[F1ANNEX X U.K. MODEL HEALTH CERTIFICATES FOR THE IMPORTATION FROM THIRD COUNTRIES AND FOR THE TRANSIT THROUGH THE EUROPEAN COMMUNITY OF CERTAIN ANIMAL BY-PRODUCTS AND PRODUCTS DERIVED THEREFROM
Textual Amendments
Notes U.K.
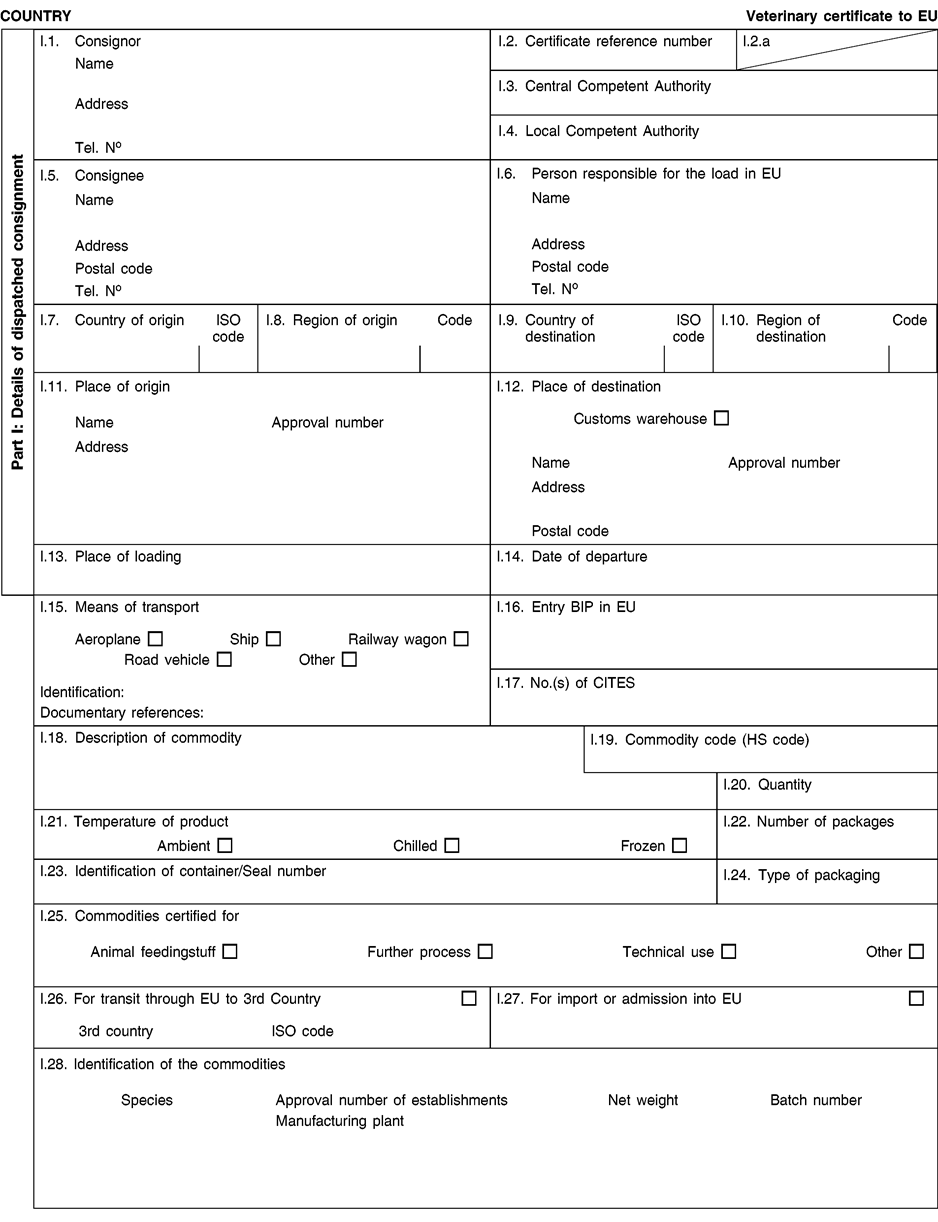
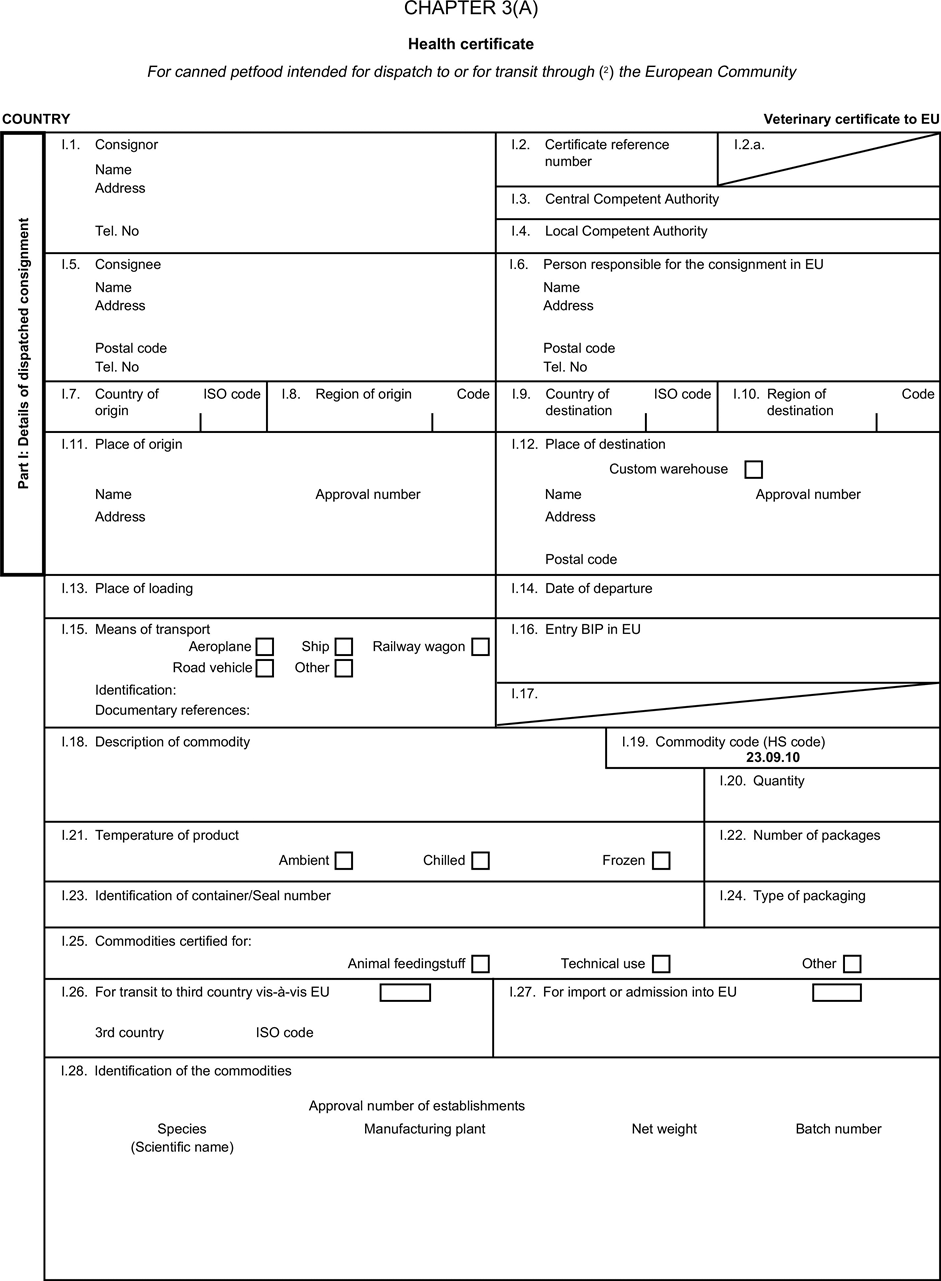
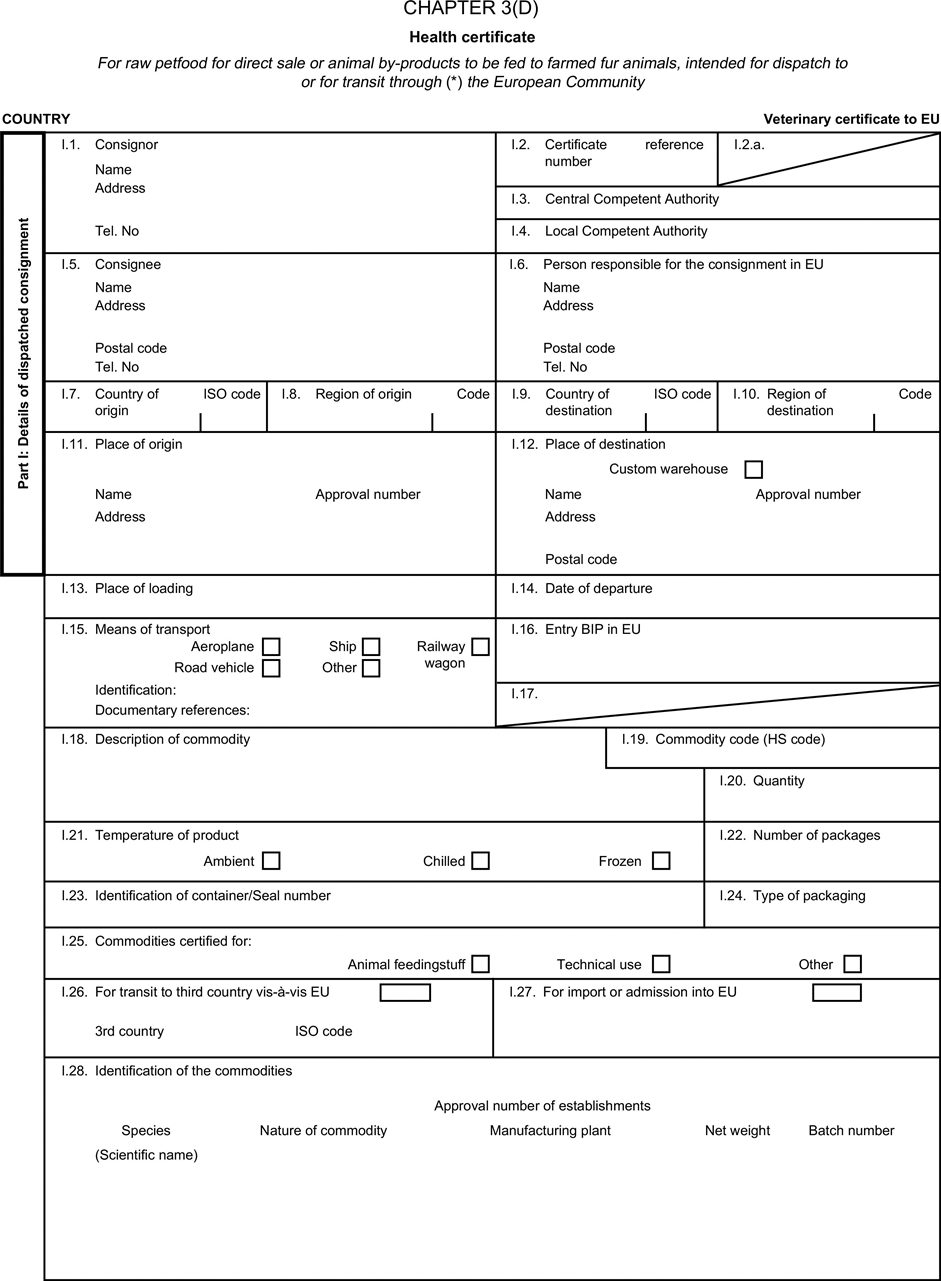
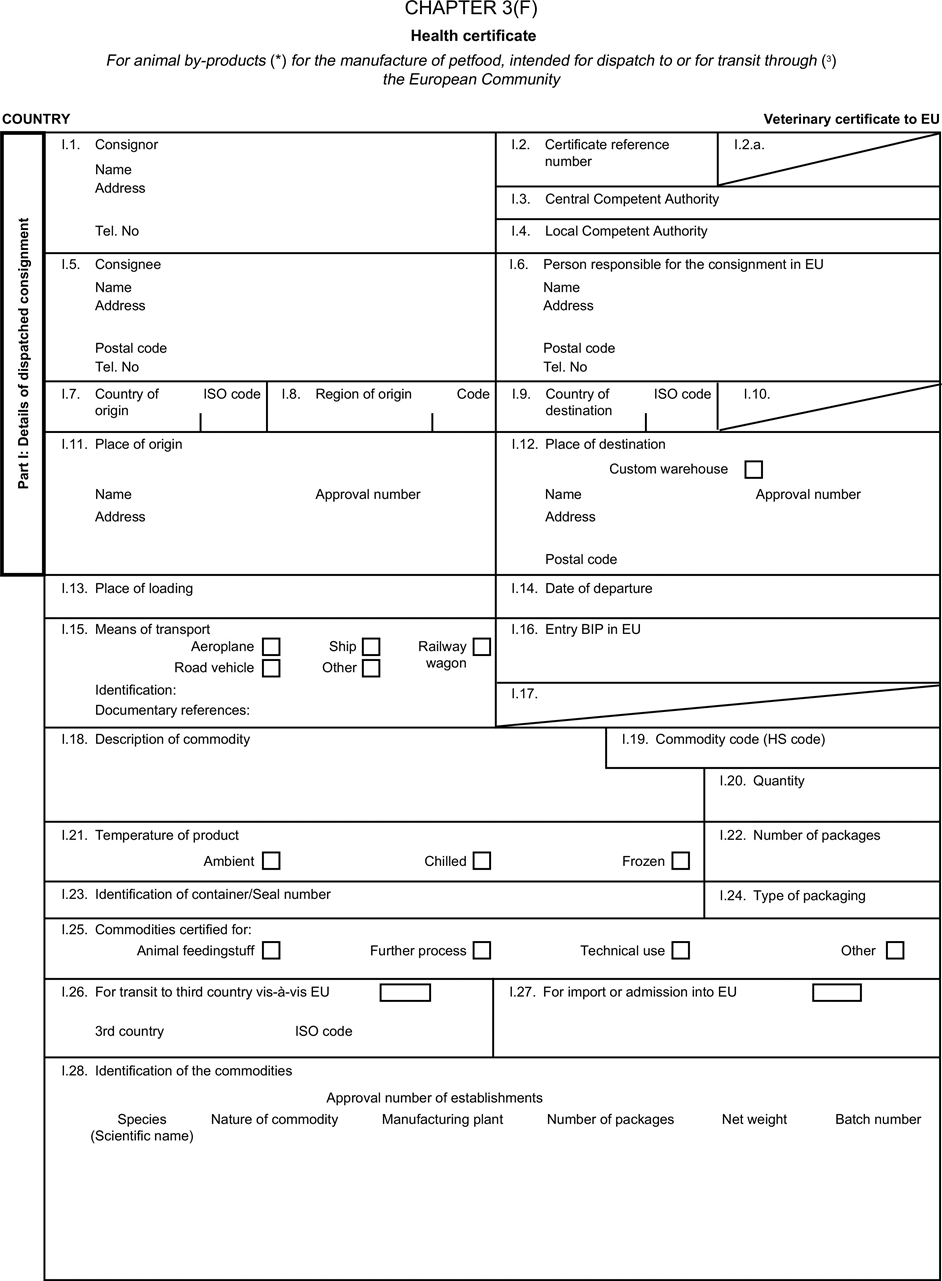
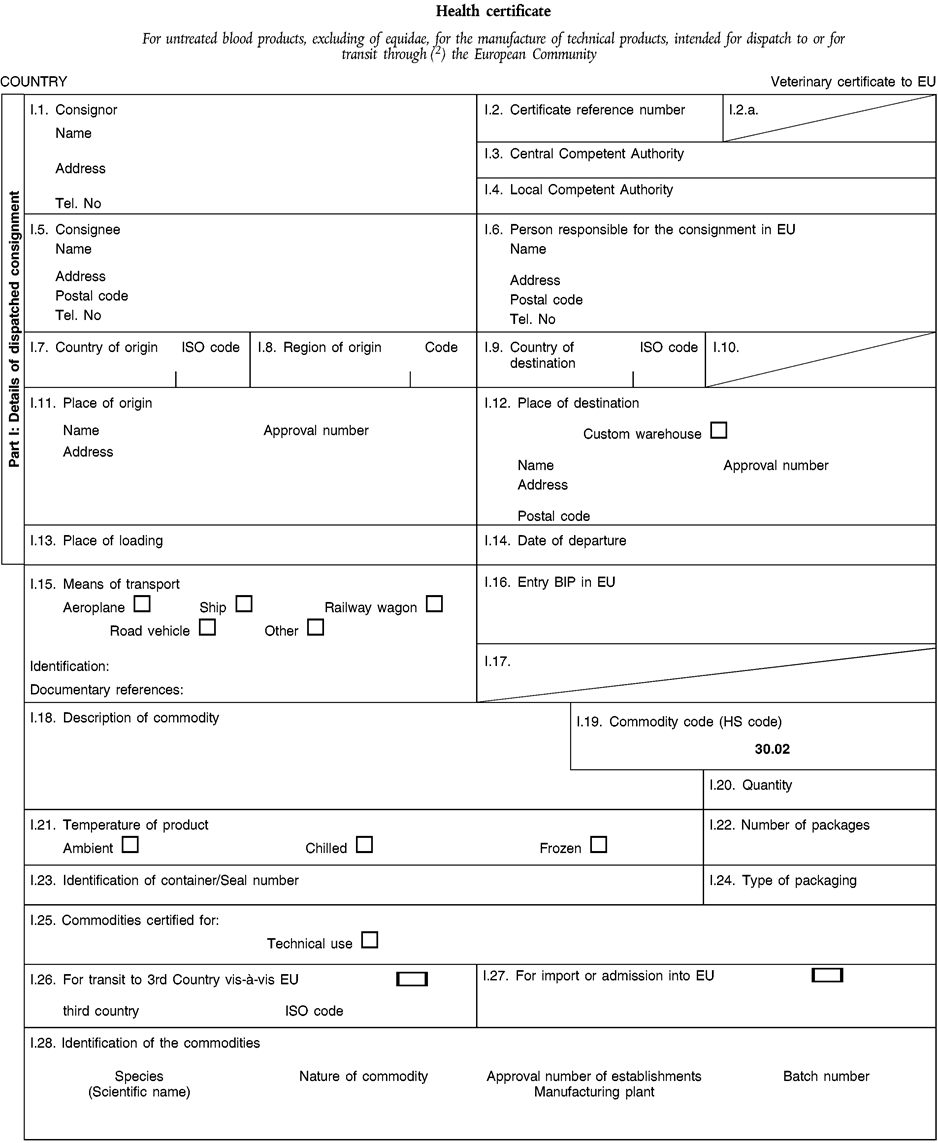
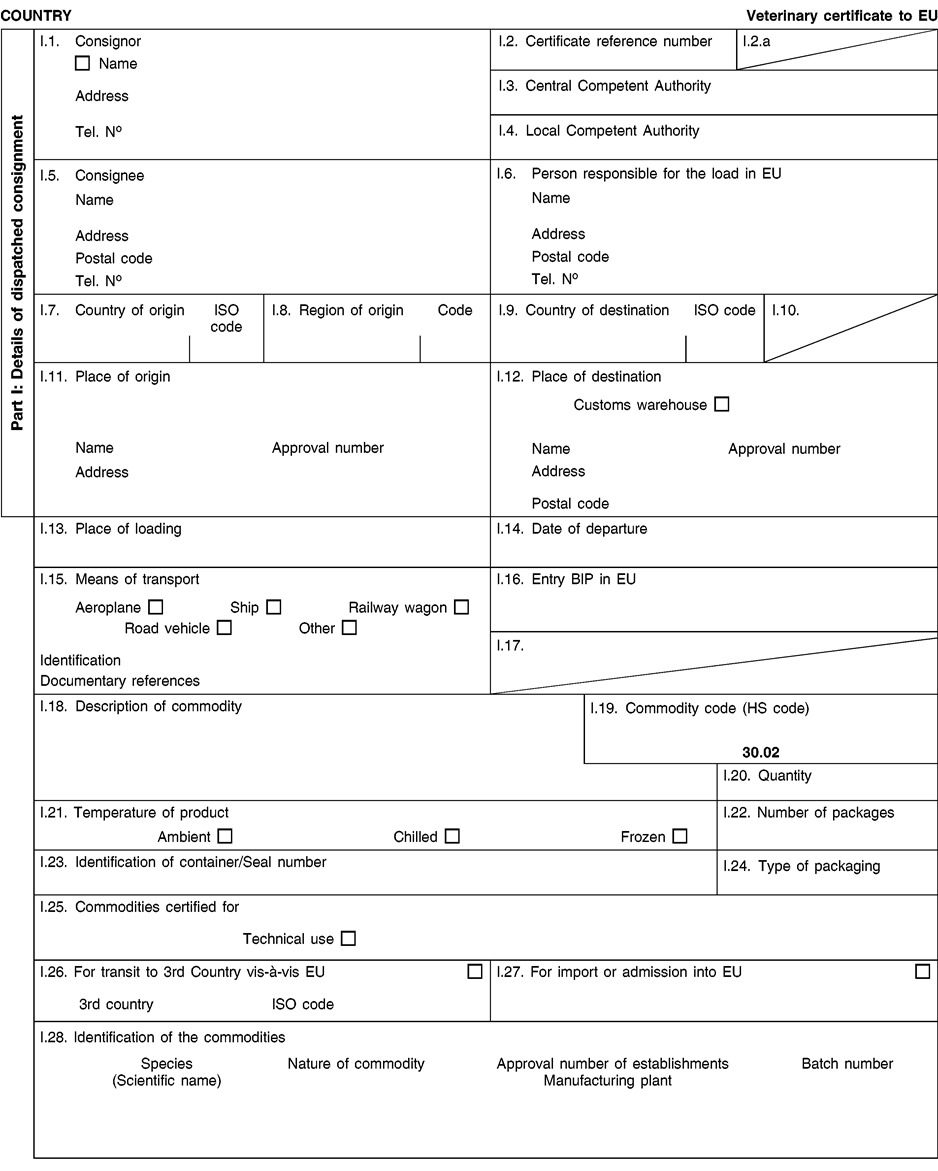
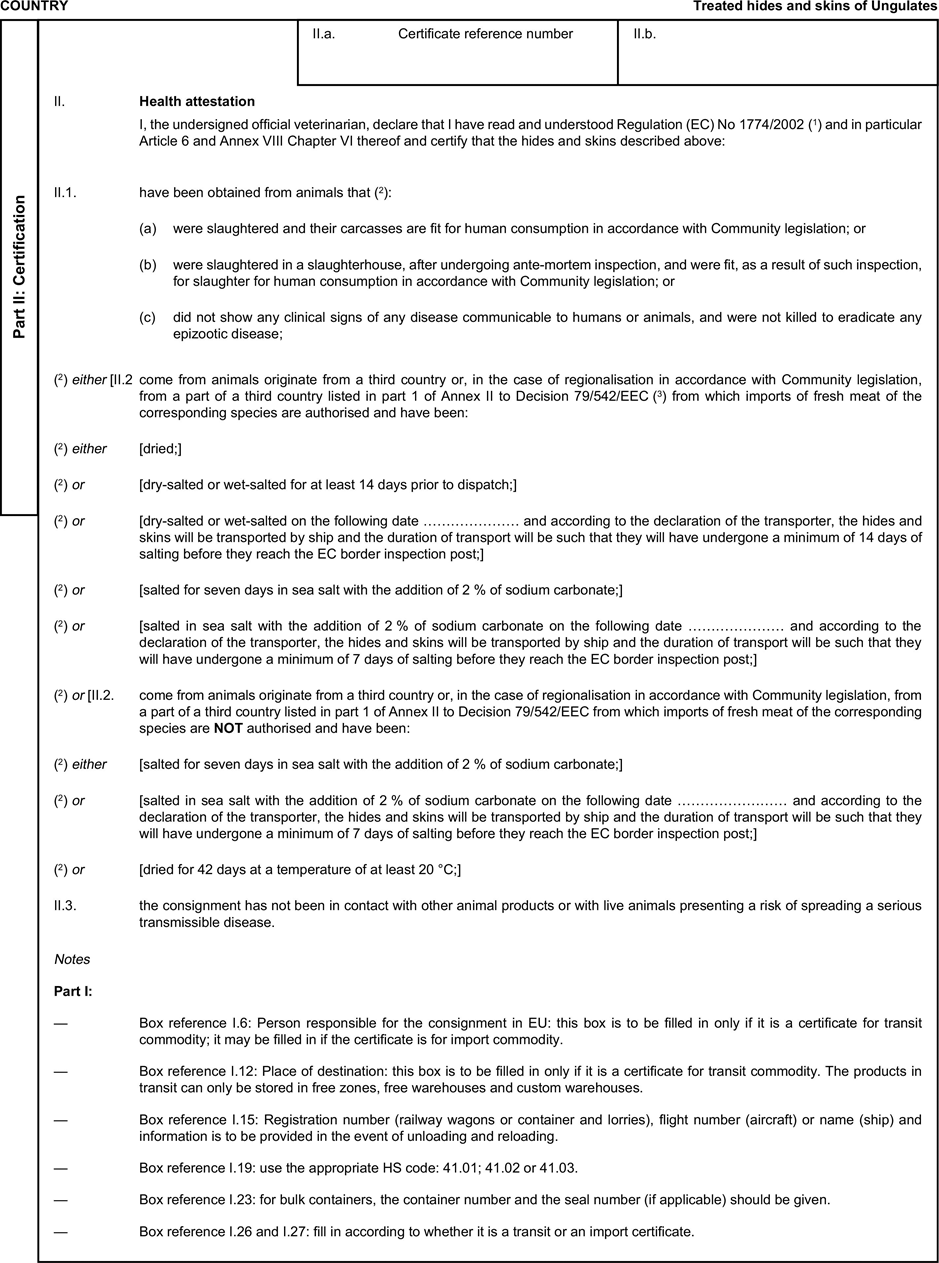
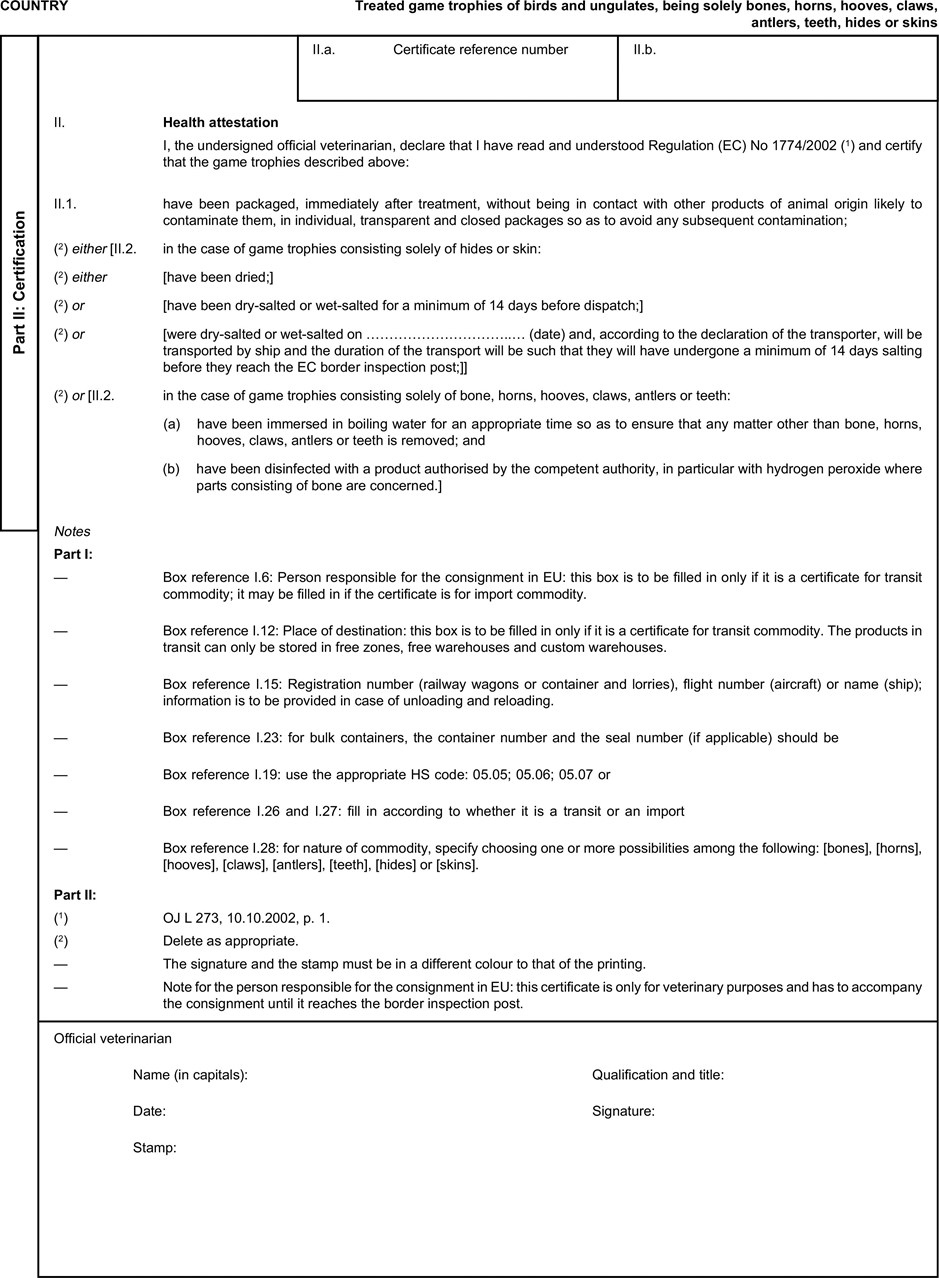
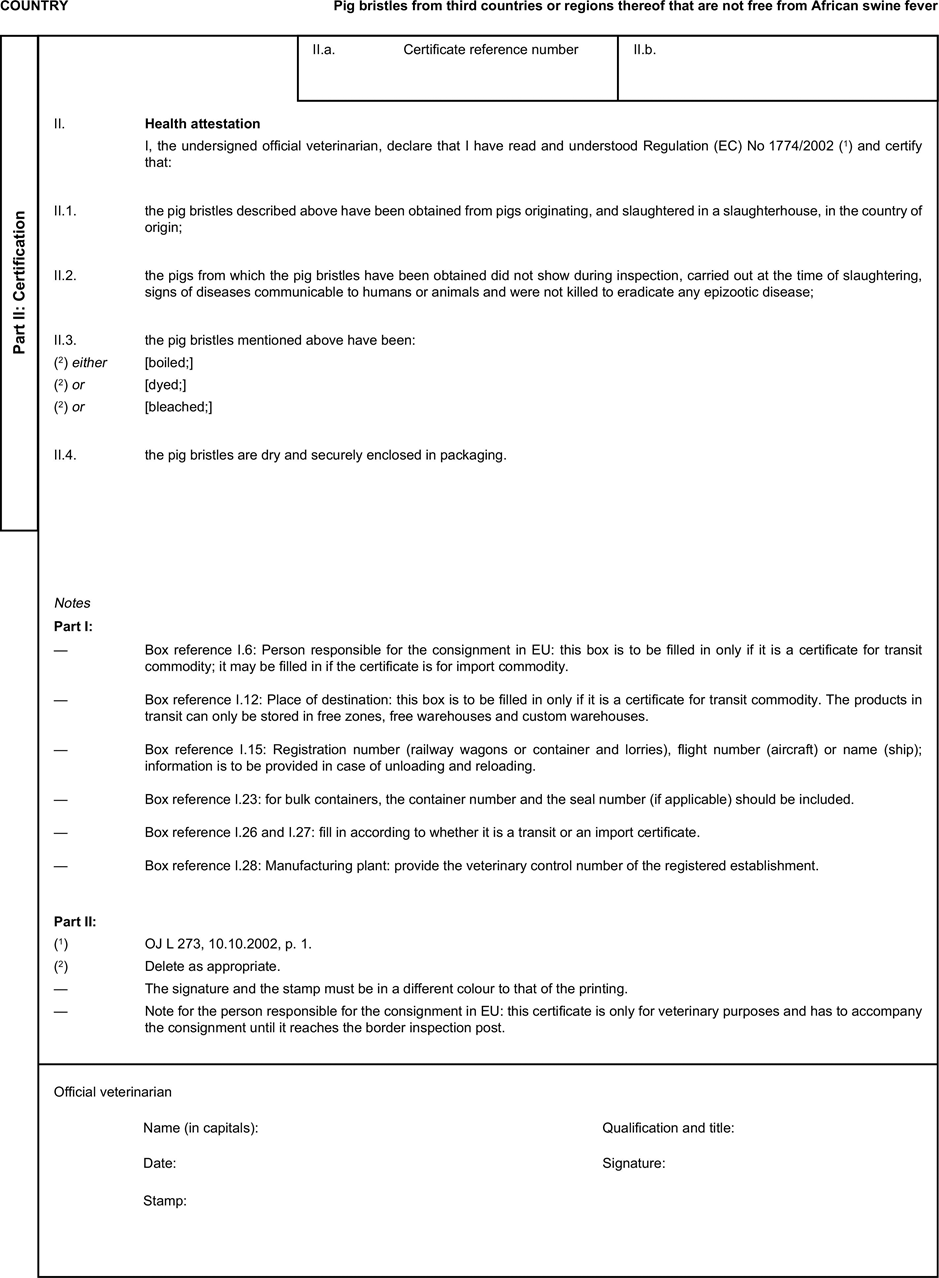
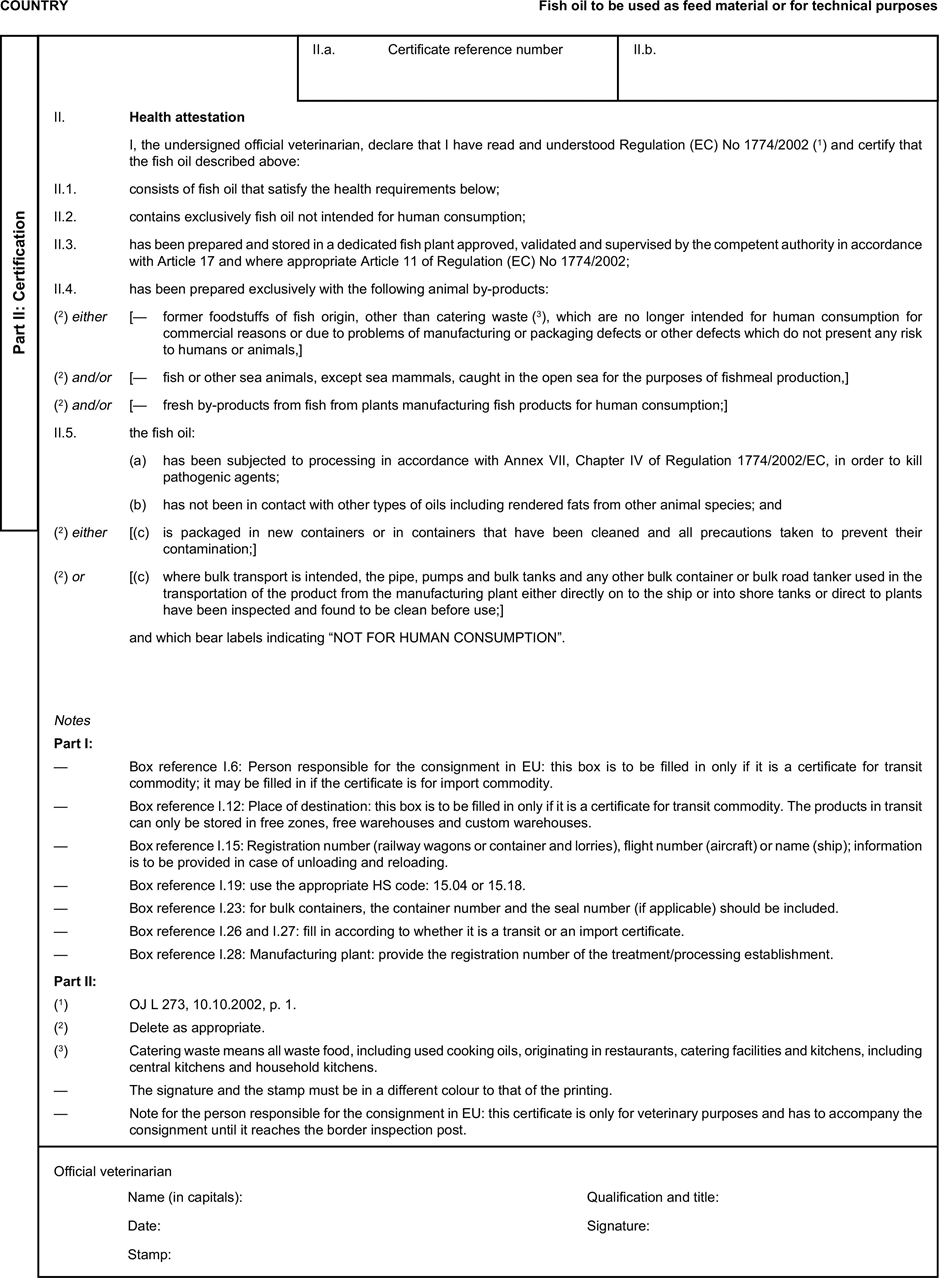
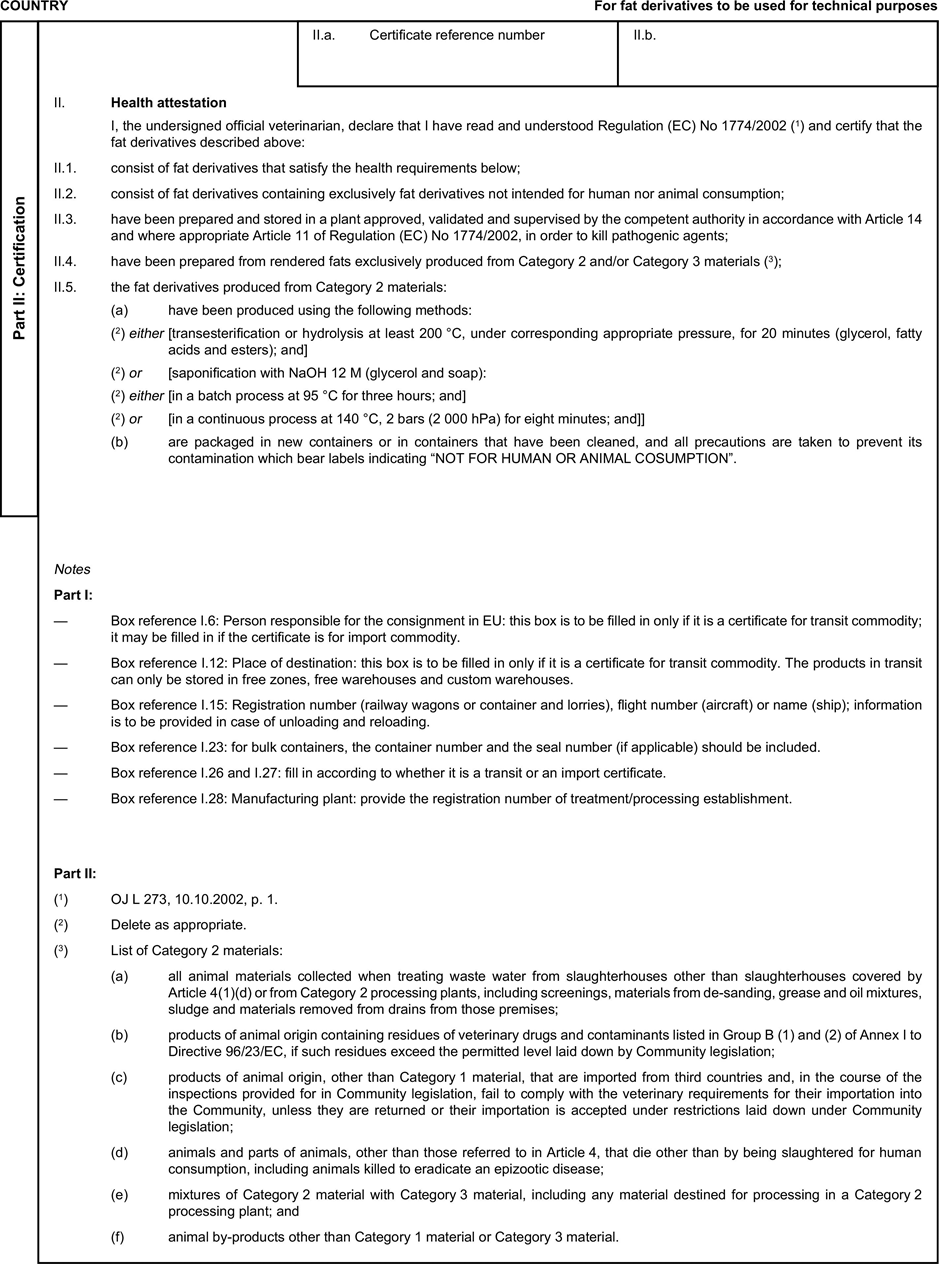
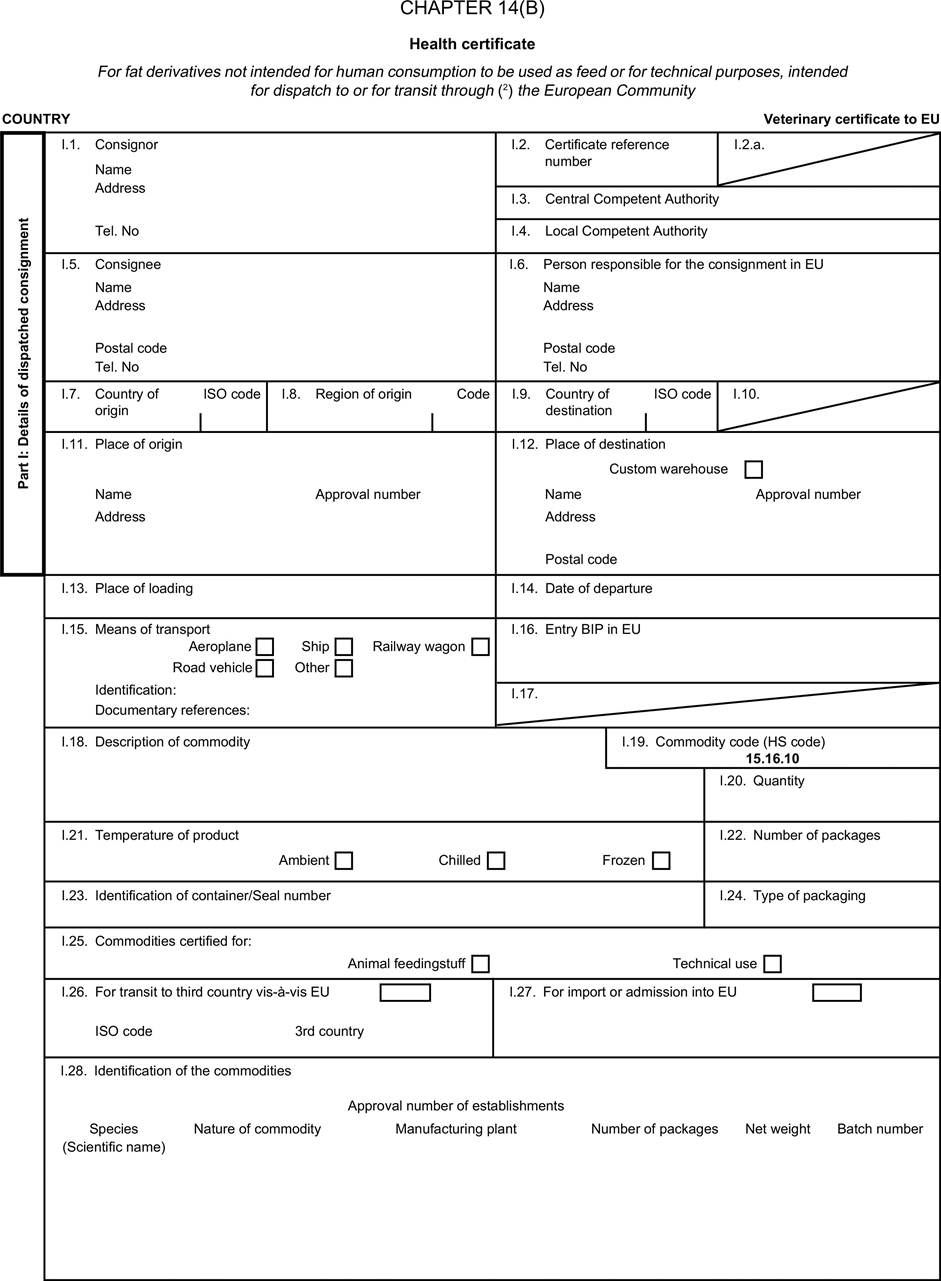
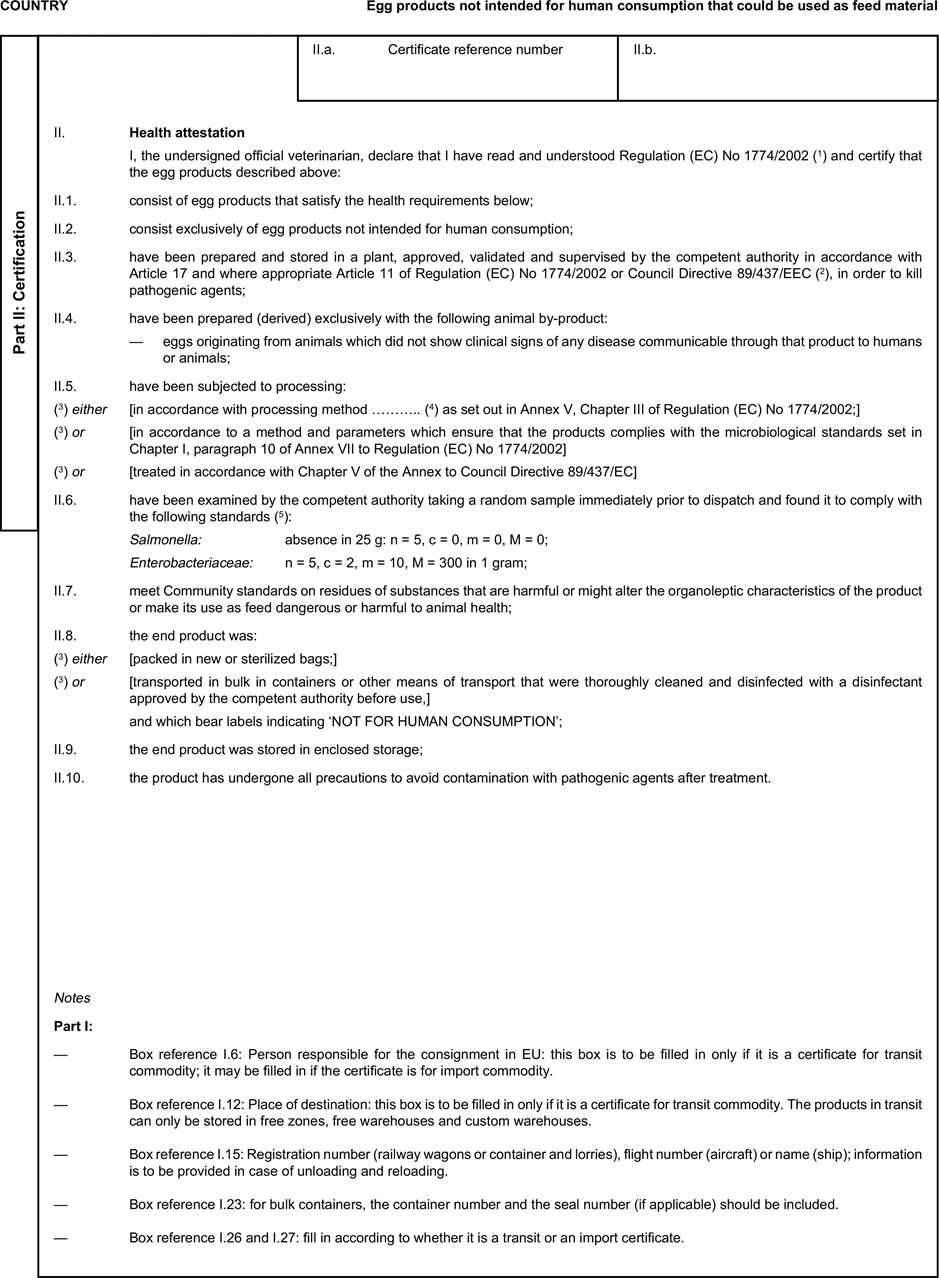
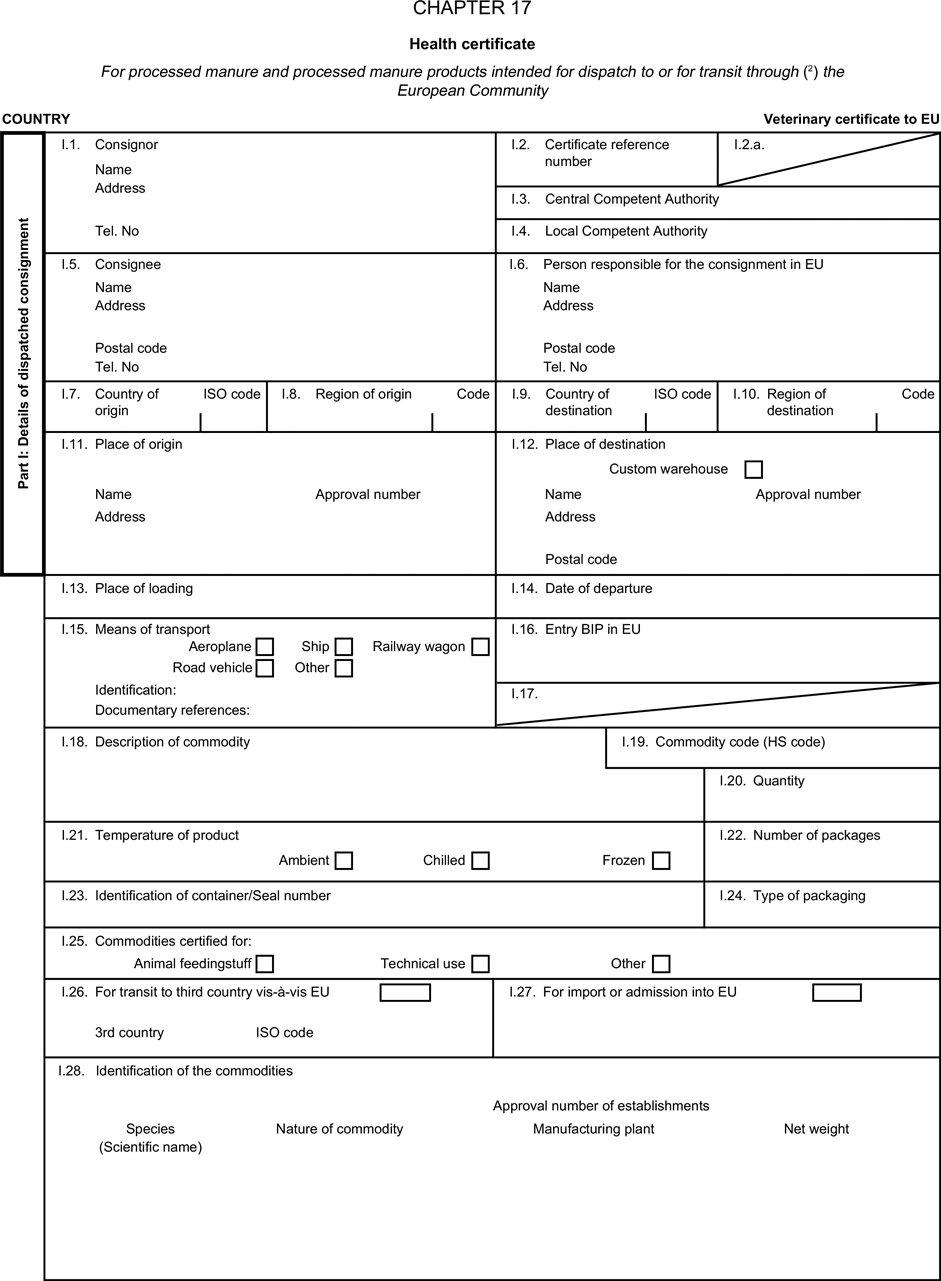
(a) Veterinary certificates shall be produced by the exporting country, based on the models appearing in this Annex X, according to the layout of the model that corresponds to the animal by-products concerned. They shall contain, in the numbered order that appears in the model, the attestations that are required for any third country and, as the case may be, those supplementary guarantees that are required for the exporting third country or part thereof. U.K.
(b) The original of each certificate shall consist of a single page, both sides, or, where more text is required, it shall be in such a form that all pages needed are part of an integrated whole and indivisible. U.K.
(c) It shall be drawn up in at least one of the official languages of the EU Member State in which the inspection at the border post shall be carried out and of the EU Member State of destination. However, these Member States may allow other languages, accompanied, if necessary, by an official translation. U.K.
(d) If for reasons of identification of the items of the consignment, additional pages are attached to the certificate, these pages shall also be considered as forming part of the original of the certificate by the application of the signature and stamp of the certifying official veterinarian, in each of the pages. U.K.
(e) When the certificate, including additional schedules referred to in d), comprises more than one page, each page shall be numbered — ( page number ) of ( total number of pages ) — on its bottom and shall bear the code number of the certificate that has been designated by the competent authority on its top. U.K.
(f) The original of the certificate must be completed and signed by an official veterinarian. In doing so, the competent authorities of the exporting country shall ensure that the principles of certification equivalent to those laid down in Council Directive 96/93/EC are followed. U.K.
(g) The colour of the signature shall be different to that of the printing. The same rule applies to stamps other than those embossed or watermark. U.K.
(h) The original of the certificate must accompany the consignment at the EU border inspection post. U.K.
(i) If health certificates are used for consignments in transit, box No I.5 (Consignee) of the relevant health certificate shall be completed with the name and address of the border inspection post through which the consignment is intended to leave the European Community. U.K.
[F2CHAPTER 2 U.K. Health certificate For milk and milk products not intended for human consumption for dispatch to or for transit through ( 2 ) the European Union]
Textual Amendments
[F2CHAPTER 4 (A) U.K. Health certificate For the import of blood and blood products from equidae for technical purposes, intended for dispatch to or for transit through ( 2 ) the European Union]
[F3CHAPTER 4(C)] U.K.
Textual Amendments
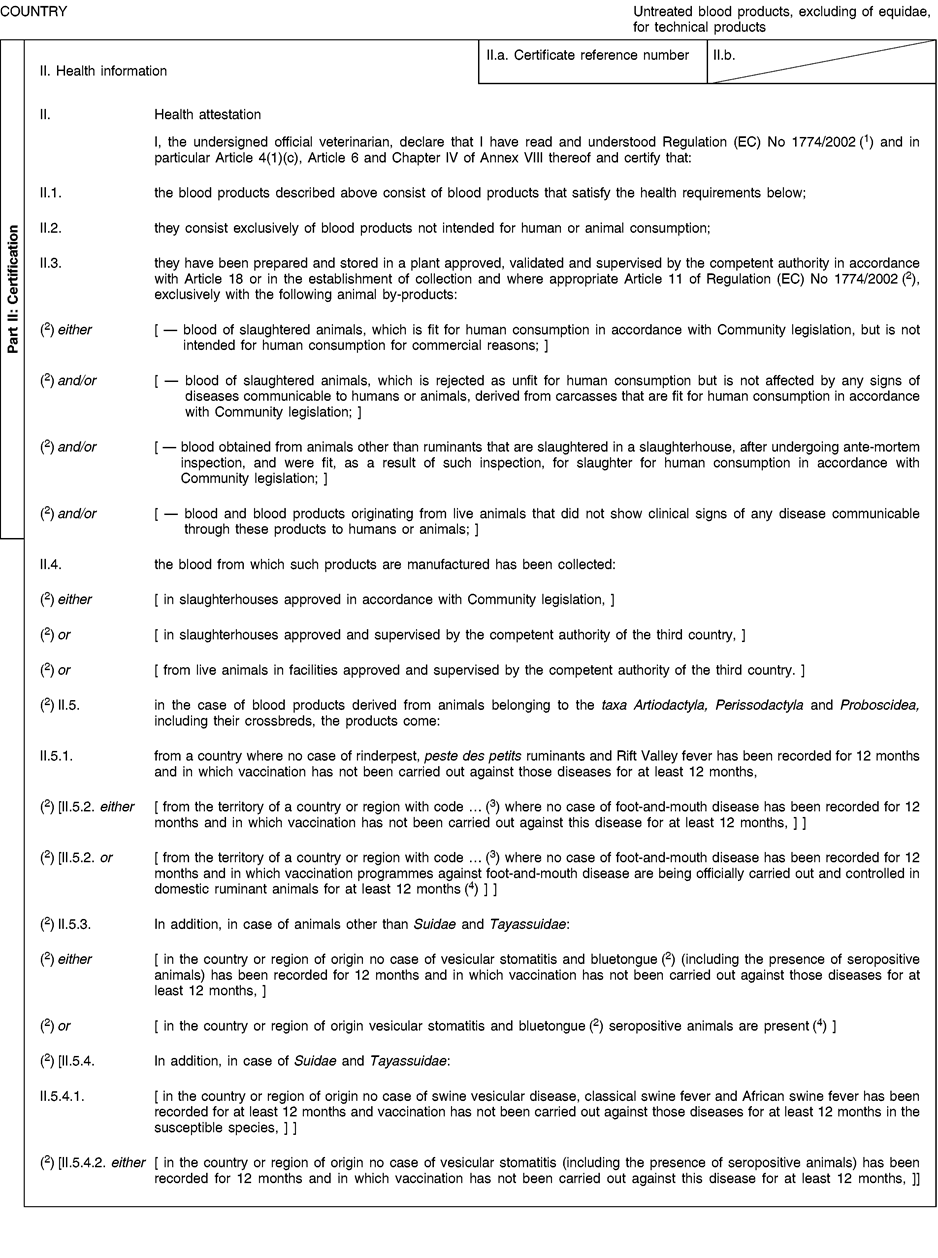
[F2CHAPTER 4 (D) U.K. Health certificate For treated blood products, excluding those of equidae, for the manufacture of technical products, intended for dispatch to or for transit through ( 2 ) the European Union]
[F4CHAPTER 18 U.K. Health certificate For horns and horn products, excluding horn meal, and hooves and hoof products, excluding hoof meal, intended for the production of organic fertilizers or soil improvers intended for dispatch to or for transit through ( 2 ) the European Union] ]
Textual Amendments