- Latest available (Revised)
- Point in Time (20/04/2022)
- Original (As adopted by EU)
Commission Regulation (EC) No 889/2008Show full title
Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control
You are here:
- Regulations originating from the EU
- 2008 No. 889
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 07/01/20201.13 MB
- Revised 12/11/20180.88 MB
- Revised 01/01/20180.84 MB
- Revised 21/05/20170.84 MB
- Revised 07/05/20170.87 MB
- Revised 07/11/20160.87 MB
- Revised 07/05/20160.84 MB
- Revised 01/01/20150.77 MB
- Revised 16/04/20140.77 MB
- Revised 01/01/20140.76 MB
- Revised 01/07/20130.70 MB
- Revised 01/01/20130.70 MB
- Revised 01/08/20120.70 MB
- Revised 10/04/20110.64 MB
- Revised 01/07/20100.41 MB
- Revised 09/08/20091.80 MB
- Revised 01/01/20091.80 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Regulation (EC) No 889/2008 (Annexes only)
Version Superseded: 31/12/2022
Alternative versions:
- 05/09/2008- Amendment
- 01/01/2009- Amendment
- 09/08/2009- Amendment
- 01/07/2010- Amendment
- 10/04/2011- Amendment
- 01/08/2012- Amendment
- 01/07/2013- Amendment
- 01/01/2014- Amendment
- 16/04/2014- Amendment
- 01/01/2015- Amendment
- 07/05/2016- Amendment
- 07/11/2016- Amendment
- 12/11/2018- Amendment
- 07/01/2020- Amendment
- Exit day: start of implementation period31/01/2020 11pm- Amendment
- End of implementation period31/12/2020- Amendment
- 13/11/2021- Amendment
- 20/04/2022- Amendment
- 20/04/2022
Point in time
Status:
Point in time view as at 20/04/2022.
Changes to legislation:
There are currently no known outstanding effects for the Commission Regulation (EC) No 889/2008.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
[F1ANNEX I U.K. Fertilisers, soil conditioners and nutrients referred to in Article 3(1) and Article 6d(2)
Textual Amendments
F1Substituted by Commission Implementing Regulation (EU) 2019/2164 of 17 December 2019 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control (Text with EEA relevance).
Note: U.K.
A: authorised under Regulation (EEC) No 2092/91 and carried over by Article 16(3)(c) of Regulation (EC) No 834/2007 U.K.
B: authorised under Regulation (EC) No 834/2007 U.K.
a Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/ 2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive ( OJ L 54, 26.2.2011, p. 1 ). | ||
b Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 relating to fertilisers ( OJ L 304, 21.11.2003, p. 1 ). ] | ||
| Authorisation | Name Compound products or products containing only materials listed hereunder: | Description, compositional requirements, conditions for use |
|---|---|---|
| A | Farmyard manure | Product comprising a mixture of animal excrements and vegetable matter (animal bedding). Factory farming origin forbidden |
| A | Dried farmyard manure and dehydrated poultry manure | Factory farming origin forbidden |
| A | Composted animal excrements, including poultry manure and composted farmyard manure included | Factory farming origin forbidden |
| A | Liquid animal excrements | Use after controlled fermentation and/or appropriate dilution Factory farming origin forbidden |
| B | Composted or fermented mixture of household waste | Product obtained from source separated household waste, which has been submitted to composting or to anaerobic fermentation for biogas production Only vegetable and animal household waste Only when produced in a closed and monitored collection systemF2... Maximum concentrations in mg/kg of dry matter: cadmium: 0,7; copper: 70; nickel: 25; lead: 45; zinc: 200; mercury: 0,4; chromium (total): 70; chromium (VI): not detectable |
| A | Peat | Use limited to horticulture (market gardening, floriculture, arboriculture, nursery) |
| A | Mushroom culture wastes | The initial composition of the substrate shall be limited to products of this Annex |
| A | Dejecta of worms (vermicompost) and insects | |
| A | Guano | |
| A | Composted or fermented mixture of vegetable matter | Product obtained from mixtures of vegetable matter, which have been submitted to composting or to anaerobic fermentation for biogas production |
| B | Biogas digestate containing animal by-products co-digested with material of plant or animal origin as listed in this Annex | Animal by-products (including by-products of wild animals) of category 3 and digestive tract content of category 2 (categories 2 and 3 as defined in Regulation (EC) No 1069/2009 of the European Parliament and of the Council a ) must not be from factory farming origin. The Processes have to be in accordance with Commission Regulation (EU) No 142/2011. Not to be applied to edible parts of the crop |
| B | Products or by-products of animal origin as below:
| (1) Maximum concentration in mg/kg of dry matter of chromium (VI): not detectable (2) Not to be applied to edible parts of the crop |
| A | Products and by-products of plant origin for fertilisers | Examples: oilseed cake meal, cocoa husks, malt culms |
| B | Hydrolysed proteins of plant origin | |
| A | Seaweeds and seaweed products | As far as directly obtained by: (i) physical processes including dehydration, freezing and grinding (ii) extraction with water or aqueous acid and/or alkaline solution (iii) fermentation |
| A | Sawdust and wood chips | Wood not chemically treated after felling |
| A | Composted bark | Wood not chemically treated after felling |
| A | Wood ash | From wood not chemically treated after felling |
| A | Soft ground rock phosphate | Product as specified in point 7 of Annex IA.2. to Regulation (EC) No 2003/2003 of the European Parliament and of the Council b . Cadmium content less than or equal to 90 mg/kg of P205 |
| A | Aluminium-calcium phosphate | Product as specified in point 6 of Annex IA.2. to Regulation (EC) No 2003/2003, Cadmium content less than or equal to 90 mg/kg of P205 Use limited to basic soils (pH > 7,5) |
| A | Basic slag | Products as specified in point 1 of Annex IA.2. to Regulation (EC) No 2003/2003 |
| A | Crude potassium salt or kainit | Products as specified in point 1 of Annex IA.3. to Regulation (EC) No 2003/2003 |
| A | Potassium sulphate, possibly containing magnesium salt | Product obtained from crude potassium salt by a physical extraction process, containing possibly also magnesium salts |
| A | Stillage and stillage extract | Ammonium stillage excluded |
| A | Calcium carbonate, for instance: chalk, marl, ground limestone, Breton ameliorant, (maerl), phosphate chalk | Only of natural origin |
| B | Mollusc waste | Only from sustainable fisheries, as defined in Article 4 (1) (7) of Regulation (EU) No 1380/2013 or organic aquaculture |
| B | Egg shells | Factory farming origin forbidden. |
| A | Magnesium and calcium carbonate | Only of natural origin e.g. magnesian chalk, ground magnesium, limestone |
| A | Magnesium sulphate (kieserite) | Only of natural origin |
| A | Calcium chloride solution | Foliar treatment of apple trees, after identification of deficit of calcium |
| A | Calcium sulphate (gypsum) | Products as specified in point 1 of Annex ID. to Regulation (EC) No 2003/2003 Only of natural origin |
| A, B | Industrial lime from sugar production | By-product of sugar production from sugar beet and sugar cane |
| A | Industrial lime from vacuum salt production | By-product of the vacuum salt production from brine found in mountains |
| A | Elemental sulphur | Products as specified in Annex ID.3 to Regulation (EC) No 2003/2003 |
| A | Trace elements | Inorganic micronutrients listed in part E of Annex I to Regulation (EC) No 2003/2003 |
| A | Sodium chloride | |
| A | Stone meal and clays | |
| B | Leonardite (Raw organic sediment rich in humic acids) | Only if obtained as a by-product of mining activities |
| B | Humic and fulvic acids | Only if obtained by inorganic salts/solutions excluding ammonium salts; or obtained from drinking water purification |
| B | Xylite | Only if obtained as a by-product of mining activities (e.g. by-product of brown coal mining) |
| B | Chitin (Polysaccharide obtained from the shell of crustaceans) | Only if obtained from sustainable fisheries, as defined in Article 4(1)(7) of Regulation (EU) No 1380/2013 or organic aquaculture |
| B | Organic rich sediment from fresh water bodies formed under exclusion of oxygen (e.g. sapropel) | Only organic sediments that are by-products of fresh water body management or extracted from former freshwater areas When applicable, extraction should be done in a way to cause minimal impact on the aquatic system Only sediments derived from sources free from contaminations of pesticides, persistent organic pollutants and petrol like substances Maximum concentrations in mg/kg of dry matter: cadmium: 0,7; copper: 70; nickel: 25; lead: 45; zinc: 200; mercury: 0,4; chromium (total): 70; chromium (VI): not detectable |
| B | Biochar — pyrolysis product made from a wide variety of organic materials of plant origin and applied as a soil conditioner | Only from plant materials, untreated or treated with products included in Annex II. Maximum value of 4 mg polycyclic aromatic hydro-carbons (PAHs) per kg dry matter (DM). This value shall be reviewed every second year, taking into account the risk of accumulation due to multiple applications |
Textual Amendments
F2Words in Annex 1 Table omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(55); 2020 c. 1, Sch. 5 para. 1(1)
[F1ANNEX II U.K. Pesticides — Plant protection products referred to in Article 5(1)
All the substances listed in this Annex have to comply at least with the conditions for use as specified in the Annex to Commission Implementing Regulation (EU) No 540/2011 (1) . More restrictive conditions for use for organic production are specified in the second column of each table.
1. Substances of plant or animal origin U.K.
| Name | Description, compositional requirement, conditions for use |
|---|---|
| Allium sativum (Garlic extract) | |
| Azadirachtin extracted from Azadirachta indica (Neem tree) | |
| Beeswax | Only as pruning agent/wound protectant |
| COS-OGA | |
| Hydrolysed proteins excluding gelatine | |
| Laminarin | Kelp shall be either grown organically in accordance with Article 6d or harvested in a sustainable way in accordance with Article 6c |
| Maltodextrin | |
| Pheromones | Only in traps and dispensers. |
| Plant oils | All uses authorised, except herbicide . |
| Pyrethrins | Only from plant origin |
| Quassia extracted from Quassia amara | Only as insecticide, repellent |
| Repellents by smell of animal or plant origin/sheep fat | Only on non-edible parts of the crop and where crop material is not ingested by sheep or goats |
| Salix spp. Cortex (a.k.a. willow bark) | |
| Terpenes (eugenol, geraniol and thymol) |
2. Basic substances U.K.
| a Obtained from sustainable fisheries or organic aquaculture. | |
| b Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market ( OJ L 309, 24.11.2009, p. 1 ). | |
| Basic substances based on food (including: Lecithins, sucrose, fructose, vinegar, whey, chitosan hydrochloride a , and Equisetum arvense etc.) | Only those basic substances as defined by Article 23 of Regulation (EC) No 1107/2009 b which are food as defined in Article 2 of Regulation (EC) No 178/2002 and have plant or animal origin Substances not to be used as herbicides |
3. Micro-organisms or substances produced by or derived from micro-organisms U.K.
| Name | Description, compositional requirement, conditions for use |
|---|---|
| Micro-organisms | Not from GMO origin |
| Spinosad | |
| Cerevisane |
4. Substances other than those mentioned in Sections 1, 2 and 3 U.K.
| Name | Description, compositional requirement, conditions or restrictions to use |
|---|---|
| Aluminium silicate (Kaolin) | |
| Calcium hydroxide | When used as fungicide, only in fruit trees, including nurseries, to control Nectria galligena |
| Carbon dioxide | |
| Copper compounds in the form of: copper hydroxide, copper oxychloride, copper oxide, Bordeaux mixture, and tribasic copper sulphate | |
| Diammonium phosphate | Only as attractant in traps |
| Ethylene | |
| Fatty acids | All uses authorised, except herbicide |
| Ferric phosphate (iron (III) orthophosphate) | Preparations to be surface-spread between cultivated plants |
| Hydrogen peroxide | |
| Kieselgur (diatomaceous earth) | |
| Lime sulphur (calcium polysulphide) | |
| Paraffin oil | |
| Potassium and sodium hydrogen carbonate (a.k.a. potassium /sodium bicarbonate) | |
| Pyrethroids (only deltamethrin or lambda-cyhalothrin) | Only in traps with specific attractants; only against Bactrocera oleae and Ceratitis capitata Wied |
| Quartz sand | |
| Sodium chloride | All uses authorised, except herbicide |
| Sulphur | ] |
ANNEX IIIU.K.Minimum surface areas indoors and outdoors and other characteristics of housing in the different species and types of production referred to in Article 10(4)
1.Bovines, equidae, ovine, caprine and porcineU.K.
| Indoors area(net area available to animals) | Outdoors area(exercise area, excluding pasturage) | ||
|---|---|---|---|
| Live weight minimum (kg) | M2/head | M2/head | |
| Breeding and fattening bovine and equidae | up to 100 | 1,5 | 1,1 |
| up to 200 | 2,5 | 1,9 | |
| up to 350 | 4,0 | 3 | |
| over 350 | 5 with a minimum of 1 m2/100 kg | 3,7 with a minimum of 0,75 m2/100 kg | |
| Dairy cows | 6 | 4,5 | |
| Bulls for breeding | 10 | 30 | |
| Sheep and goats | 1,5 sheep/goat | 2,5 | |
| 0,35 lamb/kid | 0,5 | ||
| Farrowing sows with piglets up to 40 days | 7,5 sow | 2,5 | |
| Fattening pigs | up to 50 | 0,8 | 0,6 |
| up to 85 | 1,1 | 0,8 | |
| up to 110 | 1,3 | 1 | |
| [F3Over 110 kg | 1,5 | 1,2] | |
| Piglets | over 40 days and up to 30 kg | 0,6 | 0,4 |
| Brood pigs | 2,5 female | 1,9 | |
| 6 male If pens are used for natural service: 10 m2/boar | 8,0 | ||
Textual Amendments
2.PoultryU.K.
| a Only in the case of mobile houses not exceeding 150 m2 floor space. | ||||
| Indoors area(net area available to animals) | Outdoors area(m2 of area available in rotation/head) | |||
|---|---|---|---|---|
| No animals/m2 | cm perch/animal | nest | ||
| Laying hens | 6 | 18 | 7 laying hens per nest or in case of common nest 120 cm2/bird | 4, provided that the limit of 170 kg of N/ha/year is not exceeded |
| Fattening poultry (in fixed housing) | 10 with a maximum of 21 kg liveweight/m2 | 20 (for guinea fowl only) | 4 broilers and guinea fowl 4,5 ducks 10 turkey 15 geese In all the species mentioned above the limit of 170 kg of N/ha/year is not exceeded | |
| Fattening poultry in mobile housing | 16a in mobile poultry houses with a maximum of 30 kg liveweight/m2 | 2,5, provided that the limit of 170 kg of N/ha/year is not exceeded | ||
ANNEX IVU.K.Maximum number of animals per hectare referred to in Article 15 (2)
| Class or species | Maximum number of animals per haequivalent to 170 kg N/ha/year |
|---|---|
| Equines over six months old | 2 |
| Calves for fattening | 5 |
| Other bovine animals less than one year old | 5 |
| Male bovine animals from one to less than two years old | 3,3 |
| Female bovine animals from one to less than two years old | 3,3 |
| Male bovine animals two years old or over | 2 |
| Breeding heifers | 2,5 |
| Heifers for fattening | 2,5 |
| Dairy cows | 2 |
| Cull dairy cows | 2 |
| Other cows | 2,5 |
| Female breeding rabbits | 100 |
| Ewes | 13,3 |
| Goats | 13,3 |
| Piglets | 74 |
| Breeding sows | 6,5 |
| Pigs for fattening | 14 |
| Other pigs | 14 |
| Table chickens | 580 |
| Laying hens | 230 |
[F4ANNEX V U.K. Feed materials as referred to in Article 22(d), Article 24(2) and Article 25m(1)
Textual Amendments
F4Substituted by Commission Implementing Regulation (EU) No 505/2012 of 14 June 2012 amending and correcting Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control.
[F51. FEED MATERIALS OF MINERAL ORIGIN U.K.
| A | Calcareous marine shells | |
| A | Maerl | |
| A | Lithotamn | |
| A | Calcium gluconate | |
| A | Calcium carbonate | |
| A | Defluorinated monocalciumphosphate | |
| A | Defluorinated dicalciumphosphate | |
| A | Magnesium oxide (anhydrous magnesia) | |
| A | Magnesium sulphate | |
| A | Magnesium chloride | |
| A | Magnesium carbonate | |
| A | Calcium magnesium phosphate | |
| A | Magnesium phosphate | |
| A | Monosodium phosphate | |
| A | Calcium sodium phosphate | |
| A | Sodium chloride | |
| A | Sodium bicarbonate | |
| A | Sodium carbonate | |
| A | Sodium sulphate | |
| A | Potassium chloride | ] |
Textual Amendments
F5Substituted by Commission Implementing Regulation (EU) No 354/2014 of 8 April 2014 amending and correcting Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control.
2. OTHER FEED MATERIALS U.K.
Fermentation (by-)products from microorganisms the cells of which have been inactivated or killed:
| A | Saccharomyces cerevisiae | |
| A | Saccharomyces carlsbergiensis | ] |
[F1ANNEX VI U.K. Feed additives used in animal nutrition referred to in Article 22(g), Article 24(2) and Article 25m(2)
Feed additives listed in this Annex must be authorised under Regulation (EC) No 1831/2003 of the European Parliament and of the Council.
1. TECHNOLOGICAL ADDITIVES U.K.
(a) Preservatives U.K.
| ID numbers or Functional groups | Substance | Description, conditions for use | |
|---|---|---|---|
| E 200 | Sorbic acid | ||
| E 236 | Formic acid | ||
| E 237 | Sodium formate | ||
| E 260 | Acetic acid | ||
| E 270 | Lactic acid | ||
| E 280 | Propionic acid | ||
| E 330 | Citric acid | ||
(b) Antioxidants U.K.
| ID number or Functional groups | Substance | Description, conditions for use | |
|---|---|---|---|
| 1b306(i) | Tocopherol extracts from vegetable oils | ||
| 1b306(ii) | Tocopherol-rich extracts from vegetable oils (delta rich) | ||
(c) Emulsifiers, stabilisers, thickeners and gelling agents U.K.
| ID numbers or Functional groups | Substance | Description, conditions for use | |
|---|---|---|---|
| 1c322 | Lecithins | Only when derived from organic raw material. | |
| Use restricted to aquaculture animal feed. | |||
(d) Binders and anti-caking agents U.K.
| ID number or Functional groups | Substance | Description, conditions for use | |
|---|---|---|---|
| E 412 | Guar gum | ||
| E 535 | Sodium ferrocyanide | Maximum dose rate of 20 mg/kg NaCl calculated as ferrocyanide anion. | |
| E 551b | Colloidal silica | ||
| E 551c | Kieselgur (diatomaceous earth, purified) | ||
| 1m558i | Bentonite | ||
| E 559 | Kaolinitic clays, free of asbestos | ||
| E 560 | Natural mixtures of steatites and chlorite | ||
| E 561 | Vermiculite | ||
| E 562 | Sepiolite | ||
| E 566 | Natrolite-Phonolite | ||
| 1g568 | Clinoptilolite of sedimentary origin | ||
| E 599 | Perlite | ||
(e) Silage additives U.K.
| ID number or Functional groups | Substance | Description, conditions for use |
|---|---|---|
| 1k 1k236 | Enzymes, micro-organisms Formic acid, | Use restricted to production of silage when weather conditions do not allow for adequate fermentation. The use of formic, propionic acid and their sodium salts in the production of silage shall only be permitted when weather conditions do not allow for adequate fermentation |
| 1k237 | Sodium formate | |
| 1k280 | Propionic acid | |
| 1k281 | Sodium propionate |
2. SENSORY ADDITIVES U.K.
| ID number or Functional groups | Substance | Description, conditions for use |
|---|---|---|
| 2b | Flavouring compounds | Only extracts from agricultural products. |
| Castanea sativa Mill.: Chestnut extract |
3. NUTRITIONAL ADDITIVES U.K.
(a) Vitamins, pro-vitamins and chemically well-defined substances having similar effect U.K.
| ID number or Functional groups | Substance | Description, conditions for use |
|---|---|---|
| 3a | Vitamins and provitamins | Derived from agricultural products. If derived synthetically, only those identical to vitamins derived from agricultural products may be used for monogastric animals and aquaculture animals. If derived synthetically, only vitamins A, D and E identical to vitamins derived from agricultural products may be used for ruminants; the use is subject to prior authorisation of the [F6relevant authority] based on the assessment of the possibility for organic ruminants to obtain the necessary quantities of the said vitamins through their feed rations. |
| 3a920 | Betaine anhydrous | Only for monogastric animals Only from natural origin and when available from organic origin |
Textual Amendments
F6Words in Annex 6 para. 3(a) Table substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(57); 2020 c. 1, Sch. 5 para. 1(1)
(b) Compounds of trace elements U.K.
| ID number or Functional groups | Substance | Description, conditions for use | |
|---|---|---|---|
| E1 Iron | |||
| 3b101 | Iron(II) carbonate (siderite) | ||
| 3b103 | Iron(II) sulphate monohydrate | ||
| 3b104 | Iron(II) sulphate heptahydrate | ||
| 3b201 | Potassium iodide | ||
| 3b202 | Calcium iodate, anhydrous | ||
| 3b203 | Coated granulated calcium iodate anhydrous | ||
| 3b301 | Cobalt(II) acetate tetrahydrate | ||
| 3b302 | Cobalt(II) carbonate | ||
| 3b303 | Cobalt(II) carbonate hydroxide (2:3) monohydrate | ||
| 3b304 | Coated granulated cobalt(II) carbonate hydroxide (2:3) monohydrate | ||
| 3b305 | Cobalt(II) sulphate heptahydrate | ||
| 3b402 | Copper(II) carbonate dihydroxy monohydrate | ||
| 3b404 | Copper (II) oxide | ||
| 3b405 | Copper(II) sulphate pentahydrate | ||
| 3b409 | Dicopper chloride trihydroxide (TBCC) | ||
| 3b502 | Manganese (II) oxide | ||
| 3b503 | Manganous sulfate, monohydrate | ||
| 3b603 | Zinc oxide | ||
| 3b604 | Zinc sulphate heptahydrate | ||
| 3b605 | Zinc sulphate monohydrate | ||
| 3b609 | Zinc chloride hydroxide monohydrate (TBZC) | ||
| 3b701 | Sodium molybdate dihydrate | ||
| 3b801 | Sodium selenite | ||
3b810, 3b811, 3b812, 3b813 and 3b817 | Selenised yeast inactivated |
4. ZOOTECHNICAL ADDITIVES U.K.
| ID number or Functional groups | Substance | Description, conditions for use |
|---|---|---|
| 4a, 4b, 4c and 4d | Enzymes and microorganism in the category of ‘ Zootechnical additives ’ | ] |
[F7ANNEX VII U.K. Products for cleaning and disinfection
Textual Amendments
1. Products for cleaning and disinfection of buildings and installations for livestock production referred to in Article 23(4): U.K.
Potassium and sodium soap
Water and steam
Milk of lime
Lime
Quicklime
Sodium hypochlorite (e.g. as liquid bleach)
Caustic soda
Caustic potash
Hydrogen peroxide
Natural essences of plants
Citric, peracetic acid, formic, lactic, oxalic and acetic acid
Alcohol
Nitric acid (dairy equipment)
Phosporic acid (dairy equipment)
Formaldehyde
Cleaning and disinfection products for teats and milking facilities
Sodium carbonate
[F82. Products for cleaning and disinfection for aquaculture animals and seaweed production referred to in Articles 6e(2), 25s(2) and 29a. U.K.
2.1. Subject to compliance with relevant [F9retained EU law] as referred to in Article 16(1) of Regulation (EC) No 834/2007, and in particular with Regulation (EU) No 528/2012 of the European Parliament and of the Council (2) , products used for cleaning and disinfection of equipment and facilities in the absence of aquaculture animals may contain the following active substances: U.K.Textual Amendments
F9Words in Annex 7 para. 2.1 substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(58)(a)(i); 2020 c. 1, Sch. 5 para. 1(1)
Textual Amendments
F9Words in Annex 7 para. 2.1 substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(58)(a)(i); 2020 c. 1, Sch. 5 para. 1(1)
ozone
sodium hypochlorite
calcium hypochlorite
calcium hydroxide
calcium oxide
caustic soda
alcohol
F10...
potassium permanganate
tea seed cake made of natural camelia seed (use restricted to shrimp production)
mixtures of potassium peroxomonosulphate and sodium chloride producing hypochlorous acid.
Textual Amendments
F10Words in Annex 7 para. 2.1 omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(58)(a)(ii); 2020 c. 1, Sch. 5 para. 1(1)
2.2. Subject to compliance with relevant [F11retained EU law] as referred to in Article 16(1) of Regulation (EC) No 834/2007, and in particular with Regulation (EU) No 528/2012 and Directive 2001/82/EC of the European Parliament and of the Council (3) , products used for cleaning and disinfection of equipment and facilities in the presence as well as in the absence of aquaculture animals may contain the following active substances: U.K.Textual Amendments
F11Words in Annex 7 para. 2.2 substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(58)(b); 2020 c. 1, Sch. 5 para. 1(1)
Textual Amendments
F11Words in Annex 7 para. 2.2 substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(58)(b); 2020 c. 1, Sch. 5 para. 1(1)
limestone (calcium carbonate) for pH control
dolomite for pH correction (use restricted to shrimp production)
sodium chloride
hydrogen peroxide
sodium percarbonate
organic acids (acetic acid, lactic acid, citric acid)
humic acid
peroxyacetic acids
peracetic and peroctanoic acids
iodophores (only in the presence of eggs).]]
Textual Amendments
F8Substituted by Commission Implementing Regulation (EU) No 1358/2014 of 18 December 2014 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 as regards the origin of organic aquaculture animals, aquaculture husbandry practices, feed for organic aquaculture animals and products and substances allowed for use in organic aquaculture (Text with EEA relevance).
[F1ANNEX VIII U.K. Certain products and substances for use in production of processed organic food, yeast and yeast products referred to in Article 27(1)(a) and Article 27a(a)
SECTION A — FOOD ADDITIVES, INCLUDING CARRIERS U.K.
For the purpose of the calculation referred to in Article 23(4)(a)(ii) of Regulation (EC) No 834/2007, food additives marked with an asterisk in the column of the code number, shall be calculated as ingredients of agricultural origin
a Commission Implementing Directive (EU) 2017/1279 of 14 July 2017 amending Annexes I to V to Council Directive 2000/29/EC on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community ( OJ L 184, 15.7.2017, p. 33 ). | ||||
| Code | Name | Preparation of foodstuffs of | Specific conditions and restrictions in addition to Regulation (EC) No 1333/2008 | |
|---|---|---|---|---|
| plant origin | Animal origin | |||
| E 153 | Vegetable carbon | X | Ashy goat cheese Morbier cheese | |
| E 160b* | Annatto, Bixin, Norbixin | X | Red Leicester cheese Double Gloucester cheese Cheddar Mimolette cheese | |
| E 170 | Calcium carbonate | X | X | Shall not be used for colouring or calcium enrichment of products |
| E 220 | Sulphur dioxide | X | X(Only for mead) | In fruit wines (wine made from fruits other than grapes, including cider and perry) and mead with and without added sugar: 100 mg/l (Maximum levels available from all sources, expressed as SO 2 in mg/l) |
| E 223 | Sodium metabisulphite | X | Crustaceans | |
| E 224 | Potassium metabisulphite | X | X (Only for mead) | In fruit wines (wine made from fruits other than grapes, including cider and perry) and mead with and without added sugar: 100 mg/l (Maximum levels available from all sources, expressed as SO 2 in mg/l) |
| E250 | Sodium nitrite | X | For meat products. May only be used, if it has been demonstrated to the satisfaction of the competent authority that no technological alternative, giving the same guarantees and/or allowing to maintain the specific features of the product, is available. Not in combination with E252. Indicative ingoing amount expressed as NaNO 2 : 80 mg/kg, maximum residual amount expressed as NaNO 2 : 50 mg/kg | |
| E252 | Potassium nitrate | X | For meat products. May only be used, if it has been demonstrated to the satisfaction of the competent authority that no technological alternative, giving the same guarantees and/or allowing to maintain the specific features of the product, is available. Not in combination with E250. Indicative ingoing amount expressed as NaNO 3 : 80 mg/kg, maximum residual amount expressed as NaNO 3 : 50 mg/kg | |
| E 270 | Lactic acid | X | X | |
| E 290 | Carbon dioxide | X | X | |
| E 296 | Malic acid | X | ||
| E 300 | Ascorbic acid | X | X | With regard to foodstuffs of animal origin: Meat products |
| E 301 | Sodium ascorbate | X | With regard to foodstuffs of animal origin: Meat products in connection with nitrates and nitrites | |
| E 306(*) | Tocopherol-rich extract | X | X | Anti-oxidant |
| E 322(*) | Lecithins | X | X | With regard to foodstuffs of animal origin: Milk products. Only when derived from organic production. Applicable as of 1 January 2022 . Until that date, only when derived from organic raw material. |
| E 325 | Sodium lactate | X | Milk-based and meat products | |
| E 330 | Citric acid | X | X | |
| E 331 | Sodium citrates | X | X | |
| E 333 | Calcium citrates | X | ||
| E 334 | Tartaric acid (L(+)-) | X | X(Only for mead) | With regard to foodstuffs of animal origin: Mead. |
| E 335 | Sodium tartrates | X | ||
| E 336 | Potassium tartrates | X | ||
| E 341 (i) | Monocalcium phosphate | X | Raising agent for self-raising flour | |
| E 392* | Extracts of Rosemary | X | X | Only when derived from organic production |
| E 400 | Alginic acid | X | X | With regard to foodstuffs of animal origin: milk-based products |
| E 401 | Sodium alginate | X | X | With regard to foodstuffs of animal origin: milk-based products |
| E 402 | Potassium alginate | X | X | With regard to foodstuffs of animal origin: milk-based products |
| E 406 | Agar | X | X | With regard to foodstuffs of animal origin: milk-based products and meat products |
| E 407 | Carrageenan | X | X | With regard to foodstuffs of animal origin: milk-based products |
| E 410* | Locust bean gum | X | X | Only when derived from organic production. Applicable as of 1 January 2022. |
| E 412* | Guar gum | X | X | Only when derived from organic production. Applicable as of 1 January 2022. |
| E 414* | Arabic gum | X | X | Only when derived from organic production. Applicable as of 1 January 2022. |
| E 415 | Xanthan gum | X | X | |
| E 417 | Tara gum powder | X | X | Thickener Only when derived from organic production. Applicable as of 1 January 2022. |
| E 418 | Gellan gum | X | X | High-acyl form only Only when derived from organic production. Applicable as of [F121 January 2023]. |
| E 422 | Glycerol | X | X | Only from plant origin Only when derived from organic production. Applicable as of 1 January 2022. For plant extracts, flavourings, humectant in gel capsules and as a surface coating of tablets |
| E 440 (i)* | Pectin | X | X | With regard to foodstuffs of animal origin: milk-based products |
| E 464 | Hydroxypropyl methyl cellulose | X | X | Encapsulation material for capsules |
| E 500 | Sodium carbonates | X | X | |
| E 501 | Potassium carbonates | X | ||
| E 503 | Ammonium carbonates | X | ||
| E 504 | Magnesium carbonates | X | ||
| E 509 | Calcium chloride | X | Milk coagulation | |
| E 516 | Calcium sulphate | X | Carrier | |
| E 524 | Sodium hydroxide | X | Surface treatment of ‘ Laugengebäck ’ and regulation of acidity in organic flavourings | |
| E 551 | Silicon dioxide | X | X | For herbs and spices in dried powdered form, flavourings and propolis |
| E 553b | Talc | X | X | With regard to foodstuffs of animal origin: surface treatment of sausages |
| E 901 | Beeswax | X | As a glazing agent for confectionary only. Beeswax from organic production | |
| E 903 | Carnauba wax | X | As a glazing agent for confectionary As a mitigating method for mandatory extreme cold treatment of fruit as a quarantine measure against harmful organisms (Commission Implementing Directive (EU) 2017/1279) a Only when derived from organic production. Applicable as of 1 January 2022 . Until that date, only when derived from organic raw material. | |
| E 938 | Argon | X | X | |
| E 939 | Helium | X | X | |
| E 941 | Nitrogen | X | X | |
| E 948 | Oxygen | X | X | |
| E 968 | Erythritol | X | X | Only when derived from organic production without using ion exchange technology |
Textual Amendments
F12Words in Annex 8 s. A Table substituted (20.4.2022) by The Organic Production (Amendment) Regulations 2022 (S.I. 2022/360), regs. 1(1), 2(3)
SECTION B — PROCESSING AIDS AND OTHER PRODUCTS, WHICH MAY BE USED FOR PROCESSING OF INGREDIENTS OF AGRICULTURAL ORIGIN FROM ORGANIC PRODUCTION U.K.
| Name | Preparation of all foodstuffs of plant origin | Preparation of all foodstuffs of animal origin | Specific conditions and restrictions in addition to Regulation (EU) No 1333/2008 |
|---|---|---|---|
| Water | X | X | Drinking water within the meaning of Council Directive 98/83/EC |
| Calcium chloride | X | Coagulation agent | |
| Calcium carbonate | X | ||
| Calcium hydroxide | X | ||
| Calcium sulphate | X | Coagulation agent | |
| Magnesium chloride (or nigari) | X | Coagulation agent | |
| Potassium carbonate | X | With regard to foodstuffs of plant origin: drying of grapes | |
| Sodium carbonate | X | X | |
| Lactic acid | X | With regard to foodstuffs of animal origin: for the regulation of the pH of the brine bath in cheese production | |
| L(+)lactic acid from fermentation | X | With regard to foodstuffs of plant origin: for the preparation of plant protein extracts | |
| Citric acid | X | X | |
| Sodium hydroxide | X | With regard to foodstuffs of plant origin: for sugar(s) production; for oil production excluding olive oil production; for the preparation of plant protein extracts | |
| Sulphuric acid | X | X | Gelatine production Sugar(s) production |
| Hop extract | X | With regard to foodstuffs of plant origin: only for antimicrobial purposes in production of sugar. When available from organic production | |
| Pine rosin extract | X | With regard to foodstuffs of plant origin: only for antimicrobial purposes in production of sugar. When available from organic production | |
| Hydrochloric acid | X | With regard to foodstuffs of animal origin: Gelatine production; for the regulation of the pH of the brine bath in the processing of Gouda-, Edam and Maasdammer cheeses, Boerenkaas, Friese and Leidse Nagelkaas | |
| Ammonium hydroxide | X | With regard to foodstuffs of animal origin: gelatine production | |
| Hydrogen peroxide | X | With regard to foodstuffs of animal origin: gelatine production | |
| Carbon dioxide | X | X | |
| Nitrogen | X | X | |
| Ethanol | X | X | Solvent |
| Tannic acid | X | Filtration aid | |
| Egg white albumin | X | ||
| Casein | X | ||
| Gelatin | X | ||
| Isinglass | X | ||
| Vegetable oils | X | X | Greasing, releasing or anti-foaming agent. Only when derived from organic production |
| Silicon dioxide gel or colloidal solution | X | ||
| Activated carbon | X | ||
| Talc | X | In compliance with the specific purity criteria for food additive E 553b | |
| Bentonite | X | X | With regard to foodstuffs of animal origin: as a sticking agent for mead |
| Cellulose | X | X | With regard to foodstuffs of animal origin: Gelatine production |
| Diatomaceous earth | X | X | With regard to foodstuffs of animal origin: Gelatine production |
| Perlite | X | X | With regard to foodstuffs of animal origin: Gelatine production |
| Hazelnut shells | X | ||
| Rice meal | X | ||
| Beeswax | X | Releasing agent. Beeswax from organic production | |
| Carnauba wax | X | Releasing agent. Only when derived from organic production. Applicable as of 1 January 2022 . Until that date, only when derived from organic raw material | |
| Acetic acid/vinegar | X | Only when derived from organic production. For fish processing only. From natural fermentation, Not to be produced by or from GMO | |
| Thiamin hydrochloride | X | X | Only for use in processing of fruit wines, including cider and perry and mead |
| Diammonium phosphate | X | X | Only for use in processing of fruit wines, including cider and perry and mead |
| Wood fibre | X | X | The source of timber should be restricted to certified, sustainably harvested wood. Wood used must not contain toxic components (post-harvest treatment, naturally occurring toxins or toxins from micro-organisms) |
SECTION C — PROCESSING AIDS FOR THE PRODUCTION OF YEAST AND YEAST PRODUCTS U.K.
| Name | Primary yeast | Yeast confections/ formulations | Specific conditions |
|---|---|---|---|
| Calcium chloride | X | ||
| Carbon dioxide | X | X | |
| Citric acid | X | For the regulation of the pH in yeast production | |
| Lactic acid | X | For the regulation of the pH in yeast production | |
| Nitrogen | X | X | |
| Oxygen | X | X | |
| Potato starch | X | X | For filtering Only when derived from organic production |
| Sodium carbonate | X | X | For the regulation of the pH |
| Vegetable oils | X | X | Greasing, releasing or anti-foaming agent Only when derived from organic production] |
[F1ANNEX VIIIa U.K. Products and substances authorised for use or addition in organic products of the wine sector referred to in Article 29c
| a For the individual yeast strains: if available, derived from organic raw material. | ||
| b Derived from organic raw material if available.] | ||
| Type of treatment in accordance with Annex I A to Regulation (EC) No 606/2009 | Name of products or substances | Specific conditions, restrictions within the limits and conditions set out in Regulation (EC) No 1234/2007 and Regulation (EC) No 606/2009 |
|---|---|---|
| Point 1: Use for aeration or oxygenation |
| |
| Point 3: Centrifuging and filtration |
| Use only as an inert filtering agent |
| Point 4: Use in order to create an inert atmosphere and to handle the product shielded from the air |
| |
| Points 5, 15 and 21: Use |
| |
| Point 6: Use |
| |
| Point 7: Use |
| (a) The maximum sulphur dioxide content shall not exceed 100 milligrams per litre for red wines as referred to in point 1(a) of Part A of Annex I B to Regulation (EC) No 606/ 2009 and with a residual sugar level lower than 2 grams per litre; (b) The maximum sulphur dioxide content shall not exceed 150 milligrams per litre for white and rosé wines as referred to in point 1(b) of Part A of Annex I B to Regulation (EC) No 606/2009 and with a residual sugar level lower than 2 grams per litre; (c) For all other wines, the maximum sulphur dioxide content applied in accordance with Annex I B to Regulation (EC) No 606/2009 on 1 August 2010 , shall be reduced by 30 milligrams per litre. |
| Point 9: Use |
| |
| Point 10: Clarification | ||
| Point 12: Use for acidification purposes |
| |
| Point 13: Use for deacidification purposes |
| |
| Point 14: Addition |
| |
| Point 17: Use |
| |
| Point 19: Addition |
| |
| Point 22: Use for bubbling |
| |
| Point 23: Addition |
| |
| Point 24: Addition for wine stabilisation purposes |
| |
| Point 25: Addition |
| |
| Point 27: Addition |
| |
| Point 28: Use |
| |
| Point 30: Use |
| |
| Point 31: Use |
| |
| Point 35: Use |
| |
| Point 38: Use |
| |
| Point 39: Use |
| |
| Point 44: Use |
| |
| Point 51: Use |
| |
| Type of treatment in accordance with Annex III, point A(2)(b) to Regulation (EC) No 606/2009 |
| Only for ‘ vino generoso ’ or ‘ vino generoso de licor ’ |
ANNEX IXU.K.Ingredients of agricultural origin which have not been produced organically referred to in Article 28
1.UNPROCESSED VEGETABLE PRODUCTS AS WELL AS PRODUCTS DERIVED THEREFROM BY PROCESSESU.K.
1.1.Edible fruits, nuts and seeds:U.K.
| Quercus spp. |
| Cola acuminata |
| Ribes uva-crispa |
| Passiflora edulis |
| Rubus idaeus |
| Ribes rubrum |
1.2.Edible spices and herbs:U.K.
| Schinus molle L. |
| Armoracia rusticana |
| Alpinia officinarum |
| Carthamus tinctorius |
| Nasturtium officinale |
1.3.Miscellaneous:U.K.
Algae, including seaweed, permitted in non-organic foodstuffs preparation
2.VEGETABLE PRODUCTSU.K.
2.1.Fats and oils whether or not refined, but not chemically modified, derived from plants other than:U.K.
| Theobroma cacao |
| Cocos nucifera |
| Olea europaea |
| Helianthus annuus |
| Elaeis guineensis |
| Brassica napus, rapa |
| Carthamus tinctorius |
| Sesamum indicum |
| Glycine max |
2.2.The following sugars, starches and other products from cereals and tubers:U.K.
fructose
rice paper
unleavened bread paper
starch from rice and waxy maize, not chemically modified
2.3.Miscellaneous:U.K.
pea protein Pisum spp.
rum, only obtained from cane sugar juice
kirsch prepared on the basis of fruits and flavourings as referred to in Article 27(1)(c).
3.ANIMAL PRODUCTSU.K.
aquatic organisms, not originating from aquaculture, and permitted in no-organic foodstuffs preparation
gelatin
whey powder ‘herasuola’
casings
ANNEX XU.K.Species for which organically produced seed or seed potatoes are available in sufficient quantities and for a significant number of varieties in all parts of the Community referred to in Article 45(3)
[F13ANNEX XI U.K.
Textual Amendments
F14A. Organic logo of the EU, referred to in Article 57 U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F14Annex 11 Pt. A omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(a); 2020 c. 1, Sch. 5 para. 1(1)
B. Code numbers referred to in Article 58 U.K.
The general format of the code numbers is as follows:
AB-CDE-999 U.K.
Where:
‘ AB ’ is the ISO code as specified in Article 58(1)(a) for the country where the controls take place; and
‘ CDE ’ is a term, indicated in three letters to be decided by the [F15relevant authority], like ‘ bio ’ or ‘ öko ’ or ‘ org ’ or ‘ eko ’ establishing a link with the organic production method as specified in Article 58(1)(b); and
‘ 999 ’ is the reference number, indicated in maximum three digits, to be attributed, as specified in Article 58(1)(c) by:
[F16the] competent authority to the Control Authorities or Control Bodies to which they have delegated control tasks in accordance with Article 27 of Regulation (EC) No 834/2007;
the [F17relevant authority], to:
the Control Authorities and Control Bodies referred to in Article 3(2)(a) of Commission Regulation (EC) No 1235/2008 (4) and listed in [F18the Article 3 list];
the third countries’ competent authorities or Control Bodies referred to in Article 7(2)(f) of Regulation (EC) No 1235/2008 and listed in [F19the Article 7 list];
the Control Authorities and Control Bodies referred to in Article 10(2)(a) of Regulation (EC) No 1235/2008, and listed in [F20the Article 10 list];
F21...
Textual Amendments
F15Words in Annex 11 Pt. B para. 2 substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(b)(i); 2020 c. 1, Sch. 5 para. 1(1)
F16Word in Annex 11 Pt. B para. 3(a) substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(b)(ii)(aa); 2020 c. 1, Sch. 5 para. 1(1)
F17Words in Annex 11 Pt. B para. 3(b) substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(b)(ii)(bb); 2020 c. 1, Sch. 5 para. 1(1)
F18Words in Annex 11 Pt. B para. 3(b)(i) substituted (13.11.2021) by The Organics (Equivalence and Control Bodies Listing) (Amendment) Regulations 2021 (S.I. 2021/1266), regs. 1, 3(3)(a)
F19Words in Annex 11 Pt. B para. 3(b)(ii) substituted (13.11.2021) by The Organics (Equivalence and Control Bodies Listing) (Amendment) Regulations 2021 (S.I. 2021/1266), regs. 1, 3(3)(b)
F20Words in Annex 11 Pt. B para. 3(b)(iii) substituted (13.11.2021) by The Organics (Equivalence and Control Bodies Listing) (Amendment) Regulations 2021 (S.I. 2021/1266), regs. 1, 3(3)(c)
F21Annex 11 Pt. B para. 3(c) omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(b)(ii)(cc); 2020 c. 1, Sch. 5 para. 1(1)
The [F22relevant authority] shall make the code numbers available to the public by any appropriate technical means, including publication on the Internet.]
Textual Amendments
F22Words in Annex 11 Pt. B substituted (31.12.2020) by The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(59)(c); 2020 c. 1, Sch. 5 para. 1(1)
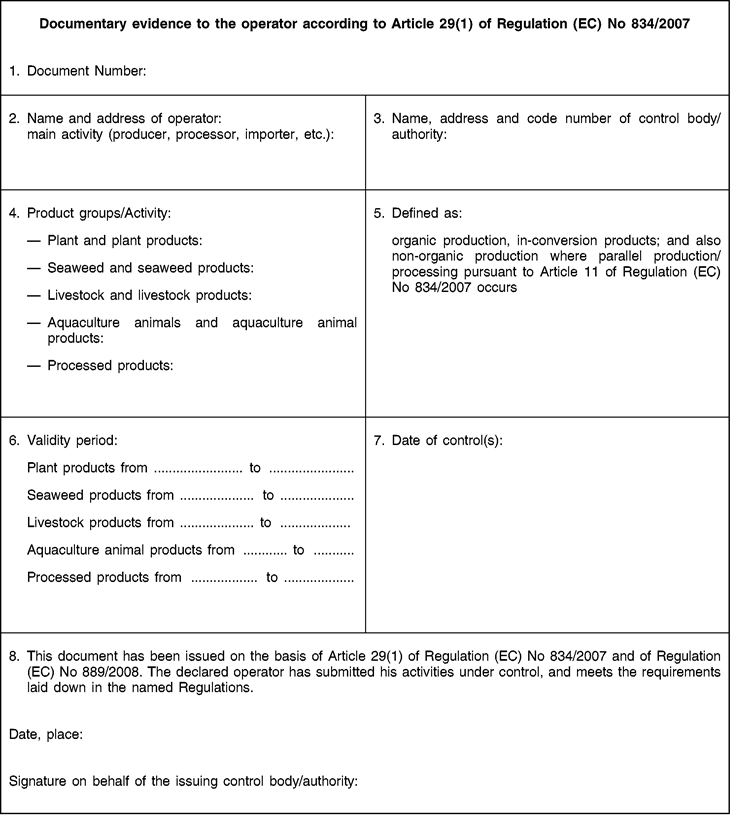
[F7ANNEX XII U.K. Model of documentary evidence to the operator according to Article 29(1) of Regulation (EC) No 834/2007 referred to in [F23Article 68(1)] of this Regulation]
Textual Amendments
F23Substituted by Commission Implementing Regulation (EU) No 126/2012 of 14 February 2012 amending Regulation (EC) No 889/2008 as regards documentary evidence and amending Regulation (EC) No 1235/2008 as regards the arrangements for imports of organic products from the United States of America (Text with EEA relevance).
[F24ANNEX XIIa U.K. Model of complementary documentary evidence to the operator according to Article 29(1) of Regulation (EC) No 834/2007 referred to in Article 68(2) of this Regulation
Textual Amendments
F24Inserted by Commission Implementing Regulation (EU) No 126/2012 of 14 February 2012 amending Regulation (EC) No 889/2008 as regards documentary evidence and amending Regulation (EC) No 1235/2008 as regards the arrangements for imports of organic products from the United States of America (Text with EEA relevance).
ANNEX XIIb U.K.
Entry referred to in the second subparagraph of Article 68(2):
:
Animal products produced without the use of antibiotics
Textual Amendments
F25Words in Annex 12b omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(60); 2020 c. 1, Sch. 5 para. 1(1)
ANNEX XIIIU.K.Model of a vendor declaration referred to in Article 69
| Vendor declaration according to Article 9(3) of Council Regulation (EC) No 834/2007 | |
| Name, address of vendor: | |
| Identification (e.g. lot or stock number): | Product name: |
| Components: (Specify all components existing in the product/used the last in the production process) ……………… ……………… ……………… ……………… ……………… | |
| I declare that this product was manufactured neither ‘from’ nor ‘by’ GMOs as those terms are used in Articles 2 and 9 of Council Regulation (EC) No 834/2007. I do not have any information which could suggest that this statement is inaccurate. Thus, I declare that the above named product complies with Article 9 of Regulation (EC) No 834/2007 regarding the prohibition on the use of GMOs. I undertake to inform our customer and its control body/authority immediately if this declaration is withdrawn or modified, or if any information comes to light which would undermine its accuracy. I authorise the control body or control authority, as defined in Article 2 of Council Regulation (EC) No 834/2007, which supervises our customer to examine the accuracy of this declaration and if necessary to take samples for analytic proof. I also accept that this task may be carried out by an independent institution which has been appointed in writing by the control body. The undersigned takes responsibility for the accuracy of this declaration. | |
| Country, place, date, signature of vendor: | Company stamp of vendor (if appropriate): |
[F3ANNEX XIIIa U.K.
Section 1 U.K.
Organic production of salmonids in fresh water:
Brown trout (Salmo trutta) — Rainbow trout (Oncorhynchus mykiss) — American brook trout (Salvelinus fontinalis) — Salmon (Salmo salar) — Charr (Salvelinus alpinus) — Grayling (Thymallus thymallus) — American lake trout (or grey trout) (Salvelinus namaycush) — Huchen (Hucho hucho)
| Production system | Ongrowing farm systems must be fed from open systems. The flow rate must ensure a minimum of 60 % oxygen saturation for stock and must ensure their comfort and the elimination of farming effluent. |
|---|---|
| Maximum stocking density | Salmonid species not listed below 15 kg/m 3 Salmon 20 kg/m 3 Brown trout and Rainbow trout 25 kg/m 3 [F8Arctic charr 25 kg/m 3] |
Section 2 U.K.
Organic production of salmonids in sea water:
Salmon (Salmo salar) , Brown trout (Salmo trutta) — Rainbow trout (Oncorhynchus mykiss)
| Maximum stocking density | 10 kg/m 3 in net pens |
|---|
Section 3 U.K.
Organic production of cod (Gadus morhua) and other Gadidae, sea bass (Dicentrarchus labrax) , sea bream (Sparus aurata) , meagre (Argyrosomus regius) , turbot (Psetta maxima [= Scopthalmus maximux]) , red porgy (Pagrus pagrus [= Sparus pagrus]) , red drum (Sciaenops ocellatus) and other Sparidae, and spinefeet ( Siganus spp.)
| Production system | In open water containment systems (net pens/cages) with minimum sea current speed to provide optimum fish welfare or in open systems on land. |
|---|---|
| Maximum stocking density | For fish other than turbot: 15 kg/m 3 For turbot: 25 kg/m 2 |
Section 4 U.K.
Organic production of sea bass, sea bream, meagre, mullets (Liza, Mugil) and eel ( Anguilla spp. ) in earth ponds of tidal areas and costal lagoons
| Containment system | Traditional salt pans transformed into aquaculture production units and similar earth ponds in tidal areas |
|---|---|
| Production system | There shall be adequate renewal of water to ensure the welfare of the species, At least 50 % of the dikes must have plant cover Wetland based depuration ponds required |
| Maximum stocking density | 4 kg/m 3 |
Section 5 U.K.
Organic production of Sturgeon in fresh water:
Species concerned: Acipenser family
| Production system | Water flow in each rearing unit shall be sufficient to ensure animal welfare Effluent water to be of equivalent quality to incoming water |
|---|---|
| Maximum stocking density | 30 kg/m 3 |
Section 6 U.K.
Organic production of fish in inland waters:
Species concerned: Carp family (Cyprinidae) and other associated species in the context of polyculture, including perch, pike, catfish, coregonids, sturgeon.
| Production system | In fishponds which shall periodically be fully drained and in lakes. Lakes must be devoted exclusively to organic production, including the growing of crops on dry areas. The fishery capture area must be equipped with a clean water inlet and of a size to provide optimal comfort for the fish. The fish must be stored in clean water after harvest. Organic and mineral fertilisation of the ponds and lakes shall be carried out in compliance with Annex I to Regulation (EC) No 889/2008 with a maximum application of 20 kg Nitrogen/ha. Treatments involving synthetic chemicals for the control of hydrophytes and plant coverage present in production waters are prohibited. Areas of natural vegetation shall be maintained around inland water units as a buffer zone for external land areas not involved in the farming operation in accordance with the rules of organic aquaculture. For grow-out ‘ polyculture ’ shall be used on condition that the criteria laid down in the present specifications for the other species of lakes fish are duly adhered to. |
|---|---|
| Farming yield | The total production of species is limited to 1 500 kg of fish per hectare per year. |
Section 7 U.K.
Organic production of penaeid shrimps and freshwater prawns ( Macrobrachium spp.):
| Establishment of production unit/s | Location to be in sterile clay areas to minimise environmental impact of pond construction. Ponds to be built with the natural pre-existing clay. Mangrove destruction is not permitted. |
|---|---|
| Conversion time | Six months per pond, corresponding to the normal lifespan of a farmed shrimp. |
| Broodstock origin | A minimum of half the broodstock shall be domesticated after three years operating The remainder is to be pathogen free wild broodstock originating from sustainable fisheries. A compulsory screening to be implemented on the first and second generation prior to introducing to the farm. |
| Eyestalk ablation | Is prohibited. |
| Maximum on farm stocking densities and production limits | Seeding: maximum 22 post larvae/m 2 Maximum instantaneous biomass: 240 g/m 2 |
[F26Section 7a U.K.
Organic production of crayfish:
Species concerned: Astacus astacus , Pacifastacus leniusculus .
| Maximum stocking density: | For small-sized crayfish (< 20 mm): 100 individuals per m 2 . For crayfish of intermediate size (20-50 mm): 30 individuals per m 2 . For adult crayfish (> 50 mm): 10 individuals per m 2 , provided that adequate hiding places are available.] |
Textual Amendments
F26 Inserted by Commission Implementing Regulation (EU) No 1358/2014 of 18 December 2014 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 as regards the origin of organic aquaculture animals, aquaculture husbandry practices, feed for organic aquaculture animals and products and substances allowed for use in organic aquaculture (Text with EEA relevance).
Section 8 U.K.
Molluscs and echinoderms:
| Production systems | Long-lines, rafts, bottom culture, net bags, cages, trays, lantern nets, bouchot poles and other containment systems. For mussel cultivation on rafts the number of drop-ropes shall not exceed one per square meter of surface area. The maximum drop-rope length shall not exceed 20 metres. Thinning-out of drop-ropes shall not take place during the production cycle, however sub-division of drop ropes shall be permitted without increasing stocking density at the outset. |
|---|
Section 9 U.K.
Tropical fresh water fish: milkfish (Chanos chanos) , tilapia ( Oreochromis spp.), siamese catfish ( Pangasius spp.):
| Production systems | Ponds and net cages |
|---|---|
| Maximum stocking density | Pangasius: 10 kg/m 3 Oreochromis: 20 kg/m 3 |
Section 10 U.K.
Other aquaculture animal species: none]
[F27ANNEX XIIIb U.K. Topics to be covered by the national competent authority in the organic data referred to in Article 92f
Textual Amendments
1. Information on the competent authority for the organic production U.K.
which body is the competent authority
resources available to the competent authority
description of audits performed by the competent authority (how, by whom)
documented procedure of the competent authority
2. Description of the control system for organic production U.K.
system of control bodies and/or control authorities
registered operators covered by the control system — minimum annual inspection
how is the risk based approach applied
3. Information on control bodies/authorities U.K.
list of control bodies/authorities
tasks delegated to the control bodies/conferred to control authorities
supervision of delegated control bodies (by whom and how)
coordination of activities in case of more than one control body/authority
training of staff performing the controls
announced/unannounced inspections and visits]
F28 ANNEX XIIIc U.K. Templates for the organic data referred to under Article 92f
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F28Annex 13c omitted (31.12.2020) by virtue of The Organic Production and Control (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/693), regs. 1(2), 3(61); 2020 c. 1, Sch. 5 para. 1(1)
ANNEX XIVU.K.Correlation Table referred to in Article 96
| Regulation (EEC) No 2092/91 | (1) Regulation (EC) No 207/93(2) Regulation (EC) No 223/2003(3) Regulation (EC) No 1452/2003 | This Regulation |
|---|---|---|
| — | Article 1 | |
| — | Article 2(a) | |
| Article 4(15) | Article 2(b) | |
| Annex III, C (first indent) | Article 2(c) | |
| Annex III, C (second indent) | Article 2(d) | |
| — | Article 2(e) | |
| — | Article 2(f) | |
| — | Article 2(g) | |
| — | Article 2(h) | |
| Article 4(24) | Article 2(i) | |
| — | Article 3(1) | |
| Annex I.B, 7.1 and 7.2 | Article 3(2) | |
| Annex I.B, 7.4 | Article 3(3) | |
| Annex I.A, 2.4 | Article 3(4) | |
| Annex I.A, 2.3 | Article 3(5) | |
| — | Article 4 | |
| Article 6(1), Annex I.A, 3 | Article 5 | |
| Annex I.A, 5 | Article 6 | |
| Annex I.B and C (titles) | Article 7 | |
| Annex I.B, 3.1 | Article 8(1) | |
| Annex I.C, 3.1 | Article 8(2) | |
| Annex I.B, 3.4, 3.8, 3.9, 3.10, 3.11 | Article 9(1) to (4) | |
| Annex I.C, 3.6 | Article 9(5) | |
| Annex I.B, 8.1.1 | Article 10(1) | |
| Annex I.B, 8.2.1 | Article 10(2) | |
| Annex I.B, 8.2.2 | Article 10(3) | |
| Annex I.B, 8.2.3 | Article 10(4) | |
| Annex I.B, 8.3.5 | Article 11(1) | |
| Annex I.B, 8.3.6 | Article 11(2) | |
| Annex I.B, 8.3.7 | Article 11(3) | |
| Annex I.B, 8.3.8 | Article 11(4), (5) | |
| Annex I.B, 6.1.9, 8.4.1 to 8.4.5 | Article 12(1) to (4) | |
| Annex I.B, 6.1.9 | Article 12(5) | |
| Annex I.C, 4, 8.1 to 8.5 | Article 13 | |
| Annex I.B, 8.1.2 | Article 14 | |
| Annex I.B, 7.1, 7.2 | Article 15 | |
| Annex I.B, 1.2 | Article 16 | |
| Annex I.B, 1.6 | Article 17(1) | |
| Annex I.B, 1.7 | Article 17(2) | |
| Annex I.B, 1.8 | Article 17(3) | |
| Annex I.B, 4.10 | Article 17(4) | |
| Annex I.B, 6.1.2 | Article 18(1) | |
| Annex I.B, 6.1.3 | Article 18(2) | |
| Annex I.C, 7.2 | Article 18(3) | |
| Annex I.B, 6.2.1 | Article 18(4) | |
| Annex I.B, 4.3 | Article 19(1) | |
| Annex I.C, 5.1, 5.2 | Article 19(2) to (4) | |
| Annex I.B, 4.1, 4.5, 4.7 and 4.11 | Article 20 | |
| Annex I.B, 4.4 | Article 21 | |
| Article 7 | Article 22 | |
| Annex I.B, 3.13, 5.4, 8.2.5 and 8.4.6 | Article 23 | |
| Annex I.B, 5.3, 5.4, 5.7 and 5.8 | Article 24 | |
| Annex I.C, 6 | Article 25 | |
| Annex III, E.3 and B | Article 26 | |
| Article 5(3) and Annex VI, part A and B | Article 27 | |
| Article 5(3) | Article 28 | |
| Article 5(3) | (1): Article 3 | Article 29 |
| Annex III, B.3 | Article 30 | |
| Annex III.7 | Article 31 | |
| Annex III, E.5 | Article 32 | |
| Annex III.7a | Article 33 | |
| Annex III, C.6 | Article 34 | |
| Annex III.8 and A.2.5 | Article 35 | |
| Annex I.A, 1.1 to 1.4 | Article 36 | |
| Annex I.B, 2.1.2 | Article 37 | |
| Annex I.B, 2.1.1, 2.2.1,2.3 and Annex I.C, 2.1, 2.3 | Article 38 | |
| Annex I.B, 6.1.6 | Article 39 | |
| Annex III, A1.3 and b | Article 40 | |
| Annex I.C, 1.3 | Article 41 | |
| Annex I.B, 3.4 (first indent and 3.6(b)) | Article 42 | |
| Annex I.B, 4.8 | Article 43 | |
| Annex I.C, 8.3 | Article 44 | |
| Article 6(3) | Article 45 | |
| (3): Article 1(1), (2) | Article 45(1), (2) | |
| (3): Article 3(a) | Article 45(1) | |
| (3): Article 4 | Article 45(3) | |
| (3): Article 5(1) | Article 45(4) | |
| (3): Article 5(2) | Article 45(5) | |
| (3): Article 5(3) | Article 45(6) | |
| (3): Article 5(4) | Article 45(7) | |
| (3): Article 5(5) | Article 45(8) | |
| Annex I.B, 8.3.4 | Article 46 | |
| Annex I.B, 3.6(a) | Article 47(1) | |
| Annex I.B, 4.9 | Article 47(2) | |
| Annex I.C, 3.5 | Article 47(3) | |
| (3): Article 6 | Article 48 | |
| (3): Article 7 | Article 49 | |
| (3): Article 8(1) | Article 50(1) | |
| (3): Article 8(2) | Article 50(2) | |
| (3): Article 9(1) | Article 51(1) | |
| (3): Article 9(2), (3) | Article 51(2) | |
| Article 51(3) | ||
| (3): Article 10 | Article 52 | |
| (3): Article 11 | Article 53 | |
| (3): Article 12(1) | Article 54(1) | |
| (3): Article 12(2) | Article 54(2) | |
| (3): Article 13 | Article 55 | |
| (3): Article 14 | Article 56 | |
| Article 57 | ||
| Article 58 | ||
| (2): Article 1 and Article 5 | Article 59 | |
| (2): Article 5 and 3 | Article 60 | |
| (2): Article 4 | Article 61 | |
| Article 5(5) | Article 62 | |
| Annex III.3 | Article 63 | |
| Annex III.4 | Article 64 | |
| Annex III.5 | Article 65 | |
| Annex III.6 | Article 66 | |
| Annex III.10 | Article 67 | |
| — | Article 68 | |
| — | Article 69 | |
| Annex III, A.1. | Article 70 | |
| Annex III, A.1.2. | Article 71 | |
| — | Article 72 | |
| Annex III, A.1.3 | Article 73 | |
| Annex III, A.2.1 | Article 74 | |
| Annex III, A.2.2 | Article 75 | |
| Annex III, A.2.3 | Article 76 | |
| Annex I.B, 5.6 | Article 77 | |
| Annex I.C, 5.5,6.7,7.7,7.8 | Article 78 | |
| Annex III, A.2.4 | Article 79 | |
| Annex III, B.1 | Article 80 | |
| Annex III, C | Article 81 | |
| Annex III, C.1 | Article 82 | |
| Annex III, C.2 | Article 83 | |
| Annex III, C.3 | Article 84 | |
| Annex III, C.5 | Article 85 | |
| Annex III, D | Article 86 | |
| Annex III, E | Article 87 | |
| Annex III, E.1 | Article 88 | |
| Annex III, E.2 | Article 89 | |
| Annex III, E.4 | Article 90 | |
| Annex III, 9 | Article 91 | |
| Annex III, 11 | Article 92 | |
| Article 93 | ||
| — | Article 94 | |
| Annex I.B, 6.1.5 | Article 95(1) | |
| Annex I.B, 8.5.1 | Article 95(2) | |
| — | Article 95(3)-(8) | |
| — | Article 95 | |
| — | Article 96 | |
| — | Article 97 | |
| Annex II, part A | Annex I | |
| Annex II, part B | Annex II | |
| Annex VIII | Annex III | |
| Annex VII | Annex IV | |
| Annex II, part C | Annex V | |
| Annex II, part D | Annex VI | |
| Annex II, part E | Annex VII | |
| Annex VI, part A and B | Annex VIII | |
| Annex VI, part C | Annex IX | |
| — | Annex X | |
| — | Annex XI | |
| — | Annex XIII | |
| — | Annex IX |
[F1Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances ( OJ L 153, 11.6.2011, p. 1 ).]
[F7 [F8Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products ( OJ L 167, 27.6.2012, p. 1 ).] ]
[F7 [F8Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products ( OJ L 311, 28.11.2001, p. 1 ).] ]
Textual Amendments
F1Substituted by Commission Implementing Regulation (EU) 2019/2164 of 17 December 2019 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and control (Text with EEA relevance).
F7Substituted by Commission Regulation (EC) No 710/2009 of 5 August 2009 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007, as regards laying down detailed rules on organic aquaculture animal and seaweed production.
F8Substituted by Commission Implementing Regulation (EU) No 1358/2014 of 18 December 2014 amending Regulation (EC) No 889/2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 as regards the origin of organic aquaculture animals, aquaculture husbandry practices, feed for organic aquaculture animals and products and substances allowed for use in organic aquaculture (Text with EEA relevance).
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Annexes only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources