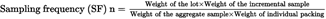- Latest available (Revised)
- Original (As adopted by EU)
Commission Regulation (EC) No 401/2006Show full title
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs (Text with EEA relevance)
You are here:
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 01/07/20140.53 MB
- Revised 13/03/20100.16 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Division A.
Changes to legislation:
There are currently no known outstanding effects for the Commission Regulation (EC) No 401/2006, Division A..![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
A.GENERAL PROVISIONSU.K.
Official controls shall be performed in accordance with the provisions of Regulation (EC) No 882/2004. The following general provisions shall apply without prejudice to the provisions in Regulation (EC) No 882/2004.
A.1.Purpose and scopeU.K.
Samples intended for official control of the levels of mycotoxins content in foodstuffs shall be taken according to the methods set out in this Annex. Aggregate samples thus obtained shall be considered as representative of the lots. Compliance with maximum limits laid down in Regulation (EC) No 466/2001 shall be established on the basis of the levels determined in the laboratory samples.
A.2.DefinitionsU.K.
For the purpose of this Annex, the following definitions shall apply:
‘lot’ means an identifiable quantity of a food commodity delivered at one time and determined by the official to have common characteristics, such as origin, variety, type of packing, packer, consignor or markings;
‘sublot’ means a designated part of a large lot in order to apply the sampling method on that designated part; each sublot must be physically separate and identifiable;
‘incremental sample’ means a quantity of material taken from a single place in the lot or sublot;
‘aggregate sample’ means the combined total of all the incremental samples taken from the lot or sublot;
‘laboratory sample’ means a sample intended for the laboratory.
A.3.General provisionsU.K.
A.3.1.PersonnelU.K.
Sampling shall be performed by an authorised person F1....
Textual Amendments
F1Words in Annex 1 para. A.3.1 omitted (31.12.2020) by virtue of The Contaminants in Food (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/639), regs. 1, 13; 2020 c. 1, Sch. 5 para. 1(1)
A.3.2.Material to be sampledU.K.
Each lot which is to be examined shall be sampled separately. In accordance with the specific sampling provisions for the different mycotoxins, large lots shall be subdivided into sublots to be sampled separately.
A.3.3.Precautions to be takenU.K.
In the course of sampling and preparation of the samples, precautions shall be taken to avoid any changes, which would affect:
the mycotoxin content, adversely affect the analytical determination or make the aggregate samples unrepresentative;
the food safety of the lots to be sampled.
Also, all measures necessary to ensure the safety of the persons taking the samples shall be taken.
A.3.4.Incremental samplesU.K.
As far as possible incremental samples shall be taken at various places distributed throughout the lot or sublot. Departure from such procedure shall be recorded in the record provided for under part A.3.8. of this Annex I.
A.3.5.Preparation of the aggregate sampleU.K.
The aggregate sample shall be made up by combining the incremental samples.
A.3.6.Replicate samplesU.K.
The replicate samples for enforcement, trade (defence) and reference (referee) purposes shall be taken from the homogenised aggregate sample, unless such procedure conflicts with [F2the rules applicable in each constituent territory of Great Britain] as regards the rights of the food business operator.
Textual Amendments
F2Words in Annex 1 para. A.3.6 substituted (31.12.2020) by The Contaminants in Food (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/639), regs. 1, 14 (as substituted by S.I. 2020/1504, regs. 1(2), 8(4)); 2020 c. 1, Sch. 5 para. 1(1)
A.3.7.Packaging and transmission of samplesU.K.
Each sample shall be placed in a clean, inert container offering adequate protection from contamination and against damage in transit. All necessary precautions shall be taken to avoid any change in composition of the sample, which might arise during transportation or storage.
A.3.8.Sealing and labelling of samplesU.K.
Each sample taken for official use shall be sealed at the place of sampling and identified following the rules [F3applicable in each constituent territory of Great Britain].
Textual Amendments
F3Words in Annex 1 para. A.3.8 substituted (31.12.2020) by The Contaminants in Food (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/639), regs. 1, 15 (as substituted by S.I. 2020/1504, regs. 1(2), 8(5)); 2020 c. 1, Sch. 5 para. 1(1)
A record shall be kept of each sampling, permitting each lot to be identified unambiguously and giving the date and place of sampling together with any additional information likely to be of assistance to the analyst.
A.4.Different types of lotsU.K.
Food commodities may be traded in bulk, containers, or individual packings, such as sacks, bags, retail packings. The method of sampling may be applied to all the different forms in which the commodities are put on the market.
Without prejudice to the specific provisions set out in other parts of this Annex, the following formula may be used as a guide for the sampling of lots traded in individual packs, such as sacks, bags, retail packings.
weight: in kg
sampling frequency (SF): every nth sack or bag from which an incremental sample must be taken (decimal figures should be rounded to the nearest whole number).
A guidance document for competent authorities for the control of compliance with EU legislation on aflatoxins is available at http://europa.eu.int/comm/food/food/chemicalsafety/contaminants/aflatoxin_guidance_en.pdf. The guidance document provides additional practical information but the information contained in the guidance document is subordinate to the provisions in this Regulation.
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Whole Annex
PrintThis Division only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
The Schedules you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources