- Latest available (Revised)
- Original (As adopted by EU)
Commission Implementing Regulation (EU) 2020/2236Show full title
Commission Implementing Regulation (EU) 2020/2236 of 16 December 2020 laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 of the European Parliament and of the Council as regards model animal health certificates for the entry into the Union and movements within the Union of consignments of aquatic animals and of certain products of animal origin from aquatic animals, official certification regarding such certificates and repealing Regulation (EC) No 1251/2008 (Text with EEA relevance)
You are here:
- Regulations originating from the EU
- 2020 No. 2236
- Whole Regulation
- Previous
- Next
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
This item of legislation originated from the EU
Legislation.gov.uk publishes the UK version. EUR-Lex publishes the EU version. The EU Exit Web Archive holds a snapshot of EUR-Lex’s version from IP completion day (31 December 2020 11.00 p.m.).
Changes over time for: Commission Implementing Regulation (EU) 2020/2236
Changes to legislation:
This version of this Regulation was derived from EUR-Lex on IP completion day (31 December 2020 11:00 p.m.). It has not been amended by the UK since then. Find out more about legislation originating from the EU as published on legislation.gov.uk.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
Commission Implementing Regulation (EU) 2020/2236
of 16 December 2020
laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 of the European Parliament and of the Council as regards model animal health certificates for the entry into the Union and movements within the Union of consignments of aquatic animals and of certain products of animal origin from aquatic animals, official certification regarding such certificates and repealing Regulation (EC) No 1251/2008
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (Animal Health Law)(1), and in particular Articles 213(2), 224(4), 238(3) and 239(3) thereof,
Having regard to Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation)(2), and in particular Article 90 thereof,
Whereas:
(1) Regulation (EU) 2016/429 lays down rules on animal diseases that are transmissible to animals or to humans, including rules for the animal health certificates required to accompany the entry into the Union and movements within the Union of consignments of aquatic animals and certain products of animal origin from aquatic animals. It also empowers the Commission to adopt implementing acts laying down rules for model forms of those animal health certificates, as well rules concerning the information to be contained in certain documents and declarations required for the entry into the Union of such consignments.
(2) In addition, that Regulation empowers the Commission to lay down special rules concerning model forms of animal health certificates, declarations and other documents for aquatic animals for which the application of the rules set out in that Regulation may not be adequate, taking into account, inter alia, the final destination of the consignment. Regulation (EU) 2016/429 also provides that those animal health certificates may include other information required under other Union legislation. Accordingly, the model animal health certificate should be set out in this Regulation.
(3) Article 213(2) and Article 224(4) of Regulation (EU) 2016/429 also empower the Commission to lay down by means of implementing acts, rules concerning the model animal health certificates, for certain movements of aquatic animals and certain products thereof. Therefore, the model of certificates for the movements of such consignments within the Union should be set out this Regulation.
(4) Articles 238(3) and 239(3) of Regulation (EU) 2016/429 empower the Commission to lay down by means of implementing acts, rules concerning models of animal health certificates, declarations and other documents for the entry into the Union of aquatic animals of which the Union is not the final destination. Accordingly, the model animal health certificate should be set out in this Regulation.
(5) Model animal health certificates set out in this Regulation should contain relevant guarantees in order to ensure that consignments of aquatic animals and of certain products of animal origin from aquatic animals entering the Union and moving within the Union do not pose a significant risk for animal or public health. Such guarantees depend on, inter alia, the relevant diseases listed in Article 5 and in Annex II to Regulation (EU) 2016/429 as well as on their categorisation as provided for in Article 9(1) of that Regulation and in the Annex to Commission Implementing Regulation (EU) 2018/1882(3).
(6) Regulation (EU) 2017/625 lays down rules for the performance of official controls and other official activities performed by the competent authorities of the Member States in order to verify compliance by operators with Union rules in the areas referred to in Article 1(2) of that Regulation, which include, inter alia, food and food safety, as well as animal health and welfare. Official certificates are defined in Regulation (EU) 2017/625 as a paper or electronic document signed by the certifying officer, and providing assurance concerning compliance with one or more requirements laid down in the rules referred to in Article 1(2) thereof. That Regulation empowers the Commission to lay down by means of implementing acts, rules concerning model official certificates and for the issuance of such certificates.
(7) Point (c) of the first paragraph of Article 90 of Regulation (EU) 2017/625 empowers the Commission to lay down by means of implementing acts rules concerning the procedures to be followed for the issuance of replacement certificates. Therefore, it is appropriate to establish common requirements as regards the replacement of animal health certificates in this Regulation.
(8) To avoid misuse and abuse, it is important to lay down rules concerning the cases where a replacement animal health certificate may be issued and the requirements that need to be met for replacing animal health certificates. The cases should be limited to administrative errors, or in cases where the initial certificate has been damaged or lost.
(9) Commission Delegated Regulation (EU) 2020/691(4) lays down supplementing rules regarding registered and approved aquaculture establishments keeping aquaculture animals and transporters of aquatic animals. In particular, that Regulation provides supplementary rules as regards the approval of aquaculture establishments keeping aquaculture animals which pose a significant risk for the transmission of diseases affecting aquatic animals. Therefore, certain model animal health certificates set out in this Regulation should include the relevant guarantees in order to ensure that the establishment has been approved in accordance with the rules laid down in Delegated Regulation (EU) 2020/691. In addition, this Regulation should also have regard to the definitions laid down in Delegated Regulation (EU) 2020/691.
(10) In addition, Commission Delegated Regulations (EU) 2020/692(5) and (EU) 2020/990(6) lay down rules supplementing those laid down in Regulation (EU) 2016/429. Delegated Regulation (EU) 2020/692 lays down, inter alia, relevant animal health requirements for entry into the Union of aquatic animals and certain products of animal origin from aquatic animals. Therefore, model animal health certificates set out in this Regulation should take into account relevant guarantees laid down in those Regulations. In addition, this Regulation should also have regard to the definitions laid down in Delegated Regulation (EU) 2020/692.
(11) Delegated Regulation (EU) 2020/990 lays down specific rules regarding movements within the Union of consignments of aquatic animals and products of animal origin from aquatic animals, including requirements for animal health and certification in this regard. In particular, that Regulation lays down certain rules on the contents of animal health certificates for aquatic animals and certain products of animal origin from aquatic animals. Therefore, the model animal health certificates set out in this Regulation should take into account the supplementing rules laid down in Regulations (EU) 2020/990. In addition, this Regulation should also have regard to the definitions laid down in Delegated Regulation (EU) 2020/990.
(12) Implementing Regulation (EU) 2018/1882 lays down the definition of ‘category D disease’ for which measures are needed to prevent such disease from spreading. Furthermore, that Implementing Regulation lays down that the disease prevention and control rules for the listed diseases referred to in Article 9(1) of Regulation (EU) 2016/429 shall apply to the categories of listed diseases for the listed species and groups of listed species referred to in the table set out in the Annex to that Regulation. That table lists, inter alia, vector species for diseases affecting aquatic animals. Accordingly, this should be taken into account in the model animal health certificates set out in this Regulation.
(13) Model animal health certificates for movements within the Union and the entry into the Union of consignments of aquatic animals and certain products of animal origin from aquatic animals should contain details of the consignment and specific animal health information certified by an official veterinarian. In the case of movements within the Union, model animal health certificates should also contain a part designated to record official controls carried out during such movements and at the place of destination, as well as the results of those official controls.
(14) Commission Implementing Regulation (EU) 2020/2235(7) lays down the model animal health certificates, model official certificates and model animal health/official certificates for the movement within the Union of consignments of animals and products. That Implementing Regulation provides for the compatibility of such certificates with the Trade Control and Expert System (TRACES) and it facilitates the system of certification in the Union. Therefore, the model animal health certificates set out in this Regulation for the movement within the Union of consignments of aquatic animals and certain products of animal origin from aquatic animals should be drawn up on the basis of the model animal health certificate for the movement within the Union of animals and products set out in Chapter 1 of Annex I to Implementing Regulation (EU) 2020/2235.
(15) Furthermore, in order to ensure consistency and to improve the efficiency of animal health certification, the model animal health certificate for the entry into the Union of consignments of aquatic animals set out in this Regulation should be drawn up on the basis of the model animal health certificate for the entry into the Union of animals, products of animal origin, composite products, germinal products, animal by-products, sprouts for human consumption and seeds intended for the production of sprouts for human consumption set out in Chapter 3 of Annex I to Implementing Regulation (EU) 2020/2235.
(16) Regulation (EU) 2017/625 provides that consignments of animals and goods are to be accompanied by an official certificate issued either on paper or in electronic form. Furthermore, Regulation (EU) 2016/429 provides that animal health certificates issued in electronic form, including those animal health certificates accompanying consignments entering the Union, may replace animal health certificates issued on paper. Therefore, it is appropriate to establish common requirements as regards the issuance of animal health certificates for consignments of aquatic animals and certain products of animal origin from aquatic animals, both in paper and in electronic form, in addition to the requirements laid down in Article 217 of Regulation (EU) 2016/429 and Chapter VII of Title II of Regulation (EU) 2017/625. These common requirements should be set out in this Regulation.
(17) Regulation (EU) 2017/625 provides that the Information Management System for Official Controls (IMSOC) is to allow for the production, handling and transmission of official certificates, including in electronic form. Commission Implementing Regulation (EU) 2019/1715(8) provides that the TRACES is the IMSOC component enabling the entire process of certificate production to be performed electronically, thus preventing possible fraudulent or deceptive practices in respect of the animal health or official certificates. Therefore, in order to ensure an adequate level of security of electronic means of electronic certification and taking into account the aim to harmonise the process of certification, model animal health certificates set out in this Regulation should be compatible with TRACES.
(18) Commission Regulation (EC) No 1251/2008(9) lays down specific rules for certification requirements for the placing on the market and import into Union of aquaculture animals and products thereof. However, the rules laid down in that Regulation have already been replaced by the rules laid down in Delegated Regulations (EU) 2020/990 and (EU) 2020/692, and the model certificates laid down in Regulation (EC) No 1251/2008 are to be replaced by the model animal health certificates laid down in this Regulation. Accordingly, in order to avoid a duplication of rules, Regulation (EC) No 1251/2008 should be repealed.
(19) It is appropriate to introduce a transitional period to take into account the specific situation of competent authorities in third countries that need to make the necessary arrangements to ensure compliance with this Regulation and the specific situation of shipments of consignments of aquatic animals and products of animal origin from aquatic animals accompanied by animal health certificates issued in accordance with Regulation (EC) No 1251/2008 before the date of application of this Regulation.
(20) As Regulation (EU) 2016/429 applies with effect from 21 April 2021, this Regulation should also apply from that date.
(21) The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed,
HAS ADOPTED THIS REGULATION:
Article 1U.K.Subject matter and scope
1.This Regulation lays down rules concerning the animal health certificates provided for in Regulation (EU) 2016/429 and official certification provided for in Regulation (EU) 2017/625 as regards the issuance and replacement of animal health certificates required for the entry into the Union(10) and movements within the Union of certain consignments of aquatic animals and of products of animal origin from aquatic animals.
2.It establishes model animal health certificates and a model declaration for the following:
(a)model animal health certificates for movements within the Union of consignments of certain categories of aquatic animals and certain products of animal origin from aquaculture animals (Annex I);
(b)a model animal health certificate for the entry into the Union of consignments of aquatic animals intended for certain aquaculture establishments, for release into the wild, or for other purposes excluding human consumption (Annex II);
(c)a model declaration by the master of vessel: addendum for transport of consignments of certain aquatic animals entering the Union by sea (Annex III).
Article 2U.K.Definitions
For the purposes of this Regulation, the following definitions shall apply:
‘container’ means container as defined in point (1) of Article 2 of Delegated Regulation (EU) 2020/990;
‘well-boat’ means a well-boat as defined in point (2) of Article 2 of Delegated Regulation (EU) 2020/990;
‘fishing bait’ means fishing bait as defined in point (4) of Article 2 of Delegated Regulation (EU) 2020/990;
‘national measures’ means national measures as defined in point (5) of Article 2 of Delegated Regulation (EU) 2020/990;
‘habitat’ means habitat as defined in point (6) of Article 2 of Delegated Regulation (EU) 2020/990;
‘listed third country, territory or zone thereof’ means a third country, territory, zone or compartment as defined in point (1) of Article 2 of Delegated Regulation (EU) 2020/692;
‘disease-free Member State, zone or compartment’ means a Member State, zone or compartment as defined in point (7) of Article 2 of Delegated Regulation (EU) 2020/990;
‘eradication programme’ means an eradication programme as defined in point (8) of Article 2 of Delegated Regulation (EU) 2020/990.
Article 3U.K.Completion of animal health certificates for consignments of aquatic animals and products of animal origin from aquatic animals
1.Animal health certificates for the movement within the Union of consignments of aquatic animals and consignments of products of animal origin from aquatic animals, set out in Annex I to this Regulation, shall be duly completed and signed by an official veterinarian in accordance with the explanatory notes provided for in Chapter 2 of Annex I to Implementing Regulation (EU) 2020/2235.
2.Animal health certificates for the entry into the Union of consignments of aquatic animals and consignments of products of animal origin from aquatic animals, set out in Annex II to this Regulation, shall be duly completed and signed by an official veterinarian in accordance with the explanatory notes provided for in Chapter 4 of Annex I to Implementing Regulation (EU) 2020/2235.
3.Operators responsible for consignments referred to in paragraphs 1 and 2 shall provide the competent authority the information on the description of such consignments as described in Part I of the model animal health certificates set out in Annexes I and II.
Article 4U.K.Requirements for animal health certificates for consignments of aquatic animals and products of animal origin from aquatic animals
1.The official veterinarian shall complete animal health certificates for consignments of aquatic animals and products of animal origin from aquatic animals in accordance with the following requirements:
(a)the animal health certificate must bear the signature of the official veterinarian and the official stamp; the colour of the signature and the colour of stamp, other of an than embossed or watermarked stamp, must be different to the colour of the printing;
(b)where the animal health certificate contains multiple or alternative statements, the statements which are not relevant must be crossed out, initialled and stamped by the official veterinarian, or completely removed from the animal health certificate;
(c)the animal health certificate must consist of one of the following:
a single sheet of paper;
several sheets of paper where all sheets are indivisible and constitute an integrated whole;
a sequence of pages with each page numbered so as to indicate that it is a particular page in a finite sequence;
(d)where the animal health certificate consists of a sequence of pages as referred to in point (c)(iii), of this paragraph, each page must bear the unique code referred to in Article 89(1)(a) of Regulation (EU) 2017/625, the signature of the official veterinarian and the official stamp;
(e)in the case of animal health certificates for movements of consignments within the Union, the animal health certificate must accompany the consignment until it reaches the place of destination in the Union;
(f)in the case of animal health certificates for the entry into the Union of consignments, the animal health certificate must be presented to the competent authority of the border control post of entry into the Union where the consignment is subjected to official controls;
(g)the animal health certificate must be issued before the consignment to which it relates leaves the control of the competent authority issuing the animal health certificate;
(h)in the case of animal health certificates for the entry into the Union of consignments, the animal health certificate must be drawn up in the official language, or in one of the official languages, of the Member State of the border control post of entry into the Union.
2.By way of derogation from paragraph 1(h), a Member State may consent to animal health certificates being drawn up in another official language of the Union, and accompanied, if necessary, by an authenticated translation.
3.Points (a) to (e) of paragraph 1 shall not apply to electronic certificates issued in accordance with the requirements of Article 39(1) of Implementing Regulation (EU) 2019/1715.
4.Points (b), (c) and (d) of paragraph 1 shall not apply to animal health certificates issued in paper and completed in, and printed from, TRACES.
Article 5U.K.Replacement of animal health certificates for consignments of aquatic animals and products of animal origin from aquatic animals
1.Competent authorities shall only issue replacement animal health certificates for consignments of aquatic animals and products of animal origin from aquatic animals in the case of administrative errors in the initial animal health certificate or where the initial animal health certificate has been damaged or lost.
2.In the replacement animal health certificate, the competent authority shall not modify information in the initial animal health certificate concerning the identification of the consignment, its traceability and the guarantees provided for in the initial animal health certificate for the consignment.
3.In the replacement animal health certificate, the competent authority shall:
(a)make clear reference to the unique code referred to in Article 89(1)(a) of Regulation (EU) 2017/625 and the date of issue of the initial animal health certificate, and clearly state that it replaces the initial animal health certificate;
(b)indicate a new animal health certificate number different to that of the initial animal health certificate;
(c)indicate the date when it was issued, as opposed to the date of issue of the initial animal health certificate;
(d)produce an original document issued in paper, except in the case of electronic replacement animal health certificates submitted in TRACES.
4.In the case of entry into the Union of consignments, the competent authority of the border control post of entry into the Union may refrain from requesting the operator responsible for the consignment to provide a replacement animal health certificate when information concerning the consignee, the importer, the border control post of entry into the Union or the means of transport changes after the certificate has been issued and such new information is provided by the operator responsible for the consignment.
Article 6U.K.Model animal health certificates for movements within the Union of certain categories of aquatic animals and products of animal origin from aquatic animals
The animal health certificates referred to in Article 1(2)(a) to be used for movements within the Union of consignments of certain categories of aquatic animals and certain products of animal origin from aquatic animals shall correspond to one of the following models, depending on the aquatic animals and categories of products concerned:
AQUA-INTRA-ESTAB drawn up in accordance with the model set out in Chapter 1 of Annex I, for aquatic animals intended for aquaculture establishments;
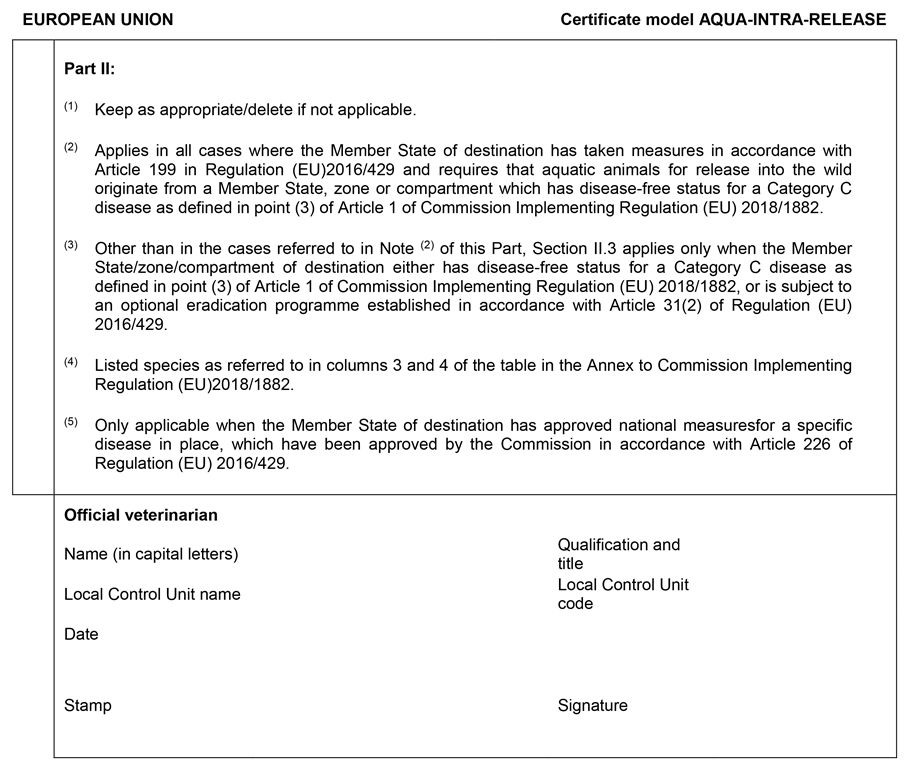
AQUA-INTRA-RELEASE drawn up in accordance with the model set out in Chapter 2 of Annex I, for aquatic animals intended for release into the wild;
AQUA-INTRA-HC drawn up in accordance with the model set out in Chapter 3 of Annex I, for aquatic animals intended for human consumption;
AQUA-INTRA-RESTRICT drawn up in accordance with the model set out in Chapter 4 of Annex I, for aquatic animals subjected to movement restrictions or emergency measures regarding listed or emerging diseases;
AQUA-INTRA-BAIT drawn up in accordance with the model set out in Chapter 5 of Annex I, for aquatic animals intended for use as live fishing bait;
PAO-AQUA-INTRA-PROCESS drawn up in accordance with the model set out in Chapter 6 of Annex I, for products of animal origin from aquaculture animals other than live aquaculture animals, intended for further processing;
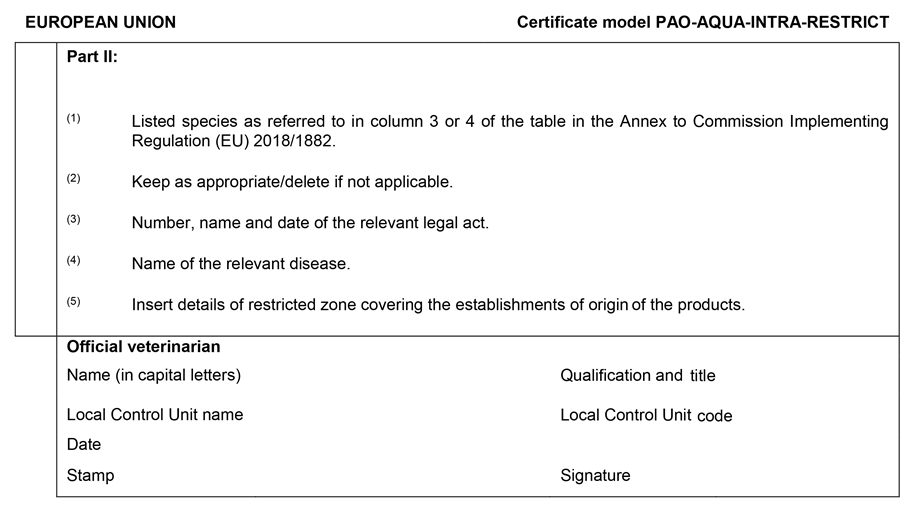
PAO-AQUA-INTRA-RESTRICT drawn up in accordance with the model set out in Chapter 7 of Annex I, for products of animal origin from aquaculture animals other than live aquaculture animals subjected to movement restrictions or emergency measures regarding listed diseases or emerging diseases.
Article 7U.K.Model animal health certificate for the entry into the Union of aquatic animals intended for aquaculture establishments, for release into the wild, or for other purposes, excluding direct human consumption
The animal health certificate referred to in Article 1(2)(b) to be used for the entry into the Union of consignments of aquatic animals intended for aquaculture establishments, for release into the wild, or for other purposes, excluding direct human consumption shall correspond to the model AQUA-ENTRY-ESTAB/RELEASE/OTHER drawn up in accordance with the model set out in Annex II.
Article 8U.K.Model declaration for the transport of certain aquatic animals entering the Union by sea
The declaration referred to in Article 1(2)(c) to be used for the transport of certain aquatic animals entering the Union by sea shall correspond to the model addendum AT-AQUA-SEA drawn up in accordance with the model set out in Annex III.
That addendum shall be completed by the master of the vessel and attached to the relevant animal health certificate.
Article 9U.K.Repeal of Commission Regulation (EC) No 1251/2008
1.Regulation (EC) No 1251/2008 is repealed with effect from 21 April 2021.
2.References to the Regulation (EC) No 1251/2008 shall be construed as references to this Regulation and read in accordance with the correlation table in Annex IV.
Article 10U.K.Transitional provisions
Consignments of aquatic animals and products of animal origin from aquatic animals accompanied by the appropriate animal health certificates issued in accordance with Regulation (EC) No 1251/2008, before the date of application of this Regulation, shall be accepted for the entry into the Union until 20 October 2021 provided that the animal health certificate was signed by an official inspector before 21 August 2021.
Article 11U.K.Entry into force and application
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 21 April 2021.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 16 December 2020.
For the Commission
The President
Ursula VON DER LEYEN
ANNEX IU.K.
Annex I contains the following models animal health certificates:
MODEL
| AQUA-INTRA-ESTAB | Chapter 1: Model animal health certificate for the movement within the Union of aquatic animals intended for aquaculture establishments |
| AQUA-INTRA-RELEASE | Chapter 2: Model animal health certificate for the movement within the Union of aquatic animals intended for release into the wild |
| AQUA-INTRA-HC | Chapter 3: Model animal health certificate for the movement within the Union of aquatic animals intended for human consumption |
| AQUA-INTRA-RESTRICT | Chapter 4: Model animal health certificate for the movement within the Union of aquatic animals subjected to movement restrictions or emergency measures regarding listed or emerging diseases |
| AQUA-INTRA-BAIT | Chapter 5: Model animal health certificate for the movement within the Union of aquatic animals intended for use as live fishing bait |
| PAO-AQUA-INTRA-PROCESS | Chapter 6: Model animal health certificate for the movement within the Union of products of animal origin from aquaculture animals other than live aquaculture animals, intended for further processing |
| PAO-AQUA-INTRA-RESTRICT | Chapter 7: Model animal health certificate for the movement within the Union of products of animal origin from aquaculture animals other than live aquaculture animals subjected to movement restrictions or emergency measures regarding listed or emerging diseases |
CHAPTER 1U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF AQUATIC ANIMALS INTENDED FOR AQUACULTURE ESTABLISHMENTS (MODEL ‘AQUA-INTRA-ESTAB’)
CHAPTER 2U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF AQUATIC ANIMALS INTENDED FOR RELEASE INTO THE WILD (MODEL ‘AQUA-INTRA-RELEASE’)
CHAPTER 3U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF AQUATIC ANIMALS INTENDED FOR HUMAN CONSUMPTION (model ‘AQUA-INTRA-HC’’)
CHAPTER 4U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF AQUATIC ANIMALS SUBJECTED TO MOVEMENT RESTRICTIONS OR EMERGENCY MEASURES REGARDING LISTED OR EMERGING DISEASES (MODEL ‘AQUA-INTRA-RESTRICT’)
CHAPTER 5U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF AQUATIC ANIMALS INTENDED FOR USE AS LIVE FISHING BAIT (MODEL ‘AQUA-INTRA-BAIT’)
CHAPTER 6U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF PRODUCTS OF ANIMAL ORIGIN FROM AQUACULTURE ANIMALS OTHER THAN LIVE AQUACULTURE ANIMALS, INTENDED FOR FURTHER PROCESSING (MODEL ‘PAO-AQUA-INTRA-PROCESS’)
CHAPTER 7U.K. MODEL ANIMAL HEALTH CERTIFICATE FOR THE MOVEMENT WITHIN THE UNION OF PRODUCTS OF ANIMAL ORIGIN FROM AQUACULTURE ANIMALS OTHER THAN LIVE AQUACULTURE ANIMALS SUBJECTED TO MOVEMENT RESTRICTIONS OR EMERGENCY MEASURES REGARDING LISTED OR EMERGING DISEASES (MODEL ‘PAO-AQUA-INTRA-RESTRICT’)
ANNEX IIU.K.
Annex II contains the following model animal health certificate:
MODEL
| AQUA-ENTRY-ESTAB/RELEASE/OTHER | Model animal health certificate for the entry into the Union of aquatic animals intended for certain aquaculture establishments, for release into the wild, or for other purposes, excluding direct human consumption |
MODEL ANIMAL HEALTH CERTIFICATE FOR THE ENTRY INTO THE UNION OF AQUATIC ANIMALS INTENDED FOR CERTAIN AQUACULTURE ESTABLISHMENTS, FOR RELEASE INTO THE WILD OR FOR OTHER PURPOSES, EXCLUDING DIRECT HUMAN CONSUMPTION (MODEL ‘AQUA-ENTRY-ESTAB/RELEASE/OTHER’) U.K.
ANNEX IIIU.K.
Annex III contains the following model declaration:
MODEL
| AT-AQUA-SEA | Model declaration by the master of the vessel: Addendum for transport of certain aquatic animals entering the Union by sea |
MODEL DECLARATION BY THE MASTER OF THE VESSEL: ADDENDUM FOR TRANSPORT OF CERTAIN AQUATIC ANIMALS ENTERING THE UNION BY SEA (MODEL ‘AT-AQUA-SEA’) U.K.
To be completed and attached to the relevant animal health certificate for entry into the Union where transported by vessel, even for part of the journey, other than fishing vessels landing wild aquatic animals and products of animal origin from wild aquatic animals for direct human consumption referred in Article 1(6) of Commission Delegated Regulation (EU) 2020/692
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland / Northern Ireland in conjunction with Annex 2 to that Protocol, references to European Union in this certificate include the United Kingdom in respect of Northern Ireland.
ANNEX IVU.K.
Correlation table referred to in Article 9(2)
| Commission Regulation (EU) No 1251/2008 | This Regulation |
|---|---|
| Article 1 | Article 1 |
| Article 2 | Article 2 |
| Article 3 to 17 | — |
| Annex I | — |
| Annex II Parts A and Part B | Annex I |
| Annex II Part C | — |
| Annex III | — |
| Annex IV Parts A, B and C | Annex II |
| Annex IV Part D | Annex III |
| Annex V | Article 3 |
Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018 on the application of certain disease prevention and control rules to categories of listed diseases and establishing a list of species and groups of species posing a considerable risk for the spread of those listed diseases (OJ L 308, 4.12.2018, p. 21).
Commission Delegated Regulation (EU) 2020/691 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of Council as regards rules for aquaculture establishments and transporters of aquatic animals (OJ L 174, 3.6.2020, p. 345).
Commission Delegated Regulation (EU) 2020/692 of 30 January 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for entry into the Union, and the movement and handling after entry of consignments of certain animals, germinal products and products of animal origin (OJ L 174, 3.6.2020, p. 379).
Commission Delegated Regulation (EU) 2020/990 of 28 April 2020 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council, as regards animal health and certification requirements for movements within the Union of aquatic animals and products of animal origin from aquatic animals (OJ L 221, 10.7.2020, p. 42).
Commission Implementing Regulation (EU) 2020/2235 of 16 December 2020 laying down rules for the application of Regulations (EU) 2016/429 and (EU) 2017/625 of the European Parliament and of the Council as regards model animal health certificates, model official certificates and model animal health/official certificates, for the entry into the Union and movements within the Union of consignments of certain categories of animals and goods, official certification regarding such certificates and repealing Regulation (EC) No 599/2004, Implementing Regulations (EU) No 636/2014 and (EU) 2019/628, Directive 98/68/EC and Decisions 2000/572/EC, 2003/779/EC and 2007/240/EC (OJ L 442, 30.12.2020, p. 1).
Commission Implementing Regulation (EU) 2019/1715 of 30 September 2019 laying down rules for the functioning of the information management system for official controls and its system components (‘the IMSOC Regulation’) (OJ L 261, 14.10.2019, p. 37).
Commission Regulation (EC) No 1251/2008 of 12 December 2008 implementing Council Directive 2006/88/EC as regards conditions and certification requirements for the placing on the market and the import into the Community of aquaculture animals and products thereof and laying down a list of vector species (OJ L 337, 16.12.2008, p. 41).
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Regulation references to ‘Union’ include the United Kingdom in respect of Northern Ireland.
Options/Help
Print Options
PrintThe Whole Regulation
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As adopted by EU): The original version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the EU Official Journal
- lists of changes made by and/or affecting this legislation item
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different versions taken from EUR-Lex before exit day and during the implementation period as well as any subsequent versions created after the implementation period as a result of changes made by UK legislation.
The dates for the EU versions are taken from the document dates on EUR-Lex and may not always coincide with when the changes came into force for the document.
For any versions created after the implementation period as a result of changes made by UK legislation the date will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. For further information see our guide to revised legislation on Understanding Legislation.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as adopted version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources