- Latest available (Revised)
- Point in Time (13/12/2020)
- Original (As adopted by EU)
Regulation (EC) No 273/2004 of the European Parliament and of the CouncilShow full title
Regulation (EC) No 273/2004 of the European Parliament and of the Council of 11 February 2004 on drug precursors (Text with EEA relevance)
You are here:
- Regulations originating from the EU
- 2004 No. 273
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 07/07/20180.31 MB
- Revised 21/09/20160.31 MB
- Revised 30/12/20130.29 MB
- Revised 20/04/20090.12 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Regulation (EC) No 273/2004 of the European Parliament and of the Council (Annexes only)
Status:
Point in time view as at 13/12/2020.
Changes to legislation:
There are outstanding changes not yet made to Regulation (EC) No 273/2004 of the European Parliament and of the Council. Any changes that have already been made to the legislation appear in the content and are referenced with annotations.![]()
Changes to Legislation
Changes and effects yet to be applied by the editorial team are only applicable when viewing the latest version or prospective version of legislation. They are therefore not accessible when viewing legislation as at a specific point in time. To view the ‘Changes to Legislation’ information for this provision return to the latest version view using the options provided in the ‘What Version’ box above.
ANNEX IU.K. [F1List of scheduled substances]
Textual Amendments
CATEGORY 1
b The CAS No is the ‘chemical abstracts service registry number’, which is a unique numeric identifier specific to each substance and its structure. The CAS No is specific to each isomer and to each salt of each isomer. It must be understood that the CAS Nos for the salts of the substances listed above will be different to those given. | |||
c Also named (+)-norpseudoephedrine, CN code 2939 43 00, CAS No 492-39-7. | |||
| Substance | CN designation(if different) | CN codea | CAS Nob |
|---|---|---|---|
| 1-phenyl-2-propanone | Phenylacetone | 2914 31 00 | 103-79-7 |
| [F2Methyl alpha-phenylacetoacetate (MAPA) | 2918 30 00 | 16648-44-5 | |
Methyl 2-methyl-3-phenyloxirane-2-carboxylate (BMK methyl glycidate) | 2918 99 90 | 80532-66-7 | |
2-methyl-3-phenyloxirane-2-carboxylic acid (BMK glycidic acid) | 2918 99 90 | 25547-51-7] | |
| N-acetylanthranilic acid | 2-acetamidobenzoic acid | 2924 23 00 | 89-52-1 |
| [F3Alpha-phenylacetoacetamide (APAA) | 2924 29 70 | 4433-77-6] | |
| [F4Alpha-phenylacetoacetonitrile (APAAN) | 2926 40 00 | 4468-48-8] | |
| Isosafrol (cis + trans) | 2932 91 00 | 120-58-1 | |
| 3,4-methylenedioxyphenylpropan-2-one | 1-(1,3-Benzodioxol-5-yl)propan-2-one | 2932 92 00 | 4676-39-5 |
| Piperonal | 2932 93 00 | 120-57-0 | |
| Safrole | 2932 94 00 | 94-59-7 | |
| [F5Methyl 3-(1,3-benzodioxol-5-yl)-2-methyloxirane-2-carboxylate (PMK methyl glycidate | 2932 99 00 | 13605-48-6 | |
| 3-(1,3-benzodioxol-5-yl)-2-methyloxirane-2-carboxylic acid (PMK glycidic acid) | 2932 99 00 | 2167189-50-4] | |
| [F64-anilino- N -phenethylpiperidine (ANPP) | 2933 39 99 | 21409-26-7 | |
| N -phenethyl-4-piperidone (NPP) | 2933 39 99 | 39742-60-4] | |
| Ephedrine | 2939 41 00 | 299-42-3 | |
| Pseudoephedrine | 2939 42 00 | 90-82-4 | |
| Norephedrine | [F12939 44 00] | 14838-15-4 | |
| Ergometrine | 2939 61 00 | 60-79-7 | |
| Ergotamine | 2939 62 00 | 113-15-5 | |
| Lysergic acid | 2939 63 00 | 82-58-6 | |
| The stereoisomeric forms of the substances listed in this category not being cathinec, whenever the existence of such forms is possible. | |||
| The salts of the substances listed in this category, whenever the existence of such salts is possible and not being the salts of cathine. | |||
| [F7(1R,2S)-(-)-chloroephedrine | [F82939 79 90] | 110925-64-9 | |
| (1S,2R)-(+)-chloroephedrine | [F92939 79 90] | 1384199-95-4 | |
| (1S,2S)-(+)-chloropseudoephedrine | [F102939 79 90] | 73393-61-0 | |
| (1R,2R)-(-)-chloropseudoephedrine | [F112939 79 90] | 771434-80-1] | |
Textual Amendments
F2Inserted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F3Inserted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F4Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F5Inserted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F6Inserted by Commission Delegated Regulation (EU) 2018/729 of 26 February 2018 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance).
F7Inserted by Commission Delegated Regulation (EU) 2016/1443 of 29 June 2016 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance).
F8Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F9Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F10Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
F11Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
[F1CATEGORY 2
| SUBCATEGORY 2A | |||
| Substance | CN designation (if different) | CN code a | CAS No b |
|---|---|---|---|
| Acetic anhydride | 2915 24 00 | 108-24-7 | |
| The salts of the substances listed in this category, whenever the existence of such salts is possible. | |||
| SUBCATEGORY 2B | |||
b The CAS No is the ‘ chemical abstracts service registry number ’ , which is a unique numeric identifier specific to each substance and its structure. The CAS No is specific to each isomer and to each salt of each isomer. It must be understood that the CAS Nos for the salts of the substances listed above will be different to those given. ] | |||
| Substance | CN designation (if different) | CN code a | CAS No b |
|---|---|---|---|
| Phenylacetic acid | 2916 34 00 | 103-82-2 | |
| Anthranilic acid | [F12ex 2922 43 00] | 118-92-3 | |
| Piperidine | 2933 32 00 | 110-89-4 | |
| Potassium permanganate | 2841 61 00 | 7722-64-7 | |
| The salts of the substances listed in this category, whenever the existence of such salts is possible. | |||
Textual Amendments
F12Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
CATEGORY 3
b The CAS No is the ‘chemical abstracts service registry number’, which is a unique numeric identifier specific to each substance and its structure. The CAS No is specific to each isomer and to each salt of each isomer. It must be understood that the CAS Nos for the salts of the substances listed above will be different to those given. | |||
| Substance | CN designation(if different) | CN codea | CAS Nob |
|---|---|---|---|
| Hydrochloric acid | Hydrogen chloride | 2806 10 00 | 7647-01-0 |
| Sulphuric acid | [F132807 00 00] | 7664-93-9 | |
| Toluene | 2902 30 00 | 108-88-3 | |
| Ethyl ether | Diethyl ether | 2909 11 00 | 60-29-7 |
| Acetone | 2914 11 00 | 67-64-1 | |
| Methylethylketone | Butanone | 2914 12 00 | 78-93-3 |
| The salts of the substances listed in this category, whenever the existence of such salts is possible and not being the salts of hydrochloric acid and sulphuric acid. | |||
Textual Amendments
F13Substituted by Commission Delegated Regulation (EU) 2020/1737 of 14 July 2020 amending Regulation (EC) No 273/2004 of the European Parliament and of the Council and Council Regulation (EC) No 111/2005 as regards the inclusion of certain drug precursors in the list of scheduled substances (Text with EEA relevance)
ANNEX IIU.K.
| Substance | Threshold |
|---|---|
| Acetic anhydride | 100 l |
| Potassium permanganate | 100 kg |
| Anthranilic acid and its salts | 1 kg |
| Phenylacetic acid and its salts | 1 kg |
| Piperidine and its salts | 0,5 kg |
ANNEX IIIU.K.
1.Model declaration relating to individual transactions (category 1 or 2)U.K.
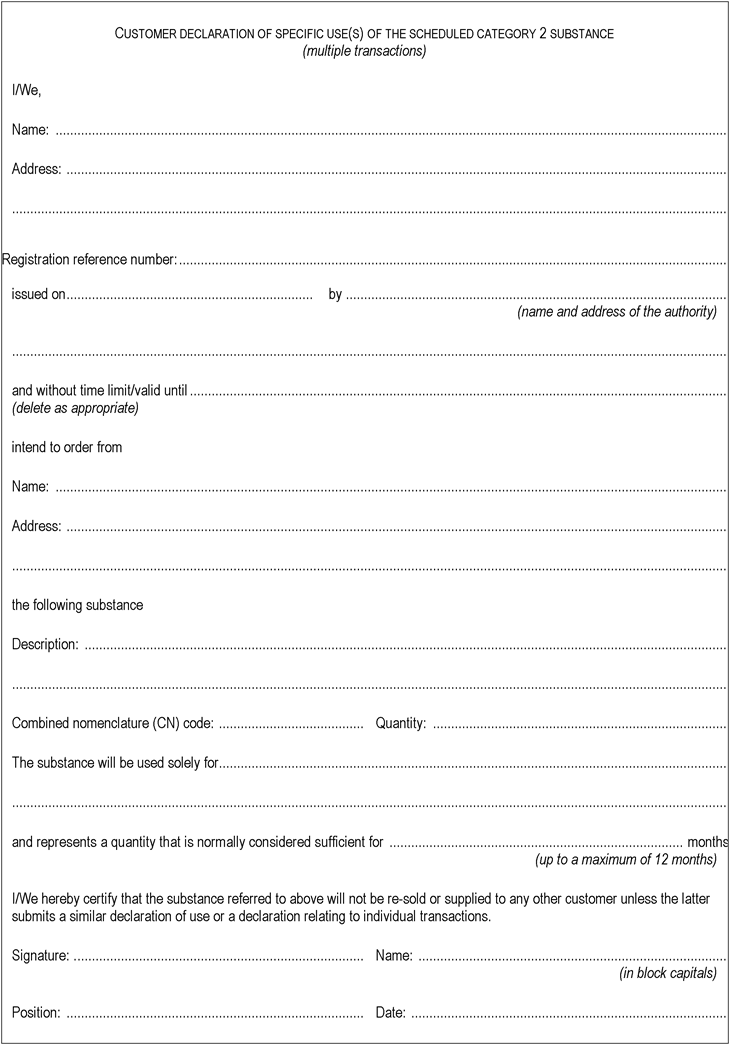
2.Model declaration relating to multiple transactions (category 2)U.K.
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Annexes only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources