- Latest available (Revised)
- Original (As adopted by EU)
Commission Regulation (EU) 2015/9Show full title
Commission Regulation (EU) 2015/9 of 6 January 2015 amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (Text with EEA relevance)
You are here:
- Regulations originating from the EU
- 2015 No. 9
- Whole Regulation
- Previous
- Next
More Resources
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Status:
This is the original version as it was originally adopted in the EU.
This legislation may since have been updated - see the latest available (revised) version
Commission Regulation (EU) 2015/9
of 6 January 2015
amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to the Act of Accession of Croatia, and in particular Article 50 thereof,
Having regard to Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation)(1), and in particular Article 15(1)(b), (c), (d) and (g), Article 18(3)(b)(i), Article 19(4)(c), Article 20(11), Article 21(6)(d), Article 23(3), Article 27(c), Article 31(2), Article 40(f), Article 41(3) and Article 42(2) thereof,
Whereas:
(1) Regulation (EC) No 1069/2009 lays down public and animal health rules for animal by-products and derived products, in order to prevent and minimise risks to public and animal health arising from those products. It also determines an end point in the manufacturing chain for certain derived products, beyond which they are no longer subject to the requirements of that Regulation.
(2) Commission Regulation (EU) No 142/2011(2) lays down implementing rules for Regulation (EC) No 1069/2009, including rules on the adoption of alternative methods of use or disposal of animal by-products or derived products and the requirements for placing on the market of organic fertilisers and certain other animal by-products.
(3) In accordance with Article 19(1)(d) of Regulation (EC) No 1069/2009, Member States may authorise the collection, transport and disposal of Category 3 materials, as referred to in Article 10(f) of that Regulation, by the other means set out in Chapter IV of Annex VI to Regulation (EU) No 142/2011. In accordance with Article 36(3) of Regulation (EU) No 142/2011, this possibility was limited to the transitional period until 31 December 2014. Certain Member States authorise the collection, transport and disposal by the other means set out in Chapter IV of Annex VI to Regulation (EU) No 142/2011 of small quantities of former foodstuffs up to 20 kg per week.
(4) In the absence of reported negative consequences for animal health and taking into account that in certain instances the disposal in accordance with Article 14 of Regulation (EC) No 1069/2009 would be unacceptably onerous compared to local disposal, it appears justified to establish the transitional derogation as a permanent option, provided such disposal does not cause unacceptable health risks. Article 15 of Regulation (EU) No 142/2011, providing special rules for the application of Article 19(1)(a), (b), (c), (e) and (f) of Regulation (EC) No 1069/2009, should therefore be supplemented with reference to the measures provided for in Chapter IV of Annex VI to Regulation (EU) No 142/2011, which should also be amended accordingly. After consultation with Member Stats and stakeholders organisations, the option that Member States may decide to increase the volume to a maximum of 50 kg per week shall be removed when the transitional derogation becomes a permanent option. In addition, paragraph 3 of Article 36 of Regulation (EU) No 142/2011 should be deleted.
(5) Given the low risk of possible contacts of farmed animals with organic fertilisers and soil improvers handled by certain operators and users, in particular when they operate outside the food and feed chain, the competent authorities should be allowed to exempt those operators and users from the registration obligation under Article 23 of Regulation (EC) No 1069/2009. Those operators and users should be added to the list of operators exempted from the obligation to notify the competent authorities in accordance with Article 20(4) of Regulation (EU) No 142/2011. Article 20(4) of Regulation (EU) No 142/2011 should be amended accordingly.
(6) Growing media, including potting soil, with a small content of animal by-products or derived products packaged for use by the final consumer do not present a risk of being used as feed for farmed animals. The limitation to a content of less than 5 % in volume of derived products of Category 2 or 3 materials in the growing media, including potting soil, mitigates the risk to use it as feed for farmed animals, since the high content of soil and other materials renders such products unpalatable for farmed animals. In the production of growing media, processed manure may be used. However, the processed manure shall not be the only component of the growing media. It should present not more than 50 % in volume in the growing media. Processed manure shall not be used for the production of growing media when the place of origin is subject to prohibition due to a suspected or confirmed outbreak of a serious transmissible disease affecting farmed animals. Therefore, such products may be exempted from veterinary controls for placing on the market other than imports. Article 22(2) of Regulation (EU) No 142/2011 should be amended accordingly.
(7) The definitions of ‘intermediate products’ and ‘trade samples’ in points 35 and 39 respectively of Annex I to Regulation (EU) No 142/2011 should be clarified in order to avoid unjustified trade barriers. The definition of ‘intermediate products’ includes also a destination of those intermediate products. It is justified to extend the current definition with possible additional uses in the cosmetic industry. Derived products which comply with the requirements of Council Directive 76/768/EEC(3) may be in accordance with Article 5(1) of Regulation (EC) No 1069/2009 declared as the end point in the manufacturing chain. Furthermore, it is necessary to clarify that pet food may be introduced into the EU as a trade sample for purposes of feeding trials, testing of machinery or of equipment. The definition of ‘intermediate products’ and ‘trade samples’ in points 35 and 39 of Annex I to Regulation (EU) No 142/2011 should be amended accordingly.
(8) Although, in accordance with Article 3(6) of Regulation (EC) No 1069/2009, equidae are considered to be farmed animals, certain individual equine animals enjoy a particular close relationship to their keepers. It is therefore justified to provide the possibility of cremating dead equidae in incinerators approved for that purpose by the competent authority, provided the equidae originate from holdings which are not subject to prohibition orders for notifiable diseases. Council Directive 2009/156/EC(4) provides for animal health conditions governing amongst others the movement of equidae, including conditions for the identification of equidae. Only dead equidae which comply with that Directive may be individually cremated in low-capacity incinerators. Chapter III of Annex III to Regulation (EU) No 142/2011 should be amended accordingly.
(9) Article 13(g) of Regulation (EC) No 1069/2009 provides that animal by-products originating from aquatic animals of Category 2 material may be ensiled, composted or transformed into biogas. The European Food Safety Authority (‘EFSA’) published a Scientific Opinion on the evaluation of a new processing method for animal by-products of Category 2 materials of fish origin(5). According to EFSA's opinion, risks arising from Category 2 materials of fish origin are adequately reduced by the processing method, and derived products may therefore be used for the production of organic fertilisers, composted, transformed into biogas or used for the manufacture of feed for fur animals or other animals not intended for human consumption. EFSA's opinion concludes that there is no increase in risk, if the processing method is also applied for the processing of by-products originating from aquatic animals of Category 3 materials. Category 3 material obtained from aquatic animals may therefore be destined for purposes listed in Article 14 of Regulation (EC) No 1069/2009.
(10) Following the successful outcome of EFSA's risk assessment, the ensilage of fish material should be added to the list of alternative processing methods in Chapter IV of Annex IV to Regulation (EU) No 142/2011. Annex IV to Regulation (EU) No 142/2011 should be amended accordingly.
(11) Digestion residues and compost of animal origin may in practice be mixed with materials of non-animal origin. Operators should know which rules apply for the disposal of such digestion residues and compost. In addition, it is necessary to clarify in which cases compost and digestion residues derived from catering waste may be disposed of in an authorised landfill. Chapter III of Annex V to Regulation (EU) No 142/2011 should be amended accordingly.
(12) Croatia notified a list of species of wild necrophagous birds which should be subject to the derogation on special feeding purposes laid down in Article 18 of Regulation (EC) No 1069/2009. The list of species of necrophagous birds in Annex VI to Regulation (EU) No 142/2011 should be amended accordingly.
(13) EFSA assessed the risk posed by composting containment and subsequent incineration of dead-on-farm porcine animals(6) and concluded that the composting containment as referred to in the alternative parameters laid down in Section 2 of Chapter III of Annex V to Regulation (EU) No 142/2011 is not a sufficient treatment for the safe disposal of Category 2 material and can therefore not be described as an alternative processing method in Chapter IV of Annex IV to that Regulation. Following the aforementioned EFSA's assessment the ‘aerobic maturation and storage of dead-on-farm pigs with subsequent incineration or co-incineration’ should be seen as a specific containment method for the storage of animal by-products pending their subsequent disposal in accordance with Regulation (EC) No 1069/2009. In order to differentiate that method from the approved methods of composting and to avoid the approval procedure required for composting plants laid down in Annex V to Regulation (EU) No 142/2011, it is appropriate to include that method in a new Chapter in Annex IX to that Regulation together with the method ‘Hydrolysis with subsequent disposal’, currently referred to in point H of Section II of Chapter IV of Annex IV, which is based on the same principles. Furthermore the reference to Annex IV in Section 11 of Chapter II of Annex XVI should be adapted accordingly. Annexes IV, IX and XVI to Regulation (EU) No 142/2011 should therefore be amended accordingly.
(14) Rendered fats from Category 3 material are subject to specific requirements under Section 3 of Chapter II of Annex X to Regulation (EU) No 142/2011. However, there are no animal health grounds to prohibit the processing of Category 3 material from aquatic animals and animal by-products from aquatic animals as referred to in Article 10(i) and (j) of Regulation (EC) No 1069/2009 together with Category 3 animal by-products obtained from terrestrial animals into mixed rendered fats. Therefore it should be possible to use Category 3 materials from aquatic animals and animal by-products from aquatic animals as referred to in Article 10(i) and (j) of Regulation (EC) No 1069/2009 for the production of rendered fat. Point A(1) of Section 3 of Chapter II of Annex X to Regulation (EU) No 142/2011 should be amended accordingly.
(15) Requirements for the heat treatment of centrifuge or separator sludge which may be later used as or in the production of organic fertilisers and placed on the market are set out in Part III of Section 4 of Chapter II in Annex X to Regulation (EU) No 142/2011. It is opportune to introduce a derogation that the competent authority may authorise alternative parameters for the heat treatment of centrifuge or separator sludge destined for uses within the Member States, provided that the operators can demonstrate that the heat treatment carried out according to the alternative parameters guarantees at least the same risk reduction as the treatment carried out according to the already established parameters applicable for placing on the market. Part III of Section 4 of Chapter II of Annex X to Regulation (EU) No 142/2011 should be amended accordingly.
(16) Intermediate products may be used, inter alia, for the production of laboratory reagents or in vitro diagnostic for animal purposes. After checks at the border inspection post in accordance with Article 4 of Council Directive 97/78/EC(7) the product has to be transported directly to the registered establishment or plant of destination. In order to clarify the requirements for the importation of intermediate products, Annex XII to Regulation (EU) No 142/2011 should be amended accordingly.
(17) Blood products intended for the production of feed for farmed animals, including spray dried blood and blood plasma of porcine animals, must have been produced in accordance with Section 2 of Chapter II of Annex X to Commission Regulation (EU) No 142/2011. With reference to point B of that Section, blood products are to be submitted to any of the processing methods 1 to 5 or processing method 7 as set out in Chapter III of Annex IV to that Regulation, or another method which ensures that the blood products comply with the microbiological standards for derived products set out in Chapter I of Annex X to Commission Regulation (EU) No 142/2011. Regulation (EU) No 142/2011 also provides, in particular in column 6 of row 2 in Table 1 of Section 1 of Chapter I of Annex XIV, that blood products not intended for human consumption that could be used as feed are to be accompanied by a health certificate in accordance with the model health certificate set out in Chapter 4(B) of Annex XV when they are intended for dispatch to or transit through the Union.
(18) Porcine epidemic diarrhoea, including infection of pigs with the porcine epidemic diarrhoea virus (PEDv) and swine delta coronavirus (SDCv), has been reported in Asia, North America, the Caribbean, Central and South America. SDCv has never been detected in the Union. Inappropriate heat treatment or contamination after heat treatment of spray dried blood and blood plasma of porcine animals, a traditional ingredient for feed for piglets, is incriminated in the spread of the virus.
(19) The Commission, acting on its own initiative, adopted Commission Implementing Regulation (EU) No 483/2014(8) as an interim safeguard measure in respect of the safety of spray dried blood and blood plasma of porcine animals intended for the production of feed for animals of the porcine species. Since the risk for animal health will remain, it is necessary to review the requirements for imports of spray dried blood and blood plasma of porcine animals intended for the production of feed for animals of the porcine species and implement the interim measures as a permanent requirement.
(20) Scientific observation indicates that porcine coronaviruses are inactivated in swine faeces if heated to and held at a temperature of 71 °C for 10 minutes or left at room temperature of 20 °C for 7 days. The virus did not survive in experimentally infected dry feed stored at room temperature of 24 °C for at least two weeks. In the Union and in third countries the commonly applied temperature for spray drying of blood and blood plasma is 80 °C throughout the substance.
(21) Based on the available information, it appears opportune to require that spray dried blood and blood plasma of porcine origin introduced from third countries and intended for feeding of porcine animals has been subjected to a high temperature treatment followed by subsequent storage for a certain time at room temperature in order to mitigate the risk of contamination after the treatment.
(22) Imports of bones and bone products (excluding bone meal), horns and horn products (excluding horn meal) and hooves and hoof products (excluding hoof meal) intended for uses other than as feed material, organic fertilisers or soil improvers should also be authorised where those materials are transported by plane, provided they comply with requirements laid down in Article 41 of Regulation (EC) No 1069/2009. Annex XIV to Regulation (EU) No 142/2011 should be amended accordingly.
(23) Following the amendments of the definition of ‘intermediate products’ and the additional requirements for imports of blood products, the model of declaration to be used for imports from third countries of intermediate products and the model of health certificate for imports of blood products intended as feed material should be modified accordingly. Chapter 4(B) and Chapter 20 of Annex XV to Regulation (EU) No 142/2011 should be amended accordingly.
(24) In order to avoid disruptions of trade, a transitional period should be laid down during which imports of the intermediate products to which the provisions of Regulation (EU) No 142/2011 apply, as amended by this Regulation, should be accepted by Member States in accordance with the rules in force prior to the entry into force of this Regulation.
(25) The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health,
HAS ADOPTED THIS REGULATION:
Article 1
Regulation (EU) No 142/2011 is amended as follows:
In Article 15, the following paragraph is added:
‘By way of derogation from Article 14 of Regulation (EC) No 1069/2009, Member States may authorise the collection, transport and disposal of small quantities of Category 3 materials as referred to in Article 10(f) of that Regulation by means referred to in Article 19(1)(d) of that Regulation, subject to compliance with the requirements for disposal by other means set out in Chapter IV of Annex VI hereto.’
In Article 19, point (c) is replaced by the following:
Chapter III, where they store derived products for certain intended purposes as referred to in Article 24(1)(j) of that Regulation;
Chapter V, where they store on the farm animal by-products intended for subsequent disposal as referred to in Article 4 of that Regulation.’
Article 20(4) is amended as follows:
point (d) is replaced by the following:
‘(d)operators using small quantities of Categories 2 and 3 materials referred to in Articles 9 and 10 of Regulation (EC) No 1069/2009 or of products derived therefrom, for the purpose of direct supply of the products within the region to the final user, on the local market or to local retail establishments, if the competent authority does not consider such activity to present a risk of spreading any serious transmissible disease to humans or animals; this point shall not apply where those materials are used as feed for farmed animals other than fur animals;’
the following points (e) and (f) are added:
users of organic fertilisers or soil improvers at premises where farmed animals are not kept;
operators handling and distributing organic fertilisers or soil improvers exclusively in ready-to-sell retail packaging of not more than 50 kg in weight for uses outside the feed and food chain.’
In Article 22, paragraph 2 is replaced by the following:
‘2.The placing on the market of the following is not subject to any animal health conditions:
(a)guano from wild sea birds, collected in the Union or imported from third countries;
(b)ready-to-sell growing media, other than that imported, with a content of less than:
5 % in volume of derived products of Category 3 material or of Category 2 material other than processed manure;
50 % in volume of processed manure.’
In Article 23, paragraph 3 is replaced by the following:
‘3.The operator or owner of the establishment or plant of destination of intermediate products or his representative shall use and/or dispatch the intermediate products exclusively for use in manufacturing according to the definition of intermediate products under Point 35 of Annex I.’
In Article 36, paragraph 3 is deleted.
Annexes I, III, IV, V, VI, IX, X, XI, XII, XIV, XV and XVI are amended in accordance with the Annex to this Regulation.
Article 2
For a transitional period until 27 September 2015, consignments of animal by-products and of derived products accompanied by a model declaration, which has been completed and signed in accordance with the model set out in Chapter 20 of Annex XV to Regulation (EU) No 142/2011 in its version before the date of entry into force of this Regulation, shall continue to be accepted for importation into the Union, provided that such model declarations were completed and signed before 27 July 2015.
Article 3
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 23 February 2015.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 6 January 2015.
For the Commission
The President
Jean-Claude Juncker
ANNEX
Annexes I, III, IV, V, VI, IX, X, XI, XII, XIV, XV and XVI to Regulation (EU) No 142/2011 are amended as follows:
Annex I is amended as follows:
point 35 is replaced by the following:
‘35.“intermediate product” means a derived product:
which is intended for uses within the manufacturing of medicinal products, veterinary medicinal products, medical devices for medical and veterinary purposes, active implantable medical devices, in vitro diagnostic medical devices for medical and veterinary purposes, laboratory reagents or cosmetic products as follows:
as material in a manufacturing process or in the final production of a finished product;
in validation or verification during a manufacturing process; or
in quality control of a finished product;
whose design, transformation and manufacturing stages have been sufficiently completed in order to be regarded as a derived product and to qualify the material directly or as a component of a product for the purposes referred to in point (a);
which however requires some further manufacturing or transformation, such as mixing, coating, assembling or packaging to make it suitable for placing on the market or putting into service, as applicable, a medicinal product, veterinary medicinal product, medical device for medical and veterinary purposes, active implantable medical device, in vitro diagnostic medical devices for medical and veterinary purposes, laboratory reagent or cosmetic products;’
point 39 is replaced by the following:
‘39.“trade samples” means animal by-products or derived products intended for particular studies or analyses authorised by the competent authority in accordance with Article 17(1) of Regulation (EC) No 1069/2009 with a view to carrying out a production process, including the processing of animal by-products or derived products, the development of feedingstuff, pet food or derived products, or the testing of machinery or equipment;’
In Annex III, Chapter III, point (a) is replaced by the following:
‘(a)only be used for the disposal of:
dead pet animals referred to in Article 8(a)(iii) of Regulation (EC) No 1069/2009;
Category 1 materials referred to in Article 8(b), (e) and (f), Category 2 materials referred to in Article 9 or Category 3 materials referred to in Article 10 of that Regulation; and
dead individually identified equine animals from holdings not subject to health restrictions in accordance with Article 4(5) or 5 of Directive 2009/156/EC, if authorised by the Member State;’
In Annex IV, Chapter IV is amended as follows:
Section 2 is amended as follows:
point H is deleted;
the following point is added:
‘K.Ensilage of fish material
1.Starting materials
For this process, only the following by-products obtained from aquatic animals may be used:
Category 2 materials referred to in Article 9(f)(i) and (iii) of Regulation (EC) No 1069/2009;
Category 3 materials.
2.Processing method
2.1.The materials to be treated shall be collected at aquaculture farms and food processing establishments on a daily basis and without undue delays, ground or chopped, and thereafter subjected to ensiling at a pH of 4 or below, with formic acid or other organic acid authorised in accordance with the feed legislation. The resulting fish silage must be a suspension of parts of aquatic animals liquefied by the action of endogenous enzymes in the presence of the added acid. The proteins of aquatic animals must be reduced into smaller soluble units, by the enzymes and the acid, in order to prevent microbial spoilage. The ensiled material is transported to the processing plant.
2.2.At the processing plant the ensiled material of aquatic animals must be piped into closed storage tanks. The incubation time must be at least 24 hours at a pH of 4 or below before heat treatment can be conducted. Before the heat treatment the ensilage of aquatic animals must have a pH of 4 or below and have a particle size of less than 10 mm following a filtration or maceration at the plant. During processing it must be subjected to preheating to a temperature above 85 °C, followed by incubation in an insulated container to obtain 85 °C throughout the fish material for 25 minutes. The process must take place in a closed production line with tanks and pipelines.
2.3.Before authorisation is given, the operator's permanent written procedure referred to in Article 29(1) to (3) of Regulation (EC) No 1069/2009 must be assessed by the competent authority.’
In Section 3, point 2(d) is replaced by the following:
‘(d)the lime-treated mixture of pig and poultry manure may be applied to land as processed manure;’
In Section 3, the following point 2(e) is added:
‘(e)The final product derived from the ensilaging of fish material may:
for Category 2 materials, be used for purposes referred to in Article 13(a) to (d) and (g) to (i) of Regulation (EC) No 1069/2009 without further processing or as feed for animals referred to in Article 18 or Article 36(a)(ii) of that Regulation; or
for Category 3 materials, be used for purposes referred to in Article 14 of Regulation (EC) No 1069/2009.’
In Annex V, Chapter III, Section 2 is amended as follows:
in point 2(b), point (x) is replaced by the following:
‘(x)animal by-products referred to in Article 10(f) of Regulation (EC) No 1069/2009, which have undergone processing as defined in Article 2(1)(m) of Regulation (EC) No 852/2004;’
in point 2(b), the following point (xi) is added:
‘(xi)mixture of animal by-products referred to in point 2(b) with non-animal by-product materials.’
in point 3, point (b) is replaced by the following:
‘(b)considers that the digestion residues or compost are unprocessed material and obliges operators to handle them in accordance with Regulation (EC) No 1069/2009, with this Regulation or, in the case of compost or digestion residues derived from catering waste, to recover or dispose of in accordance with the environmental legislation.’
Annex VI is amended as follows:
in Chapter II, Section 2, point 1(a)(i) is replaced by the following:
‘(i)one of the following species of necrophagous birds in the following Member States:
| Country code | Member State | Animal species | |
|---|---|---|---|
| Local name | Latin name | ||
| BG | Bulgaria | bearded vulture black vulture Egyptian vulture griffon vulture golden eagle imperial eagle white-tailed eagle black kite red kite | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos Aquila helíaca Haliaeetus albicilla Milvus migrans Milvus milvus |
| EL | Greece | bearded vulture black vulture Egyptian vulture griffon vulture golden eagle imperial eagle white-tailed eagle black kite | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos Aquila heliaca Haliaeetus albicilla Milvus migrans |
| ES | Spain | bearded vulture black vulture Egyptian vulture griffon vulture golden eagle Spanish imperial eagle black kite red kite | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos Aquila adalberti Milvus migrans Milvus milvus |
| FR | France | bearded vulture black vulture Egyptian vulture griffon vulture golden eagle white-tailed eagle black kite red kite | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos Haliaeetus albicilla Milvus migrans Milvus milvus |
| HR | Croatia | bearded vulture black vulture Egyptian vulture griffon vulture | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus |
| IT | Italy | bearded vulture black vulture Egyptian vulture griffon vulture golden eagle black kite red kite | Gypaetus barbatus Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos Milvus migrans Milvus milvus |
| CY | Cyprus | black vulture griffon vulture | Aegypius monachus Gyps fulvus |
| PT | Portugal | black vulture Egyptian vulture griffon vulture golden eagle | Aegypius monachus Neophron percnopterus Gyps fulvus Aquila chrysaetos |
| SK | Slovakia | golden eagle imperial eagle white-tailed eagle black kite red kite | Aquila chrysaetos Aquila heliaca Haliaeetus albicilla Milvus migrans Milvus milvus’ |
In Chapter IV, the second paragraph is deleted.
In Annex IX, the following Chapter V is added:
‘CHAPTER V CONTAINMENT METHODS
Section 1 General provisions
1.Materials resulting from a containment method may be used or disposed of only within the Member State where that containment method is authorised by the competent authority.
2.The competent authority of a Member State shall make the results of official controls available to the competent authority of another Member State upon request, where a containment method is used for the first time in that Member State, in order to facilitate the introduction of the new containment method.
Section 2 Methodology
A.Aerobic maturation and storage of dead-on-farm pigs and certain other porcine material with subsequent incineration or co-incineration.
1.Member States concerned
The process of aerobic maturation and storage of dead-on-farm pigs and certain other porcine material with subsequent incineration or co-incineration may be used in France, Ireland, Latvia, Portugal and the United Kingdom.
Following aerobic maturation and storage of material, the competent authority of the Member State concerned must ensure that the materials are collected and disposed of within the territory of that Member State.
2.Starting materials
For this process, only the following materials of animals of the porcine species may be used:
Category 2 materials referred to in Article 9(f)(i) to (iii) of Regulation (EC) No 1069/2009;
Category 3 materials referred to in Article 10(h) of Regulation (EC) No 1069/2009.
This method is only applicable to the disposal of animals of the porcine species originating in the same holding, provided this holding is not subject to restrictions due to a suspected or confirmed outbreak of a serious transmissible disease affecting animals of the porcine species. This method may not be used for animals which have died due to those diseases or have been killed for diseases control purposes, or parts of those animals.
3.Methodology
3.1.General principles
The method is a process authorised by the competent authority.
The site must be constructed and laid out in accordance with Union legislation for the protection of the environment, in order to prevent odours and risks to soil and groundwater.
The operator must:
take preventive measures against access of animals and put in place a documented pest control programme;
put in place procedures to prevent the spreading of diseases;
put in place procedures to prevent the spreading of used sawdust outside the closed system.
The process must be carried out in a closed system which consist of several cells, with a waterproof floor and delimited by solid walls. Any waste water must be collected; the cells must be connected with a drainpipe fitted with a 6 mm grid to capture solids.
Size and number of the cells must be adapted to the mortality level defined in the permanent written procedure referred to in Article 29(1) to (3) of Regulation (EC) No 1069/2009 with sufficient capacity for farm mortalities occurring during an eight-month period at least.
3.2.Phases
3.2.1.Filling and storage phase
The fallen pigs and other porcine material must be individually covered in sawdust and piled up until the cell is full. First a layer of at least 30 centimetres of sawdust must be placed on the ground. The carcasses and other porcine material must then be placed on this first layer of sawdust and each layer of carcasses and other porcine material must be covered with a layer of sawdust at least 30 cm thick.
Personnel must not walk on the stored material.
3.2.2.Maturing phase
When the cell is full and a rise in temperature allows the degradation of all the soft tissues, the maturation period starts and must last at least 3 months.
At the end of the filling and storage phase and during all of the maturation phase, the operator must monitor the temperature in each cell with a temperature sensor placed between 40 cm and 60 cm beneath the pile surface of the latest built layer.
The electronic reading and monitoring of the temperature must be recorded by the operator.
At the end of the filling and storage phase, the temperature monitoring is an indicator of a satisfactory pile layout. The temperature must be measured by an automatic recording device. The aim is to reach 55 °C during 3 consecutive days, revealing that the maturing process is active and that the pile layout is effective and that the maturing phase has started.
The operator must monitor the temperature once a day and the following measures shall be taken depending on the outcome of these measurements:
where the temperature of 55 °C or more is maintained during 3 consecutive days, the pile may be removed after a 3 consecutive months maturing phase, or may remain stored on the premises awaiting a later removal;
where the temperature of 55 °C is not reached during 3 consecutive days, measures defined in the permanent written procedure referred to in Article 29(1) to (3) of Regulation (EC) No 1069/2009 must be set by the operator; if needed, the competent authority may stop the processing method and the material must be disposed of in compliance with Article 13 of the aforementioned Regulation.
A time limit for the storage phase may be determined by the competent authority.
3.2.3.Transport and incineration or co-incineration
The transport of the resulted material after the maturation phase to the approved incineration or co-incineration plant is subject to controls referred to in Regulation (EC) No 1069/2009 or Directive 2008/98/EC.
B.Hydrolysis with subsequent disposal
1.Member States concerned
The process of hydrolysis with subsequent disposal may be used in Ireland, Spain, Latvia, Portugal and the United Kingdom.
Following hydrolysis, the authorising competent authority must ensure that the materials are collected and disposed of within the same Member State referred to above.
2.Starting materials
For this process, only the following materials of porcine origin may be used:
Category 2 materials referred to in Article 9(f)(i) to (iii) of Regulation (EC) No 1069/2009;
Category 3 materials referred to in Article 10(h) of that Regulation.
This method is only applicable to the disposal of animals of the porcine species originating in the same holding and provided this holding is not subject to prohibition due to a suspected or confirmed outbreak of a serious transmissible disease affecting animals of the porcine species, or animals that have been killed for disease control purposes.
3.Methodology
Hydrolysis with subsequent disposal is a temporary storage on the spot. It shall be carried out according to the following standards:
Following their collection on a holding for which the competent authority has authorised the use of the processing method, based on an assessment of the animal density of the holding, the likely mortality rate and the potential risks for public and animal health which may arise, the animal by-products must be placed into a container which has been constructed in accordance with point (b) (“the container”) and which has been placed at a dedicated site in accordance with points (c) and (d) (“the dedicated site”).
The container must:
have a device to close it;
be waterproof, leak-proof and hermetically sealed;
be coated in a way which prevents corrosion;
be equipped with a device for controlling emissions in accordance with point (e).
The container must be placed in a dedicated site which is physically separate from the holding.
That site must have dedicated access routes for the movement of materials and for collection vehicles.
The container and the site must be constructed and laid out in accordance with Union legislation for the protection of the environment, in order to prevent odours and risks to soil and groundwater.
The container must be linked to a pipe for gaseous emissions, which must be equipped with appropriate filters to prevent the transmission of diseases communicable to humans and animals.
The container must be closed for the process of hydrolysis for a period of at least three months, in such a way that any unauthorised opening is prevented.
The operator must put in place procedures to prevent the transmission of diseases communicable to humans or animals by movements of personnel.
The operator must:
take preventive measures against birds, rodents, insects and other vermin;
put in place a documented pest control programme.
The operator must keep records of:
any placing of material into the container;
any collection of hydrolysed material from the container.
The operator must empty the container at regular intervals for a check:
for the absence of corrosion;
to detect and prevent possible leakage of liquid materials into the ground.
Following hydrolysis, the materials must be collected, used and disposed of in accordance with Article 13(a), (b), (c) or Article 13(e)(i) of Regulation (EC) No 1069/2009 or Article 14 of that Regulation for Category 3 materials.
The process must be carried out in a batch mode.
Any other handling or use of the hydrolysed materials, including their application to land, shall be prohibited.’
In Annex X, Chapter II is amended as follows:
in Section 3, point A, point 1 is replaced by the following:
‘1.Rendered fats
Only Category 3 material, other than Category 3 materials referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009, may be used for the production of rendered fat.’
in Section 4, Part III, the following paragraph is added:
‘By way of derogation from the first paragraph, the competent authority may authorise alternative parameters for the heat treatment of centrifuge or separator sludge destined for uses within Member States which have authorised those alternative parameters, provided operators can demonstrate that the heat treatment according to the alternative parameters guarantees at least the same risk reduction as the treatment carried out according to the parameters set out in the first paragraph.’
In Annex XI, Chapter II, a new Section 3 is added:
‘Section 3 Requirements for approval of establishments or plants
In order to be approved in accordance with Article 24(1)(f) of Regulation (EC) No 1069/2009, operators shall ensure that establishments or plants carrying out the activities referred to in point 1 of Section 1 meet the requirements laid down in Article 8 of this Regulation and:
have adequate facilities for storage of incoming ingredients to prevent cross-contamination and avoid contamination during storage;
dispose of unused animal by-products or derived products in accordance with Articles 13 and 14 of Regulation (EC) No 1069/2009.’
In Annex XII, point 3(a) is replaced by the following:
‘3.The intermediate products imported into the Union shall be checked at the border inspection post in accordance with Article 4 of Directive 97/78/EC and transported directly from the border inspection post either to:
a registered establishment or plant for the production of laboratory reagents, medical devices and in vitro diagnostic medical devices for veterinary purposes or the derived products referred to in Article 33 of Regulation (EC) No 1069/2009, where the intermediate products must be further mixed, used for coating, assembled or packaged before they are placed on the market or put into services in accordance with the Union legislation applicable to the derived product;’.
Annex XIV is amended as follows:
Chapter I is amended as follows:
In Section 1, in row 2 of Table 1, the text in the fourth column is replaced by the following:
‘The blood products must have been produced in accordance with Section 2 of Chapter II of Annex X and Section 5 of Chapter I of Annex XIV.’
a new Section 5 is added:
‘Section 5 Imports of blood products for the feeding of farmed animals
The following requirements shall apply to the importation of blood products, including spray dried blood and blood plasma which have been derived from porcine animals intended for the feeding of porcine animals:
These derived products must be:
subjected to a heat treatment at a temperature of at least 80 °C throughout the substance and the dry blood and blood plasma is of not more than 8 % moisture with a water activity (Aw) of less than 0,60;
stored in dry warehouse conditions under room temperature for at least 6 weeks.’
Annex XV is amended as follows:
Chapter 4(B) is replaced by the following:
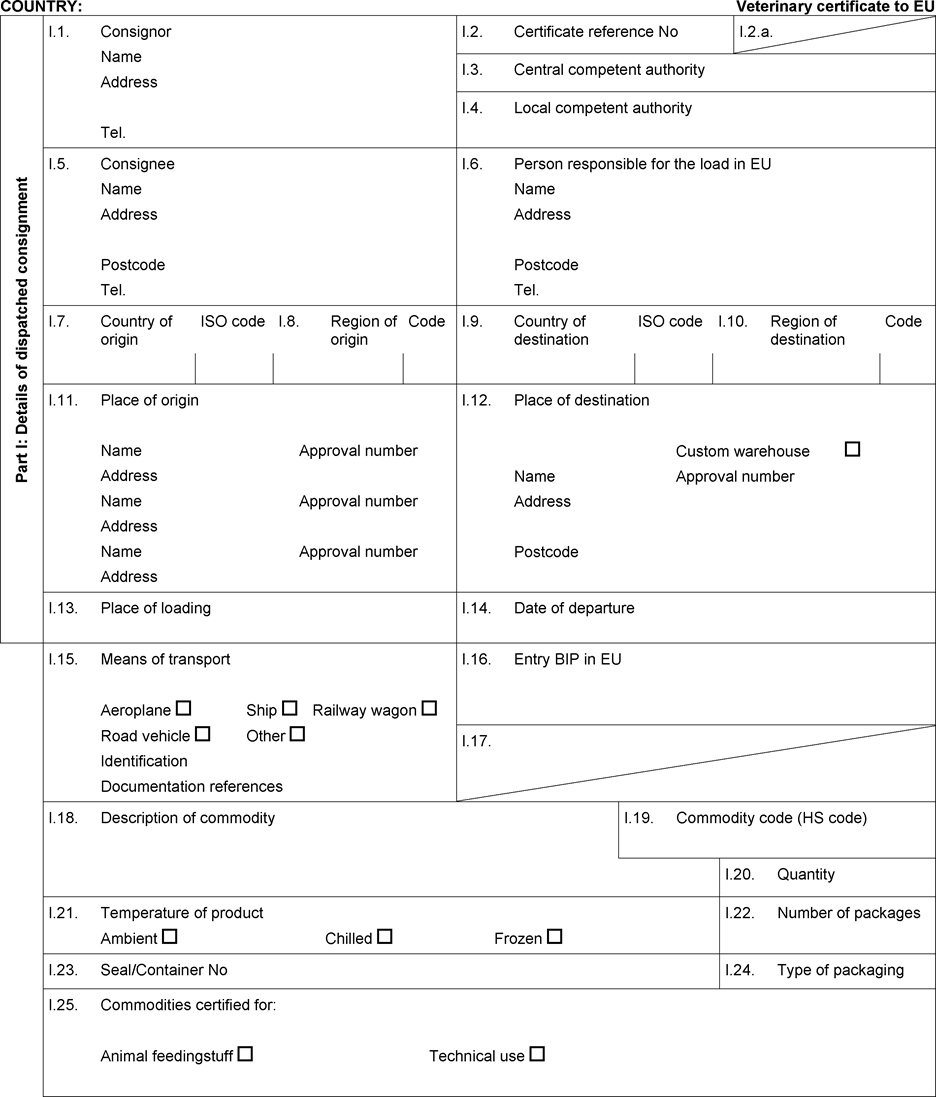
‘CHAPTER 4(B) Health certificate For blood products not intended for human consumption that could be used as feed material, intended for dispatch to or for transit through (2) the European Union
’
Chapter 20 is replaced by the following:
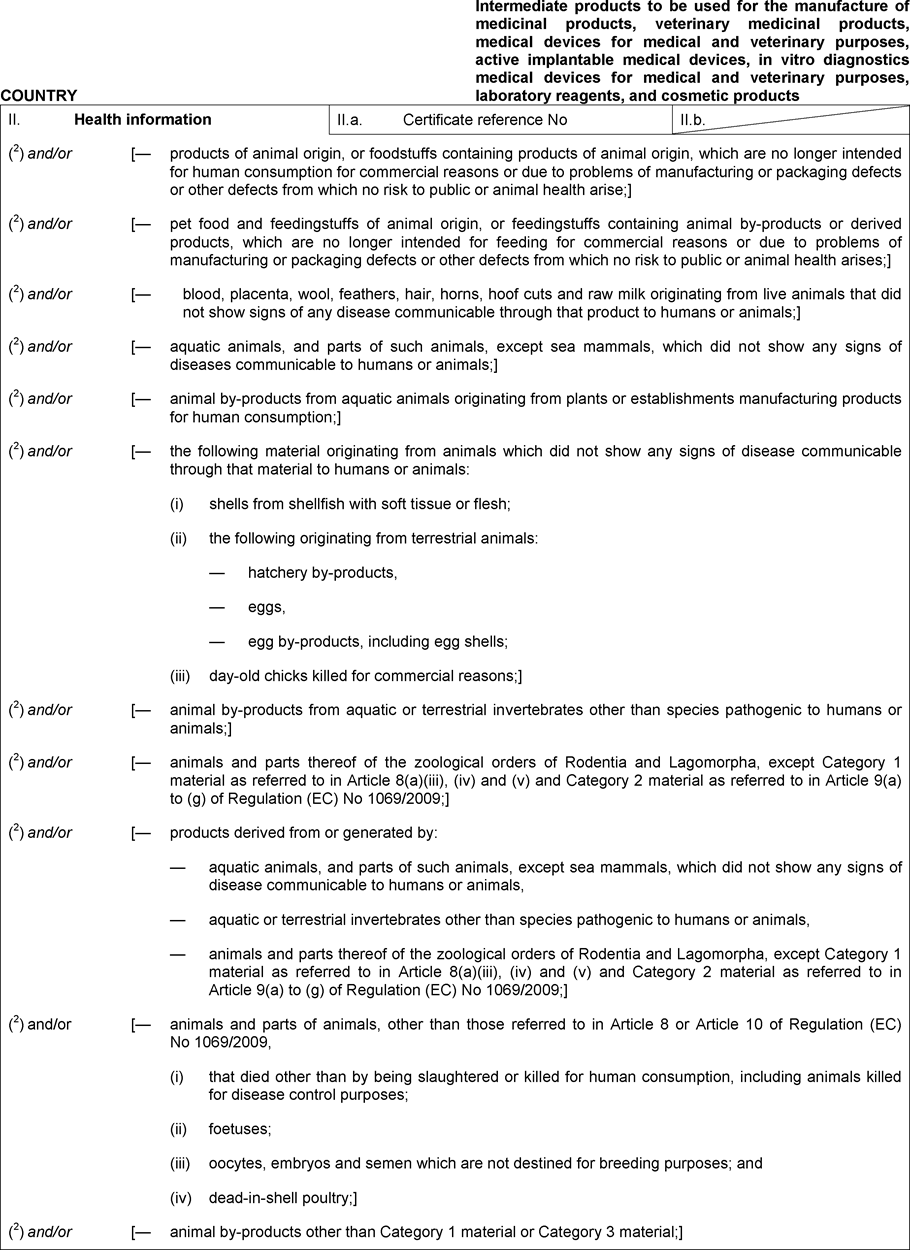
‘CHAPTER 20 Model declaration Declaration for the import from third countries and for the transit through the European Union of intermediate products to be used for the manufacture of medicinal products, veterinary medicinal products, medical devices for medical and veterinary purposes, active implantable medical devices, in vitro diagnostics medical devices for medical and veterinary purposes, laboratory reagents and cosmetic products
’
In Annex XVI, Chapter III, Section 11 is replaced by the following:
‘Section 11 Official controls regarding hydrolysis with subsequent disposal
The competent authority shall carry out controls at sites where hydrolysis with subsequent disposal is carried out in accordance with point B of Section 2 of Chapter V of Annex IX.
Such controls shall, for the purpose of reconciliation of the quantities of hydrolysed materials dispatched and disposed of, include documentary checks:
of the amount of materials which are hydrolysed at the site;
in the establishments or plants where the hydrolysed materials are disposed of.
Controls shall be carried out regularly on the basis of a risk assessment.
During the period of the first 12 months of operation, a control visit to a site, where a container for the hydrolysis is located, shall be carried out every time hydrolysed material is collected from the container.
Following the period of the first 12 months of operation, a control visit to such sites shall be carried out every time the container is emptied and checked for the absence of corrosion and leaking in accordance with point B(3)(j) of Section 2 of Chapter V of Annex IX.’
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (OJ L 54, 26.2.2011, p. 1).
Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products (OJ L 262, 27.9.1976, p. 169).
Council Directive 2009/156/EC of 30 November 2009 on animal health conditions governing the movement and importation from third countries of equidae (codified version) (OJ L 192, 23.7.2010, p. 1).
EFSA Journal 2011; 9(9):2389 (11 pp.).
EFSA Journal 2012; 10(2):2559 (11 pp.).
Council Directive 97/78/EC of 18 December 1997 laying down the principles governing the organisation of veterinary checks on products entering the Community from third countries (OJ L 24, 30.1.1998, p. 9).
Commission Implementing Regulation (EU) No 483/2014 of 8 May 2014 on protection measures in relation to porcine diarrhoea caused by a deltacoronavirus as regards the animal health requirements for the introduction into the Union of spray dried blood and blood plasma of porcine origin intended for the production of feed for farmed porcine animals (OJ L 138, 13.5.2014, p. 52).
Options/Help
Print Options
PrintThe Whole Regulation
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources
The data on this page is available in the alternative data formats listed: