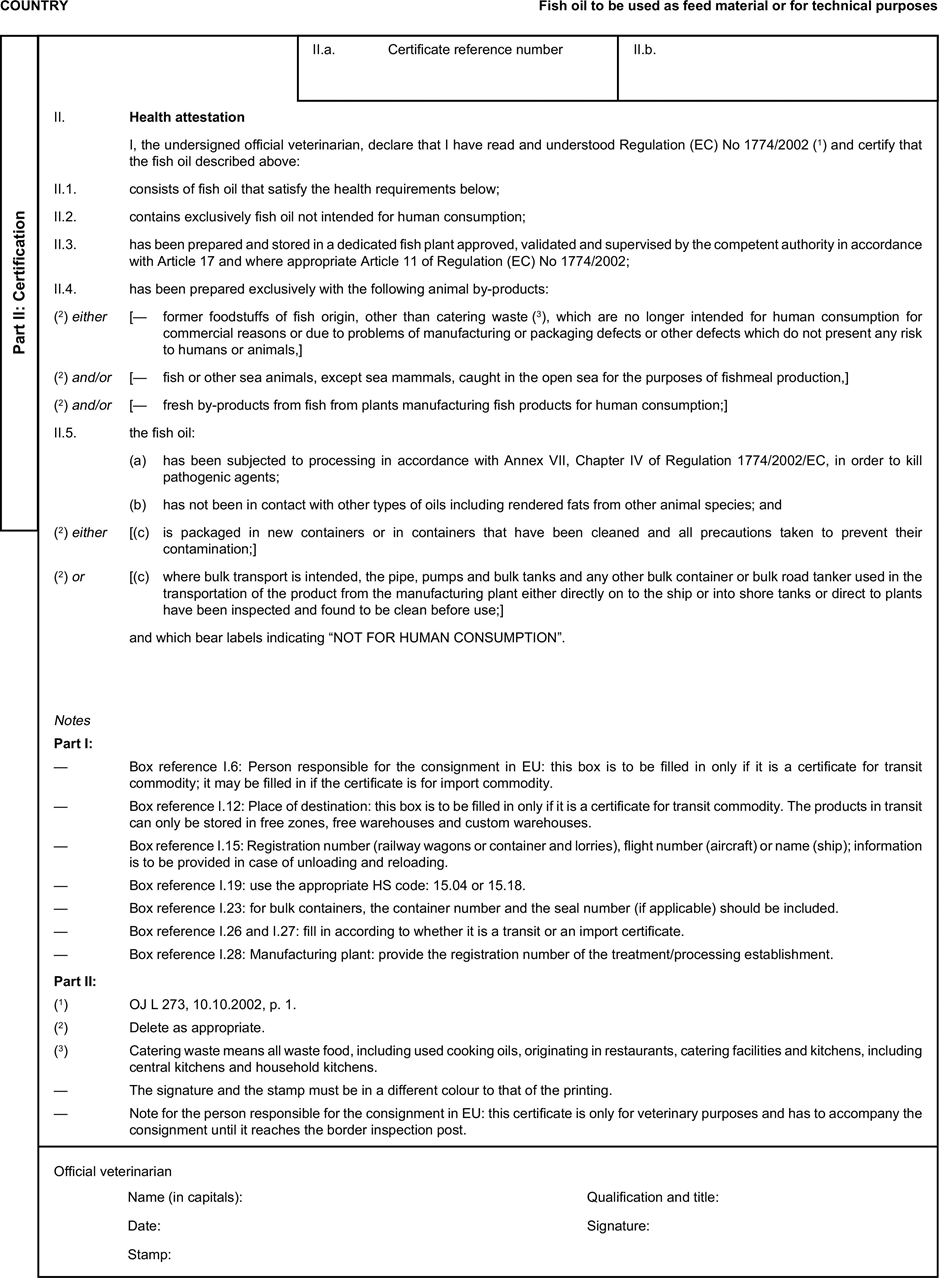
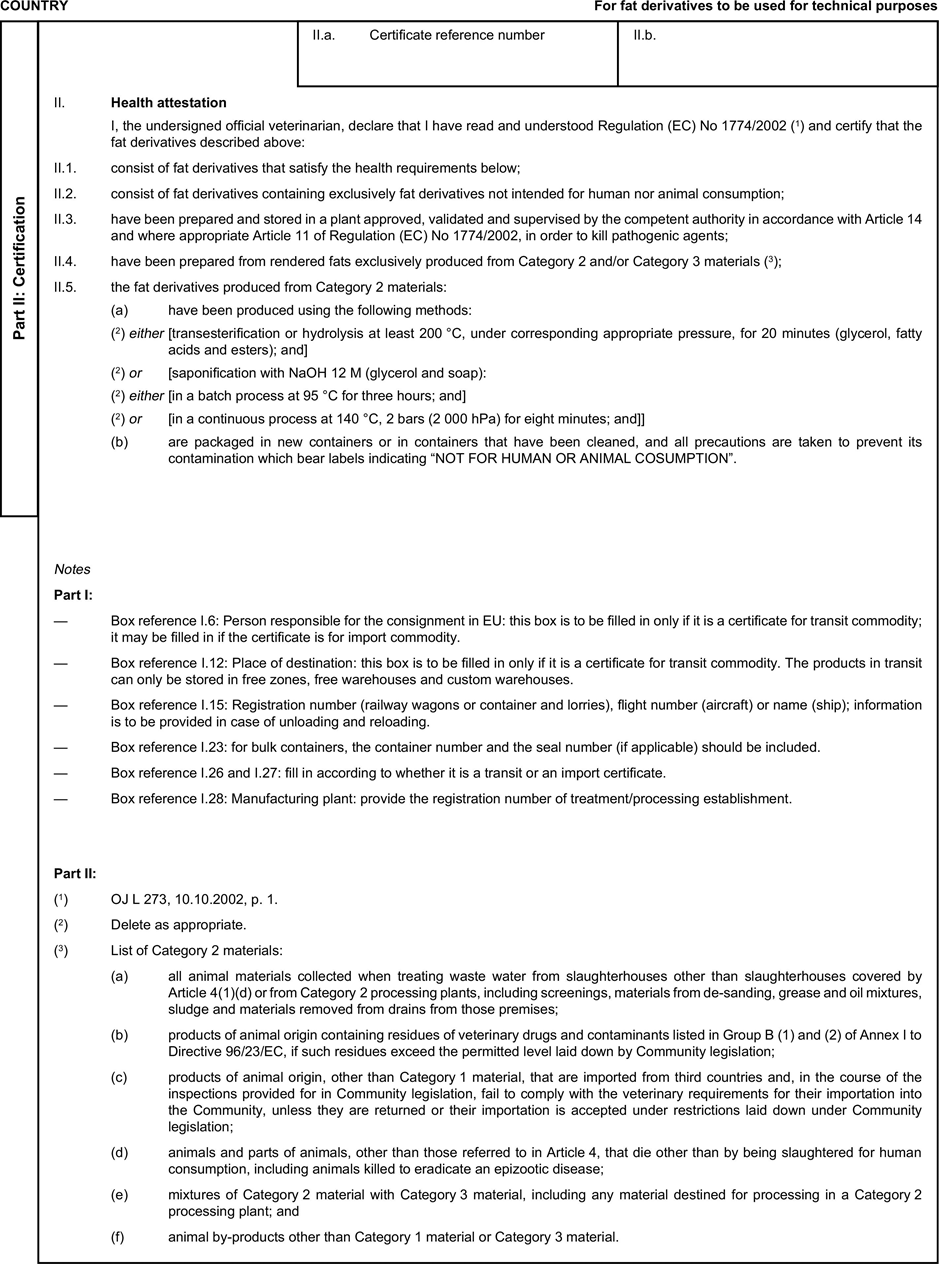
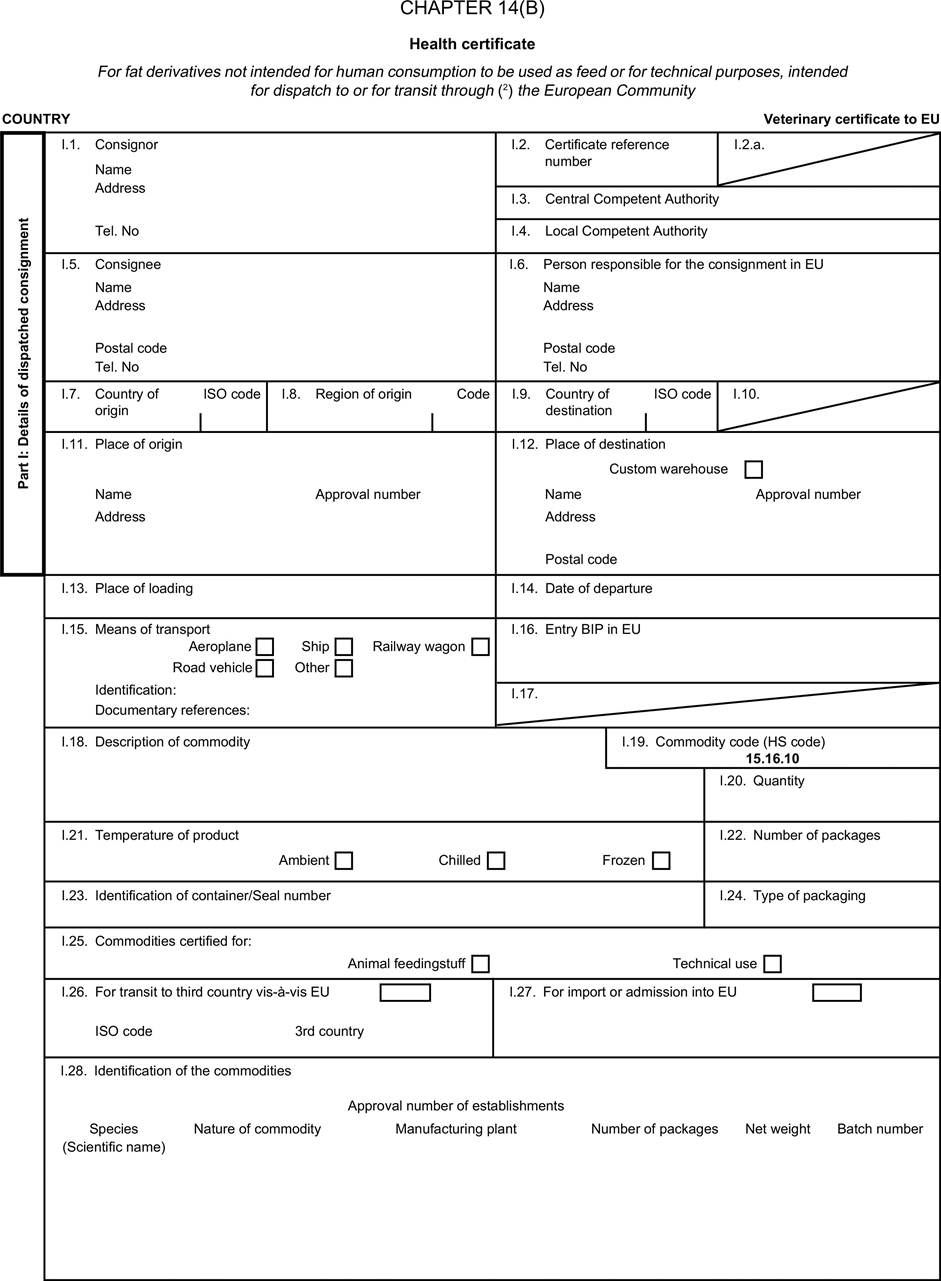
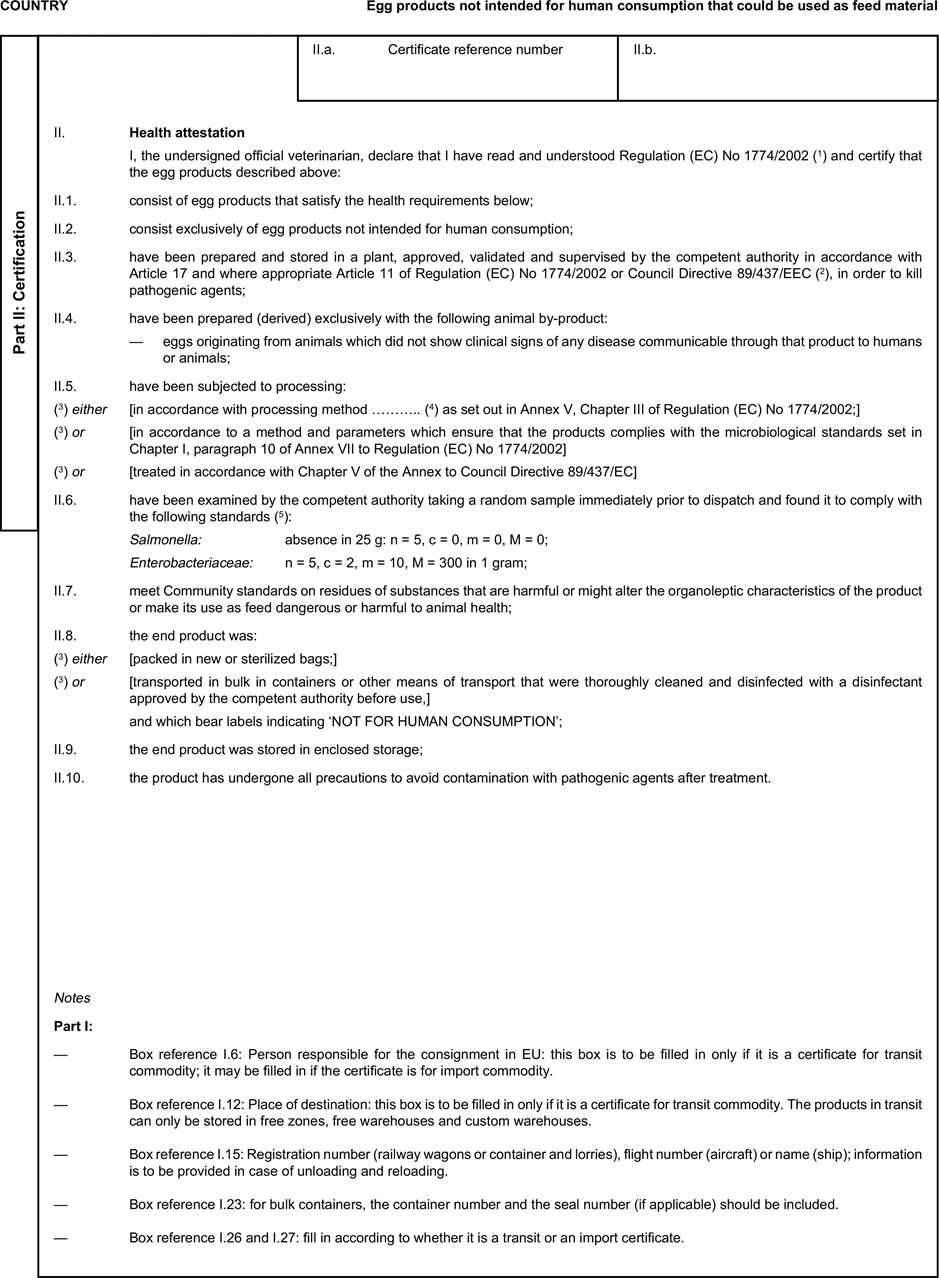
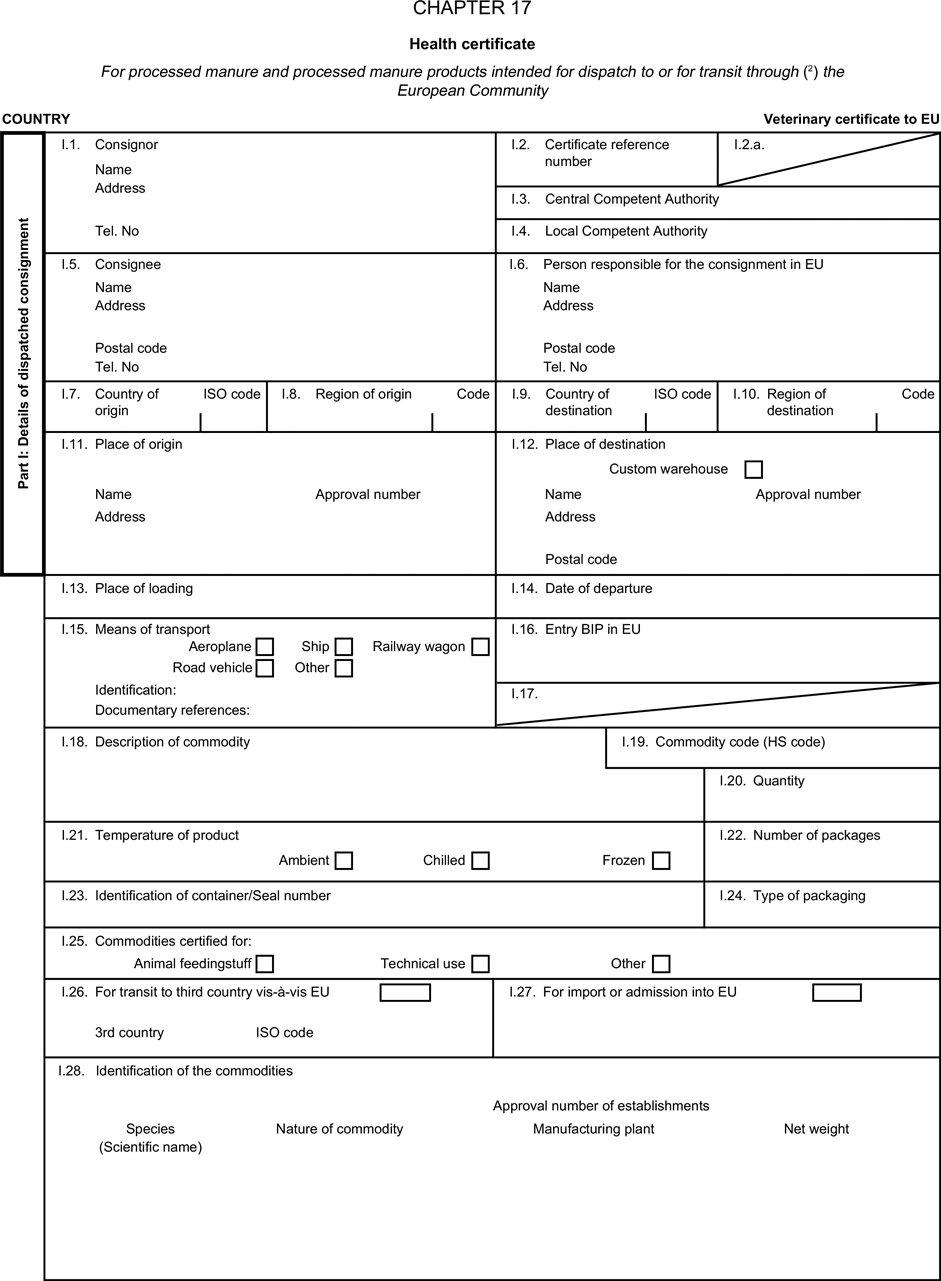
[F1ANNEX X U.K. MODEL HEALTH CERTIFICATES FOR THE IMPORTATION FROM THIRD COUNTRIES AND FOR THE TRANSIT THROUGH THE EUROPEAN COMMUNITY OF CERTAIN ANIMAL BY-PRODUCTS AND PRODUCTS DERIVED THEREFROM
Textual Amendments
Notes U.K.
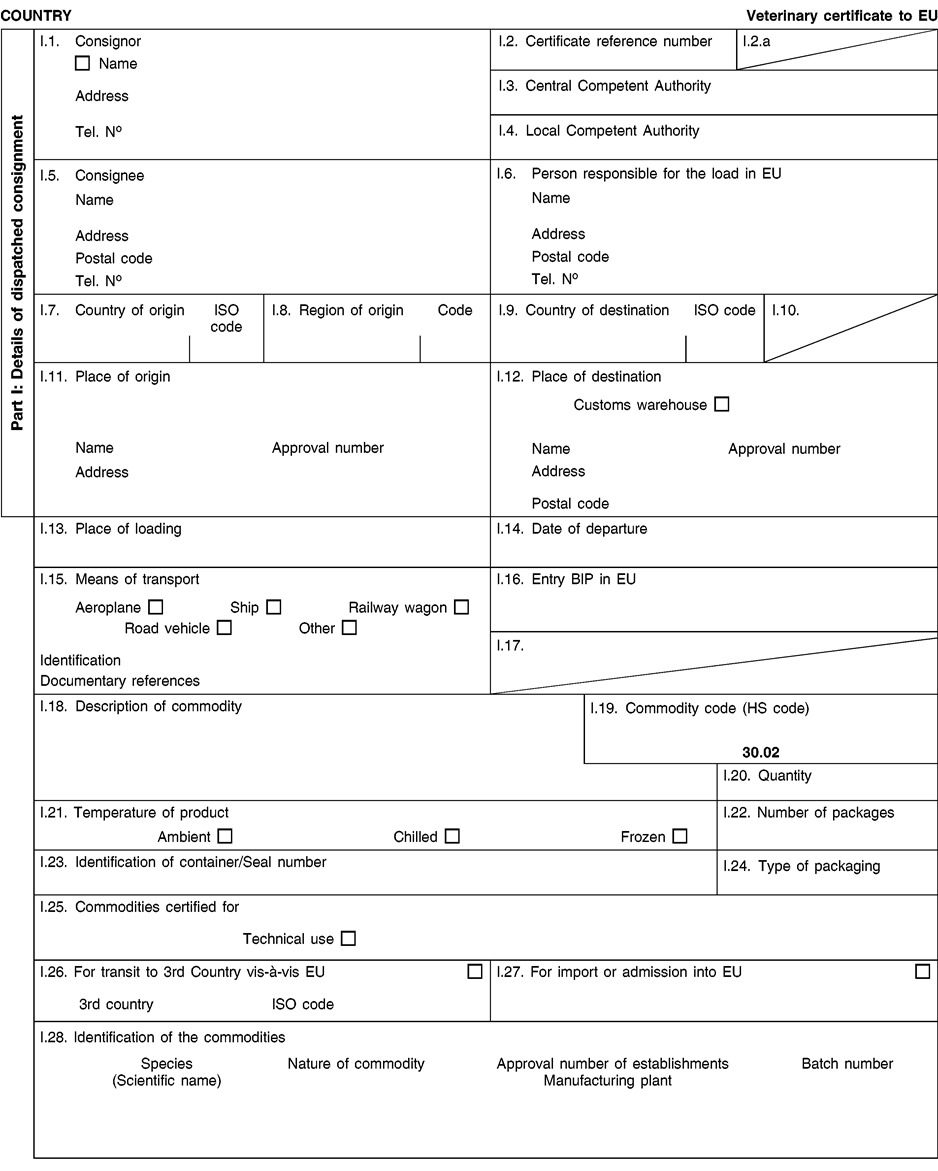
(a) Veterinary certificates shall be produced by the exporting country, based on the models appearing in this Annex X, according to the layout of the model that corresponds to the animal by-products concerned. They shall contain, in the numbered order that appears in the model, the attestations that are required for any third country and, as the case may be, those supplementary guarantees that are required for the exporting third country or part thereof. U.K.
(b) The original of each certificate shall consist of a single page, both sides, or, where more text is required, it shall be in such a form that all pages needed are part of an integrated whole and indivisible. U.K.
(c) It shall be drawn up in at least one of the official languages of the EU Member State in which the inspection at the border post shall be carried out and of the EU Member State of destination. However, these Member States may allow other languages, accompanied, if necessary, by an official translation. U.K.
(d) If for reasons of identification of the items of the consignment, additional pages are attached to the certificate, these pages shall also be considered as forming part of the original of the certificate by the application of the signature and stamp of the certifying official veterinarian, in each of the pages. U.K.
(e) When the certificate, including additional schedules referred to in d), comprises more than one page, each page shall be numbered — ( page number ) of ( total number of pages ) — on its bottom and shall bear the code number of the certificate that has been designated by the competent authority on its top. U.K.
(f) The original of the certificate must be completed and signed by an official veterinarian. In doing so, the competent authorities of the exporting country shall ensure that the principles of certification equivalent to those laid down in Council Directive 96/93/EC are followed. U.K.
(g) The colour of the signature shall be different to that of the printing. The same rule applies to stamps other than those embossed or watermark. U.K.
(h) The original of the certificate must accompany the consignment at the EU border inspection post. U.K.
(i) If health certificates are used for consignments in transit, box No I.5 (Consignee) of the relevant health certificate shall be completed with the name and address of the border inspection post through which the consignment is intended to leave the European Community. U.K.
[F2CHAPTER 2 U.K. Health certificate For milk and milk products not intended for human consumption for dispatch to or for transit through ( 2 ) the European Union]
Textual Amendments
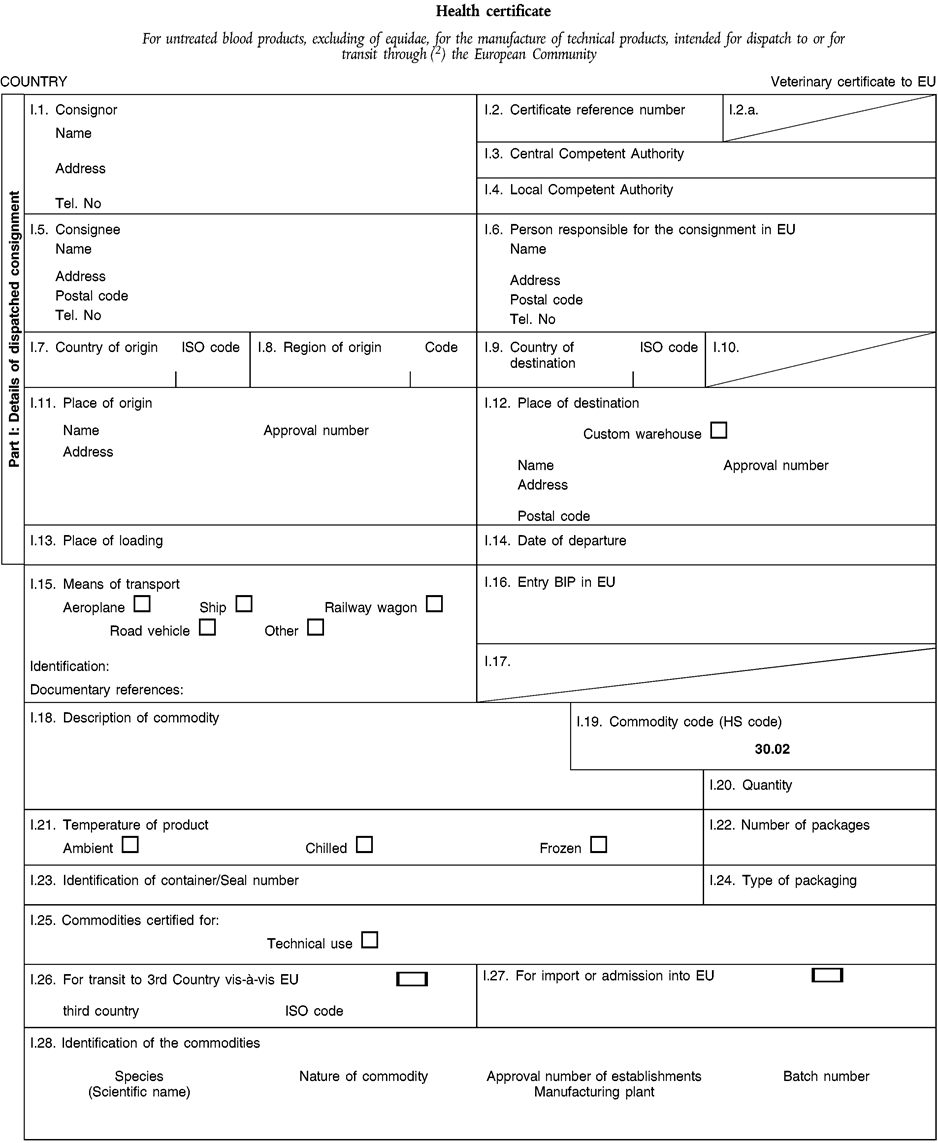
[F2CHAPTER 4 (A) U.K. Health certificate For the import of blood and blood products from equidae for technical purposes, intended for dispatch to or for transit through ( 2 ) the European Union]
[F3CHAPTER 4(C)] U.K.
Textual Amendments
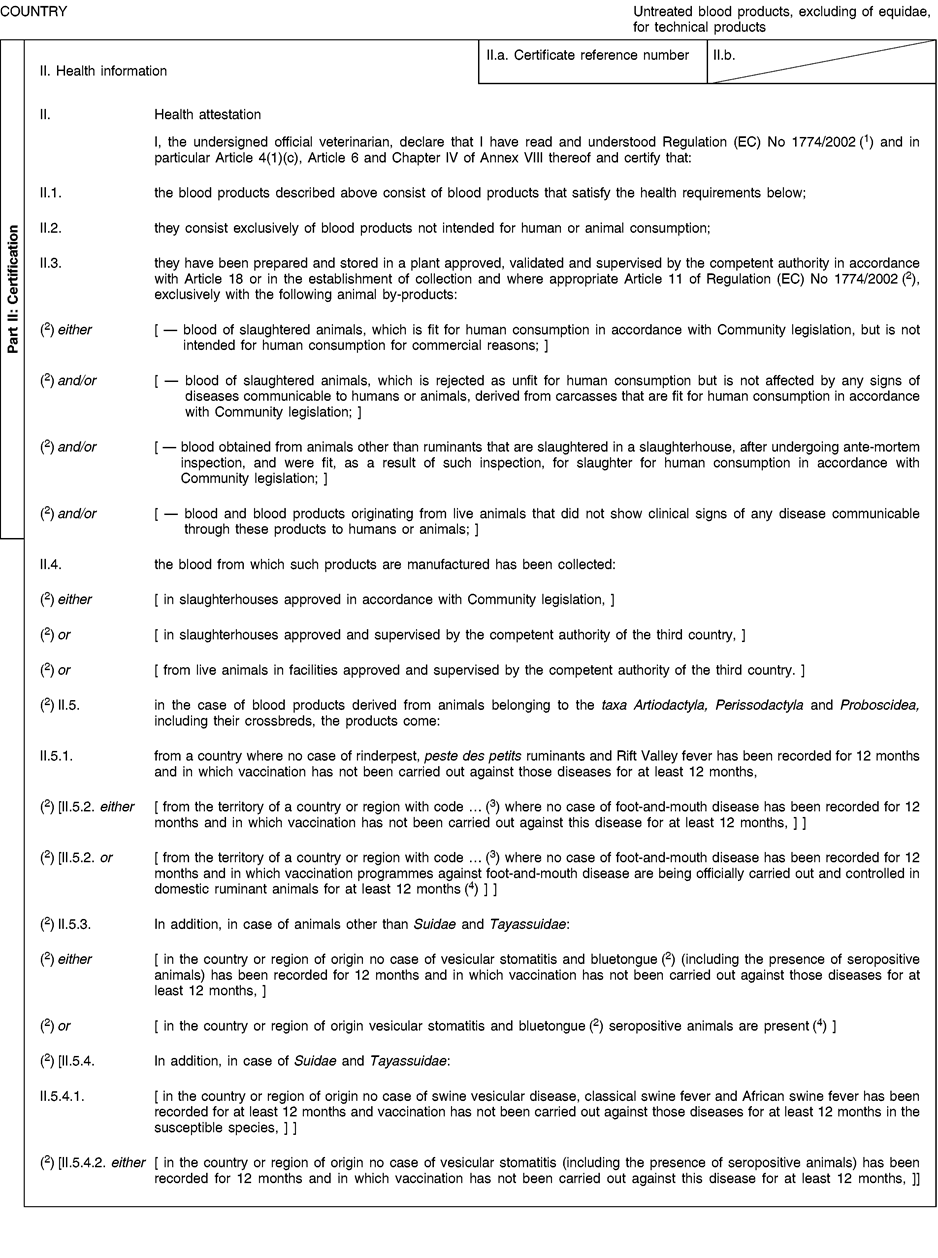
[F2CHAPTER 4 (D) U.K. Health certificate For treated blood products, excluding those of equidae, for the manufacture of technical products, intended for dispatch to or for transit through ( 2 ) the European Union]
[F4CHAPTER 18 U.K. Health certificate For horns and horn products, excluding horn meal, and hooves and hoof products, excluding hoof meal, intended for the production of organic fertilizers or soil improvers intended for dispatch to or for transit through ( 2 ) the European Union] ]
Textual Amendments
Options/Help
Print Options
PrintThe Whole Regulation
PrintThis Annex only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources