- Latest available (Revised)
- Point in Time (08/04/2019)
- Original (As adopted by EU)
Commission Implementing Regulation (EU) 2019/628Show full title
Commission Implementing Regulation (EU) 2019/628 of 8 April 2019 concerning model official certificates for certain animals and goods and amending Regulation (EC) No 2074/2005 and Implementing Regulation (EU) 2016/759 as regards these model certificates (Text with EEA relevance)
You are here:
- Regulations originating from the EU
- 2019 No. 628
- Annexes only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Revised version PDFs
- Revised 17/05/20193.26 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Implementing Regulation (EU) 2019/628 (Annexes only)
Version Superseded: 31/12/2020
Alternative versions:
Status:
Point in time view as at 08/04/2019.
Changes to legislation:
Commission Implementing Regulation (EU) 2019/628 is up to date with all changes known to be in force on or before 15 August 2024. There are changes that may be brought into force at a future date. Changes that have been made appear in the content and are referenced with annotations.![]()
Changes to Legislation
Changes and effects yet to be applied by the editorial team are only applicable when viewing the latest version or prospective version of legislation. They are therefore not accessible when viewing legislation as at a specific point in time. To view the ‘Changes to Legislation’ information for this provision return to the latest version view using the options provided in the ‘What Version’ box above.
ANNEX IU.K. MODEL OFFICIAL CERTIFICATES FOR THE ENTRY INTO THE UNION OF ANIMALS, PRODUCTS OF ANIMAL ORIGIN, COMPOSITE PRODUCTS, GERMINAL PRODUCTS AND ANIMAL BY-PRODUCTS
ANNEX IIU.K. NOTES ON THE COMPLETION OF THE MODEL OFFICIAL CERTIFICATES FOR THE ENTRY INTO THE UNION OF ANIMALS, PRODUCTS OF ANIMAL ORIGIN, COMPOSITE PRODUCTS, GERMINAL PRODUCTS, AND ANIMAL BY-PRODUCTS
General U.K.
To positively select any option, please tick or mark the relevant box with a cross (X).
Whenever mentioned, ‘ISO’ means the international standard two-letter code for a country, in accordance with the international standard ISO 3166 alpha-2(1).
Only one of the options may be selected in boxes I.15, I.18, I.20 and I.22.
If the consignee, the entry border control post (BCP) or the transport details (that is to say, the means and date) change after the certificate has been issued, the operator responsible for the consignment must advise the competent authority of the Member State of entry. Such a change shall not result in a request for a replacement certificate.
Part I: Details of the dispatched consignment U.K.
Country:The name of the third country issuing the certificate.U.K.
Box I.1.Consignor/Exporter: the name and address (street, city and region, province or state, as appropriate) of the natural or legal person dispatching the consignment that must be located in the third country, except for the re-entry of consignments originating from the European Union.U.K.
Box I.2.Certificate reference No: the unique mandatory code assigned by the competent authority of the third country in accordance with its own classification. This box is compulsory for all certificates not submitted in IMSOC.U.K.
Box I.2.aIMSOC reference No: the unique reference code automatically assigned by IMSOC, if the certificate is registered in IMSOC. This box must not be completed if the certificate is not submitted in IMSOC.U.K.
Box I.3.Central competent authority: name of the central authority in the third country issuing the certificate.U.K.
Box I.4.Local competent authority: if applicable, the name of the local authority in the third country issuing the certificate.U.K.
Box I.5.Consignee/Importer: name and address of the natural or legal person to whom the consignment is intended in the Member State or third country of destination in the case of transit. However, this information is not compulsory for consignments in transit through the European Union.U.K.
Box I.6.Operator responsible for the consignment:U.K.
The name and address of the person in the European Union in charge of the consignment when presented to the BCP and who makes the necessary declarations to the competent authorities either as the importer or on behalf of the importer.
For products in transit through the European Union: the name and address are compulsory.
For certain animals: the name and address are compulsory if required by the relevant European Union legislation.
For animals and products for the placing on the market: the name and address are optional.
Box I.7.Country of origin:U.K.
For products: the name and ISO code of the country where the goods were produced, manufactured and packaged (labelled with the identification mark).
For animals: the country of residence during the required period as set out in the relevant European Union health certificate. For registered horses re-entering the European Union, the country of origin means the country from which they were last consigned.
In the case of trade involving more than one third country (triangular trade), a separate certificate must be completed for each country of origin.
Box I.8.Region of origin: if applicable, for animals or products affected by the regionalisation measures in accordance with European Union legislation. The code of approved regions, zones or compartments must be stated as defined in the relevant European Union legislation.U.K.
Box I.9.Country of destination: the name and ISO code of the European Union country of destination of the animals or products.U.K.
If the products are in transit, the name and ISO code of the third country of destination is required.
Box I.10.Region of destination: see box I.8.U.K.
Box I.11.Place of dispatch: the name, address and approval number, if required by the European Union legislation, of the holdings or establishments from which the animals or the products come from.U.K.
For animals: a holding or any other officially monitored agricultural, industrial or commercial establishment, including zoos, amusement parks, wildlife and hunting reserves, where animals are regularly kept or bred.
For germinal products: semen collection or storage centres, or embryo collection or production teams.
For other products: any unit of a company in the food or animal by-product sector. Only the establishment shipping the products is to be named. In the case of trade involving more than one third country (triangular trade), the place of dispatch is the last third-country establishment of the export chain from which the final consignment is transported to the European Union.
Box I.12.Place of destination:U.K.
Except in the case of storage of products in transit, this information is optional.
For the placing on the market: the place where the animals or products are sent for final unloading. Give the name, address and approval number of the holdings or establishments of the place of destination, if applicable.
For storage of products in transit: the name, address and approval number of the warehouse in a free zone, the customs warehouse or the ship supplier.
Box I.13.Place of loading:U.K.
For animals: the name of the city or the place where the animals are loaded and if they are assembled beforehand, the details of the official assembly centre.
For products: the name of the city and category (for example, establishment, holding, port or airport) of the final place where the products are to be loaded in the means of transport for the journey to the European Union. In the case of a container, state where it is to be placed aboard the final means of transport to the European Union. In the case of a ferry, indicate the place where the truck embarked.
Box I.14.Date and time of departure:U.K.
For animals: the date and time at which the animals are scheduled to leave in their means of transport (aeroplane, vessel, railway or road vehicle).
For products: the date when the means of transport departs (aeroplane, vessel, railway or road vehicle).
Box I.15.Means of transport: means of transport leaving the country of dispatch.U.K.
Mode of transport: aeroplane, vessel, railway, road vehicle or other. ‘Other’ means modes of transport not covered by Council Regulation (EC) No 1/2005(2).
Identification of the means of transport: for aeroplanes the flight number, for vessels the ship name(s), for railways the train identity and wagon number, for road transports the registration number plate with trailer number plate if applicable.
In the case of a ferry, state the identification of the road vehicle, the registration number plate with trailer number plate if applicable, and the name of the scheduled ferry.
Box I.16.Entry BCP: state the name of the BCP and its identification code assigned by IMSOC.U.K.
Box I.17.Accompanying documents:U.K.
The type and reference number of document must be stated when a consignment is accompanied by the other documents such as CITES permit, permit for invasive alien species (IAS) or a commercial document (for example, the airway bill number, the bill of lading number or the commercial number of the train or road vehicle)
Box I.18.Transport conditions: category of required temperature during the transport of products (ambient, chilled, frozen). Only one category may be selected.U.K.
Box I.19.Container No/Seal No: if applicable, the corresponding numbers.U.K.
The container number must be provided if the goods are transported in closed containers.
Only the official seal number must be stated. An official seal applies if a seal is affixed to the container, truck or rail wagon under the supervision of the competent authority issuing the certificate.
Box I.20.Goods certified as: state the purpose for the placing on the market of the animals or intended use for products as specified in the relevant European Union health certificate.U.K.
Animal feedingstuffs: concerns only animal by-products intended for animal feed as referred to in Regulation (EC) No 1069/2009 of the European Parliament and of the Council(3).
Approved body: movement of animals to an approved body, an institute or a centre in accordance with Council Directive 92/65/EEC(4).
Artificial reproduction: concerns only germinal products.
Breeding/production: for breeding and production animals, including aquaculture animals intended for farming.
Canning industry: concerns, for example, tuna intended for the canning industry.
Circus/exhibition: for registered circus and exhibition animals and aquatic animals for aquariums or similar businesses not for further sale.
Fattening: concerns ovine and caprine animals only.
Further process: concerns only products which have to be further processed before being placed on the market.
Game restocking: concerns only game for the purpose of rebuilding stocks.
Human consumption: concerns only products intended for human consumption for which a health or veterinary certificate is required by European Union legislation.
Other: intended for purposes not listed elsewhere in this classification, including aquatic animals intended for put-and-take fisheries.
Pets: commercial movements into the Union of dogs, cats, ferrets and birds. For ornamental aquatic animals intended for pet shops or similar businesses for further sale.
Pharmaceutical use: animal by-products unfit for human or animal consumption, as referred to in Regulation (EC) No 1069/2009.
Quarantine: refers to Commission Implementing Regulation (EU) No 139/2013(5) for birds other than poultry, to Directive 92/65/EEC for carnivores, primates and bats, and to Council Directive 2006/88/EC(6) for aquaculture animals.
Registered equidae: in accordance with Council Directive 2009/156/EC(7).
Relaying: concerns only aquaculture animals.
Slaughter: for animals destined directly or via an assembly centre to a slaughterhouse.
Technical use: animal by-products unfit for human or animal consumption, as referred to in Regulation (EC) No 1069/2009.
Trade samples: as defined in point 39 of Annex I to Commission Regulation (EU) No 142/2011(8).
Box I.21.For transit: only for the transit of animals or products through the European Union from one third country to another third country or from one part of a third country to another part of the same third country. State the name and ISO code of the third country of destination.U.K.
Box I.22.For internal market: for all consignments destined to the European Union market.U.K.
Definitive import: this option must only be used for consignments intended to be placed under the customs procedure ‘release for free circulation’ in the European Union.
For certain animals (for example, registered equidae) only one of the following options must be selected:
Re-entry: this option must only be used for animals authorised for re-entry, such as registered horses for racing, competition and cultural events re-entering the European Union after their temporary export.
Temporary admission: this option must only be used for the entry of animals authorised for temporary entry into the European Union, such as registered horses for a period of less than 90 days.
Box I.23.Total number of packages: the number of boxes, cages or stalls, in which the animals are being transported, the number of cryogenic containers for germinal products or the number of packages for products. In the case of bulk consignments, this box is optional.U.K.
Box I.24.Quantity:U.K.
For animals: the total number of heads or straws expressed as units.
For germinal products: the total number of straws expressed as units.
For products and aquatic animals, except ornamental fish: the total gross and net weight in kilograms.
Total net weight: this is defined as the mass of the goods themselves without immediate containers or any packaging.
Total gross weight: overall weight in kilograms. This is defined as the aggregate mass of the products and of the immediate containers and all their packaging, but excluding transport containers and other transport equipment.
Box I.25.Description of goods: State the relevant Harmonised System code (HS code) and the title defined by the World Customs Organisation as referred to in Council Regulation (EEC) No 2658/87(9). This customs description shall be supplemented, if necessary, by additional information required to classify the animals or the products in veterinary terms. In addition, state any specific requirements relating to the animals or to the nature/processing of the products as defined in the relevant European Union model health or veterinary certificate.U.K.
Zone: for animals or products affected by the setting up of approved zones or compartments in accordance with European Union legislation. The zones or production areas (for example, in the case of bivalve molluscs) must be indicated as published in the European Union lists of approved establishments.
For animals: the species, breed or category, identification method, identification number, age, sex, quantity or net weight, and test.
For germinal products: collection or production date, approval number of the centre or team, identification of the straw, and quantity. In addition, as regards donor animals, the species, breed or category, and identification.
For products: the species, types of products, type of treatment, approval number of establishments together with ISO country code (slaughter house, processing plant, cold store), number of packages, type of packaging, batch number, net weight, and final consumer (i.e. products are packed for final consumer).
Species: the scientific name or as defined in accordance with European Union legislation.
Type of packaging: identify the type of packaging according to the definition given in Recommendation No 21(10) of UN/CEFACT (United Nations Centre for Trade Facilitation and Electronic Business).
Part II: Certification U.K.
This part must be completed by an official veterinarian or an official inspector.
Box II.Health information: please complete this part in accordance with the specific European Union health requirements relating to the animal species or to the nature of the products and as defined in the equivalence agreements with certain third countries or in other European Union legislation, such as that for certification.U.K.
Where there are no animal or public health attestations for the consignment, then the whole of this section shall be deleted or invalidated or not be present at all in accordance with the footnotes for Part II of the specific European Union health certificates.
Box II.a.Certificate reference No: same reference code as in box I.2.U.K.
Box II.b.IMSOC reference No: same reference code as in box I.2.a.U.K.
| Certifying officer: | Official veterinarian or official inspector as defined by the relevant European Union legislation: the name in capital letters, qualification and title, where applicable, identification number and original stamp of the competent authority and date of signature. |
ANNEX IIIU.K. MODEL OFFICIAL CERTIFICATES FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF ANIMALS AND GOODS INTENDED FOR HUMAN CONSUMPTION
PART IU.K.
CHAPTER A: MODEL OFFICIAL CERTIFICATE FOR THE ENTRY IN THE UNION FOR PLACING ON THE MARKET OF LIVE BIVALVE MOLLUSCS, ECHINODERMS, TUNICATES AND MARINE GASTROPODS U.K.
CHAPTER B: ADDITIONAL MODEL OFFICIAL CERTIFICATION FOR PROCESSED BIVALVE MOLLUSCS BELONGING TO THE SPECIES ACANTHOCARDIA TUBERCULATUM U.K.
PART IIU.K.
CHAPTER A: MODEL OFFICIAL CERTIFICATE FOR THE ENTRY IN THE UNION FOR PLACING ON THE MARKET OF FISHERY PRODUCTS U.K.
CHAPTER B: MODEL OF OFFICIAL CERTIFICATE FOR FISHERY PRODUCTS CAUGHT BY VESSELS FLYING THE FLAG OF A MEMBER STATE AND TRANSFERRED IN THIRD COUNTRIES WITH OR WITHOUT STORAGE U.K.
CHAPTER C: MODEL OF OFFICIAL CERTIFICATE TO BE SIGNED BY THE CAPTAIN ACCOMPANYING FROZEN FISHERY PRODUCTS WHEN ENTERING THE UNION FOR PLACING ON THE MARKET DIRECTLY FROM A FREEZER, REEFER OR FACTORY VESSEL U.K.
PART IIIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF CHILLED, FROZEN OR PREPARED FROGS' LEGS INTENDED FOR HUMAN CONSUMPTION
PART IVU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF CHILLED, FROZEN, SHELLED, COOKED, PREPARED OR PRESERVED SNAILS INTENDED FOR HUMAN CONSUMPTION
PART VU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF RENDERED ANIMAL FATS AND GREAVES INTENDED FOR HUMAN CONSUMPTION
PART VIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF GELATINE INTENDED FOR HUMAN CONSUMPTION
PART VIIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF COLLAGEN INTENDED FOR HUMAN CONSUMPTION
PART VIIIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF RAW MATERIALS FOR THE PRODUCTION OF GELATINE AND COLLAGEN INTENDED FOR HUMAN CONSUMPTION
PART IXU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF TREATED RAW MATERIALS FOR THE PRODUCTION OF GELATINE AND COLLAGEN INTENDED FOR HUMAN CONSUMPTION
PART XU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF HONEY AND OTHER APICULTURE PRODUCTS INTENDED FOR HUMAN CONSUMPTION
PART XIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF HIGHLY REFINED CHONDROITIN SULPHATE, HYALURONIC ACID, OTHER HYDOLYSED CARTILAGE PRODUCTS, CHITOSAN, GLUCOSAMINE, RENNET, ISINGLASS AND AMINO ACIDS INTENDED FOR HUMAN CONSUMPTION
PART XIIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF REPTILE MEAT INTENDED FOR HUMAN CONSUMPTION
PART XIIIU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF INSECTS INTENDED FOR HUMAN CONSUMPTION
PART XIVU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF OTHER PRODUCTS OF ANIMAL ORIGIN INTENDED FOR HUMAN CONSUMPTION NOT COVERED BY ARTICLES 7 TO 25 OF COMMISSION IMPLEMENTING REGULATION (EU) 2019/628
PART XVU.K. MODEL OFFICIAL CERTIFICATE FOR THE ENTRY INTO THE UNION FOR PLACING ON THE MARKET OF SPROUTS AND SEEDS INTENDED FOR THE PRODUCTION OF SPROUTS
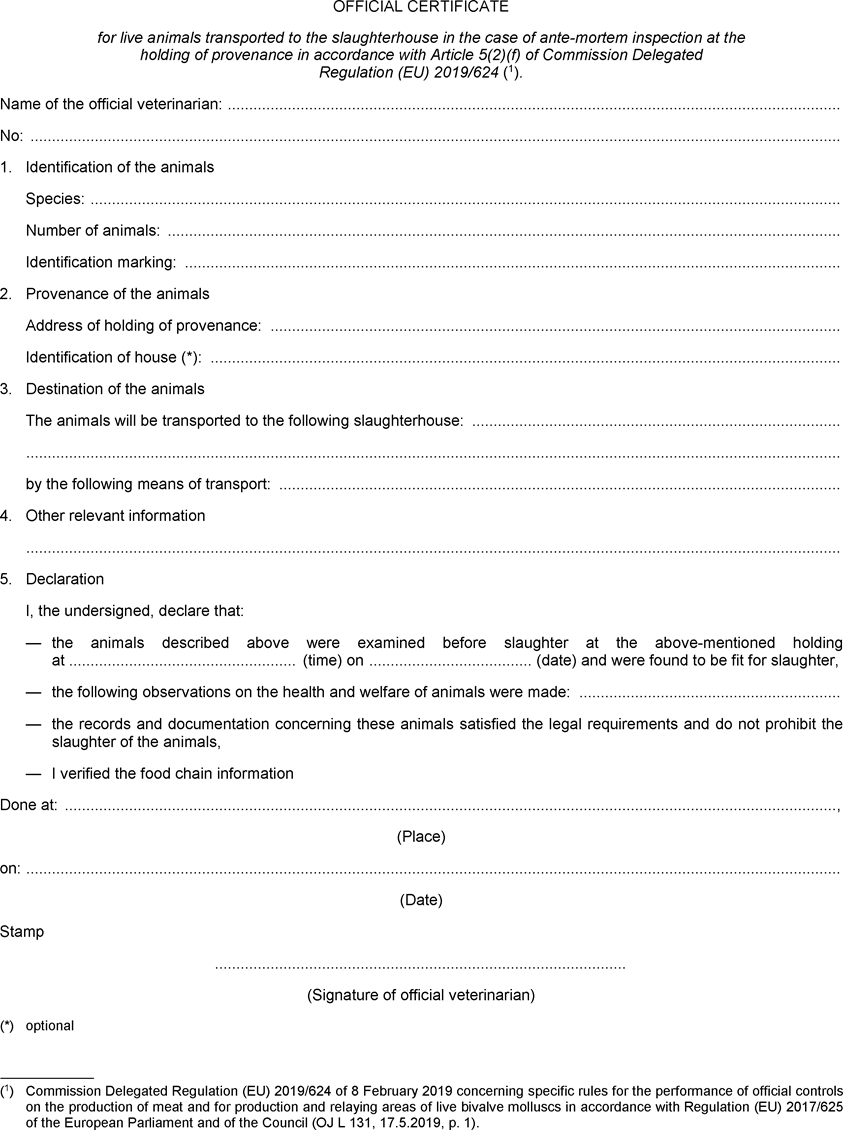
ANNEX IVU.K. MODEL OFFICIAL CERTIFICATES IN THE CASE OF ANTE-MORTEM INSPECTION AT THE HOLDING OF PROVENANCE
Part I: MODEL OFFICIAL CERTIFICATE FOR LIVE ANIMALSU.K.
Part II: MODEL OFFICIAL CERTIFICATE FOR POULTRY INTENDED FOR THE PRODUCTION OF FOIE GRAS AND DELAYED EVISCERATED POULTRYU.K.
Part III: MODEL OFFICIAL CERTIFICATE FOR FARMED GAME SLAUGHTERED AT THE HOLDING OF PROVENANCEU.K.
Part IV: MODEL OFFICIAL CERTIFICATE FOR FARMED GAME SLAUGHTERED AT THE HOLDING in accordance with point 3a of Section III of Annex III to Regulation (EC) No 853/2004U.K.
ANNEX VU.K. MODEL OFFICIAL CERTIFICATE IN THE CASE OF EMERGENCY SLAUGHTER OUTSIDE THE SLAUGHTERHOUSE IN ACCORDANCE WITH ARTICLE 4 OF COMMISSION DELEGATED REGULATION (EU) 2019/624 (11)
MODEL OFFICIAL CERTIFICATE IN THE CASE OF EMERGENCY SLAUGHTER OUTSIDE THE SLAUGHTERHOUSEU.K.
ANNEX VIU.K. CORRELATION TABLE REFERRED TO IN ARTICLE 32
| Regulation (EU) No 211/2013 | This Regulation |
|---|---|
| Article 1 | Article 1(2)(b)(ii) |
| Article 2 | Article 2(2) |
| Article 3 | Article 27 |
| Article 4 | — |
| Article 5 | — |
| Annex | Part XV of Annex III |
List of country names and code elements under: http://www.iso.org/iso/country_codes/iso-3166-1_decoding_table.htm
Council Regulation (EC) No 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97 (OJ L 3, 5.1.2005, p. 1).
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation) (OJ L 300, 14.11.2009, p. 1).
Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A (I) to Directive 90/425/EEC (OJ L 268, 14.9.1992, p. 54).
Commission Implementing Regulation (EU) No 139/2013 of 7 January 2013 laying down animal health conditions for imports of certain birds into the Union and the quarantine conditions thereof (OJ L 47, 20.2.2013, p. 1).
Council Directive 2006/88/EC of 24 October 2006 on animal health requirements for aquaculture animals and products thereof, and on the prevention and control of certain diseases in aquatic animals (OJ L 328, 24.11.2006, p. 14).
Council Directive 2009/156/EC of 30 November 2009 on animal health conditions governing the movement and importation from third countries of equidae (OJ L 192, 23.7.2010, p. 1).
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (OJ L 54, 26.2.2011, p. 1).
Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff (OJ L 256, 7.9.1987, p. 1).
Last version: Revision 9 Annexes V and VI as published on: http://www.unece.org/tradewelcome/un-centre-for-trade-facilitation-and-e-business-uncefact/outputs/cefactrecommendationsrec-index/list-of-trade-facilitation-recommendations-n-21-to-24.html
Commission Delegated Regulation (EU) 2019/624 of 8 February 2019 concerning specific rules for the performance of official controls on the production of meat and for production and relaying areas of live bivalve molluscs in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council (OJ L 131, 17.5.2019, p. 1).
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Annexes only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources