- Latest available (Revised)
- Point in Time (01/04/2023)
- Original (As made)
The Blood Safety and Quality Regulations 2005
You are here:
- UK Statutory Instruments
- 2005 No. 50
- Schedules only
- Show Geographical Extent(e.g. England, Wales, Scotland and Northern Ireland)
- Show Timeline of Changes
More Resources
Changes over time for: The Blood Safety and Quality Regulations 2005 (Schedules only)
Alternative versions:
- 08/02/2005- Amendment
- 31/08/2006- Amendment
- 18/07/2016- Amendment
- 31/12/2020- Amendment
- 01/04/2023
Point in time
Status:
Point in time view as at 01/04/2023.
Changes to legislation:
There are currently no known outstanding effects for the The Blood Safety and Quality Regulations 2005.![]()
Changes to Legislation
Revised legislation carried on this site may not be fully up to date. At the current time any known changes or effects made by subsequent legislation have been applied to the text of the legislation you are viewing by the editorial team. Please see ‘Frequently Asked Questions’ for details regarding the timescales for which new effects are identified and recorded on this site.
Regulations 4(4)(b), 7(1)(c), 2(a), (b) and (d), and (3)(b) and (c), 9(1)(c) and (h) and 13.
SCHEDULEU.K.
PART 1U.K.
DefinitionsU.K.
The following definitions apply for the purposes of this Schedule.
1. “Autologous donation” means blood and blood components collected from an individual and intended solely for subsequent autologous transfusion or other human application to that same individual.U.K.
2. “Allogeneic donation” means blood and blood components collected from an individual and intended for transfusion to another individual, for use in medical devices or as starting material or raw material for manufacturing into medicinal products.U.K.
3. “Whole blood” means a single blood donation.U.K.
4. “Cryopreservation” means prolongation of the storage life of blood components by freezing.U.K.
5. “Plasma” means the liquid portion of the blood in which the cells are suspended. Plasma may be separated from the cellular portion of a whole blood collection for therapeutic use as fresh-frozen plasma or further processed to cryoprecipitate and cryoprecipitate-depleted plasma for transfusion. It may be used for the manufacture of medicinal products derived from human blood and human plasma or used in the preparation of pooled platelets, or pooled, leucocyte-depleted platelets. It may also be used for re-suspension of red cell preparations for exchange transfusion or perinatal transfusion.U.K.
6. “Cryoprecipitate” means a plasma component prepared from plasma, fresh-frozen, by freeze-thaw precipitation of proteins and subsequent concentration and re-suspension of the precipitated proteins in a small volume of the plasma.U.K.
7. “Washed” means a process of removing plasma or storage medium from cellular products by centrifugation, decanting of the supernatant liquid from the cells and addition of an isotonic suspension fluid, which in turn is generally removed and replaced following further centrifugation of the suspension. The centrifugation, decanting, replacing process may be repeated several times.U.K.
8. “Red cells” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed.U.K.
9. “Red cells, buffy coat removed” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed. The buffy coat, containing a large proportion of the platelets and leucocytes in the donated unit, is removed.U.K.
10. “Red cells, leucocyte-depleted” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed, and from which leucocytes are removed.U.K.
11. “Red cells in additive solution” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed. A nutrient or preservative solution is added.U.K.
12. “Additive solution” means a solution specifically formulated to maintain beneficial properties of cellular components during storage.U.K.
13. “Red cells, buffy coat removed, in additive solution” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed. The buffy coat, containing a large proportion of the platelets and leucocytes in the donated unit, is removed. A nutrient or preservative solution is added.U.K.
14. “Buffy coat” means a blood component prepared by centrifugation of a unit of whole blood, and which contains a considerable proportion of the leucocytes and platelets.U.K.
15. “Red cells, leucocyte-depleted, in additive solution” means the red cells from a single whole blood donation, with a large proportion of the plasma from the donation removed, and from which leucocytes are removed. A nutrient or preservative solution is added.U.K.
16. “Red cells, apheresis” means the red cells from an apheresis red cell donation.U.K.
17. “Apheresis” means a method of obtaining one or more blood components by machine processing of whole blood in which the residual components of the blood are returned to the donor during or at the end of the process.U.K.
18. “Platelets, apheresis” means a concentrated suspension of blood platelets obtained by apheresis.U.K.
19. “Platelets, apheresis, leucocyte-depleted” means a concentrated suspension of blood platelets, obtained by apheresis, and from which leucocytes are removed.U.K.
20. “Platelets, recovered, pooled” means a concentrated suspension of blood platelets, obtained by processing of whole blood units and pooling the platelets from the units during or after separation.U.K.
21. “Platelets, recovered, pooled, leucocyte-depleted” means a concentrated suspension of blood platelets, obtained by processing of whole blood units and pooling the platelets from the units during or after separation, and from which leucocytes are removed.U.K.
22. “Platelets, recovered, single unit” means a concentrated suspension of blood platelets, obtained by processing of a single unit of whole blood.U.K.
23. “Platelets, recovered, single unit, leucocyte-depleted” means a concentrated suspension of blood platelets, obtained by processing of a single whole blood unit from which leucocytes are removed.U.K.
24. “Plasma, fresh-frozen” means the supernatant plasma separated from a whole blood donation or plasma collected by apheresis, frozen and stored.U.K.
25. “Plasma, cryoprecipitate-depleted for transfusion” means a plasma component prepared from a unit of plasma, fresh-frozen. It comprises the residual portion after the cryoprecipitate has been removed.U.K.
26. “Granulocytes, apheresis” means a concentrated suspension of granulocytes obtained by apheresis.U.K.
27. “Statistical process control” means a method of quality control of a product or a process that relies on a system of analysis of an adequate sample size without the need to measure every product of the process.U.K.
PART 2U.K.INFORMATION REQUIREMENTS FOR DONORS
Part A – Information to be provided to prospective donors of blood or blood componentsU.K.
1. Accurate educational materials, which are written in terms which can be understood by members of the general public, about the essential nature of blood, the blood donation procedure, the components derived from whole blood and apheresis donations, and the important benefits to patients.U.K.
2. For both allogeneic and autologous donations, the reasons for requiring an examination and health and medical history, and the testing of donations, and the significance of “informed consent”.U.K.
3. For allogeneic donations, the criteria for self-deferral, and temporary and permanent deferral, and the reasons why individuals are not to donate blood or blood components if there could be a risk for the recipient.U.K.
4. For autologous donations, the possibility of deferral and the reasons why the donation procedure would not take place in the presence of a health risk to the individual whether as donor or recipient of the autologous blood or blood components.U.K.
5. Information on the protection of personal data, including confirmation that there will be no disclosure of the identity of the donor, of information concerning the donor's health, and of the results of the tests performed, other than in accordance with the requirements of these Regulations.U.K.
6. The reasons why individuals are not to make donations which may be detrimental to their health.U.K.
7. Specific information on the nature of the procedures involved either in the allogeneic or autologous donation process and their respective associated risks. For autologous donations, the possibility that the autologous blood and blood components may not suffice for the intended transfusion requirements.U.K.
8. Information on the option for donors to change their mind about donating prior to proceeding further, or the possibility of withdrawing or self-deferring at any time during the donation process, without any undue embarrassment or discomfort.U.K.
9. The reasons why it is important that donors inform the blood establishment of any subsequent event that may render any prior donation unsuitable for transfusion.U.K.
10. Information on the responsibility of the blood establishment to inform the donor, through an appropriate mechanism, if test results show any abnormality of significance to the donor's health.U.K.
11. Information as to why unused autologous blood and blood components will be discarded and not transfused to other patients.U.K.
12. Information that test results detecting markers for viruses, such as HIV, HBV, HCV or other relevant blood transmissible microbiologic agents, will result in donor deferral and destruction of the collected unit.U.K.
13. Information on the opportunity for donors to ask questions at any time.U.K.
Part B – Information to be obtained from donors by blood establishments at every donationU.K.
Identification of the donorU.K.
14. Personal data uniquely, and without any risk of mistaken identity, distinguishing the donor, as well as contact details.
Health and medical history of the donorU.K.
15. Health and medical history, provided on a questionnaire and through a personal interview performed by a qualified health professional, that includes relevant factors that may assist in identifying and screening out persons whose donation could present a health risk to others, such as the possibility of transmitting diseases, or health risks to themselves.
Signature of the donorU.K.
16. Signature of the donor, on the donor questionnaire, countersigned by the qualified health professional responsible for obtaining the health history confirming that the donor has—
(a)read and understood the educational materials provided;
(b)had an opportunity to ask questions;
(c)been provided with satisfactory responses to any questions asked;
(d)given informed consent to proceed with the donation process;
(e)been informed, in the case of autologous donations, that the donated blood and blood components may not be sufficient for the intended transfusion requirements; and
(f)acknowledged that all the information provided by the donor is true to the best of his knowledge.
PART 3U.K.ELIGIBILITY CRITERIA FOR DONORS OF WHOLE BLOOD AND BLOOD COMPONENTS
Acceptance criteria for donors of whole blood and blood componentsU.K.
1.
Under exceptional circumstances, individual donations from donors who do not comply with following criteria may be authorised by a qualified healthcare professional in the blood establishment. [F1All such cases must be clearly documented and subject to—
(a)in relation to Great Britain, the requirements in regulation 7;
(b)in relation to Northern Ireland, the quality management provisions in Articles 11, 12, and 13 of Directive 2002/98/EC.]
The criteria in this paragraph do not apply to autologous donations.
Textual Amendments
F1Words in Sch. 1 Pt. 3 para. 1 substituted (31.12.2020) by The Blood Safety and Quality (Amendment) (EU Exit) Regulations 2019 (S.I. 2019/4), reg. 14 (as substituted by S.I. 2020/1304, regs. 1, 14); 2020 c. 1, Sch. 5 para. 1(1)
1.1. Age and body weight of donorsU.K.
| Age | 18 to 65 years | |
|---|---|---|
| 17 years | Where, in the opinion of a qualified health professional, the donor has sufficient knowledge and understanding of what is involved in the process of blood donation to give their informed consent, or otherwise with the written consent of a person with parental responsibility. | |
| First time donors over 60 years | — at the discretion of the doctor in the blood establishment | |
| Over 65 years | — with permission of the doctor in the blood establishment, given annually | |
| Body weight | ≥ 50 kg for donors either of whole blood or apheresis blood components | |
1.2. Haemoglobin levels in donor's bloodU.K.
| Haemoglobin | For females ≥ 125 g/l | For males ≥ 135 g/l | Applicable to allogeneic donors of whole blood and cellular components |
1.3. Protein levels in donor's bloodU.K.
| Protein | ≥ 60 g/l | The protein analysis for apheresis plasma donations must be performed at least annually |
1.4. Platelet levels in donor's bloodU.K.
| Platelets | Platelet number greater than or equal to 150 x 109 /1 | Level required for apheresis platelet donors |
DEFERRAL CRITERIA FOR DONORS OF WHOLE BLOOD AND BLOOD COMPONENTS
Deferral criteria for donors of whole blood and blood componentsU.K.
2.1. Permanent deferral criteria for donors of allogeneic donations
| Cardiovascular disease | Prospective donors with active or past serious cardiovascular disease, except congenital abnormalities with complete cure |
| Central nervous system disease | A history of serious CNS disease |
| Abnormal bleeding tendency | Prospective donors who give a history of a coagulopathy |
| Repeated episodes of syncope, or a history of convulsions | Other than childhood convulsions or where at least three years have elapsed since the date the donor last took anticonvulsant medication without any recurrence of convulsions |
| Gastrointestinal. Genitourinary, haematological, immunological, metabolic, renal, or respiratory system diseases | Prospective donors with serious active, chronic, or relapsing disease |
| Diabetes | If being treated with insulin |
| Infectious diseases | Hepatitis B, except for HBsAg-negative persons who are demonstrated to be immune |
| Hepatitis C | |
| HIV – 1 and 2 | |
| HTLV I/II | |
| Babesiosis (*) | |
| Kala Azar (visceral leishmaniasis) (*) | |
| Trypanosomiasis cruzi (Chagas' disease) (*) | |
| Malignant diseases | Except in situ cancer with complete recovery |
| Transmissible spongiform encephalopathies (TSEs) (e.g. Creutzfeldt Jakob Disease, variant Creutzfeldt Jakob Disease) | Persons who have a family history which places them at risk of developing a TSE, or persons who have received a corneal or dura mater graft, or who have been treated in the past with medicines made from human pituitary glands. For variant Creutzfeldt Jacob disease, further precautionary measures may be recommended. |
| Intravenous (IV) or intramuscular (IM) drug use | Any history of non-prescribed IV or IM drug use, including body-building steroids or hormones |
| Xenotransplant recipients | |
| Sexual behaviour | Persons whose sexual behaviour puts them at high risk of acquiring severe infectious diseases that can be transmitted by blood |
2.2. Temporary deferral criteria for donors of allogeneic donationsU.K.
2.2.1. InfectionsU.K.
Duration of deferral periodU.K.
After an infectious illness, prospective donors shall be deferred for at least two weeks following the date of full clinical recovery.
However, the following deferral periods shall apply for the infections listed in the table:
| Brucellosis (*) | 2 years following the date of full recovery |
| Osteomyelitis | 2 years after confirmed cured |
| Q fever (*) | 2 years following the date of confirmed cure |
| Syphilis (*) | 1 year following the date of confirmed cure |
| Toxoplasmosis (*) | 6 months following the date of clinical recovery |
| Tuberculosis | 2 years following the date of confirmed cure |
| Rheumatic fever | 2 years following the date of cessation of symptoms, unless evidence of chronic heart disease |
Fever >38°C | 2 weeks following the date of cessation of symptoms |
| Flu-like illness | 2 weeks after cessation of symptoms |
Malaria (*) | |
| — individuals who have lived in a malarial area within the first five years of life | 3 years following return from last visit to any endemic area, provided person remains symptom free; may be reduced to 4 months if an immunologic or molecular genomic test is negative at each donation. |
| — individuals with a history of malaria | 3 years following cessation of treatment and absence of symptoms. Donations may be accepted thereafter only if an immunologic or molecular genomic test is negative |
| — asymptomic visitors to endemic areas | 6 months after leaving the endemic area unless an immunologic or molecular genomic test is negative |
| — individuals with a history of undiagnosed febrile illness during or within six months of a visit to an endemic area | 3 years following resolution of symptoms; may be reduced to 4 months if an immunologic or molecular test is negative |
| [F2West Nile Virus (WNV) (*) | 28 days after leaving a risk area of locally acquired West Nile Virus unless an individual Nucleic Acid Test (NAT) is negative] |
Textual Amendments
F2Words in Sch. Pt. 3 para. 2.2.1 Table substituted (18.7.2016) by The Blood Safety and Quality (Amendment) Regulations 2016 (S.I. 2016/604), regs. 1, 2(2)
2.2.2. Exposure to risk of acquiring a transfusion-transmissible infectionU.K.
— Endoscopic examination using flexible instruments, — mocusal splash with blood or needlestick injury, — transfusion of blood components, — tissue or cell transplant of human origin, — major surgery, — tattoo or body piercing, — acupuncture unless performed by a qualified practitioner and with sterile single-use needles, — persons at risk due to close household contact with persons with hepatitis B. | Defer 6 months, or 4 months provided a NAT test for hepatitis C is negative |
| Persons whose behaviour or activity places them at risk of acquiring infectious diseases that may be transmitted by blood. | Defer after cessation of risk behaviour for a period determined by the disease in question, and by the availability of appropriate tests. |
2.2.3. VaccinationU.K.
| Attenuated viruses or bacteria | 4 weeks |
| Inactivated/killed viruses, bacteria or rickettsiae | No deferral if well |
| Toxoids | No deferral if well |
| Hepatitis A or hepatitis B vaccines | No deferral if well and if no exposure |
| Rabies | No deferral if well and if no exposure If vaccination is given following exposure defer for one year |
| Tick-borne encephalitis vaccines | No deferral if well and if no exposure |
2.2.4. Other temporary deferralsU.K.
| Pregnancy | 6 months after delivery or termination, except in exceptional circumstances and at the discretion of a physician |
| Minor surgery | 1 week |
| Dental treatment | Minor treatment by dentist or dental hygienist – defer until next day (NB: Tooth extraction, root-filling and similar treatment is considered as minor surgery) |
| Medication | Based on the nature of the prescribed medicine, its mode of action an the disease being treated |
2.3. Deferral for particular epidemiological situationsU.K.
| Particular epidemiological situations (e.g. disease outbreaks) | Deferral consistent with the epidemiological situation |
2.4. Deferral criteria for donors of autologous donationsU.K.
| Serious cardiac disease | Depending on the clinical setting of the blood collection |
| Active bacterial infection |
PART 4U.K.STORAGE, TRANSPORT AND DISTRIBUTION CONDITIONS FOR BLOOD AND BLOOD COMPONENTS
1. STORAGEU.K.
1.1. Liquid storageU.K.
| Component | Temperature of storage | Maximum storage time |
|---|---|---|
| Red cell preparations and whole blood (if used for transfusion as whole blood) | +2 to +6°C | 28 to 49 days according to the processes used for collection, processing and storage |
| Platelet preparations | +20 to +24°C | 5 days, may be stored for 7 days in conjunction with detection or reduction of bacterial contamination |
| Granulocytes | +20 to +24°C | 24 hours |
1.2. CryopreservationU.K.
| Component | Storage conditions and duration |
|---|---|
| Red blood cells | Up to 30 years according to processes used for collection, processing and storage |
| Platelets | Up to 24 months according to processes used for collection, processing and storage |
| Plasma and cryoprecipitate | Up to 36 months according to processes used for collection, processing and storage |
| Cryopreserved red blood cells and platelets must be formulated in a suitable medium after thawing. The allowable storage period after thawing to depend on the method used. | |
TRANSPORT AND DISTRIBUTIONU.K.
2. Transport and distribution of blood and blood components at all stages of the transfusion chain must be under conditions that maintain the integrity of the product.
ADDITIONAL REQUIREMENTS FOR AUTOLOGOUS DONATIONSU.K.
3.
3.1. Autologous blood and blood components must be clearly identified as such and stored, transported and distributed separately from allogeneic blood and blood components.U.K.
3.2. Autologous blood and blood components must be labelled as required by regulation 8, and, in addition, the label must include the identification of the donor and the warning “FOR AUTOLOGOUS TRANSFUSION ONLY”.U.K.
PART 5U.K.QUALITY AND SAFETY REQUIREMENTS FOR BLOOD AND BLOOD COMPONENTS
1. THE BLOOD COMPONENTSU.K.
| 1. Red cell preparations | The components listed in points 1.1 to 1.8 may be further processed within blood establishments and must be labelled accordingly |
| 1.1 | Red cells |
| 1.2 | Red cells, buffy coat removed |
| 1.3 | Red cells, leucocyte-depleted |
| 1.4 | Red cells, in additive solution |
| 1.5 | Red cells, buffy coat removed, in additive solution |
| 1.6 | Red cells, leucocyte-depleted, in additive solution |
| 1.7 | Red cells, apheresis |
| 1.8 | Whole blood |
| 2. Platelet preparations | The components listed in points 2.1 to 2.6 may be further processed within blood establishments and must be labelled accordingly |
| 2.1 | Platelets, apheresis |
| 2.2 | Platelets, apheresis, leucocyte-depleted |
| 2.3 | Platelets, recovered, pooled |
| 2.4 | Platelets, recovered, pooled, leucocyte-depleted |
| 2.5 | Platelets, recovered, single unit |
| 2.6 | Platelets, recovered, single unit, leucocyte-depleted |
| 3. Plasma preparations | The components listed in 3.1 to 3.3 may be further processed within blood establishments and must be labelled accordingly |
| 3.1 | Fresh-frozen plasma |
| 3.2 | Fresh-frozen plasma, cryoprecipitate-depleted |
| 3.3 | Cryoprecipitate |
| 4. | Granulocytes, apheresis |
2. QUALITY CONTROL REQUIREMENTS FOR BLOOD AND BLOOD COMPONENTSU.K.
2.1. Blood and blood components must comply with the following technical quality measurements and meet the acceptable results.U.K.
2.2. Appropriate bacteriological control of the collection and manufacturing process must be performed.U.K.
2.3. For autologous donations, the measures marked with an asterisk (*) are recommendations only.U.K.
| Component | Quality measures required | Acceptable results for quality measures |
|---|---|---|
| The required frequency of sampling for all measurements shall be determined using statistical process control | ||
| Red cells | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 45g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at end of the shelf life | |
| Red cells, buffy coat removed | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 43 g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Red cells, leucocyte-depleted | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 40g per unit | |
| Leucocyte content | Less than 1 x 106 per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Red cells, in additive solution | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 45g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at end of the shelf life | |
| Red cells, buffy coat removed, in additive solution | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 43g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Red cells, leucocyte-depleted, in additive solution | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 40g per unit | |
| Leucocyte content | Less than 1 x 106 per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Red cells, apheresis | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis |
| Haemoglobin (*) | Not less than 40g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Whole blood | Volume | Valid for storage characteristics to maintain product within specifications for haemoglobin and haemolysis 450ml +/- 50ml For paediatric autologous whole blood collections – not to exceed 10.5ml per kg body weight |
| Haemoglobin (*) | Not less than 45g per unit | |
| Haemolysis | Less than 0.8% of red cell mass at the end of the shelf life | |
| Platelets, apheresis | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per single donation are permitted within the limits that comply with validated preparation and preservation conditions | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Platelets, apheresis, leucocyte-depleted | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per single donation are permitted within the limits that comply with validated preparation and preservation conditions | |
| Leucocyte content | Less than 1 x 106 per unit | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Platelets, recovered, pooled | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per pool are permitted within limits that comply with validated preparation and preservation conditions | |
| Leucocyte content | Less than 0.2 x 109 per single unit (platelet-rich plasma method) Less than 0.05 x 109 per single unit (buffy coat method) | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Platelets, recovered, pooled, leucocyte-depleted | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per pool are permitted within limits that comply with validated preparation and preservation conditions | |
| Leucocyte content | Less than 1 x 106 per pool | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Platelets, recovered, single unit | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per single unit are permitted within limits that comply with validated preparation and preservation conditions | |
| Leucocyte content | Less than 0.2 x 109 per single unit (platelet-rich plasma method) Less than 0.05 x 109 per single unit (buffy coat method) | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Platelets, recovered, single unit, leucocyte-depleted | Volume | Valid for storage characteristics to maintain product within specifications for pH |
| Platelet content | Variations in platelet content per single unit are permitted within limits that comply with validated preparation and preservation conditions | |
| Leucocyte content | Less than 1 x 106 per unit | |
| pH | [F3Minimum 6.4 corrected for 22°C, at the end of the shelf life] | |
| Plasma, fresh-frozen | Volume | Stated volume +/- 10% |
| Factor VIIIc(*) | Average (after freezing and thawing): 70% or more of the value of the freshly collected plasma unit | |
| Total protein | Not less than 50g/l | |
| Residual cellular content(*) | Red cells: less than 6.0 x 109/l Leucocytes: less than 0.1 x 109/l Platelets: less than 50 x 109/l | |
| Plasma, fresh-frozen, cryoprecipitate-depleted | Volume | Stated volume +/-10% |
| Residual cellular content(*) | Red cells: less than 6.0 x 109/l Leucocytes: less than 0.1 x 109/l Platelets: less than 50 x 109/l | |
| Cryoprecipitate | Fribrinogen content(*) | Greater than or equal to 140mg per unit |
| Fractor VIIIc content (*) | Greater than or equal to 70 international units per unit | |
| Granulocytes, apheresis | Volume | Less than 500ml |
| Granulocyte content | Greater than 1 x 1010 granulocytes per unit |
Textual Amendments
F3Words in Sch. Pt. 5 para. 2 Table substituted (18.7.2016) by The Blood Safety and Quality (Amendment) Regulations 2016 (S.I. 2016/604), regs. 1, 2(3)
[F4PART 6U.K.RECORD OF DATA ON TRACEABILITY
Textual Amendments
F4Sch. Pts. 6-8 inserted (31.8.2006) by The Blood Safety and Quality (Amendment) Regulations 2006 (S.I. 2006/2013), regs. 1(1), 15
A. BY BLOOD ESTABLISHMENTSU.K.
1. Blood establishment identificationU.K.
2. Blood donor identificationU.K.
3. Blood unit identificationU.K.
4. Individual blood component identificationU.K.
5. Date of collection (year/month/day)U.K.
6. Facilities to which blood units or blood components are distributed, or subsequent disposition.U.K.
B. BY FACILITIESU.K.
1. Blood component supplier identificationU.K.
2. Issued blood component identificationU.K.
3. Transfused recipient identificationU.K.
4. For blood units not transfused, confirmation of subsequent dispositionU.K.
5. Date of transfusion or disposition (year/month/day)U.K.
6. Lot number of the component, if relevant.U.K.
PART 7U.K.NOTIFICATION OF SERIOUS ADVERSE REACTIONS
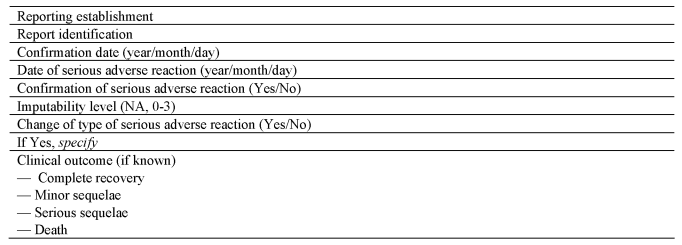
SECTION AU.K.Rapid notification format for suspected serious adverse reactions
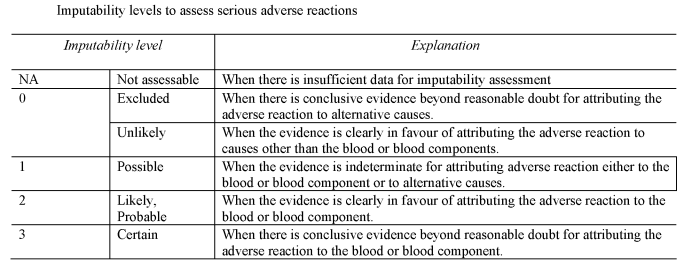
SECTION BU.K.Serious adverse reactions – imputability levels
SECTION CU.K.Confirmation format for serious adverse reactions
SECTION DU.K.Annual notification format for serious adverse reactions
PART 8U.K.NOTIFICATION OF SERIOUS ADVERSE EVENTS
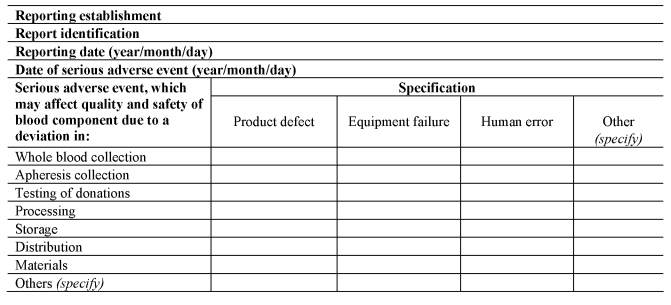
SECTION AU.K.Rapid Notification Format for Serious Adverse Events
SECTION BU.K.Confirmation Format for Serious Adverse Events
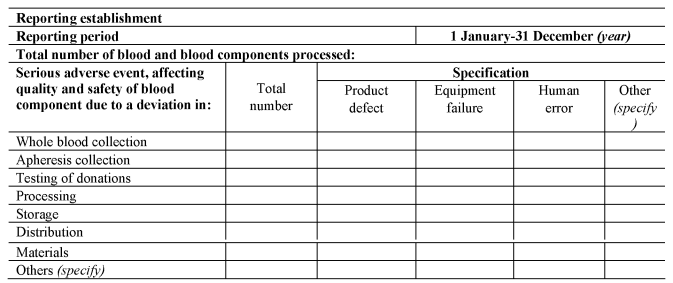
SECTION CU.K.Annual Notification Format for Serious Adverse Events]
Options/Help
Print Options
PrintThe Whole Instrument
PrintThe Schedules only
Legislation is available in different versions:
Latest Available (revised):The latest available updated version of the legislation incorporating changes made by subsequent legislation and applied by our editorial team. Changes we have not yet applied to the text, can be found in the ‘Changes to Legislation’ area.
Original (As Enacted or Made): The original version of the legislation as it stood when it was enacted or made. No changes have been applied to the text.
Point in Time: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
See additional information alongside the content
Geographical Extent: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Show Timeline of Changes: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Explanatory Memorandum
Explanatory Memorandum sets out a brief statement of the purpose of a Statutory Instrument and provides information about its policy objective and policy implications. They aim to make the Statutory Instrument accessible to readers who are not legally qualified and accompany any Statutory Instrument or Draft Statutory Instrument laid before Parliament from June 2004 onwards.
More Resources
Access essential accompanying documents and information for this legislation item from this tab. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as enacted version that was used for the print copy
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- correction slips
- links to related legislation and further information resources
Timeline of Changes
This timeline shows the different points in time where a change occurred. The dates will coincide with the earliest date on which the change (e.g an insertion, a repeal or a substitution) that was applied came into force. The first date in the timeline will usually be the earliest date when the provision came into force. In some cases the first date is 01/02/1991 (or for Northern Ireland legislation 01/01/2006). This date is our basedate. No versions before this date are available. For further information see the Editorial Practice Guide and Glossary under Help.
More Resources
Use this menu to access essential accompanying documents and information for this legislation item. Dependent on the legislation item being viewed this may include:
- the original print PDF of the as made version that was used for the print copy
- correction slips
Click 'View More' or select 'More Resources' tab for additional information including:
- lists of changes made by and/or affecting this legislation item
- confers power and blanket amendment details
- all formats of all associated documents
- links to related legislation and further information resources