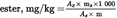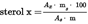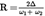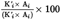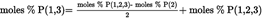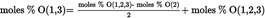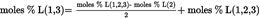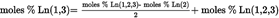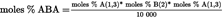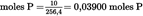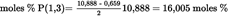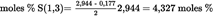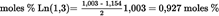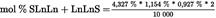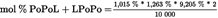- Y Diweddaraf sydd Ar Gael (Diwygiedig)
- Pwynt Penodol mewn Amser (01/10/2008)
- Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE)
Commission Regulation (EEC) No 2568/91Dangos y teitl llawn
Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis
You are here:
- Rheoliadau yn deillio o’r UE
- 1991 No. 2568
- Annexes only
- Dangos Graddfa Ddaearyddol(e.e. Lloegr, Cymru, Yr Alban aca Gogledd Iwerddon)
- Dangos Llinell Amser Newidiadau
Rhagor o Adnoddau
PDF o Fersiynau Diwygiedig
- ddiwygiedig 20/10/20193.52 MB
- ddiwygiedig 04/12/20163.60 MB
- ddiwygiedig 11/10/20163.55 MB
- ddiwygiedig 04/08/20163.60 MB
- ddiwygiedig 16/10/20153.42 MB
- ddiwygiedig 01/01/20153.68 MB
- ddiwygiedig 01/03/20143.69 MB
- ddiwygiedig 01/01/20142.84 MB
- ddiwygiedig 09/08/20122.49 MB
- ddiwygiedig 01/04/20112.46 MB
- ddiwygiedig 01/10/20081.42 MB
- ddiwygiedig 01/01/20081.42 MB
- ddiwygiedig 01/11/20035.95 MB
- ddiwygiedig 22/05/20020.48 MB
- ddiwygiedig 01/11/20010.66 MB
- ddiwygiedig 01/07/20010.66 MB
- ddiwygiedig 01/06/19990.65 MB
- ddiwygiedig 01/11/19980.65 MB
- ddiwygiedig 10/02/19980.65 MB
- ddiwygiedig 31/10/19950.59 MB
- ddiwygiedig 28/05/19950.59 MB
- ddiwygiedig 01/11/19940.55 MB
- ddiwygiedig 19/02/19940.55 MB
- ddiwygiedig 05/02/19940.55 MB
- ddiwygiedig 01/05/19930.55 MB
- ddiwygiedig 21/03/19930.56 MB
- ddiwygiedig 20/02/19930.56 MB
- ddiwygiedig 01/11/19920.55 MB
- ddiwygiedig 21/07/19920.55 MB
- ddiwygiedig 03/07/19920.54 MB
- ddiwygiedig 05/06/19920.54 MB
- ddiwygiedig 21/12/19910.52 MB
- ddiwygiedig 06/09/19910.52 MB
When the UK left the EU, legislation.gov.uk published EU legislation that had been published by the EU up to IP completion day (31 December 2020 11.00 p.m.). On legislation.gov.uk, these items of legislation are kept up-to-date with any amendments made by the UK since then.
Mae hon yn eitem o ddeddfwriaeth sy’n deillio o’r UE
Mae unrhyw newidiadau sydd wedi cael eu gwneud yn barod gan y tîm yn ymddangos yn y cynnwys a chyfeirir atynt gydag anodiadau.Ar ôl y diwrnod ymadael bydd tair fersiwn o’r ddeddfwriaeth yma i’w gwirio at ddibenion gwahanol. Y fersiwn legislation.gov.uk yw’r fersiwn sy’n weithredol yn y Deyrnas Unedig. Y Fersiwn UE sydd ar EUR-lex ar hyn o bryd yw’r fersiwn sy’n weithredol yn yr UE h.y. efallai y bydd arnoch angen y fersiwn hon os byddwch yn gweithredu busnes yn yr UE. EUR-Lex Y fersiwn yn yr archif ar y we yw’r fersiwn swyddogol o’r ddeddfwriaeth fel yr oedd ar y diwrnod ymadael cyn cael ei chyhoeddi ar legislation.gov.uk ac unrhyw newidiadau ac effeithiau a weithredwyd yn y Deyrnas Unedig wedyn. Mae’r archif ar y we hefyd yn cynnwys cyfraith achos a ffurfiau mewn ieithoedd eraill o EUR-Lex. The EU Exit Web Archive legislation_originated_from_EU_p3
Changes over time for: Commission Regulation (EEC) No 2568/91 (Annexes only)
Version Superseded: 01/04/2011
Alternative versions:
- 01/01/2008- Amendment
- 01/10/2008- Amendment
- 01/10/2008
Point in time - 01/04/2011- Amendment
- 01/01/2014- Amendment
- 01/03/2014- Amendment
- 16/10/2015- Amendment
- 04/08/2016- Amendment
- 11/10/2016- Amendment
- 04/12/2016- Amendment
- 20/10/2019- Amendment
- Exit day: start of implementation period31/01/2020 11pm- Amendment
- End of implementation period31/12/2020- Amendment
Status:
Point in time view as at 01/10/2008.
Changes to legislation:
There are outstanding changes not yet made to Commission Regulation (EEC) No 2568/91. Any changes that have already been made to the legislation appear in the content and are referenced with annotations.![]()
Changes to Legislation
Changes and effects yet to be applied by the editorial team are only applicable when viewing the latest version or prospective version of legislation. They are therefore not accessible when viewing legislation as at a specific point in time. To view the ‘Changes to Legislation’ information for this provision return to the latest version view using the options provided in the ‘What Version’ box above.
[X1ANNEXES U.K. Summary
Editorial Information
| Annex I: | Characteristics of olive oil |
| Annex Ia: | Sampling of batches of olive oil or olive-residue oil in immediate packaging not exceeding 100 litres |
| Annexe Ib: | Decision tree |
| Annex II: | [F1Determination of free fatty acids, cold method] |
| Annex III: | Determination of the peroxide value |
| Annex IV: | [F2Determination of wax content by capillary column gas-liquid chromatography] |
| Annex V: | Determination of the composition and content of sterols by capillary-column gas chromatography |
| Annex VI: | Determination of erythrodiol and uvaol |
| Annex VII: | [F1Determination of the percentage of 2-glyceryl monopalmitate] |
| Annex VIII: | Determination of composition of trilinolein |
| Annex IX: | Spectrophotometric investigation in the ultraviolet |
| Annex XA: | Analysis by gas chromatography of methyl esters of fatty acids |
| Annex XB: | Preparation of methyl esters of fatty acids |
| Annex XI: | Determination of the volatile halogenated solvents of olive oil |
| Annex XII: | Organoleptic assessment of virgin olive oil |
| Annex XIII: | [F2Neutralization and decolorization of olive oil in the laboratory] |
| Annex XIV: | Additional Notes 2, 3 and 4 to Chapter 15 of the combined nomenclature |
| Annex XIX: | Method for determining aliphatic alcohol content |
| Annex XV: | Oil content of olive residue |
| Annex XVI: | Determination of iodine value |
| Annex XVII: | Determination of stigmastadienes in vegetable oils |
| Annex XVIII: | Method for determining the content of triglycerides with ECN42] |
Textual Amendments
[F1ANNEX I U.K.
OLIVE OIL CHARACTERISTICS
| a Total isomers which could (or could not) be separated by capillary column. | |||||||||||
| b Or where the median defect is less than or equal to [F33,5] and the fruity median is equal to 0. | |||||||||||
| c Oils with a wax content of between 300 mg/kg and 350 mg/kg are considered to be lampante olive oil if the total aliphatic alcohol content is less than or equal to 350 mg/kg or if the erythrodiol and uvaol content is less than or equal to 3,5 %. | |||||||||||
| d Oils with a wax content of between 300 mg/kg and 350 mg/kg are considered to be crude olive-residue oil if the total aliphatic alcohol content is above 350 mg/kg and if the erythrodiol and uvaol content is greater than 3,5 %. | |||||||||||
| Category | Acidity (%) (*) | Peroxide index mEq O 2 /kg (*) | Waxes mg/kg (**) | 2-glyceril monopalmitate (%) | Stigmastadiene mg/kg a | Difference: ECN42 (HPLC) and ECN42 (theoretical calculation) | K 232 (*) | K 270 (*) | Delta-K (*) | Organoleptic evaluation median defect (Md) (*) | Organoleptic evaluation fruity median (Mf) (*) |
|---|---|---|---|---|---|---|---|---|---|---|---|
1. Extra virgin olive oil | ≤ 0,8 | ≤ 20 | ≤ 250 | ≤ 0,9 if total palmitic acid % ≤ 14 ≤ 1,0 if total palmitic acid % > 14 | ≤ 0,10 | ≤ 0,2 | ≤ 2,50 | ≤ 0,22 | ≤ 0,01 | Md = 0 | Mf > 0 |
2. Virgin olive oil | ≤ 2,0 | ≤ 20 | ≤ 250 | ≤ 0,9 if total palmitic acid % ≤ 14 ≤ 1,0 if total palmitic acid % > 14 | ≤ 0,10 | ≤ 0,2 | ≤ 2,60 | ≤ 0,25 | ≤ 0,01 | Md ≤ [F33,5] | Mf > 0 |
3. Lampante olive oil | > 2,0 | — | ≤ 300 c | ≤ 0,9 if total palmitic acid % ≤ 14 ≤ 1,1 if total palmitic acid % > 14 | ≤ 0,50 | ≤ 0,3 | — | — | — | Md > [F33,5] b | — |
4. Refined olive oil | ≤ 0,3 | ≤ 5 | ≤ 350 | ≤ 0,9 if total palmitic acid % ≤ 14 ≤ 1,1 if total palmitic acid % > 14 | — | ≤ 0,3 | — | ≤ 1,10 | ≤ 0,16 | — | — |
5. Olive oil composed of refined and virgin olive oils | ≤ 1,0 | ≤ 15 | ≤ 350 | ≤ 0,9 if total palmitic acid % ≤ 14 ≤ 1,0 if total palmitic acid % > 14 | — | ≤ 0,3 | — | ≤ 0,90 | ≤ 0,15 | — | — |
6. Crude olive-residue oil | — | — | > 350 d | ≤ 1,4 | — | ≤ 0,6 | — | — | — | — | — |
7. Refined olive-residue oil | ≤ 0,3 | ≤ 5 | > 350 | ≤ 1,4 | — | ≤ 0,5 | — | ≤ 2,00 | ≤ 0,20 | — | — |
8. Olive-residue oil | ≤ 1,0 | ≤ 15 | > 350 | ≤ 1,2 | — | ≤ 0,5 | — | ≤ 1,70 | ≤ 0,18 | — | — |
| a Other fatty acids content (%): palmitic: 7,5-20,0; palmitoleic: 0,3-3,5; heptadecanoic: ≤ 0,3; heptadecenoic: ≤ 0,3; stearic: 0,5-5,0; oleic: 55,0-83,0; linoleic: 3,5-21,0. | ||||||||||||||||
| b Total: Delta-5,23-stigmastadienol+chlerosterol+beta-sitosterol+sitostanol+delta-5-avenasterol+delta-5,24-stigmastadienol. | ||||||||||||||||
| c Oils with a wax content of between 300 mg/kg and 350 mg/kg are considered to be lampante olive oil if the total aliphatic alcohol content is less than or equal to 350 mg/kg or if the erythrodiol and uvaol content is less than or equal to 3,5 %. | ||||||||||||||||
| d Oils with a wax content of between 300 mg/kg and 350 mg/kg are considered to be crude olive-residue oil if the total aliphatic alcohol content is above 350 mg/kg and if the erythrodiol and uvaol content is greater than 3,5 %. | ||||||||||||||||
| Category | Acid content a | Total transoleic isomers (%) | Total translinoleic + translinolenic isomers (%) | Sterols composition | Total sterols (mg/kg) | Erythrodiol and uvaol (%) (**) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myristic (%) | Linolenic (%) | Arachidic (%) | Eicosenoic (%) | Behenic (%) | Lignoceric (%) | Cholesterol (%) | Brassicasterol (%) | Campesterol (%) | Stigmasterol (%) | Betasitosterol (%) b | Delta-7-stigmastenol (%) | |||||
1. Extra virgin olive oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,2 | ≤ 0,2 | ≤ 0,05 | ≤ 0,05 | ≤ 0,5 | ≤ 0,1 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 000 | ≤ 4,5 |
2. Virgin olive oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,2 | ≤ 0,2 | ≤ 0,05 | ≤ 0,05 | ≤ 0,5 | ≤ 0,1 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 000 | ≤ 4,5 |
3. Lampante olive oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,2 | ≤ 0,2 | ≤ 0,10 | ≤ 0,10 | ≤ 0,5 | ≤ 0,1 | ≤ 4,0 | — | ≥ 93,0 | ≤ 0,5 | ≥ 1 000 | ≤ 4,5 c |
4. Refined olive oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,2 | ≤ 0,2 | ≤ 0,20 | ≤ 0,30 | ≤ 0,5 | ≤ 0,1 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 000 | ≤ 4,5 |
5. Olive oil composed of refined and virgin olive oils | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,2 | ≤ 0,2 | ≤ 0,20 | ≤ 0,30 | ≤ 0,5 | ≤ 0,1 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 000 | ≤ 4,5 |
6. Crude olive-residue oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,3 | ≤ 0,2 | ≤ 0,20 | ≤ 0,10 | ≤ 0,5 | ≤ 0,2 | ≤ 4,0 | — | ≥ 93,0 | ≤ 0,5 | ≥ 2 500 | > 4,5 d |
7. Refined olive-residue oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,3 | ≤ 0,2 | ≤ 0,40 | ≤ 0,35 | ≤ 0,5 | ≤ 0,2 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 800 | > 4,5 |
8. Olive-residue oil | ≤ 0,05 | ≤ 1,0 | ≤ 0,6 | ≤ 0,4 | ≤ 0,3 | ≤ 0,2 | ≤ 0,40 | ≤ 0,35 | ≤ 0,5 | ≤ 0,2 | ≤ 4,0 | < Camp. | ≥ 93,0 | ≤ 0,5 | ≥ 1 600 | > 4,5 |
Textual Amendments
Notes: U.K.
(a) The results of the analyses must be expressed to the same number of decimal places as used for each characteristic. U.K.
The last digit must be increased by one unit if the following digit is greater than 4.
(b) If just a single characteristic does not match the values stated, the category of an oil can be changed or the oil declared impure for the purposes of this Regulation. U.K.
(c) If a characteristic is marked with an asterisk (*), referring to the quality of the oil, this means the following: U.K.
for lampante olive oil, it is possible for both the relevant limits to be different from the stated values at the same time;
for virgin olive oils, if at least one of these limits is different from the stated values, the category of the oil will be changed, although they will still be classified in one of the categories of virgin olive oil.
(d) If a characteristic is marked with two asterisks (**), referring to the quality of the oil, this means that for all types of olive-residue oil, it is possible for both the relevant limits to be different from the stated values at the same time.] U.K.
[F4ANNEX Ia U.K. Sampling of olive oil or olive-pomace oil delivered in immediate packaging not exceeding 100 litres
Textual Amendments
This method of sampling applies to deliveries of olive oil or olive-pomace oil not exceeding 125 000 litres, put up in immediate packaging not exceeding 100 litres.
If the delivery exceeds 125 000 litres, it is to be subdivided into batches of 125 000 litres or under. If the delivery is less than 125 000 litres it shall constitute one batch. The method shall then be applied to each batch.
The minimum number of primary samples to be taken is determined by the size of the batch in accordance with the table set out in point 1.
The size of the primary sample is determined on the basis of the capacity of the immediate packaging, in accordance with the table set out in point 2.1.
Delivery, primary sample and laboratory sample shall mean the definitions given in standard EN ISO 5555.
‘ Batch ’ shall mean a set of sales units which are produced, manufactured and packed in circumstances such that the oil contained in each sales unit is considered to be homogenous in terms of all analytical characteristics.
1. NUMBER OF PRIMARY SAMPLES TO BE TAKEN U.K.
The minimum number of primary samples to be taken will be determined by the size of the batch in accordance with the following table:
| Size of batch (litres) less than | Minimum number of primary samples |
|---|---|
| 7 500 | 2 |
| 25 000 | 3 |
| 75 000 | 4 |
| 125 000 | 5 |
The immediate packs selected to form a primary sample must be adjacent to each other in the batch.
In cases of doubt, Member States shall increase the number of primary samples to be taken.
2. CONTENT OF PRIMARY SAMPLES U.K.
2.1 Primary samples must comprise the following: U.K.
2.2 The primary samples are to be kept in the immediate packaging up to the time of analysis. The oil in the primary samples shall then, as applicable, be subdivided into three laboratory samples in order to carry out: U.K.
the analyses referred to in Annexes II, III, IX and X,
the analysis referred to in Annex XII,
the other analyses.
2.3 The packs constituting a primary sample shall be subdivided in accordance with the control procedures provided for in national law. U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3. ANALYSES AND RESULTS U.K.
Each of the primary samples referred to in point 1 shall be subdivided into laboratory samples, in accordance with point 2.5 of standard EN ISO 5555, and analysed as follows:
determination of free fatty acids, as referred to in the first indent of Article 2(1),
determination of the peroxide value, as referred to in the second indent of Article 2(1),
spectrophotometric analysis, as referred to in the eighth indent of Article 2(1),
determination of the fatty acid composition, as referred to in the ninth indent of Article 2(1).
Where one of the results of the analyses referred to in (a) for at least one of the primary samples taken from the same batch does not comply with the characteristics of the category of oil declared, the whole of the batch concerned is to be declared not to comply.
Where the results of the analyses referred to in (a) for each of the primary samples taken from the same batch are not all uniform, given the repeatability characteristics of the methods concerned, the entire batch is to be declared non-uniform and each primary sample is to be subject to the other analysis required. Otherwise, one primary sample from that batch is to be subject to the other analysis required.
Where one of the results of the analyses referred to in the second paragraph of point (b) does not comply with the characteristics of the category of oil declared, the whole of the batch concerned is to be declared not to comply.
Where all the results of the analyses referred to in the second paragraph of point (b) comply with the characteristics of the category of oil declared, the whole batch is to be declared to comply.]
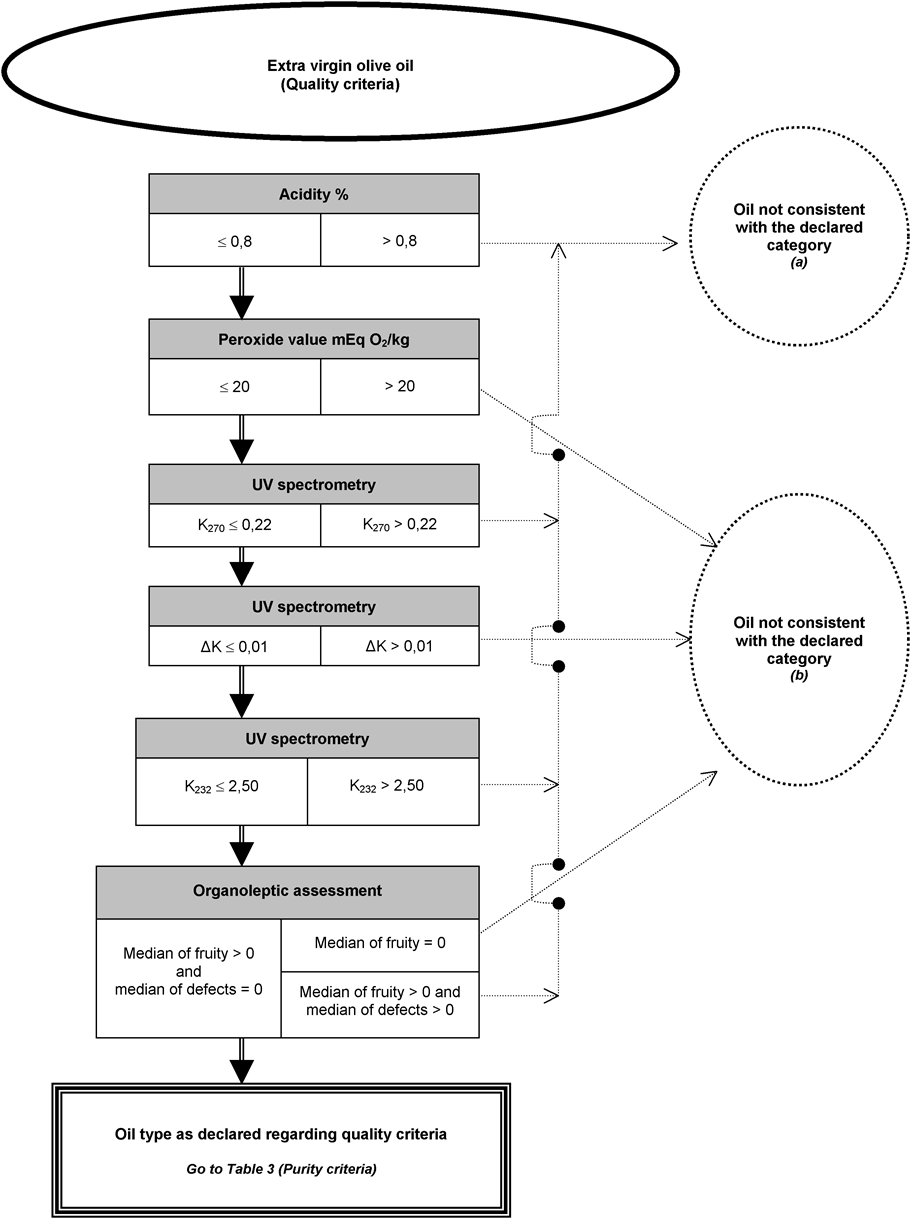
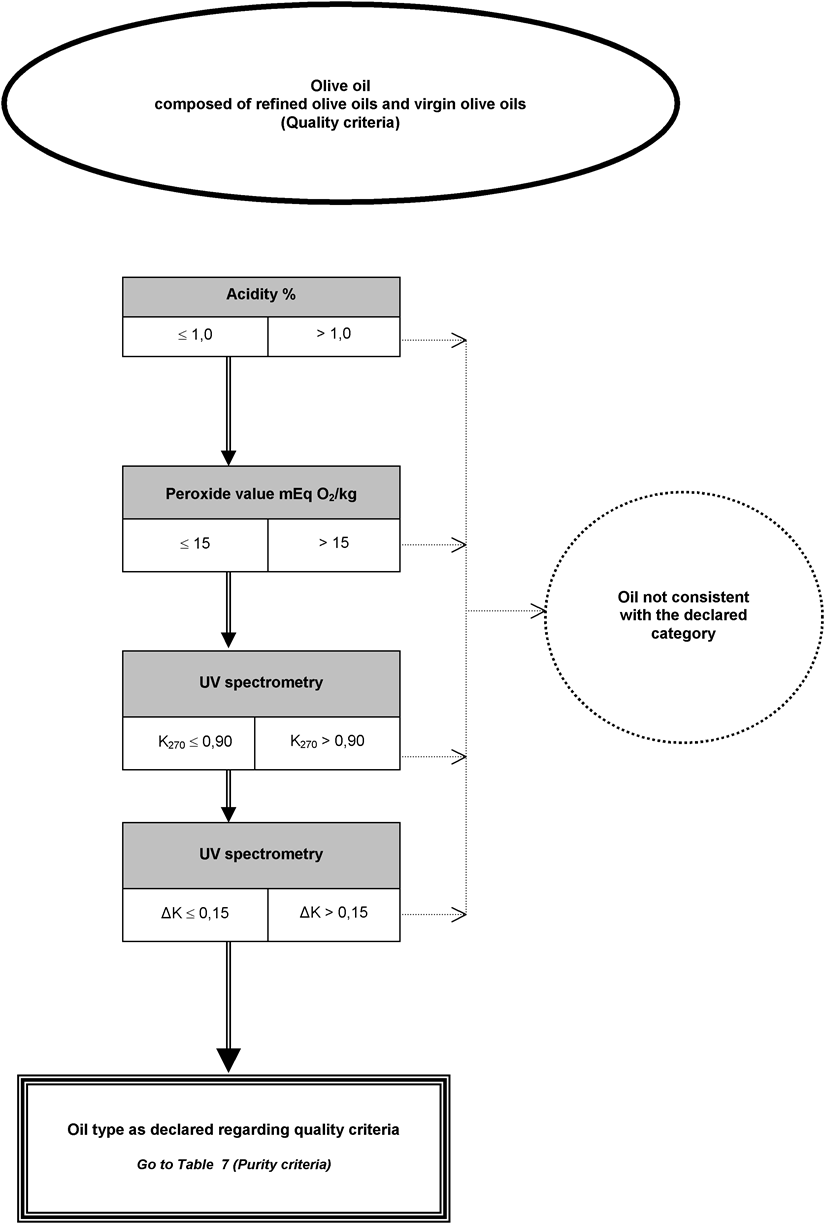
[F5ANNEX Ib U.K. DECISION TREE FOR VERIFYING WHETHER AN OLIVE OIL SAMPLE IS CONSISTENT WITH THE CATEGORY DECLARED
Textual Amendments
The analysis to verify whether an olive oil or olive-pomace oil is consistent with the category declared may be undertaken:
either by carrying out in any random order the analyses envisaged for the purpose of verifying compliance with the characteristics specified in Annex I; or
by carrying out in the order shown in the decision tree the analyses specified therein until one of the decisions appearing in the decision tree is reached.
The analyses relating to contaminants required for verifying compliance with European Community standards are to be carried out separately.
The decision tree applies to all categories of olive oil and olive-pomace oil. It consists of tables numbered 1 to 11 which must be approached on the basis of the declared category of oil concerned in the order set out in the general table.
Key to general tables to 11:
the double line (=) indicates the route to be followed in case of compliance (positive answer) with the criteria specified in the preceding box. The dotted line (…) indicates the alternative route to be followed in case of non-compliance,
the headings in the boxes in tables 1 to 11 refer to the analyses provided for in this Regulation on the basis of the table of equivalence set out in Appendix 1 to this Annex,
the letters in brackets appearing in the negative decision circles in tables 1 to 11 cross-refer to indicative information given in Appendix 2 to this Annex. The letters in themselves do not entail the obligation to pursue the analyses or imply the veracity of the assumptions stated.
Appendix 1 Table of equivalence between the annexes to this Regulation and the analyses specified in the decision tree
[F1— Acidity | Annex II | Determination of free fatty acids, cold method] |
— Peroxide value | Annex III | Determination of peroxide value |
— UV spectrometry | Annex IX | Spectrophotometric analysis |
— Organoleptic assessment | Annex XII | Organoleptic assessment of virgin olive oil |
— 3,5-Stigmastadienes | Annex XVII | Method of determining stigmastadienes in vegetable oils |
— Trans isomers of fatty acids | Annex Xa and | Analysis by gas chromatography of methyl esters of fatty acids |
| Annex Xb | Preparation of methyl esters of fatty acids | |
— Fatty acids content | Annex Xa and | Analysis by gas chromatography of methyl esters of fatty acids |
| Annex Xb | Preparation of methyl esters of fatty acids | |
— ΔECN42 | Annex XVIII | Determination of the composition of triglycerides with ECN42 (difference between the HPLC data and theoretical content) |
— Sterols composition and total sterols | Annex V | Determination of the composition and content of sterols by capillary-column gas chromatography |
— Erythrodiol and Uvaol | Annex VI | Determination of erythrodiol and uvaol |
[F1— Saturated fatty acids in position 2 | Annex VII | Determination of the percentage of 2-glyceryl monopalmitate] |
— Aliphatic alcohols | Annex XIX | Determination of aliphatic alcohols content by capillary-column gas chromatography |
— Saturated fatty acids in 2-position | Annex VII | Determination of saturated fatty acids in position 2 of the triglyceride |
Appendix 2
Table 1 U.K.
See virgin or lampante olive oil (Quality criteria Table 2 , or Quality and purity criteria Table 4 )
See lampante olive oil (Quality and purity criteria Table 4 )
Table 2 U.K.
See lampante olive oil (Quality and purity criteria Table 4 )
See extra virgin olive oil (Quality criteria Table 1 )
Table 3 U.K.
Presence of refined oil (olive or others)
Presence of olive-pomace oil
Table 4 U.K.
See extra virgin olive oil and virgin olive oil (Quality criteria Table 1 and Table 2 )
Presence of refined oil (olive or others)
Presence of olive-pomace oil
Presence of esterified oils
Table 7 U.K.
Presence of olive-pomace oil
Presence of esterified oils
Table 8 U.K.
Presence of refined oil (olive or others)
See lampante olive oil (Quality and purity criteria Table 4 )
Presence of esterified oils
Table 11 U.K.
Presence of esterified oils]
ANNEX IIU.K. [F1DETERMINATION OF FREE FATTY ACIDS, COLD METHOD]
1.DETERMINATION OF ACIDITYU.K.
The determination of free fatty acids in olive oils. The content of free fatty acids is expressed as acidity calculated conventionally.
1.1.PrincipleU.K.
A sample is dissolved in a mixture of solvents and the free fatty acids present titrated using an ethanolic solution of potassium hydroxide.
1.2.ReagentsU.K.
All the reagents should be of recognized analytical quality and the water used either distilled or of equivalent purity.
1.2.1. [X1Diethyl ether]; 95 % ethanol (v/v), mixture of equal parts by volume.U.K.
Note:U.K.
[X1Diethyl ether] is highly inflammable and may form explosive peroxides. Special care should be taken in its use.U.K.
Neutralize precisely at the moment of use with the potassium hydroxide solution (1.2.2), with the addition of 0,3 ml of the phenolpthalein solution (1.2.3) per 100 ml of mixture.
Note:U.K.
If it is not possible to use [X1diethyl ether], a mixture of solvents containing ethanol and toluene may be used. If necessary, ethanol may be replaced by propanol-2.U.K.
1.2.2.Potassium hydroxide, titrated ethanolic solution, c(KOH) about 0,1 mol/l or, if necessary, c(KOH) about 0,5 mol/l.U.K.
The exact concentration of the ethanolic solution of potassium hydroxide must be known and checked immediately prior to use. Use a solution prepared at least five days before use and decanted into a brown glass bottle with a rubber stopper. The solution should be colourless or straw coloured.
Note:U.K.
A stable colourless solution of potassium hydroxide may be prepared as follows. Bring to the boil 1 000 ml of ethanol with 8 g of potassium hydroxide and 0,5 g of aluminium shavings and continue boiling under reflux for one hour. Distill immediately. Dissolve in the distillate the required quantity of potassium hydroxide. Leave for several days and decant the clear supernatant liquid from the precipitate of potassium carbonate.U.K.
The solution may also be prepared without distillation as follows: to 1 000 ml of ethanol add 4 ml of aluminium butylate and leave the mixture for several days. Decant the supernatant liquid and dissolve the required quantity of potassium hydroxide. The solution is ready for use.
1.2.3.Phenolphthalein, 10 g/l solution in 95 to 96 % ethanol (v/v) or alkaline blue, (in the case of strongly coloured fats) 20 g/l solution in 95 to 96 % ethanol (v/v).U.K.
1.3.ApparatusU.K.
Usual laboratory equipment including:
1.3.1.analytical balance;U.K.
1.3.2.250 ml conical flask;U.K.
1.3.3.10 ml burette, graduated in 0,05 ml.U.K.
1.4.ProcedureU.K.
1.4.1.Preparation of the specimen for testingU.K.
(Carry out the test on the filtered sample. Where moisture and impurities together are less than 1 %, use the specimen without further treatment; where they exceed 1 %, it should be filtered.)
1.4.2.Taking the sampleU.K.
Take a sample depending on the presumed acid number in accordance with the following table:
| Expected acid value | Mass of sample(g) | Weighing accuracy(g) |
|---|---|---|
| < 1 | 20 | 0,05 |
| 1 to 4 | 10 | 0,02 |
| 4 to 15 | 2,5 | 0,01 |
| 15 to 75 | 0,5 | 0,001 |
| > 75 | 0,1 | 0,0002 |
Weigh the sample in the conical flask (1.3.2).
1.4.3.DeterminationU.K.
Dissolve the sample (1.4.2) in 50 to 150 ml of the previously neutralized mixture of diethyl [X1ether] and ethanol (1.2.1).
Titrate while stirring with the 0,1 mol/l solution of potassium hydroxide (1.2.2) (see Note 2) until the indicator changes (the pink colour of the phenolphtalein persists for at least 10 seconds).
Note 1.U.K.
The titrated ethanolic solution of potassium hydroxide (1.2.2) may be replaced by an aqueous solution of potassium or sodium hydroxide provided that the volume of water introduced does not induce phase separation.U.K.
Note 2.U.K.
If the quantity of 0,1 mol/l potassium hydroxide solution required exceeds 10 ml, use the 0,5 mol/l solution.U.K.
Note 3.U.K.
If the solution becomes cloudy during titration, add enough of the solvents (1.2.1) to give a clear solution.U.K.
1.5.Acidity: expressed as percentage of oleic acidU.K.
Acidity as a percentage by weight is equal to:
where:
=
the volume of titrated potassium hydroxide solution used, in millilitres;
=
the exact concentration in moles per litre of the titrated solution of potassium hydroxide used;
=
the molar weight in grams per mole of the acid used to express the result (= 282);
=
the weight in grams of the sample.
[X1Take as the result, the arithmetic mean [F2of two calculations] carried out.]
ANNEX IIIU.K.DETERMINATION OF PEROXIDE VALUE
1.SCOPEU.K.
This Standard describes a method for the determination of the peroxide value of oils and fats.
2.FIELD OF APPLICATIONU.K.
This Standard is applicable to animal and vegetable oils and fats.
3.DEFINITIONU.K.
The peroxide value is the quantity of those substances in the sample, expressed in terms of milliequivalents of active oxygen per kilogram, which oxidize potassium iodide under the operating conditions described.
4.PRINCIPLEU.K.
Treatment of the test portion, in solution in acetic acid and chloroform, by a solution of potassium iodide. Titration of the liberated iodine with standardized sodium thiosulphate solution.
5.APPARATUSU.K.
All the equipment used shall be free from reducing or oxidizing substances.
Note:U.K.
Do not grease ground surfaces.U.K.
5.1.3 ml glass scoop.U.K.
5.2.Flasks, with ground necks and stoppers, of about 250 ml capacity, dried beforehand and filled with a pure, dry inert gas (nitrogen or, preferably, carbon dioxide).U.K.
5.3.25- or 50-ml burette, graduated in 0,1 ml.U.K.
6.REAGENTSU.K.
6.1.Chloroform, analytical reagent quality, freed from oxygen by bubbling a current of pure, dry inert gas through it.U.K.
6.2.Glacial acetic acid, analytical reagent quality, freed from oxygen by bubbling a current of pure, [X1dry inert gas] through it.U.K.
6.3.Potassium iodide, saturated aqueous solution, recently prepared, free from iodine and iodates.U.K.
6.4.Sodium thiosulphate, 0,01 or 0,002 [X1Mol/L accurately] standardized aqueous solution, standardized just before use.U.K.
6.5.Starch solution, 10 g/l aqueous dispersion, recently prepared from natural soluble starch.U.K.
7.SAMPLEU.K.
Take care that the sample is taken and stored away from the light, kept cold and contained in completely filled glass containers, hermetically sealed with ground-glass or cork stoppers.
8.PROCEDUREU.K.
The test shall be carried out in diffuse daylight or in artificial light. Weigh in a glass scoop (5.1) or, failing this, in a flask (5.2), to the nearest 0,001 g, a mass of the sample in accordance with the following table, according to the expected peroxide value:
| Expected peroxide value(meq) | Weight of test portion(g) |
|---|---|
| 0 to 12 | 5,0 to 2,0 |
| 12 to 20 | 2,0 to 1,2 |
| 20 to 30 | 1,2 to 0,8 |
| 30 to 50 | 0,8 to 0,5 |
| 50 to 90 | 0,5 to 0,3 |
Unstopper a flask (5.2) and introduce the glass scoop containing the test portion. Add 10 ml of chloroform (6.1). Dissolve the test portion rapidly by stirring. Add 15 ml of acetic acid (6.2), then 1 ml of potassium iodide solution (6.3). Insert the stopper quickly, shake for one minute, and leave for exactly five minutes away from the light at a temperature from 15 to 25 °C.
Add about 75 ml of distilled water. Titrate the liberated iodine with the sodium thiosulphate solution [X1(6.4) (0,002 Mol/L solution for expected values less than 12, and 0,01 Mol/L solution] for expected values above 12) shaking vigorously, using starch solution (6.5) as indicator.
Carry out two determinations on the same test sample.
Carry out simultaneously a blank test. If the result of the blank exceeds 0,05 ml of [X10,01 mol/L sodium] thiosulphate solution (6.4), replace the impure reagents.
9.EXPRESSION OF RESULTSU.K.
The peroxide value (PV), expressed in milliequivalents of active oxygen per kilogram, is given by the formula:
where:
=
the number of ml of the standardized sodium thiosulphate solution (6.4) used for the test, corrected to take into account the blank test;
=
the weight in g, of the test portion.
Take as the result the arithmetic mean of the two determinations carried out.
[F1ANNEX IV U.K. DETERMINATION OF WAX CONTENT BY CAPILLARY COLUMN GAS CHROMATOGRAPHY
1. SUBJECT U.K.
This method describes a process for determining the wax content of olive oils. Waxes are separated according to the number of their carbon atoms. The method may be used in particular to distinguish between olive oil obtained by pressing and that obtained by extraction (olive-residue oil).
2. PRINCIPLE U.K.
Addition of a suitable internal standard to the fat or oil, then fractionation by chromatography on a hydrated silica gel column. Recovery under the test conditions of the fraction eluted first (the polarity of which is less than that of the triglycerides), then direct analysis by capillary column gas chromatography.
3. EQUIPMENT U.K.
3.1. 25 ml Erlenmeyer flask. U.K.
3.2. Glass column for gas chromatography, internal diameter 15,0 mm, length 30 to 40 cm, fitted with a stopcock. U.K.
3.3. Suitable gas chromatograph with a capillary column, equipped with a system for direct introduction into the column comprising the following: U.K.
3.3.1. Thermostatic chamber for the columns, equipped with a temperature programmer. U.K.
3.3.2. Cold injector for direct introduction into the column. U.K.
3.3.3. Flame ionisation detector and converter-amplifier. U.K.
3.3.4. Recorder-integrator capable of working with the converter-amplifier (3.3.3), rate of response no slower than 1 second, with variable paper speed. (It is also possible to use computerised systems that allow the acquisition of gas chromatography data via a PC.) U.K.
3.3.5. Glass or fused silica capillary column 8 to 12 m long and with an internal diameter of 0,25 to 0,32 mm, with liquid phase, with a uniform film thickness between 0,10 and 0,30 μm. (There are liquid phases suitable for the purpose of type SE-52 or SE-54 available on the market.) U.K.
3.4. 10 μl microsyringe for on-column injection, equipped with a hardened needle. U.K.
3.5. Electrovibrator. U.K.
3.6. Rotary evaporator. U.K.
3.7. Muffle furnace. U.K.
3.8. Analytical balance with guaranteed precision of ± 0,1 mg. U.K.
3.9. Normal laboratory glassware. U.K.
4. REAGENTS U.K.
4.1. Silica gel with a granule size of between 60 and 200 μm. U.K.
Place the gel in the furnace at 500 °C for at least four hours. After cooling, add 2 % water in relation to the quantity of sampled silica gel. Shake well to homogenise the slurry. Keep in darkness for at least 12 hours prior to use.
4.2. n-hexane, for chromatography. U.K.
4.3. Ethyl ether, for chromatography. U.K.
4.4. n-heptane, for chromatography. U.K.
4.5. Standard solution of lauryl arachidate, at 0,1 % (m/v) in hexane (internal standard). (It is also possible to use palmityl palmitate or myristyl stearate.) U.K.
4.5.1. Sudan 1 (1-phenyl-azo-2-naphthol). U.K.
4.6. Carrier gas: hydrogen or helium, gas-chromatographic purity. U.K.
4.7. Auxiliary gases: U.K.
pure hydrogen for gas chromatography,
pure air for gas chromatography.
5. PROCEDURE U.K.
5.1. Preparation of the chromatographic column. U.K.
Suspend 15 g of silica gel (4.1) in the n-hexane (4.2) and introduce it into the column (3.2). Allow to settle spontaneously. Complete settling with the aid of an electrovibrator (3.5) to make the chromatographic layer more homogeneous. Percolate 30 ml of n-hexane to remove any impurities. Using the balance (3.8) weigh exactly 500 mg of the sample into the 25 ml Erlenmeyer flask (3.1), add the appropriate quantity of the internal standard (4.5) according to the presumed wax content. For example, add 0,1 mg of lauryl arachidate for olive oil, and 0,25 to 0,5 mg for olive-residue oil. Transfer the prepared sample to the chromotography column using two 2 ml portions of n-hexane (4.2).
Allow the solvent to flow away until it reaches 1 mm above the upper level of the absorbant then percolate a further 70 ml of n-hexane in order to eliminate the n-alkanes naturally present. Then start the chromatographic elution by collecting 180 ml of the mixture of n-hexane/ethyl ether (ratio 99:1), keeping a rate of flow of approximately 15 drops every 10 seconds. Elution of the sample must be carried out at a room temperature of 22 ± 4 °C.
NB: U.K.
The n-hexane/ethyl ether mixture (99:1) must be prepared every day.
For a visual check on the correct elution of the waxes 100 μl of 1 % Sudan in the elution mixture can be added to the sample in solution. Since the colourant has an intermediate retention, between waxes and triglycerides, when the coloration has reached the bottom of the column the elution should be suspended because all the waxes will have been eluted.
Dry the fraction thus obtained in a rotary evaporator (3.6.) until virtually all the solvent has been eliminated. Eliminate the final 2 ml of solvent with the aid of a weak current of nitrogen; then add 2-4 ml n-heptane.
5.2. Analysis by gas chromatography U.K.
5.2.1. Preparatory work U.K.
Fit the column to the gas chromatograph (3.3) by connecting the inlet port to the on-column system and the outlet port to the detector. Perform a general check on the GC apparatus (operation of gas circuits, detector and recorder efficiency, etc.).
If the column is being used for the first time it should be conditioned first. Pass a little gas through the column, then turn on the GC apparatus. Heat gradually until 350 °C is reached after about four hours. Maintain that temperature for at least two hours then regulate the apparatus to operating conditions (set gas flow, light flame, connect to the electronic recorder (3.3.4), set temperature of column chamber, detector, etc.) and record the signal at a sensitivity at least twice as high as that required for the analysis. The baseline must be linear, with no peaks of any kind, and must not show any deviation.
A negative straight-line drift indicates that the column connections are not tight; a positive drift that the column has not been sufficiently conditioned.
5.2.2. Choice of operating conditions U.K.
The operating conditions are generally as follows:
column temperature:
20 °C/minute 5 °C/minute 20 °C/minute Initially 80 °C
(1′)
→ 240 °C → 325 °C
(6′)
→ 340 °C
(10′)
detector temperature: 350 °C;
quantity of substance injected: 1 μl of the n-heptane solution (2-4 ml);
carrier gas: helium or hydrogen at the correct linear velocity for the gas selected (see Appendix);
instrument sensitivity: suitable for the following conditions:
The conditions may be modified according to the characteristics of the column and the GC apparatus to obtain separation of all the waxes and a satisfactory peak resolution (see figure); the internal standard C 32 retention time must be 18 ± 3 minutes. The most representative wax peak must be at least 60 % of the full scale.
The peak integration parameters must be established so as to obtain a correct evaluation of the areas of the peaks in question.
NB : Given the high final temperature, a positive drift of no more than 10 % of the full scale is permitted. U.K.
5.3. Performance of the analysis U.K.
Sample 1 μl of the solution using the 10 μl microsyringe; withdraw the syringe plunger so that the needle is empty. Place the needle in the injector and after 1-2 seconds inject quickly; remove the needle slowly after about five seconds.
Record until the waxes are completely eluted.
The base line must always satisfy the required conditions.
5.4. Identification of peaks U.K.
Identification of the different peaks should be based on retention time by comparison with wax mixtures of known retention times analysed under the same conditions.
The figure is a chromatogram of the waxes of a virgin olive oil.
5.5. Evaluation of quantity U.K.
Calculate the areas of the peaks of the internal standard and the aliphatic esters of C 40 to C 46 using the integrator.
Calculate the wax content of each of the esters in mg/kg fat using the formula:
where:
=
area of each ester’s peak, in square millimetres;
=
area of the internal standard’s peak, in square millimetres;
=
mass of added internal standard, in milligrams;
=
mass of sample for analysis, in grams.
6. EXPRESSION OF RESULTS U.K.
Indicate the total of the contents of the various C 40 to C 46 waxes in mg/kg fat (ppm).
NB : The components to be quantified refer to the peaks with carbon pair numbers between esters C 40 and C 46 , using the example of the olive oil wax chromatogram shown in the figure below. If ester C 46 appears twice, it is recommended that to identify it the fraction of the waxes of an olive-residue oil should be analysed where the C 46 peak is easy to identify because it is in the clear majority. U.K.
The results should be expressed to one decimal place.
Figure Chromatogram of the waxes of an olive oil (1) U.K.
Appendix
Determination of the linear velocity of the gas U.K.
Inject 1-3 μl methane (or propane) into the GC apparatus after it has been regulated to normal operating conditions. Measure the time it takes for the gas to flow through the column from the time it is injected to the time the peak appears (t M ).
The linear velocity in cm/s is given by the formula L/t M , where L is the length of the column in cm and t M the time measured in seconds.]
ANNEX VU.K.DETERMINATION OF THE COMPOSITION AND CONTENT OF STEROLS BY CAPILLARY-COLUMN GAS CHROMATOGRAPHY
1.SCOPEU.K.
The method describes a procedure for determining the individual and total sterols content of fatty substances.
2.PRINCIPLE OF THE METHODU.K.
The fatty substance, with added α-cholestanol as an internal standard, is saponified with potassium hydroxide in ethanolic solution and the unsaponifiables are then extracted with [X1diethyl ether].
The sterol fraction is separated from the unsaponifiable extract by chromatography on a basic silica gel plate. The sterols recovered from the silica gel are transformed into trimethyl-silyl ethers and are analysed by capillary-column gas chromatography.
3.APPARATUSU.K.
3.1.250 ml flask fitted with a reflux condenser having ground-glass joints.U.K.
3.2.500 ml separating funnels.U.K.
3.3.250 ml flasks.U.K.
3.4.Complete apparatus for analysis by thin-layer chromatography using 20 × 20 cm glass plates.U.K.
3.5.Ultraviolet lamp having a wavelength of 366 or 254 nm.U.K.
3.6.100 μl and 500 μl microsyringes.U.K.
3.7.A cylindrical filter funnel with a G3 porous septum (porosity 15 to 40 μm) of diameter approximately 2 cm and a depth of some 5 cm, with an attachment suitable for filtration under vacuum and a 12/21 male ground glass joint.U.K.
3.8.50 ml vacuum conical flask with a 12/21 ground-glass female joint which can be fitted to the filter funnel (3.7).U.K.
3.9.A 10 ml test tube with a tapering bottom and a sealing stopper.U.K.
3.10.Gas chromatograph suitable for use with a capillary column, provided with a splitting system consisting of:U.K.
3.10.1.a thermostatic chamber for columns capable of maintaining the desired temperature with an accuracy of ± 1 °C;U.K.
3.10.2.a temperature-adjustable vaporization unit with a persilanized glass vapourizing element;U.K.
3.10.3.a flame ionization detector and converter-amplifier;U.K.
3.10.4.an integrator-recorder suitable for use with the converter-amplifier (3.10.3) having a response time of not more than one second and a variable paper speed.U.K.
3.11.A glass or fused-silica capillary column of length 20 to 30 m, internal diameter 0,25 to 0,32 mm, entirely coated with SE-52 or SE-54 liquid or equivalent in a uniform thickness between 0,10 and 0,30 μm.U.K.
3.12.A 10 μl gas chromatography microsyringe with a hardened needle.U.K.
4.REAGENTSU.K.
4.1.Potassium hydroxide, [X1approximately 2 mol/L ethanolic solution.] Dissolve 130 g of potassium hydroxide [X1(minimum concentration 85 %)] with cooling in 200 ml of distilled water and then make up to one litre with ethanol. Keep the solution in well-stoppered dark glass bottles.U.K.
4.2. [X1Diethyl ether], analytical purity.U.K.
4.3.Anhydrous sodium sulphate, analytical purity.U.K.
4.4.Glass plates coated with silica gel, without fluorescence indicator, thickness 0,25 mm (commercially available ready for use).U.K.
4.5.Potassium hydroxide, [X10,2 mol/L] ethanolic solution. Dissolve 13 g of potassium hydroxide in 20 ml of distilled water and make up to one litre with ethanol.U.K.
4.6.Benzene, for chromatography. (See 5.2.2)U.K.
4.7.Acetone, for chromatography. (See 5.2.2)U.K.
4.8.Hexane, for chromatography. (See 5.2.2)U.K.
4.9. [X1Diethyl ether], for chromatography. (See 5.2.2)U.K.
4.10.Chloroform, analytical purity. (See 5.2.2)U.K.
4.11.Reference solution for thin-layer chromatography: cholesterol or phytosterols, [F22 %] solution in chloroform.U.K.
4.12.2,7-dichlorofluorescein, 0,2 % ethanolic solution. Make slightly basic by adding a few drops of [X12 mol/L] alcoholic potassium hydroxide solution.U.K.
4.13.Anhydrous pyridine, for chromatography.U.K.
4.14.Hexamethyl disilazane.U.K.
4.15.Trimethylchlorosilane.U.K.
4.16.Reference solutions of sterol trimethylsilyl ethers. To be prepared at the time of use from pure sterols or mixtures of sterols obtained from oils containing them.U.K.
4.17. [X1β-cholestanol], 0,2 % solution (m/V) in chloroform (internal standard).U.K.
4.18.Carrier gas: hydrogen or helium, gas-chromatographic purity.U.K.
4.19.Auxiliary gases:U.K.
hydrogen, gas-chromatographic purity,
air, gas-chromatographic purity.
5.PROCEDUREU.K.
5.1.Preparation of the unsaponifiables.U.K.
5.1.1.Using the 500 μl microsyringe [X1introduce, into a 250 ml flask a volume of 0,2 % β-cholestanol solution in chloroform (4.17) containing an amount of cholestanol corresponding to approximately 10 % of the sterol content of the sample aliquot to be taken for the determination.] For example, for 5 g of sample add 500 μl of the 0,2 % α-cholestanol solution in the case of an olive oil and 1 500 μl for[F6 seed oils or] olive-pomaca oil.U.K.
Evaporate to [X1dryness in a current] of nitrogen and then weigh accurately 5 g of the dry filtered sample into the same flask.
[F2Oils] containing appreciable quantities of cholesterol may show a peak having a retention time identical to cholestanol. If this occurs the sterol fraction will have to be analyzed in duplicate with and without internal standard[F7or betulinol will have to be used instead of cholestanol].
Textual Amendments
5.1.2.Add 50 ml of [X12 mol/L] ethanolic potassium hydroxide solution, fit the reflux condenser and heat to gentle boiling on a water bath with continuous vigorous stirring until saponification takes place (the solution becomes clear). Continue heating for a further 20 minutes, then add 50 ml of [X1distilled water to the] top of the condenser, detach the condenser and cool the flask to approximately 30 °C.U.K.
5.1.3.Transfer the contents of the flask quantitatively into a 500 ml separating funnel using several rinses of distilled water, amounting in all to about 50 ml. Add approximately 80 ml of [X1diethyl ether], shake vigorously for approximately 30 seconds and allow to settle (Note 1).U.K.
Separate off the lower aqueous phase collecting it in a second separating funnel. Perform two further extractions on the aqueous phase in the same way using 60 to 70 ml of ethyl ether on each occasion.
Note 1.U.K.
Any emulsion can be destroyed by adding small quantities of ethyl or methyl alcohol by means of a spray.U.K.
5.1.4.Pool the ether extracts into a single separating funnel and wash with distilled water (50 ml at a time) until the wash water gives a neutral reaction.U.K.
When the wash water has been removed, dry with anhydrous sodium sulphate and [X1filter through anhydrous] sodium sulphate into a previously weighed 250 ml flask, washing the funnel and filter with small quantities of [X1diethyl ether.]
5.1.5.Distil the ether down to a few ml, then bring to dryness under a slight vacuum or in a current of nitrogen, completing drying in a stove at 100 °C for approximately a quarter of an hour, and then weigh after cooling in a desiccator.U.K.
5.2.Separation of the sterol fraction.U.K.
5.2.1.Preparation of the basic plates. Immerse the silica gel plates (4.4) completely in the [X10,2 mol/L] ethanolic potassium hydroxide solution (4.5) for 10 seconds, then allow to dry in a fume cupboard for two hours and finally place [X1in an oven at] 100 °C for one hour.U.K.
Remove from the stove and keep in a calcium chloride desiccator until required for use (plates treated in this way must be used within 15 days).
Note 2.U.K.
When basic silica gel plates are used to separate the sterol fraction there is no need to treat the unsaponifiables with alumina. In this way all compounds of an acid nature (fatty acids and others) are retained on the spotting line and the sterols band is clearly separated from the aliphatic and triterpene alcohols band.U.K.
5.2.2.Place a 95:5 (v/v) benzene/acetone [X1mixture into the] plate-developing chamber to a depth of approximately 1 cm. As an alternative a 65:35 (v/v) hexane/ethyl ether mixture may be used. Close the chamber with the appropriate cover and leave thus for approximately half an hour so that liquid-vapour equilibrium is established. Strips of filter paper dipping into the eluent may be placed on the internal surfaces of the chamber. This reduces developing time by approximately one-third and brings about more uniform and regular elution of the components.U.K.
Note 3.U.K.
The developing mixture should be replaced for every test in order to achieve perfectly reproducible elution conditions.U.K.
5.2.3.Prepare an approximately 5 % solution of the unsaponifiables (5.1.5) in chloroform and, using the 100 μl microsyringe, streak a chromatographic plate [X1(5.2.1) with 300 μl] approximately 2 cm from one end in a streak which is as thin and as uniform as possible. In line with the streak place 2 to 3 μl of the sterol reference solution (4.11) at one end of the plate so that the sterol band can be identified after developing.U.K.
5.2.4.Place the plate in the developing chamber prepared as specified in 5.2.2. The ambient temperature should be maintained between 15 and 20 °C. Immediately close the chamber with the cover and allow to elute until the solvent front reaches approximately 1 cm from the upper edge of the plate. Remove the plate from the developing chamber and evaporate the solvent in a flow of hot air or by leaving the plate for a short while under a hood.U.K.
5.2.5.Spray the plate lightly and uniformly with the 2,7-dichlorofluoroscein solution. When the plate is observed under ultraviolet light the sterol band can be identified through being aligned with the stain obtained from the reference solution. Mark the limits of the band along the edges of the fluorescence with a black pencil.U.K.
5.2.6.Using a metal spatula scrape off the silica gel in the marked area. Place the finely comminuted material removed into the filter funnel (3.7). Add 10 ml of hot chloroform, mix carefully with the metal spatula and filter under vacuum, collecting the filtrate in the conical flask (3.8) attached to the filter funnel.U.K.
Wash the residue [X1in the funnel three times with diethyl ether] (approximately 10 ml each time) collecting the filtrate in the same flask attached to the funnel. Evaporate the filtrate to a volume of 4 to 5 ml, transfer the residual solution to the previously weighed 10 ml test tube (3.9), evaporate to dryness by mild heating in a gentle flow of nitrogen, make up again using a few drops of acetone, evaporate again to dryness, place in a stove at 105 °C for approximately 10 minutes and then allow to cool in a desiccator and weigh.
The residue contained in the test tube consists of the sterol fraction.
5.3.Preparation of the trimethylsilyl ethers.U.K.
5.3.1.Add the silylation reagent, consisting of a 9:3:1 (v/v/v) mixture of pyridine/hexamethyl disilazane/trimethyl chlorosilane (Note 4) in the ratio of 50 μl for every milligram of sterols to the test tube containing the sterol fraction, avoiding any uptake of moisture (Note 5).U.K.
Note 4.U.K.
Solutions which are ready for use are available commercially. Other silanizing reagents such as, for example, bis-trimethylsilyl, trifluor acetamide + 1 % trimethyl chlorosilane, which has to be diluted with an equal volume of anhydrous pyridine, are also available.U.K.
5.3.2.Stopper the test tube, shake carefully (without overturning) until the sterols are completely dissolved. Stand for at least 15 minutes at ambient temperature and then centrifuge for a few minutes. The clear solution is ready for gas chromatographic analysis.U.K.
Note 5.U.K.
The slight opalescence which may form is normal and does not cause any interference. The formation of a white floc or the appearance of a pink colour are indicative of the presence of moisture or deterioration of the reagent. If these occur the test must be repeated.U.K.
5.4.Gas chromatographic analysis.U.K.
5.4.1.Preliminary operations, column packing.U.K.
5.4.1.1.Fit the column in the gas chromatograph, attaching the inlet end to the evaporator connected to the splitting system and the outlet end to the detector.U.K.
Carry out general checks on the gas chromatograph unit (leaks from the gas circuits, detector efficiency, efficiency of the splitting system and recording system, etc.).
5.4.1.2.If the column is being used for the first time it is recommended that it should be subjected to conditioning. Pass a gentle flow of gas through the column and then switch on the gas chromatography unit and begin gradual heating up to a temperature of at least 20 °C above the operating temperature (Note 6). Hold this temperature for at least two hours, then place the entire unit in operating mode (adjustment of gas flows and splitting, ignition of the flame, connection with the electronic recorder, adjustment of the column chamber, detector and injector temperature, etc.) and then record the signal with a sensitivity at least two times greater than that intended for the analysis. The course of the base line must be linear, without peaks of any kind, and must not drift.U.K.
A negative straight-line drift indicates leakage from the column connections; a positive drift indicates inadequate conditioning of the column.
Note 6.U.K.
The conditioning temperature must always be at least 20 °C less than the maximum temperature specified for the stationary phase used.U.K.
5.4.2.Choice of operating conditions.U.K.
5.4.2.1.The guideline operating conditions are as follows:U.K.
column temperature: 260 ± 5 °C,
evaporator temperature: 280 °C,
detector temperature: 290 °C,
linear velocity of the carrier gas: helium 20 to 35 cm/s, hydrogen 30 to 50 cm/s,
splitting ratio: from 1:50 to 1:100,
instrument sensitivity: from 4 to 16 times the minimum attenuation,
recording sensitivity: 1 to 2 mV f.s.,
paper speed: 30 to 60 cm/hour,
amount of substance injected: 0,5 to 1 μl of TMSE solution.
These conditions may be varied in the light of column and gas-chromatograph characteristics so as to obtain chromatograms which meet the following requirements:
the retention time for β-sitosterol should be 20 ± 5 minutes,
the campesterol peak should be: for olive oil (mean content 3 %) 15 ± 5 % of full scale; for soya oil (mean content 20 %) 80 ± 10 % of full scale,
all the sterols present must be separated. In addition to being separated the peaks must also be completely resolved, i.e. the peak trace should return to the base line before leaving for the next peak. Incomplete resolution is however tolerated provided that the peak at TRR 1,02 can be quantified using the perpendicular.
5.4.3.Analytical procedure.U.K.
5.4.3.1.Using the 10 μl microsyringe take 1 μl of hexane, draw in 0,5 μl of air and then 0,5 to 1 μl of the sample solution. Raise the plunger of the syringe further so the needle is emptied. Push the needle through the membrane of the injection unit and after one to two seconds inject rapidly, then slowly remove the needle after some five seconds.U.K.
5.4.3.2.Continue recording until the TMSE of the sterols present are completely elutedU.K.
The base line must continue to meet the requirements (5.4.1.2).
5.4.4.Peak identification.U.K.
Identify individual peaks on the basis of retention times and by comparison with mixtures of sterol TMSE analysed under the same conditions.
The sterols are eluted in the following order: cholesterol, brassicasterol, 24-methylene cholesterol, campesterol, campestanol, stigmasterol, Δ 7-campesterol, Δ 5,23-stigmastadienol, [X1chlerosterol], β-sistosterol, sitostanol, Δ 5-avenasterol, Δ 5,24-stigmastadienol[X1, Δ 7-stigmasterol,] Δ 7-avenasterol.
The retention times for sitosterol for SE-52 and SE-54 columns are shown in Table 1.
Figures 1 and 2 illustrate typical chromatograms for some oils.
5.4.5.Quantitative evaluation.U.K.
5.4.5.1.Calculate the areas of the [X1β-cholestanol and the sterol peaks using the integrator. Ignore peaks for any compounds which are not included among those listed in Table 1. The response coefficient for β-cholestanol] is to be equal to 1.U.K.
5.4.5.2.Calculate the concentration of each individual sterol in mg/100 g of fatty material as follows:U.K.
where:
=
mass of β-cholestanol] added, im milligrams;
=
mass of the sample used for determination, in grams.
6.EXPRESSION OF THE RESULTSU.K.
6.1Record individual sterol concentrations as mg/100 g of fatty material and their sum as ‘total sterols’.U.K.
6.2Calculate the percentage of each individual sterol from the ratio of the relevant peak area to the total peak area for sterols.U.K.
where:
=
peak area for x;
=
total peak area for sterols.
APPENDIX
Determination of the linear velocity of the gasU.K.
With the gas chromatograph set to normal operating conditions inject 1 to 3 μl of methane (or propane) and measure the time taken by the gas to pass through the column from the time of injection to the time at which the peak appears (tM).
The linear velocity in cm/s is given by L/tM, where L is the length of the column in centimetres and tM is the measured time in seconds.
Table I
Relative retention times for sterols
| Peak | Identification | Relative retention time | ||
|---|---|---|---|---|
| SE 54column | SE 52column | |||
| 1 | cholesterol | Δ-5-cholesten-3β-ol | 0,67 | 0,63 |
| 2 | cholestanol | 5α-cholestan-3β-ol | 0,68 | 0,64 |
| 3 | brassicasterol | [24S]-24-methyl-Δ-5,22-cholestadien-3β-ol | 0,73 | 0,71 |
| 4 | 24-methylene-cholesterol | 24-methylene-Δ-5,24-cholesten-3β-ol | 0,82 | 0,8 |
| 5 | campesterol | [24R]-24-methyl-Δ-5-cholesten-3β-ol | 0,83 | 0,81 |
| 6 | campestanol | [24R]-24-methyl-cholestan-3β-ol | 0,85 | 0,82 |
| 7 | stigmasterol | [24R]-24-ethyl-Δ-5,22-cholestadien-3β-ol | 0,88 | 0,87 |
| 8 | Δ-7-campesterol | [24R]-24-methyl-Δ-7-cholesten-3β-ol | 0,93 | 0,92 |
| 9 | Δ-5,23-stigmastadienol | [24R,S]-24-ethyl-Δ-5,23-cholestadien-3β-ol | 0,95 | 0,95 |
| 10 | chlerosterol | [24S]-24-ethyl-Δ-5,25-cholastadien-3β-ol | 0,96 | 0,96 |
| 11 | β-sitosterol | [24R]-24-ethyl-Δ-5-cholestan-3β-ol | 1,0 | 1,0 |
| 12 | sitostanol | 24-ethyl-cholestan-3β-ol | 1,02 | 1,02 |
| 13 | Δ-5-avenasterol | [24Z]-24-ethylidene-5-cholesten-3β-ol | 1,03 | 1,03 |
| 14 | Δ-5,24-stigmastadienol | [24R,S]-24-ethyl-Δ-5,24-cholestadien-3β-ol | 1,08 | 1,08 |
| 15 | Δ-7-stigmastenol | [24R,S]-24-Ethyl-Δ-7,24-cholestadien-3β-ol | 1,12 | 1,12 |
| 16 | Δ-7-avenasterol | [24Z]-24-ethyliden-Δ-7-cholesten-3β-ol | 1,16 | 1,16 |
ANNEX VIU.K.DETERMINATION OF ERYTHRODIOL AND UVAOL
INTRODUCTIONU.K.
Erythrodiol (commonly understood as the glycols erythrodiol and uvaol together) is a constituent of the unsaponifiable fraction, characteristic of some types of fatty substances. It is found at considerably higher concentrations in solvent-extracted olive oil than in other oils, such as pressed olive oil and grape pip oil, which also contain it, and so its presence may demonstrate the presence of solvent-extract olive oil.
1.SCOPEU.K.
The method describes a procedure for detecting erythrodiol in fatty substances.
2.PRINCIPLE OF THE METHODU.K.
The fatty substance is saponified with potassium [X1hydroxide in ethanolic solution. The unsaponifiable fraction is then extracted with diethyl ether] and purified by passage over a column of alumina.
The unsaponifiables are subjected to thin-layer chromatography on a silica gel plate until the bands corresponding to the sterol and erythrodiol fractions are separated. The sterols and the erythrodiol recovered from the plate are transformed into trimethylsilyl ethers and the mixture is analysed by gas chromatography.
The result is expressed as the percentage of erythrodiol in the mixture of erythrodiol and sterols.
3.APPARATUSU.K.
3.1.The apparatus described in Annex V (determination of the content of sterols).U.K.
4.REAGENTSU.K.
4.1.The reagents described in Annex V (determination of the content of sterols).U.K.
4.2.Reference solution of erythrodiol, 0,5 % solution in chloroform.U.K.
5.PROCEDUREU.K.
5.1.Preparation of the unsaponifiables.U.K.
As described at paragraph 5.1.2 of Annex V.
5.2.Separation of erythrodiol and the sterols.U.K.
5.2.1.See paragraph 5.2.1 of Annex V.U.K.
5.2.2.See paragraph 5.2.2 of Annex V.U.K.
5.2.3.Prepare a 5 % solution of the unsaponifiables in chloroform.U.K.
Using the 0,1 ml microsyringe, streak a chromatographic plate with 0,3 ml of solution approximately 1,5 cm from the lower edge in a streak which is as thin and uniform as possible.
At one end of the plate place a few microlitres of the solutions of cholesterol and erythrodiol to serve as a reference.
5.2.4.Place the plate in the developing chamber prepared as specified in 5.2.1. The ambient temperature should be about 20 °C. Immediately close the chamber with the cover and allow to elute until the solvent front reaches approximately 1 cm from the upper edge of the plate. Remove the plate from the developing chamber and evaporate the solvent in a flow of hot air.U.K.
5.2.5.Spray the plate lightly and uniformly with the alcoholic 2,7-dichlorofluoroscein solution. When the plate is observed under ultralviolet light the sterol and erythrodiol bands can be identified through being aligned with the references. Mark with a spot just outside the edges of the fluorescence.U.K.
5.2.6.Using a metal spatula scrape off the silica gel in the marked areas. Place the material from the plate in a 50 ml flask. Add 15 ml of hot chloroform, shake well and filter through a funnel with a sintered glass disc so that the silica gel is transferred to the filter. Wash three times with hot chloroform (10 ml each time) collecting the filtrate in a 100 ml flask. Evaporate the filtrate to a volume of 4 to 5 ml, transfer to a calibrated 10 ml conical-bottomed centrifuge tube, dry by gently heating in a current of nitrogen and weigh.U.K.
5.3.Preparation of the trimethylsily estersU.K.
As described in paragraph 5.3 of Annex V.
5.4.Gas chromatographic analysisU.K.
As described in paragraph 5.4 of the above method. The operating conditions of the gas chromatograph in analysis must be such as to perform the sterol analysis and separate the TMSE from the erythrodiol and uvaol.
Once the sample has been injected, continue recording until the sterols present, the erythrodiol and the uvaol have been eluted. Then identify the peaks (the retention times for erythrodiol and uvaol relative to β-sitosterol are about 1,45 and 1,55 respectively) and calculate the areas as for the sterols.
6.EXPRESSION OF THE RESULTSU.K.
where:
=
peak area for uvaol in square millimetres;
=
total peak area for sterols in square millimetres].
The result is expressed to one decimal place.
[F1ANNEX VII U.K. DETERMINATION OF THE PERCENTAGE OF 2-GLYCERYL MONOPALMITATE
1. PURPOSE AND SCOPE U.K.
This method describes the analysis procedure for determining the percentage of palmitic acid in position 2 of the triglycerides by evaluating 2-glyceryl monopalmitate.
This method can be applied to liquid vegetable oils at ambient temperature (20 °C).
2. PRINCIPLE U.K.
After preparation the oil sample is subjected to the action of pancreatic lipase: partial and specific hydrolysis in positions 1 and 3 of the triglyceride molecule causes monoglycerides to appear in position 2. The percentage of 2-glyceryl monopalmitate in the monoglyceride fraction is determined after silylation by capillary-column gas chromatography.
3. APPARATUS AND MATERIALS U.K.
3.1. 25 ml Erlenmeyer flask U.K.
3.2. 100, 250 and 300 ml beakers U.K.
3.3. Glass chromatograph column, internal diameter 21-23 mm, length 400 mm, fitted with a sintered glass disc and a stopcock U.K.
3.4. 10, 50, 100 and 200 ml measuring cylinders U.K.
3.5. 100 and 250 ml flasks U.K.
3.6. Rotary evaporator U.K.
3.7. 10 ml conical-bottomed centrifuge tubes with groundglass stopper U.K.
3.8. Centrifuge for 10 and 100 ml tubes U.K.
3.9. Thermostat permitting a stable temperature of 40 ± 0,5 °C U.K.
3.10. 1 and 2 ml graduated pipettes U.K.
3.11. 1 ml hypodermic syringe U.K.
3.12. 100 μl microsyringe U.K.
3.13. 1 000 ml funnel U.K.
3.14. Capillary gas chromatograph with an on-column cold injector for direct injection of the sample into the column and a furnace able to maintain the selected temperature to approximately 1 °C U.K.
3.15. On-column cold injector for direct injection of the sample into the column U.K.
3.16. Flame ionisation detector and electrometer U.K.
3.17. Recorder-integrator adapted to the electrometer with a response rate no greater than 1 sec and a variable paper roll rate U.K.
3.18. Capillary column made of glass or fused silica 8-12 metres long, 0,25-0,32 mm internal diameter, covered with methylpolysiloxane or phenyl methylpolysiloxane 5 %, 0,10-0,30 μm thick, useable at 370 °C U.K.
3.19. 10 μl microsyringe fitted with a hardened needle, at least 7,5 cm long for direct on-column injection. U.K.
4. REAGENTS U.K.
4.1. Silica gel with a grain size of between 0,063 and 0,200 mm (70/280 mesh) prepared as follows: Place the silica gel in a porcelain capsule, dry in an incubator at 160 °C for four hours, then leave to cool at room temperature in a desiccator. Add water equivalent to 5 % of the mass of the silica gel as follows: Weigh 152 g silica gel into an Erlenmeyer flask then add 8 g of distilled water, stopper and shake gently to distribute the water evenly. Leave to stand for at least 12 hours before use. U.K.
4.2. n-hexane (for chromatography) U.K.
4.3. Isopropanol U.K.
4.4. Isopropanol, 1/1 (v/v) aqueous solution U.K.
4.5. Pancreatic lipase. It must have an activity of between 2,0 and 10 lipase units per mg. (Pancreatic lipases with an activity of between 2 and 10 units per mg enzyme are commercially available.) U.K.
4.6. Buffer solution of trishydroxymethylaminomethane: 1 M aqueous solution adjusted to pH 8 (potentiometric control) by conc. HCl (1/1 v/v) U.K.
4.7. Enzyme-quality sodium cholate, 0,1 % aqueous solution (this solution must be used within two weeks of its preparation) U.K.
4.8. Calcium chloride, 22 % aqueous solution U.K.
4.9. Diethyl ether for chromatography U.K.
4.10. Developer solvent: mixture of n-hexane/diethyl ether (87:13 v:v) U.K.
4.11. Sodium hydroxide, 12 % by weight solution U.K.
4.12. Phenolphthalein, 1 % solution in ethanol U.K.
4.13. Carrier gas: hydrogen or helium, for gas chromatography U.K.
4.14. Auxiliary gases: hydrogen, 99 % minimum purity, free from moisture and organic substances, and air, for gas chromatography, of the same purity U.K.
4.15. Silanisation reagent: mixture of pyridine/hexamethyldisilazane, trimethylchlorosilane 9/3/1 (v/v/v). (Ready-to-use solutions are commercially available. Other silylation reagents may be used, particularly bis-trimethylsilyl trifluoracetamide + 1 % trimethylchlorosilane, diluted with an identical volume of anhydrous pyridine.) U.K.
4.16. Reference samples: pure monoglycerides or monoglyceride mixtures with a known percentage composition similar to that of the sample. U.K.
5. METHOD U.K.
5.1. Sample preparation U.K.
5.1.1. Oils with a free acidity of less than 3 % do not need to be neutralised before chromatography on a silica gel column. Oils with a free acidity of more than 3 % must be neutralised as per point 5.1.1.1. U.K.
5.1.1.1. Pour 50 g of oil and 200 ml n-hexane into the 1 000 ml funnel (3.13). Add 100 ml of isopropanol and a quantity of 12 % sodium hydroxide solution (4.11) equivalent to the free acidity of the oil plus 5 %. Shake vigorously for one minute. Add 100 ml of distilled water, shake again and leave to stand. U.K.
After decanting, remove the lower layer containing the soaps. Remove any intermediate layers (mucilage and insoluble substances). Wash the hexane solution of the neutralised oil with successive portions of 50-60 ml of the 1/1 (v/v) isopropanol/water solution (4.4) until the pink colouration of the phenolphthalein disappears.
Remove most of the hexane by vacuum distillation (use a rotary evaporator, for example) and transfer the oil into a 100 ml flask (3.5). Dry the oil in vacuum until the solvent is completely removed.
After that procedure is completed, the acidity of the oil should be less than 0,5 %.
5.1.2. Put 1,0 g of the oil prepared as above into a 25 ml Erlenmeyer flask (3.1) and dissolve in 10 ml of developer mixture (4.10). Leave the solution to stand for at least 15 minutes before silica gel column chromatography. U.K.
If the solution is cloudy centrifuge it to ensure optimum conditions for chromatography. (Ready-to-use 500 mg silica gel SPE cartridges can be used).
5.1.3. Preparation of the chromatography column U.K.
Pour about 30 ml of the developer solvent (4.10) into the column (3.3), insert a piece of cotton into the bottom part of the column using a glass rod; press to eliminate the air.
In a beaker prepare a suspension of 25 g of silica gel (4.1) in about 80 ml of developer solvent and pour it into the column using a funnel.
Check that all the silica gel is in the column; wash with developer solvent (4.10), open the stopcock and allow the liquid to reach a level about 2 mm above the level of the silica gel.
5.1.4. Column chromatography U.K.
Weigh accurately 1,0 g of sample prepared as in point 5.1 into a 25 ml Erlenmeyer flask (3.1).
Dissolve the sample in 10 ml of developer solvent (4.10). Pour the solution into the chromatography column prepared as in point 5.1.3. Avoid disturbing the surface of the column.
Open the stopcock and pour the sample solution until it reaches the level of the silica gel. Develop with 150 ml of the developer solvent. Adjust the flow rate to 2 ml/min (so that 150 ml enters the column in about 60-70 minutes).
Recover the eluate in a previously weighed 250 ml flask. Evaporate the solvent under vacuum and remove the final traces of the solvent under a nitrogen current.
Weigh the flask and calculate the recovered extract.
(If ready-to-use silica gel SPE cartridges are used use the following method: Put 1 ml of solution (5.1.2) into the prepared cartridges with 3 ml of n-hexane.
After percolating the solution develop with 4 ml of n-hexane/diethyl ether 9/1 (v/v).
Recover the eluate in a 10 ml tube and evaporate to dry in a nitrogen current.
Expose the dry residue to pancreatic lipase (5.2). (It is essential to check the fatty acid composition before and after crossing the SPE cartridge.)
5.2. Hydrolysis by pancreatic lipase U.K.
5.2.1. Weigh into the centrifuge tube 0.1 g of the oil prepared as in point 5.1. Add 2 ml of buffer solution (4.6), 0,5 ml of the sodium cholate solution (4.7) and 0,2 ml of the calcium chloride solution, stirring well after each addition. Close the tube with the groundglass stopper and place in the thermostat at 40 + 0,5 °C. U.K.
5.2.2. Add 20 mg of lipase, shake carefully (avoid wetting the stopper) and place the tube in the thermostat for exactly two minutes. Then remove it, shake vigorously for exactly 1 minute and leave to cool. U.K.
5.2.3. Add 1 ml of diethyl ether, stopper and shake vigorously, then centrifuge and transfer the ether solution into a clean, dry tube using a microsyringe. U.K.
5.3. Preparation of the silanised derivatives and gas chromatography U.K.
5.3.1. With a microsyringe insert 100 μl of solution (5.2.3) into a 10 ml conical-bottomed tube. U.K.
5.3.2. Remove the solvent under a slight nitrogen current, add 200 μl of silanisation reagent (4.15), stopper the tube and leave to stand for 20 minutes. U.K.
5.3.3. After 20 minutes, add 1 to 5 ml of n-hexane (depending on the chromatography conditions): the resulting solution is ready for gas chromatography. U.K.
5.4. Gas chromatography U.K.
Operating conditions:
Injector temperature (on-column injector) lower than solvent boiling point (68 °C);
Detector temperature: 350 °C;
Column temperature: programming of furnace temperature: 60 °C for 1 minute, increasing by 15 °C per minute up to 180 °C, then by 5 °C per minute up to 340 °C, then 340 °C for 13 minutes;
Carrier gas: hydrogen or helium, set at a linear velocity sufficient to obtain the resolution reflected in Figure 1. The retention time of the C 54 triglyceride must be 40 ± 5 minutes (see Figure 2). (The operating conditions indicated above are indicative. Operators will have to optimise them to obtain the desired resolution. The peak corresponding to 2-glyceryl monopalmitate must have a minimum height equal to 10 % of the recorder scale.)
Quantity of substance injected: 0,5-1 μl of the n-hexane solution (5 ml) (5.3.3).
5.4.1. Identification of the peaks U.K.
The individual monoglycerides are identified from their retention times and by comparison with those obtained for standard monoglyceride mixtures under the same conditions.
5.4.2. Quantitative evaluation U.K.
The area of each peak is calculated using an electronic integrator.
6. EXPRESSION OF RESULTS U.K.
The percentage of glyceryl monopalmitate is calculated from the ratio between the area of the corresponding peak and the areas of the peaks of all the monoglycerides (see Figure 2) using the formula:
where:
=
area of the peak corresponding to glyceryl monopalmitate
=
sum of the areas of all the monoglyceride peaks
The result must be to one decimal place.
7. ANALYSIS REPORT U.K.
The analysis report must specify:
reference to this method,
all the information needed for a full identification of the sample,
the analysis result,
any deviation from the method, whether as the result of a decision by the parties concerned or for another reason,
details to identify the laboratory, the date of the analysis and the signatures of those responsible for the analysis.
Figure 1
Chromatogram of the products of the silanisation reaction obtained by the action of lipase on a refined olive oil with 20 % esterified oil added (100 %)
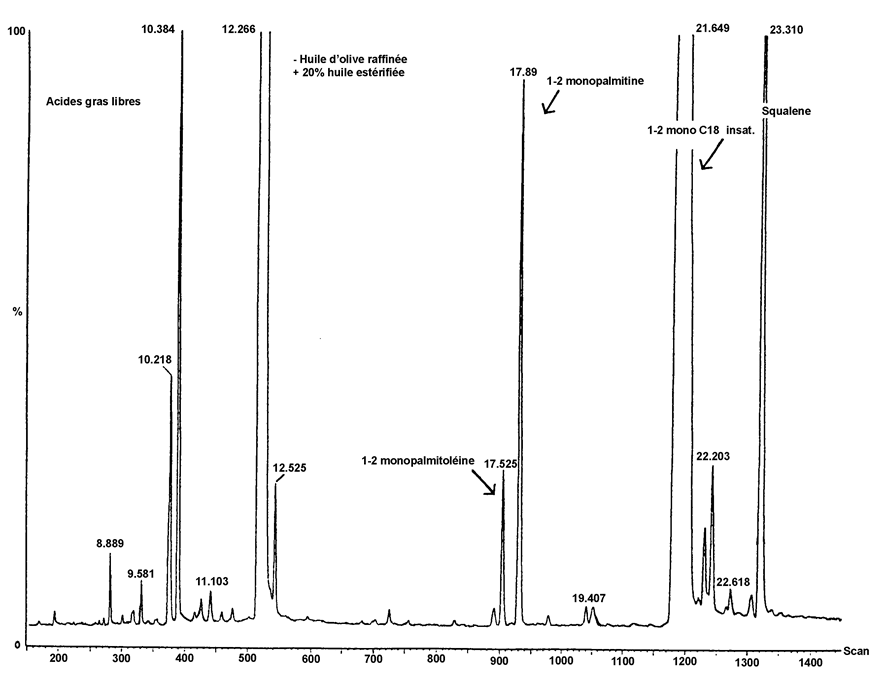
Figure 2 U.K.
Chromatogram of :
( A ) unesterified olive oil, after lipase; after silanisation; under these conditions (8-12 m capillary column) the wax fraction is eluted at the same time as the diglyceride fraction or slightly afterwards . U.K.
After lipase, the triglyceride content should not exceed 15 %
Chromatogram of :
( B ) unesterified oil after lipase; after silanisation; under these conditions (8-12 m capillary column) the wax fraction is eluted at the same time as the diglyceride fraction or slightly afterwards . U.K.
After lipase, the triglyceride content should not exceed 15 % .
8. NOTES U.K.
Note 1. PREPARATION OF THE LIPASE U.K.
Lipases with satisfactory activity are commercially available. They can also be prepared in the laboratory in the following manner:
Cool to 0 °C 5 kg of fresh pig’s pancreas. Remove the surrounding solid fat and the connective tissue and grind to a liquid paste in a blender. Stir the paste with 2,5 litres of anhydrous acetone for 4-6 hours, then centrifuge. Extract the residue three more times with the same volume of anhydrous acetone, then twice with an acetone/diethyl ether mixture (1/1 v/v) and twice with diethyl ether.
Vacuum-dry the residue for 48 hours to obtain a stable powder which can be stored for a long time in a refrigerator away from moisture.
Note 2. MONITORING LIPASE ACTIVITY U.K.
Prepare an olive oil emulsion as follows:
In a mixer stir for 10 minutes a mixture of 165 ml of a 100 g/l gum arabic solution, 15 g of crushed ice and 20 ml of a previously neutralised olive oil.
Pour 10 ml of the emulsion into a 50 ml beaker, then 0,3 ml of a 0,2 g/ml sodium cholate solution and then 20 ml of distilled water.
Put the beaker in a thermostat set at 37 °C; introduce the electrodes of the pH meter and the screw agitator.
Using a burette, add a 0,1 N sodium hydroxide solution drop by drop until a pH of 8,3 is obtained.
Add an aliquot of the lipase powder suspension in water (0,1 g/ml of lipase). As soon as the pH meter reads 8,3, start the chronometer and add the sodium hydroxide solution drop by drop at a rate which maintains the pH at 8,3. Note every minute the volume of solution consumed.
Record the data on an x/y graph with the time on the x-axis and millilitres of 0,1 N alkaline solution consumed to keep a constant pH on the y-axis. A linear graph should be obtained.
Lipase activity, expressed in lipase units per mg, is given by the following formula:
where:
is activity in lipase units/mg
is the number of millilitres of 0,1 N sodium hydroxide solution per minute (calculated on the basis of the graph)
is the titre of the sodium hydroxide solution
is the mass in mg of the test lipase.
A lipase unit is defined as the quantity of enzyme which releases 10 micro-equivalents of acid per minute.]
F8ANNEX VIIIU.K. [F8DETERMINATION OF TRILINOLEIN CONTENT]
Textual Amendments
1.SCOPEU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2.FIELD OF APPLICATIONU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3.PRINCIPLEU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4.APPARATUSU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
5.REAGENTSU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
6.PREPARATION OF SAMPLESU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7.PROCEDUREU.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
8.CALCULATION AND EXPRESSION OF RESULTSU.K.
F8. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F8. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F8. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note 1.U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note 2. Examples: U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note 3.U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note 4.U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Note 5:U.K.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F8. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . .U.K.
F8. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .]
. . . . .U.K.
ANNEX IXU.K.SPECTROPHOTOMETRIC INVESTIGATION IN THE ULTRAVIOLET
FOREWORDU.K.
Spectrophotometric examination in the ultraviolet can provide information on the quality of a fat, its state of preservation and changes brought about in it by technological processes.
The absorption at the wavelengths specified in the method is due to the presence of conjugated diene and triene systems. These absorptions are expressed as specific extinctions E1 % 1 cm (the extinction of 1 % solution of the fat in the specified solvent, in a thickness of 1 cm) conventionally indicated by K (also referred to as ‘extinction coefficient’).
1.SCOPEU.K.
The method describes the procedure for performing a spectrophotometric examination [X1of olive oil in] the ultraviolet.
2.PRINCIPLE OF THE METHODU.K.
The fat in question is dissolved in the required solvent and the extinction of the solution is then determined at the specified wavelengths with reference to pure solvent. Specific extinctions are calculated from the spectrophotometer readings.
3.EQUIPMENTU.K.
3.1.A spectrophotometer for measuring extinction in the ultraviolet between 220 and 360 nm, with the possibility of reading individual nanometric units.U.K.
3.2.Rectangular quartz cuvettes, with covers, having an optical length of 1 cm. When filled with water or other suitable solvent the cuvettes should not show differences between them of more than 0,01 extinction units.U.K.
3.3.25 ml graduated flasks.U.K.
[F23.4. Chromatography column having an upper part 270 mm in length and a diameter of 35 mm and a lower part 270 mm in length and a diameter of approximately 10mm.] U.K.
4.REAGENTSU.K.
4.1.Spectrophotometrically pure iso-octane (2,2,4-trimethylpentane). With reference to distilled water this should have a transmittance of not less than 60 % at 220 nm and not less than 95 % at 250 nm, orU.K.
spectrophotometrically pure cyclohexane: with reference to distilled water this should have a transmittance of not less than 40 % at 220 nm and not less than 95 % at 250 nm[F2.]
[F6. . . . .]
4.2.Basic alumina for column chromatography prepared and checked as described in Appendix I.U.K.
4.3.n-hexane, for chromatography.U.K.
5.PROCEDUREU.K.
5.1.The sample in question must be perfectly homogeneous and without suspected impurities. Oils which are liquid at ambient temperature are to be filtered through paper at a temperature of approximately 30 °C, hard fats are to be homogenized and filtered at a temperature of not more than 10 °C above the melting point.U.K.
5.2.Weigh accurately approximately 0,25 g of the sample so prepared into a 25 ml graduated flask, make up to the mark with the solvent specified and homogenize. The resulting solution must be perfectly clear. If opalescence or turbidity is present filter quickly through paper.U.K.
5.3.Fill a cuvette with the solution obtained and measure the extinctions at an appropriate wavelength between 232 and 276 nm, using the solvent used as a reference.U.K.
The extinction values recorded must lie within the range 0,1 to 0,8. If not the measurements must be repeated using more concentrated or more dilute solutions as appropriate.
5.4.When a determination of specific extinction is required after passage over alumina, proceed as follows. Place 30 g of basic alumina in suspension in hexane in the chromatography column. After the adsorbent has settled remove the excess hexane down to approximately 1 cm above the top of the alumina.U.K.
Dissolve 10 g of the fat, homogenized and filtered as described in 5.1, in 100 ml of hexane and pour the solution into the column. Collect the eluate and evaporate off all the solvent under vacuum at a temperature below 25 °C.
Proceed immediately as specified in 5.2 using the fat so obtained.
6.EXPRESSION OF THE RESULTSU.K.
6.1.Record the specific extinctions (extinction coefficients) at the various wavelengths calculated as follows:U.K.
where:
=
specific extinction at wavelength λ;
=
extinction measured at wavelength λ;
=
concentration of the solution in g/100 ml;
=
thickness of the cuvette in cm.
The results are to be expressed to two decimal places.
6.2.Spectrophotometric analysis of olive oil in accordance with the official method in the EEC regulations specifies determination of the specific extinction in iso-octane solution at wavelengths of 232 and 270 nm and the determination K, which is given by:U.K.
where Km is the specific extinction at wavelength m, the wavelength for maximum absorption around 270 nm.
APPENDIX IPreparation of the alumina and testing its activity
A.1.1.Preperation of the aluminaU.K.
Place alumina which has been previously desiccated in a furnace at 380 to 400 °C for three hours into a hermetically sealed container, add distilled water in the ratio of 5 ml per 100 g of alumina, immediately close the container, shake repeatedly, and then allow to rest for at least 12 hours before use.
A.1.2.Checking the activity of the aluminaU.K.
Prepare a chromatographic column with 30 g of alumina. Working as described in paragraph 5.4 pass a mixture consisting of:
95 % virgin olive oil having a specific extinction of less than 0,18 at 268 nm,
5 % ground-nut oil treated with earth in the refining process, having a specific extinction of not less than 4 at 268 nm
through the column.
If after passage through the column the mixture has a specific extinction of more than 0,11 at 268 nm the alumina is acceptable, if not the level of dehydration must be increased.
APPENDIX IICalibration of the spectrophotometer
A.2.The equipment must be checked at intervals (at least every six months) for both wavelength response and the accuracy of the response.U.K.
A.2.1.The wavelength may be checked using a mercury vapour lamp or by means of suitable filters.U.K.
A.2.2.In order to check the response of the photocell and the photomultiplier proceed as follows: weigh 0,2000 g of pure potassium chromate for spectrophotometry and dissolve in 0,05 N potassium hydroxide solution in a 1 000 ml graduated flask and make up to the mark. Take precisely 25 ml of the solution obtained, transfer to a 500 ml graduated flask and dilute up to the mark using the same potassium hydroxide solution.U.K.
Measure the extinction of the solution so obtained at 275 nm, using the potassium hydroxide solution as a reference. The extinction measured using a 1 cm cuvette should be 0,200 ± 0,005.
ANNEX X AU.K.ANALYSIS BY GAS CHROMATOGRAPHY OF METHYL ESTERS OF FATTY ACIDS
1.SCOPEU.K.
This method gives general guidance for the application of gas chromatography, using packed or capillary columns, to determine the qualitative and quantitative composition of a mixture of fatty acid methyl esters obtained in accordance with the method specified in Annex X B.
The method is not applicable to polymerized fatty acids.
2.REAGENTSU.K.
2.1.Carrier gasU.K.
Inert gas (nitrogen, helium, argon, hydrogen, etc.), thoroughly dried and with an oxygen content of less than 10 mg/kg.
Note 1.U.K.
Hydrogen, which is used as a carrier gas only with capillary columns, can double the speed of analysis but is hazardous. Safety devices are available.U.K.
2.2.Auxiliary gasesU.K.
2.2.1.Hydrogen (purity ≥ 99,9 %), feree from organic impurities.U.K.
2.2.2.Air or oxygen, free from organic impurities.U.K.
2.3.Reference standardU.K.
A mixture of methyl esters of pure fatty acids, or the methyl esters of a fat of known composition, preferably similar to that of the fatty matter to be analyzed.
Care shall be taken to prevent the oxidation of polyunsaturated fatty acids.
3.APPARATUSU.K.
The instructions given relate to the usual equipment used for gas chromatography, employing packed and/or capillary columns and a flame-ionization detector. Any apparatus giving the efficiency and resolution specified in 4.1.2 is suitable.
3.1.Gas chromatographU.K.
The gas chromatograph shall comprise the following elements.
3.1.1.Injection systemU.K.
Use an injection system either:
with packed columns, having the least deadspace possible (in this case the injection system shall be capable of being heated to a temperature 20 to 50 °C higher than that of the column); or
with capillary columns, in which case the injection system shall be specially designed for use with such columns. It may be of the split type or it may be of the splitless on column injector type.
Note 2.U.K.
In the absence of fatty acids with less than 16 carbon atoms, a moving needle injector may be used.U.K.
3.1.2.OvenU.K.
The oven shall be capable of heating the column to a temperature of at least 260 °C and of maintaining the desired temperature to within 1 °C with a packed column and within 0,1 °C with a capillary column. The last requirement is particularly important when a fused silica tube is used.
The use of temperature-programmed heating is recommended in all cases, and in particular for fatty acids with less than 16 carbon atoms.
3.1.3.Packed columnU.K.
3.1.3.1.Column, constructed of a material inert to the substances to be analyzed (i.e. glass or stainless steel) having the following dimensions:U.K.
length: 1 to 3 m. A relatively short column should be used when long-chain fatty acids (above C20) are present. When analyzing acids with 4 or 6 carbon atoms, it is recommended that a column 2 m in length is used;
internal diameter: 2 to 4 mm.
Note 3.U.K.
If polyunsaturated components with more than three double bonds are present, they may be decomposed in a stainless steel column.U.K.
Note 4.U.K.
A system with packed twin columns may be used.U.K.
3.1.3.2.Packing, comprising the following elements:U.K.
support: acid-washed and silanized diatomaceous earth, or other suitable inert support with a narrow range of grain size (25 μm range between the limits 125 to 200 μm) the average grain size being related to the internal diameter and length of the column;
stationary phase: polyester type of polar liquid (e.g. diethylene glycol polysuccinate, butanediol polysuccinate, ethyleneglycol polyadipate, etc.) cyanosilicones or any other liquid permitting the chromatographic separation required (see clause 4). The stationary phase should amount to 5 to 20 % (m/m) of the packing. A non-polar stationary phase can be used for certain separations.
3.1.3.3.Conditioning of the columnU.K.
With the column disconnected, if possible, from the detector, gradually heat the oven to 185 °C and pass a current of inert gas through the freshly prepared column at a rate of 20 to 60 ml/min for at least 16 hours at this temperature, and for a further 2 hours at 195 °C.
3.1.4.Capillary columnU.K.
3.1.4.1.Tube, made of a material inert to the substances to be analysed (usually glass or fused silica). The internal diameter shall be between 0,2 and 0,8 mm. The internal surface shall undergo an appropriate treatment (e.g. surface preparation, inactivation) before receiving the stationary phase coating. A length of 25 mm is sufficient in most cases.U.K.
3.1.4.2.Stationary phase, usually of the type polyglycol (poly(ethylene glycol) 20 000), polyester (butanediol polysuccinate) or polar polysiloxane (cyanosilicones). Bonded (cross-linked) columns are suitable.U.K.
Note 5.U.K.
There is a risk of polar polysiloxanes giving rise to difficulties in the identification and separation of linolenic acid and C20 acids.U.K.
The coatings shall be thin, i.e, 0,1 to 0,2 μm.
3.1.4.3.Assembly and conditioning of the columnU.K.
Observe the normal precautions for assembling capillary columns, i.e. arrangement of the column in the oven (support), choice and assembly of joints (leak tightness), positioning of the ends of the column in the injector and the detector (reduction of dead-spaces). Place the column under a flow of carrier gas (e.g. 0,3 bar (30 kPa) for a column of length 25 mm and internal diameter 0,3 mm).
Condition the column by temperature programming of the oven at 3 °C/min from ambient temperature to a temperature 10 °C below the decomposure limit of the stationary phase. Maintain the oven at this temperature for one hour until stabilization of the baseline. Return it to 180 °C to work under isothermal conditions.
Note 6.U.K.
Suitably pre-conditioned columns are available commercially.U.K.
3.1.5.Detector, preferably capable of being heated to a temperature above that of the column.U.K.
3.2.SyringeU.K.
The syringe shall have a maximum capacity of 10 μl, and be graduated in 0,1 μl divisions.
3.3.RecorderU.K.
If the recorder curve is to be used to calculate the composition of the mixture analysed, an electronic recorder of high precision, compatible with the apparatus used, is required. The recorder shall have the following characteristics:
rate of response, below 1,5 s, preferably 1 s (the rate of response is the time taken for the recording pen to pass from 0 to 90 % following the sudden introduction of a 100 % signal);
width of the paper, 20 cm minimum;
paper speed, adjustable to values between 0,4 and 2,5 cm/min.
3.4.IntegratorU.K.
Rapid and accurate calculation can be performed with the help of an electronic integrator. This shall give a linear response with adequate sensitivity, and the correction for deviation of the base-line shall be satisfactory.
4.PROCEDUREU.K.
The operations described in 4.1 to 4.3 relate to the use of a flame-ionization detector.
As an alternative a gas chromatograph employing a catharometer detector (working on the principle of thermal conductivity changes) may be used. The operating conditions are then modified as described in clause 6.
4.1.Test conditionsU.K.
4.1.1.Selection of optimum operating conditionsU.K.
4.1.1.1.Packed columnU.K.
In the selection of the test conditions, the following variables should be taken into account:
the length and diameter of the column;
the nature and amount of the stationary phase;
the temperature of the column;
the carrier gas flow;
the resolution required;
the size of the test portion, selected in such a way that the assembly of the detector and electrometer gives a linear response;
the duration of analysis.
In general, the values given in Table 1 and Table 2 will lead to the desired results, i.e. at least 2 000 theoretical Iplates per metre of column length for methyl stearate and its elution within about 15 minutes.
Where the apparatus allows it, the injector should be at a temperature of about 200 °C and the detector at a temperature equal to or higher than that of the column.
As a rule, the ratio of the flow-rate of the hydrogen supplied to the flame-ionization detector to that of the carrier gas varies from 1:2 to 1:1 depending on the diameter of the column. The flow of oxygen is about 5 to 10 times that of the hydrogen.
| Table 1 | |
| Internal diameter of columnmm | Carrier gas flowml/min |
|---|---|
| 2 | 15 to 25 |
| 3 | 20 to 40 |
| 4 | 40 to 60 |
| Table 2 | |
| Concentration of stationary phase% (m/m) | Column temperature°C |
|---|---|
| 5 | 175 |
| 10 | 180 |
| 15 | 185 |
| 20 | 185 |
4.1.1.2.Capillary columnU.K.
The properties of efficiency and permeability of capillary columns mean that the separation between constituents and the duration of the analysis are largely dependent on the flow-rate of the carrier-gas in the column. It will therefore be necessary to optimize the operating conditions by acting on this parameter (or more simply on the headloss of the column), according to whether one wishes to improve the separations or to make a rapid analysis.
4.1.2.Determination of the number of theoretical plates (efficiency) and resolution (See Figure 1)U.K.
Carry out the analysis of a mixture of methyl stearate and methyl oleate in about equivalent proportions (for example, methyl esters from cocoa butter).
Choose the temperature of the column and the carrier gas flow so that the maximum of the methyl stearate peak is recorded about 15 minutes after the solvent peak. Use a sufficient quantity of the mixture of methyl esters that the methyl stearate peak occupies about three-quarters of the full scale.
Calculate the number of theoretical plates, n (efficiency), using the formula:
and the resolution, R, using the formula:
where:
is the retention distance, in millimetres, from the start of the chromatogram to the maximum of the peak for methyl stearate;
are the widths, in millimetres, of the peaks for methyl stearate and methyl oleate respectively, measured between the points of intersection of the tangents at the points of inflection of the curve with the base-line;
is the distance, in millimetres, between the peak maxima for methyl stearate and methyl oleate[F9;]
Textual Amendments
[F10and the resolution index, lr, using the formula
Textual Amendments
where:
=
the height of the smallest peak, measured from the base line;
=
the height of the lowest point of the valley between the two adjacent peaks, measured from the base line.]
The operating conditions to be selected are those which will afford at least 2 000 theoretical plates per metre of column length for methyl stearate and a resolution of at least 1,25.
4.2.Test portionU.K.
Using the syringe (3.2) take 0,1 to 2 μl of the solution of methyl esters prepared according to Annex X B and inject them into the column.
In the case of esters not in solution, prepare a solution of approximately 100 mg/ml in heptane of chromatographic quality, and inject 0,1 to 1 ml of this solution.
If the analysis is for constituents present only in trace amounts, the size of the test portion may be increased (up to 10-fold).
4.3.AnalysisU.K.
Generally, the operating conditions shall be those defined in 4.1.1.
Nevertheless, it is possible to work with a lower column temperature when the determination of fatty acids with fewer than 12 carbon atoms is required, or at a higher temperature when determining fatty acids with more than 20 carbon atoms. On occasion, it is possible to employ temperature programming in both these cases. For example, if the sample contains the methyl esters of fatty acids with fewer than 12 carbon atoms, inject the sample at 100 °C (or at 50 to 60 °C if butyric acid is present) and immediately raise the temperature at a rate of 4 to 8 °C/min to the optimum. In certain cases, the two procedures can be combined.
After the programmed heating, continue the elution at a constant temperature until all the components have been eluted. If the instrument does not have programmed heating, use it at two fixed temperatures between 100 and 195 °C.
If necessary, it is recommended that an analysis be carried out on two fixed phases with different polarities to verify the absence of masked peaks, for example in the case of the simultaneous presence of C18:3 and C20:0, or C18:3 and C18:2 conjugated.
4.4.Preparation of the reference chromatogram and reference graphsU.K.
Analyze the reference standard mixture (2.3) using the same operating conditions as those employed for the sample, and measure the retention times or retention distances for the constituent fatty acids. Construct on semi-logarithmic paper, for any degree of unsaturation, the graphs showing the logarithm of retention time or distance as a function of the number of carbon atoms. In isothermal conditions, the graphs for straight-chain acids of the same degree of unsaturation should be straight lines. These lines should be approximately parallel.
It is necessary to avoid conditions such that ‘masked peaks’ exist, i.e. where the resolution is insufficient to separate two constituents.
5.EXPRESSION OF RESULTSU.K.
5.1.Qualitative analysisU.K.
Identify the methyl ester peaks for the sample from the graphs prepared in 4.4, if necessary by interpolation.
5.2.Quantitative analysisU.K.
5.2.1.Determination of the compositionU.K.
Apart from exceptional cases, use the internal normalization method, i.e. assume that the whole of the components of the sample are represented on the chromatogram, so that the total of the areas under the peaks represents 100 % of the constituents (total elution).
If the equipment includes an integrator, use the figures obtained therefrom. If not, determine the area under each peak by multiplying the height of the peak by its width at mid-height, and where necessary take into account the various attenuations used during the recording.
5.2.2.Method of calculationU.K.
5.2.2.1.General caseU.K.
Calculate the content of a given component i, expressed as a percentage by mass of methyl esters, by determining the percentage represented by the area of the corresponding peak relative to the sum of the areas of all the peaks, using the following formula:
where:
is the area under the peak corresponding to component i;
is the sum of the areas under all the peaks.
Give the result to one decimal place.
Note 7:U.K.
In this general case, the result of the calculation based on relative areas is considered to represent a percentage by mass. For the cases in which this assumption is not allowed, see 5.2.2.2.U.K.
5.2.2.2.Use of correction factorsU.K.
In certain cases, for example in the presence of fatty acids with fewer than eight carbon atoms or of acids with secondary groups, when using thermal conductivity detectors or where the highest degree of accuracy is particularly required, correction factors should be used to convert the percentages of peak areas into mass percentages of the components.
Determine the correction factors with the help of a chromatogram derived from the analysis of a reference mixture of methyl esters of known composition, carried out under operating conditions identical with those used for the sample.
For this reference mixture, the percentage by mass of component i is given by the formula:
where:
is the mass of component i in the reference mixture;
is the total of the masses of the various components of the reference mixture.
From the chromatogram of the reference mixture (4.4) calculate the percentage (area/area) for component i as follows:
where:
is the area under the peak corresponding to component i;
is the sum of the areas under all the peaks.
The correction factor is then calculated as:
Commonly, the correction factors are expressed relative to KC16, so that the relative factors become:
For the sample, the content of each component i, expressed as a percentage by mass of methyl esters, is:
Give the results to one decimal place.
5.2.2.3.Use of an internal standardU.K.
In certain analyses (for example where not all of the fatty acids are quantified, such as when acids with four and six carbons are present alongside acids with 16 and 18 carbons, or when it is necessary to determine the absolute amount of a fatty acid in a sample) it is necessary to use an Internal Standard. Fatty acids with five, 15 or 17 carbons are frequently used. The correction factor (if any) for the Internal Standard should be determined.
The percentage by mass of component i, expressed as methyl esters, is then given by the formula:
where:
is the area under the peak corresponding to component i;
is the area under the peak corresponding to the Internal Standard;
is the correction factor for component i (relative to KC1);
is the correction factor for the Internal Standard (relative to KC16);
is the mass, in milligrams, of the test portion;
is the mass, in milligrams, of the Internal Standard.
Give the results to one decimal place.
[F106. SPECIAL CASE — DETERMINATION OF TRANS-ISOMERS U.K.
It is possible to determine the content of trans-isomers in fatty acids with a number of carbon atoms between 10 and 24 by separating the methyl esters using gas chromatography capillary columns having a specific polarity.
6.1. A capillary column made of silica having an internal diameter of between 0,25 mm and 0,32 mm and a length of 50 m, coated with cyanopropisilicon, the thickness of the coating being between 0,1 and 0,3 μm (type SP 2380, C.P. sil 88, silor 10 and similar types). U.K.
[F16.2. The methyl esters are prepared using procedure B set out in Annex XB. Fatty substances having a free acidity over 3 % must first be neutralised in accordance with point 5.1.1 of Annex VII.] U.K.
6.3. The operating conditions for gas chromatography are overall as follows: U.K.
column temperature set between 150 °C and 230 °C (for example 165 °C for 15 minutes then increasing by 5 °C a minute to 200 °C);
injector temperature: 250 °C if the splitting system is used or the initial temperature of the column if the on-column system is used;
detector temperature: 260 °C;
flow rate of the carrier gas (helium and hydrogen): 1,2 ml a minute.
The quantity injected must be such that in the conditions of sensitivity employed the height of the peak corresponding to the methyl ester of the arachidic acid is equal to or greater than 20 % of the bottom of the scale.
6.4. Identification of the various methyl esters is effected on the basis of the retention times which are compared with those for the reference mixtures (as indicated at point 2.3). U.K.
The esters of trans fatty acids are eluted before the corresponding cis-isomers. An example of a chromatogram is given in figure 2.
6.5. The efficiency of the column determined in accordance with point 4.1.2 must be such as to allow separation of certain critical couples, for example the couple formed by the massif of the transisoleic acids and the oleic acid peak, trans C18:1/cis C18:1, with a resolution index greater than 2. U.K.
6.6. The percentage of the various trans fatty acids is calculated on the basis of the relationship between the surface of the relevant peak and the sum of the surfaces of all the peaks present. U.K.
The percentages of:
the trans octadecenoic acids (T 18: 1) indicated in Annex I to this Regulation as the sum of the transoleic isomers;
the cis-trans and trans-cis octadecadienoic acids [(CT/TC) 18: 2] indicated in Annex I to this Regulation as the sum of the translinoleic isomers;
the trans-cis-trans, cis-cis-trans, cis-trans-cis, trans-cis-cis, octadecatrienoic acids [(TCT + CCT + CTC + TCC) 18: 3], indicated in Annex I of this Regulation as the sum of the translinolenic isomers
are taken into account.
Note 8: U.K.
Taking into account the particular characteristics of this method, please give the results with 2 decimals.] U.K.
[F97.] SPECIAL CASE — USE OF A CATHAROMETER DETECTOR (WORKING ON THE PRINCIPLE OF THERMAL CONDUCTIVITY CHANGES)U.K.
A gas chromatograph employing a detector working on the principle of thermal conductivity changes (a catharometer) may also be used for the determination of the qualitative and quantitative composition of a mixture of fatty acid methyl esters. If it is used, the conditions specified in clause 3 and clause 4 should be modified as shown in Table 3.
For quantitative analysis, use the correction factors defined in 5.2.2.2.
| Table 3 | |
| Variable | Value/condition |
|---|---|
| Column | Length: 2 to 4 m Internal diameter: 4 mm |
| Support | Grain size between 160 and 200 μm |
| Concentration of stationary phase | 15 to 25 % (m/m) |
| Carrier gas | Helium or, failing this, hydrogen, with as low an oxygen content as possible |
| Auxiliary gases | None |
| Injector temperature | From 40 to 60 °C above that of the column |
| Column temperature | 180 to 200 °C |
| Flow of carrier gas | Usually between 60 and 80 ml/min |
| Size of test portion injected | Usually between 0,5 and 2 μl |
[F98.] TEST REPORTU.K.
The test report shall specify the methods used for the preparation of the methyl esters and for the gas chromatographic analysis, and the results obtained. It shall also mention all operating details not specified in this International Standard, or regarded as optional, together with details of any incidents which may have influenced the results.
The test report shall include all information necessary for the complete identification of the sample.
[F11ANNEX X B U.K. PREPARATION OF THE FATTY ACID METHYL ESTERS FROM OLIVE OIL AND OLIVE-POMACE OIL
Textual Amendments
F11 Substituted by Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
The following two methods are recommended for preparing the fatty acid methyl esters from olive oils and olive-pomace oils:
:
Trans-esterification with cold methanolic solution of potassium hydroxide
:
Methylation by heating with sodium methylate in methanol followed by esterification in acid medium.
Each method will be applied according to the analytical parameter to be determined and the oil category as indicated below:
determination of difference between actual and theoretical content of triglycerides with ECN42 (ΔECN42):
method A will be applied to samples of all the oil categories after purification of the oil by passing it through a silica gel column;
determination of the fatty acid composition:
method A will be applied directly to samples of the following oil categories:
virgin olive oils with an acidity of less than 3,3 %,
refined olive oil,
olive oil (blend of virgin olive oils and refined olive oil),
refined olive-pomace oil,
olive-pomace oil (blend of virgin olive oils and refined olive-pomace oil);
method B will be applied directly to samples of the following oil categories:
virgin olive oil with an acidity of more than 3,3 %,
crude olive-pomace oil;
determination of trans-isomers of fatty acids:
method A will be applied directly to samples of the following oil categories:
virgin olive oils with an acidity of less than 3,3 %,
refined olive oil,
olive oil (blend of virgin olive oils and refined olive oil),
refined olive-pomace oil,
olive-pomace oil (blend of virgin olive oils and refined olive-pomace oil);
method A will be applied to the following categories of oils after purification of the oil by passing it through a silica gel column:
virgin olive oil with an acidity of more than 3,3 %,
crude olive-pomace oil.
PURIFICATION OF OIL SAMPLES U.K.
When necessary, the samples will be purified by passing the oil through a silica gel column, eluting with hexane/diethyl ether (87:13, v/v) as described in IUPAC method 2.507.
Alternatively, solid-phase extraction on silica gel phase cartridges can be used. A silica gel cartridge (1 g, 6 ml) is placed in a vacuum elution apparatus and washed with 6 ml of hexane. The vacuum is released to prevent the column from becoming dry and then a solution of the oil (0,12 g approximately) in 0,5 ml of hexane is loaded into the column and vacuum is applied. The solution is pulled down and then eluted with 10 ml of hexane/diethyl ether (87:13 v/v) under vacuum. The combined eluates are homogenised and divided in two similar volumes. An aliquot is evaporated to dryness in a rotary evaporator under reduced pressure at room temperature. The pomace is dissolved in 1 ml of heptane and the solution is ready for fatty acid analysis by GC. The second aliquot is evaporated and the pomace is dissolved in 1 ml of acetone for triglyceride analysis by HPLC, if necessary.
METHODS FOR PREPARING THE FATTY ACID METHYL ESTERS U.K.
1. Method A: Trans-esterification with cold methanolic solution of potassium hydroxide U.K.
1.1. Purpose U.K.
This rapid method is applicable to olive oils and olive-pomace oils with a free fatty acid content of less than 3,3 %. Free fatty acids are not esterified by potassium hydroxide. Fatty acid ethyl esters are trans-esterified at a lower rate than glyceridic esters and may be only partially methylated.
1.2. Principle U.K.
Methyl esters are formed by trans-esterification with methanolic potassium hydroxide as an intermediate stage before saponification takes place (title 5 in ISO-5509:2000, title 5 in IUPAC method 2.301).
1.3. Reagents U.K.
Methanol containing not more than 0,5 % (m/m) water.
Heptane, chromatographic quality.
Potassium hydroxide, approximately 2 N methanolic solution: dissolve 11,2 g of potassium hydroxide in 100 ml of methanol.
1.4. Apparatus U.K.
Screw-top test tubes (5 ml volume) with cap fitted with a PTFE joint.
Graduated or automatic pipettes, 2 ml and 0,2 ml
1.5. Procedure U.K.
In a 5 ml screw-top test tube weigh approximately 0,1 g of the oil sample. Add 2 ml of heptane, and shake. Add 0,2 ml of 2 N methanolic potassium hydroxide solution, put on the cap fitted with a PTFE joint, tighten the cap, and shake vigorously for 30 seconds. Leave to stratify until the upper solution becomes clear. Decant the upper layer containing the methyl esters. The heptane solution is suitable for injection into the gas chromatograph. It is advisable to keep the solution in the refrigerator until gas chromatographic analysis. Storage of the solution for more than 12 hours is not recommended.
2. Method B: Methylation by heating with sodium methylate in methanol followed by esterification in acid medium U.K.
2.1. Purpose U.K.
This method is applicable to olive oils and olive-pomace oils with a free fatty acid content of more than 3,3 %.
2.2. Principle U.K.
Neutralisation of the free fatty acids and alkaline methanolysis of the glycerides, followed by esterification of the fatty acids in acid medium (title 4.2. in IUPAC method 2.301).
2.3. Reagents U.K.
heptane, chromatographic quality,
methanol containing not more than 0,05 % (m/m) water,
sodium methylate, 0,2 N methanolic solution: dissolve 5 g of sodium in 1 000 ml of methanol (this may be prepared from commercial solutions),
phenolphthalein, 0,2 % methanolic solution,
sulphuric acid, 1 N in methanolic solution: add 3 ml of 96 % sulphuric acid to 100 ml of methanol,
saturated solution of sodium chloride in water.
2.4. Apparatus U.K.
50 ml flat-bottomed volumetric flask with long, narrow, ground neck,
reflux condenser: air condenser (1 m long) with ground joint appropriate to the neck of the flask,
boiling chips,
glass funnel.
2.5. Procedure U.K.
Transfer about 0,25 g of the oil sample into a 50 ml ground-necked volumetric flask. With the aid of a funnel, add 10 ml of 0,2 N sodium methylate in methanol and the boiling chips. Fit a reflux condenser, shake, and bring to the boil. The solution should become clear, which usually occurs in about 10 minutes. The reaction is complete after 15 minutes. Remove the flask from the source of heat, wait until the reflux stops, remove the condenser, and add two drops of phenolphthalein solution. Add a few ml of 1 N sulphuric acid in methanol solution until the solution becomes colourless and then add 1 ml in excess. Fit the condenser and boil again for 20 minutes. Withdraw from the source of heat and cool the flask under running water. Remove the condenser, add 20 ml of saturated sodium chloride solution, and shake. Add 5 ml of heptane, plug the flask, and shake vigorously for 15 seconds.
Leave to settle until the two phases have separated. Add saturated sodium chloride solution again until the aqueous layer reaches the lower end of the flask neck. The upper layer containing the methyl esters fills the flask neck. This solution is ready to be injected in the GC.
Caution : Methylation by method B must be done under a hood.
2.6. Alternatives to methylation Method B U.K.
2.6.1. Method C U.K.
2.6.1.1. Principle U.K.
The fatty matter undergoing analysis is treated with methanol-hydrochloric acid, in a sealed vial, at 100 °C.
2.6.1.2. Apparatus U.K.
Strong glass vial of a capacity of about 5 ml (height 40 to 45 mm, diameter 14 to 16 mm).
1 and 2 ml graduated pipettes.
2.6.1.3. Reagents U.K.
Solution of hydrochloric acid in 2 % methanol. This is prepared from gaseous hydrochloric acid and anhydrous methanol (Note 1).
Hexane, chromatographic quality.
Note 1: U.K.
Commercial solutions of hydrogen chloride in methanol can be used. Small amounts of gaseous hydrochloric acid can easily be prepared in the laboratory by simple displacement from the commercial solution (p = 1,18) by dripping concentrated sulphuric acid. Since hydrochloric acid is very rapidly absorbed by methanol, it is advisable to take the usual precautions when dissolving it, e.g. introduce the gas through a small inverted funnel with the rim just touching the surface of the liquid. Large quantities of methanolic hydrochloric acid solution can be prepared in advance, as it keeps perfectly in glass-stoppered bottles stored in the dark. Alternatively, this reagent can be prepared by dissolution of acetyl chloride in anhydrous methanol. U.K.
2.6.1.4. Procedure U.K.
Place in the glass vial 0,2 g of the fatty matter, which has previously been dried out on sodium sulphate and filtered, and 2 ml of hydrochloric acid-methanol solution. Heat seal the vial.
Immerse the vial at 100 °C for 40 minutes.
Cool the vial under running water, open, add 2 ml of distilled water and 1 ml of hexane.
Centrifuge and remove the hexane phase, which is ready for use.
2.6.2. Method D U.K.
2.6.2.1. Principle U.K.
The fatty matter undergoing analysis is heated under reflux with methanol-hexane-sulphuric acid. The methyl esters obtained are extracted with petroleum ether.
2.6.2.2. Apparatus U.K.
Test tube of a capacity of about 20 ml, fitted with an air reflux condenser approximately 1 m in length, with ground glass joints.
5 ml graduated pipette.
50 ml separating funnel.
10 ml and 25 ml measuring beakers.
15 ml test tube with conical base.
2.6.2.3. Reagents U.K.
Methylation reagent: anhydrous methanol-hexane-concentrated sulphuric acid (p = 1,84) in the ratio 75:25:1 (V/V/V).
40 to 60 °C petroleum ether.
Anhydrous sodium sulphate.
2.6.2.4. Procedure U.K.
Place 0,1 g of oil in the 20 ml test tube and add 5 ml of methylation reagent.
Fit the reflux condenser and heat for 30 minutes in a boiling water bath (Note 2).
Transfer quantitatively the mixture into a 50 ml separating funnel, with the aid of 10 ml distilled water and 10 ml petroleum ether. Shake vigorously, and allow the phases to separate, remove the aqueous phase and wash the ether layer twice with 20 ml distilled water. Add to the separating funnel a small quantity of anhydrous sodium sulphate, shake, allow to settle for a few minutes and filter, collecting the filtrate in a 15 ml test tube with a conical base.
Evaporate the solvent over a water bath in a current of nitrogen.
Note 2: U.K.
To control boiling, insert a glass rod into the test tube and limit the temperature of the water bath to 90 °C. U.K.
3. Precision parameters U.K.
The statistical evaluation of the precision of methods A and B was published by the International Olive Oil Council in its method COI/T.20/CO. No 24.
RECOMMENDATIONS FOR GAS CHROMATOGRAPHIC ANALYSIS OF THE FATTY ACID ESTERS FROM OLIVE OIL AND OLIVE-POMACE OIL U.K.
1. Procedure U.K.
The gas chromatographic analysis of solutions of fatty esters in heptane is to be carried out according to standard ISO-5508 using a capillary column (50 m length × 0,25 or 0,32 mm i.d.) impregnated with cyanopropylsilicone phase as indicated for the determination of fatty acid trans-isomers (COI/T.20/Doc. no. 17).
Figure 1 gives the typical gas chromatographic profile of an olive-pomace oil containing methyl and ethyl esters of fatty acids, and trans-isomers of methyl esters.
2. Calculations U.K.
2.1. For the calculation of the fatty acid composition and ΔECN42, all the following fatty acids will be taken into account: U.K.
Myristic (C14:0).
Palmitic (C16:0). Sum of the areas of the peaks corresponding to the methyl and ethyl esters.
Palmitoleic (C16:1). Sum of the areas of the peaks corresponding to the ω9 and ω7 isomers of the methyl ester.
Margaric (C17:0).
Margaroleic (C17:1).
Stearic (C18:0).
Oleic (C18:1). Sum of the areas of the peaks corresponding to the ω9 and ω7 isomers of the methyl ester, ethyl ester, and trans-isomers of the methyl ester.
Linoleic (C18:2). Sum of the areas of the peaks corresponding to the methyl and ethyl esters, and the trans-isomers of the methyl ester.
Arachidic (C20:0).
Linolenic (C18:3). Sum of the areas of the methyl ester and the trans-isomers of the methyl ester.
Eicosenoic (C20:1).
Behenic (C22:0).
Lignoceric (C24:0).
Squalene will not be taken into account for the calculation of the total area.
2.2. For the calculation of the percentage of trans-C18:1 the peak corresponding to the methyl esters of this fatty acid is to be used. For the sum [trans-C18:2 + trans-C18:3], all the peaks corresponding to the trans-isomers of these two fatty acids are to be added together. For the calculation of the total area, all the peaks mentioned in 2.1. are to be taken into account (see COI/T.20/Doc. No. 17). U.K.
The calculation of the percentage of each fatty acid will be carried out according to the formula:
ANNEX XIU.K.DETERMINATION OF VOLATILE HALOGENATED SOLVENTS CONTENT OF OLIVE OIL
1.METHODU.K.
Analysis by gas chromatography using the head space technique.
2.EQUIPMENTU.K.
2.1.Gas chromatography apparatus fitted with an electron capture detector (ECD).U.K.
2.2.Head space apparatus.U.K.
2.3.Gas chromatography column, of glass, 2 m long and 2 mm in diameter, stationary phase. OV101 10 % or equivalent, impregnating a calcined diatomaceous earth, acid washed and silanised and of a particle size of 80 to 100 mesh.U.K.
2.4.Carrier and auxiliary gas: nitrogen for gas chromatography, suitable for detection by electron capture.U.K.
2.5.Glass flasks, 10 to 15 ml, with teflon coating and aluminium stopper with fitment for entry of syringe.U.K.
2.6.Hermetically sealing clamps.U.K.
2.7.Gas syringe 0,5 to 2 ml.U.K.
3.REAGENTSU.K.
Standard: halogenated solvents of a degree of purity suitable for gas chromatography.
4.PROCEDUREU.K.
4.1.Exactly weigh around 3 g of oil in a glass flask (not to be reused); hermetically seal it. Place it in a thermostat at 70 °C for one hour. Using a syringe carefully remove 0,2 to 0,5 ml of the head space. Inject this into the column of the gas chromatography apparatus regulated as follows:U.K.
injector temperature: 150 °C,
column temperature: 70 to 80 °C,
detector temperature: 200 to 250 °C.
Other temperatures may also be used provided the results remain equivalent.
4.2.Reference solutions: prepare standard solutions using refined olive oil with no trace of solvents with concentrations ranging from 0,05 to 1 ppm (mg/kg) and corresponding to the presumed content of the sample. The halogenated solvents may be diluted using pentane.U.K.
4.3.Quantitative assessment: correlate the surfaces or the elevations of the peaks of the sample and of the standard solution of the concentration presumed closest. If the deviation is greater than 10 % the analysis must be repeated in comparison with another standard solution until the deviation is within 10 %. The content is determined on the basis of the average of the elementary injections.U.K.
4.4.Expression of results: in ppm (mg/kg). The detection limit for the method is 0,01 mg/kg.U.K.
[F3ANNEX XII U.K. THE INTERNATIONAL OLIVE COUNCIL’S METHOD FOR THE ORGANOLEPTIC ASSESSMENT OF VIRGIN OLIVE OIL
1. PURPOSE AND SCOPE U.K.
This method is based on Decision No DEC-21/95-V/2007 of 16 November 2007 on the International Olive Council’s revised method for the organoleptic assessment of virgin olive oil. Its purpose is to determine the procedure for assessing the organoleptic characteristics of virgin olive oil within the meaning of point 1 of Annex XVI to Regulation (EC) No 1234/2007 and to establish the method for its grading on the basis of those characteristics. It also provides indications for optional labelling.
The method described is applicable only to virgin olive oil and to the grading or labelling of such oil according to the intensity of the defects perceived, their fruitiness and other positive attributes, as determined by a group of tasters selected, trained and monitored as a panel.
2. GENERAL U.K.
For the general basic vocabulary, the tasting room, the tasting glass and any other matters relating to this method, compliance with the stipulations of the International Olive Council, and in particular with Decision No DEC-21/95-V/2007 of 16 November 2007 on the revised method for the organoleptic assessment of virgin olive oil, is recommended.
3. SPECIFIC VOCABULARY U.K.
3.1. Positive attributes U.K.
:
range of smells (dependent on variety) characteristic of oil from healthy fresh fruit, green or ripe, perceived directly and/or retronasally.
Fruitiness is qualified as green if the range of smells is reminiscent of green fruit and is characteristic of oil from green fruit.
Fruitiness is qualified as ripe if the range of smells is reminiscent of ripe fruit and is characteristic of oil from green and ripe fruit.
:
characteristic primary taste of oil from green olives or olives turning colour. It is detected by the circumvallate papillae in the ‘ V ’ region of the tongue.
:
tingling sensation characteristic of oil made at the beginning of the season mainly from olives that are still green. It can be perceived throughout the mouth cavity, particularly in the throat.
3.2. Negative attributes U.K.
:
characteristic flavour of oil from olives that have been piled or stored in such a way as to have reached an advanced stage of anaerobic fermentation, or of oil which has been left in contact with the sediment that settles in underground tanks and vats and which has also undergone a process of anaerobic fermentation.
:
characteristic flavour of oil from olives in which large numbers of fungi and yeasts have developed as a result of storage for several days in humid conditions.
:
characteristic flavour of certain oils reminiscent of wine or vinegar. This flavour is mainly due to the aerobic fermentation of the olives or of olive paste left on pressing mats which have not been properly cleaned, leading to the formation of acetic acid, ethyl acetate and ethanol.
:
flavour reminiscent of metal, characteristic of oil that has been in prolonged contact with metallic surfaces during crushing, mixing, pressing or storage.
:
flavour of oil that has undergone an intense process of oxidation.
:
characteristic flavour caused by excessive and/or prolonged heating during production, particularly by thermo-mixing of the paste in unsuitable conditions.
:
characteristic flavour of certain oils from dry olives.
:
thick and pasty mouthfeel produced by certain old oils.
:
flavour reminiscent of diesel, grease or mineral oil.
:
flavour acquired by the oil as a result of prolonged contact with vegetable water which has undergone fermentation.
:
flavour of oil extracted from olives which have been preserved in brine.
:
characteristic flavour of oil from olives pressed in new esparto mats. The flavour may vary depending on whether the mats are made of green or dried esparto.
:
flavour of oil from olives collected with earth or mud on them and not washed.
:
flavour of oil from olives heavily attacked by grubs of the olive fly ( Bactrocera oleae ).
:
characteristic flavour of oil kept too long in hermetically sealed containers, notably in tins, attributed to formation of 2,6-nonadienal.
:
characteristic flavour of oil extracted from olives damaged by frost while on the tree.
3.3. Optional terminology for labelling purposes U.K.
Upon request, the panel head may certify that the oils which have been assessed comply with the definitions and ranges that correspond to the following adjectives according to the intensity and perception of the attributes:
for each of the positive attributes mentioned under point 3.1 ( fruity — whether green or ripe — pungent or bitter ):
the term ‘ intense ’ may be used where the median of the attribute concerned is greater than 6;
the term ‘ medium ’ may be used where the median of the attribute concerned is between 3 and 6;
the term ‘ light ’ may be used where the median of the attribute concerned is less than 3;
the attributes in question may be used without the adjectives given in points (i), (ii) and (iii) where the median of the attribute concerned is 3 or more;
the term ‘well balanced’ may be used where the oil does not display a lack of balance, which is defined as the smell, taste and feel that oil has when the median of the bitter and/or pungent attributes is two points higher than the median of its fruitiness ;
the term ‘mild oil’ may be used where the median of the bitter and pungent attributes is 2 or less.
4. PANEL U.K.
The panel consists of a panel head and from eight to 12 tasters.
The panel head must be a soundly trained expert in the various types of oil. He or she is responsible for the panel and its organisation and operation, including preparation, coding and presentation of the samples to the tasters and collection and processing of the data.
He or she selects the tasters, sees to their training and checks that their performance remains of adequate standard.
The testers must be selected and trained on account of their skill in distinguishing between similar samples. The International Olive Council’s manual on the selection, training and monitoring of qualified virgin olive oil tasters must be followed.
Panels must undertake to participate in national, Community and international organoleptic assessments organised for the purposes of periodic monitoring and harmonisation of perception criteria. In the case of panels approved in accordance with Article 4(1) of this Regulation, they must also provide the Member State concerned with full information each year on the composition of the panel and the number of assessments made in their capacity as an approved panel.
5. PROCEDURE FOR ORGANOLEPTIC ASSESSMENT AND GRADING U.K.
5.1. Use of profile sheet by taster U.K.
The profile sheet to be used by the taster is reproduced as Appendix A.
Tasters must each smell and then taste (2) the oil submitted for examination, marking the intensity of their perception of each negative and positive attribute on the 10-cm scale provided on the profile sheet. If a taster perceives the fruitiness to be of a green or ripe character, he or she must tick the corresponding box on the profile sheet.
If the tasters perceive any negative attributes not listed on the profile sheet, these must be noted under ‘ Other ’ , using the term or terms that describe them best from among those defined above.
5.2. Processing of data by panel head U.K.
The panel head collects the profile sheets completed by the tasters and scrutinises the intensities assigned to the various attributes. In the event of an anomaly he or she will ask tasters to re-examine their sheet and if necessary repeat the test.
The panel head may feed each tester’s data into a computer programme to calculate the median in accordance with Appendix B. Input of each sample shall be made with the help of a grid with nine columns for the nine sensory attributes and one line for each panel member.
If a negative attribute is perceived and mentioned under ‘ Other ’ by at least 50 % of the panel, the head must calculate the median for this attribute and grade accordingly.
The panel head may certify that the oil submitted for examination meets the conditions set out under point 3.3(a) for the use of the terms ‘ green ’ and ‘ ripe ’ only if at least 50 % of the panel perceived that the fruitiness had this character and noted it down.
For assessments intended to monitor compliance, one test shall be carried out. In the event of contradictory assessments, the panel head shall arrange for the assessment to be carried out in duplicate. For confirmation assessments, the assessment must be carried out in triplicate. In these cases, the median of the attributes shall be calculated from the average of the medians. Repeat tests must be carried out at different sessions.
5.3. Grading of oils U.K.
The oil is graded as follows in line with the median of the defects and the median for ‘ fruity ’ . The median of the defects is defined as the median of the defect perceived with the greatest intensity. The median of the defects and the median for ‘ fruity ’ are expressed to one decimal place, and the value of the robust variation coefficient which defines them must be no greater than 20 %.
The oil is graded by comparing the median value of the defects and the median for ‘ fruity ’ with the reference ranges given below. The error of the method was taken into account when determining the limits of these intervals, which are therefore considered to be absolute. The software packages allow visualised grading using a table of statistics or a graph.
:
the median of the defects is 0 and the median for ‘ fruity ’ is above 0;
:
the median of the defects is above 0 but not above 3,5 and the median for ‘ fruity ’ is above 0;
:
the median of the defects is above 3,5; or the median of the defects is not above 3,5 and the median for ‘ fruity ’ is 0.
5.4. Special case U.K.
If the median of a positive attribute other than ‘ fruity ’ is above 5,0, the panel head must note this on the analysis certificate.
Appendix A
Profile sheet for virgin olive oil U.K.
Appendix B
METHOD FOR CALCULATING THE MEDIAN AND CONFIDENCE INTERVALS U.K.
Median U.K.
The median is defined as the real number Xm characterised by the fact that the probability (P) that the distribution values (X) are below that number (Xm) is not more than 0,5 and that simultaneously the probability (P) that the distribution values (X) are not above Xm is not less than 0,5. Another definition considers the median to be the 50th percentile of a distribution of numbers ranked in ascending order. In other words, the median is the central value of an ordered series with an uneven number of values or the average of the two central values of an ordered series with an even number of values.
Robust standard deviation U.K.
To obtain a reliable estimate of the variability that arises around the median, recourse is required to the Stuart and Kendall method of estimating the robust standard deviation. The following formula gives the asymptotic standard deviation, i.e. the robust estimate of the variability of the data under consideration, where N is the number of observations and IQR the interquartile range, which covers exactly 50 % of the cases of any probability distribution.
The interquartile range is obtained by calculating the magnitude of the deviation between the 75th and the 25th percentiles.
IQR = 75th percentile – 25th percentile
The percentile is the value Xpc characterised by the fact that the probability (P) that the distribution values are below Xpc is not more than a determined hundredth and that simultaneously the probability (P) that the distribution values are not above Xpc is not less than the said hundredth. The hundredth indicates the distribution fraction used. For the median, this is 50/100.
In practice, the percentile is the distribution value corresponding to a determined area plotted from the distribution or density curve. For example, the 25th percentile is the distribution value corresponding to an area equal to 0,25 or 25/100.
Robust variation coefficient % U.K.
The rVC % is a pure number, i.e. without dimension, that indicates the percentage of variability of the series of numbers analysed. For that reason, it is very useful for verifying the reliability of the panel members.
Confidence intervals at 95 % on the median U.K.
The confidence intervals at 95 % (value of the error of first kind equal to 0,05 or 5 %) represent the range in which the value of the median would be able to vary should it be possible to repeat the experiment an infinite number of times. In practice, this interval indicates the range of variability of the test under the operating conditions selected, based on the assumption that it is possible to repeat the test several times. As with the rVC %, the interval helps evaluate the reliability of the test.
Upper C.I. = Me + (c.S*)
Lower C.I. = Me – (c.S*)
where c is equal to 1,96 for a confidence interval of 0,95.]
F8ANNEX XIIIU.K. [F8NEUTRALIZATION AND DECOLORIZATION OF OLIVE OIL IN THE LABORATORY]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
F12ANNEX XIVU.K. [F12 [F2ADDITIONAL NOTES 2, 3 AND 4 TO CHAPTER 15 OF THE COMBINED NOMENCLATURE] ]
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Textual Amendments
F12 Deleted by Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
ANNEX XVU.K.
1.OIL CONTENT OF OLIVE RESIDUEU.K.
1.1.ApparatusU.K.
suitable extraction apparatus fitted with a 200 to 250 ml round-bottomed flask,
electrically heated bath (e.g., sand bath, water bath) or hotplate,
analytical balance,
oven regulated to a maximum of 80° C,
electrically heated oven fitted with a thermostatic device regulated to 103 ± 2° C and one that can be swept with a stream of air or operated at reduced pressure,
mechanical mill, easy to clean, and one that allows the olive residues to be ground without a rise in their temperature or any appreciable alteration in their content of moisture, volatile matter or substances extractable with hexane,
extraction thimble and cotton wool or filter paper from which substances extractable with hexane have already been removed,
dessicator,
sieve with 1 mm diameter apertures,
small particles of previously dried pumice stone.
1.2.ReagentU.K.
Normal hexane, technical grade, which must leave a residue of less than 0,002 g per 100 ml, on complete evaporation.
2.PROCEDUREU.K.
2.1.Preparation of the test sampleU.K.
If necessary, use the mechanical mill, which has previously been properly cleaned, to grind the laboratory sample in order to reduce it to particles that can pass completely through the sieve.
Use about one twentieth of the sample to complete the process of cleaning the mill, discard the ground material, grind the remainder and collect, mix carefully and analyze without delay.
2.2.Test portionU.K.
As soon as the grinding operation has been completed, weigh out about 10 g of the sample to the nearest 0,01 g for testing.
2.3.Preparation of the extraction thimbleU.K.
Place the test portion in the thimble and plug with cotton wool. If a filter paper is used, envelope the test portion in it.
2.4.Peliminary dryingU.K.
If the olive residues are very moist (i.e., moisture and volatile matter content more than 10 %), carry out preliminary drying by placing the loaded thimble (or filter paper) in the oven heated for an appropriate time at not more than 80° C in order to reduce the moisture and volatile matter content to less than 10 %.
2.5.Preparation of the round-bottomed flaskU.K.
Weigh to the nearest 1 mg the flask containing one or two particles of pumice stone, previously dried in the stove at 103 ± 2° C and then cooled in a dessicator for not less than one hour.
2.6.Initial extractionU.K.
Into the extraction apparatus insert the thimble (or filter paper) containing the test portion. Pour into the flask the requisite quantity of hexane. Fit the flask to the extraction apparatus and place the whole on the electrically heated bath. Adjust the rate of heating in such a way that the reflux rate is not less than three drops per second (moderate, not violent boiling). After four hours extraction, allow to cool. Remove the thimble from the extraction apparatus and place it in a stream of air in order to drive off most of the impregnating solvent.
2.7.Second extractionU.K.
Tip the contents of the thimble into the micro-grinder and grind as finely as possible. Return the ground mixture to the thimble without loss and place it back in the extraction apparatus.
Continue the extraction for a further two hours using the same round-bottomed flask containing the initial extract.
The resultant solution in the extraction flask must be clear. If not, filter it through a filter paper and wash the original flask and the filter paper several times with hexane. Collect the filtrate and the washing solvent in a second round-bottomed flask which has been dried and tared to the nearest 1 mg.
2.8.Removal of solvent and weighing of extractU.K.
Remove the greater part of the solvent by distillation on an electrically heated bath. Remove the last traces of solvent by heating the flask in the oven at 103 ± 2° C for 20 minutes. Assist the elimination process either by blowing in air, or preferably an inert gas, at intervals or by using reduced pressure.
Leave the flask in a dessicator to cool for at least one hour and weigh to the nearest 1 mg.
Heat again for 10 minutes under the same conditions, cool in a dessicator and reweigh.
The difference between the two weighings shall not exceed 10 mg. If it does, heat again for periods of 10 minutes followed by cooling and weighing until the weight difference is 10 mg or less. Note the last weight of the flask.
Carry out duplicate determinations on the test sample.
3.EXPRESSION OF RESULTSU.K.
3.1.Method of calculation and formulaU.K.
The extract expressed as a percentage by mass of the product as received is equal to:
| where: | S = is the percentage by mass of extract of the product as received, m0 = is the mass, in grams, of the test portion, m1 = is the mass, in grams, of the extract after drying. |
Take as the result the arithmetic mean of the duplicate determinations, providing the repeatability conditions are satisfied.
Express the result to the first decimal place.
3.2.RepeatabilityU.K.
The difference between the duplicate determinations carried out simultaneously or in rapid sucession by the same analyst shall not exceed 0,2 g of hexane extract per 100 g of sample.
If this condition is not satisfied, repeat the analysis on two other test portions. If, in this case too, the difference exceeds 0,2 g, take as the result the arithmetic mean of the four determinations.
ANNEX XVIU.K.DETERMINATION OF IODINE VALUE
1.SCOPEU.K.
This International Standard specifies a method for the determination of the iodine value of animal and vegetable fats and oils, referred to hereafter as fats.
2.DEFINITIONU.K.
For the purposes of this International Standard, the following definition applies:
2.1. iodine value. The mass of iodine absorbed by the sample under the operating conditions specified in this International Standard.U.K.
The iodine value is expressed as grams of iodine per 100 g of sample.
3.PRINCIPLEU.K.
Dissolution of a test portion in solvent and addition of Wijs reagent. After a specified time, addition of potassium iodide solution and water, and titration of the liberated iodine with sodium thiosulfate solution.
4.REAGENTSU.K.
All reagents shall be of recognized analytical grade:
4.1. water, complying with the requirements of ISO 3696, Grade 3.U.K.
4.2. potassium iodide, 100 g/l solution, not containing iodate or free iodine.U.K.
4.3. starch, solution.U.K.
Mix 5 g of soluble starch in 30 ml of water, add this mixture to 1 000 ml of boiling water, boil for three minutes and allow to cool.
4.4. sodium thiosulfate, standard volumetric solution c (Na2S2O3.5H2O) = 0,1 mol/l, standardized not more than seven days before use.U.K.
4.5. solvent, prepared by mixing equal volumes of cyclohexane and acetic acid.U.K.
4.6. Wijs reagent, containing iodine monochloride in acetic acid. Commercially available Wijs reagent shall be used.U.K.
5.APPARATUSU.K.
Usual laboratory apparatus and, in particular, the following:
5.1. glass weighing scoops, suitable for the test portion and for inserting into the flasks (6.2).U.K.
5.2. conical flasks, of 500 ml capacity, fitted with ground glass stoppers and completely dry.U.K.
6.PREPARATION OF THE TEST SAMPLEU.K.
The homogenized sample is dried over sodium sulphate and filtered.
7.PROCEDUREU.K.
7.1.Test portionU.K.
The mass of the test portion varies according to its expected iodine value as shown in Table 1.
| Table 1 | |
| Expected iodine value | Mass of test portion(g) |
|---|---|
| less than 5 | 3,0 |
| 5 to 20 | 1,0 |
| 21 to 50 | 0,4 |
| 51 to 100 | 0,2 |
| 101 to 150 | 0,13 |
| 151 to 200 | 0,1 |
Weigh the test portion to the nearest 0,1 mg in a glass weighing scoop (5.1).
7.2.DeterminationU.K.
Place the test portion in a 500 ml flask (6.2). Add 20 ml of the solvent (4.5) to dissolve the fat. Add exactly 25 ml of the Wijs reagent (4.6), insert the stopper, swirl the contents and place the flask in the dark. Do not use a mouth pipette for the Wijs reagent.
Similarily, prepare a blank with the solvent and the reagent but omitting the test portion.
For samples having an iodine valve below 150, leave the flasks in the dark for one hour; for those with an iodine value above 150 and for polymerized products or products oxidized to a considerable extent, leave for two hours.
At the end of the time, add 20 ml of the potassium iodide solution (4.2) and 150 ml of water (4.1) to each of the flasks.
Titrate with the standard volumetric sodium thiosulfate solution (4.4) until the yellow colour due to iodine has almost disappeared. Add a few drops of the starch solution (4.3) and continue the titration until the blue colour just disappears after very vigorous shaking.
Note:U.K.
Potentiometric determination of the end point is permissible.U.K.
7.3.Number of determinationsU.K.
Carry out two determinations on the same test sample.
8.EXPRESSION OF RESULTSU.K.
The iodine value is given by the expression
where:
=
is the numerical value of the exact concentration, in moles per litre, of the standard volumetric sodium thiosulfate solution (4.4) used;
=
is the numerical value of the volume, in millilitres, of the standard volumetric sodium thiosulfate solution (4.4) used for the blank test;
=
is the numerical value of the volume, in millilitres, of the standard volumetric sodium thiosulfate solution (4.4) used for the determination;
=
is the numerical value of the mass, in grams, of the test portion (7.1).
Take as the result the arithmetic mean of the two determinations, provided that the requirement for repeatability (9.2) is satisfied.
[F13ANNEX XVII U.K. METHOD FOR THE DETERMINATION OF STIGMASTADIENES IN VEGETABLE OILS
Textual Amendments
F13 Inserted by Commission Regulation (EC) No 656/95 of 28 March 1995 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis and Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
1. PURPOSE U.K.
Determination of stigmastadienes in vegetable oils containing low concentrations of these hydrocarbons, particularly in virgin olive oil and crude olive-residue oil.
2. SCOPE U.K.
The standard may be applied to all vegetable oils although measurements are reliable only where the content of these hydrocarbons lies between 0,01 and 4,0 mg/kg. The method is particularly suited to detecting the presence of refined vegetable oils (olive, olive residue, sunflower, palm, etc.) in virgin olive oil since refined oils contained stigmastadienes and virgin oils do not.
3. PRINCIPLE U.K.
Isolation of unsaponifiable matter. Separation of steroidal hydrocarbon fraction by column chromatography on silica gel and analysis by capillary gas chromatography.
4. APPARATUS U.K.
4.1. 250 ml flasks suitable for use with a reflux condenser. U.K.
4.2. Separating funnels of 500 ml capacity. U.K.
4.3. 100 ml round-bottom flasks. U.K.
4.4. Rotary evaporator. U.K.
4.5. Glass chromatography column (1,5 to 2,0 cm internal diameter by 50 cm length) with Teflon tap and a plug of glass wool fibre or sintered glass disc at the bottom. To prepare silica gel column, pour hexane into the chromatography column to a depth of approximately 5 cm and then fill with a slurry of silica gel in hexane (15 g in 40 ml) with the help of hexane portions. Allow to settle and finish settling by applying slight vibration. Add anhydrous sodium sulphate to a height of approximately 0,5 cm, finally elute the excess hexane. U.K.
4.6. Gas chromatograph with flame ionization detector, split or cold on-column injector and oven programmable to within ± 1 °C. U.K.
4.7. Fused silica capillary column for gas chromatography (0,25 or 0,32 mm internal diameter by 25 m length) coated with 5 %-phenylmethylsilicone phase, 0,25 mm film thickness. U.K.
Note 1: U.K.
Other columns of similar or lower polarity can be used. U.K.
4.8. Integrator-recorder with possibility of valley-valley integration mode. U.K.
4.9. 5 to 10 ml microsyringe for gas chromatography with cemented needle. U.K.
4.10. Electrical heating mantle or hot place. U.K.
5. REAGENTS U.K.
All reagents should be of analytical grade unless otherwise specified. The water used should be distilled water, or water of at least equivalent purity.
5.1. Hexane or mixture of alkanes of bp interval 65 to 70 °C, distilled with rectifying column. U.K.
Note 2: U.K.
The solvent must be distilled to remove impurities. U.K.
5.2. 96 v/v ethanol. U.K.
5.3. Anhydrous sodium sulphate. U.K.
5.4. Alcoholic potassium hydroxide solution at 10 %. Add 10 ml of water to 50 g potassium hydroxide, stir, and then dissolve the mixture in ethanol to 500 ml. U.K.
Note 3: U.K.
Alcoholic potash turns brown on standing. It should be prepared freshly each day and kept in well stoppered dark glass bottles. U.K.
5.5. Silica gel 60 for column chromatography, 70 to 230 mesh, (Merck, reference 7734 or similar). U.K.
Note 4: U.K.
Usually, silica gel can be used directly from the container without any treatment. However, some batches of silica gel may show low activity resulting in bad chromatographic separations. Under this circumstance, the silica gel should be treated in the following way: Activate the silica gel by heating for a minimum of four hours at 550 °C. After heating, place the silica gel in a desiccator while the gel is cooling and then transfer the silica gel to a stoppered flask. Add 2 % of water and shake until no lumps can be seen and the powder flows freely. U.K.
If batches of silica gel result in chromatograms with interfering peaks, the silica gel should be treated as above. An alternative could be the use of extra pure silica gel 60 (Merck, reference 7754).
5.6. Stock solution (200 ppm) of cholesta-3,5-diene (Sigma, 99 % purity) in hexane (10 mg in 50 ml). U.K.
5.7. Standard solution of cholesta-3,5-diene hexane at concentration of 20 ppm, obtained by dilution of above solution. U.K.
Note 5: U.K.
The solutions 5.6 and 5.7 are stable for a period of at least four months if kept at less than 4 °C. U.K.
5.8. Solution of n-nonacosane in hexane at concentration of approximately 100 ppm. U.K.
5.9. Carrier gas for chromatography: helium or hydrogen of 99,9990 % purity. U.K.
5.10. Auxiliary gases for flame ionization detector: hydrogen of 99,9990 % purity and purified air. U.K.
6. PROCEDURE U.K.
6.1. Preparation of unsaponifiable matter U.K.
6.1.1. Weigh 20 ± 0,1 g of oil into a 250-ml flask (4.1), add 1 ml of the standard solution of cholesta-3,5-diene (20μg) and 75 ml of alcoholic potash at 10 %, fit reflux condenser, and heat to slight boiling for 30 minutes, Remove the flask containing the sample from the heat and allow the solution to cool slightly (do not allow to cool completely as the sample will set). Add 100 ml of water and transfer the solution to a separating funnel (4.2) with the aid of 100 ml of hexane. Shake the mixture vigorously for 30 seconds and allow the separate. U.K.
Note 6: U.K.
If an emulsion is produced which does not rapidly disappear, add small quantities of ethanol. U.K.
6.1.2. Transfer the aqueous phase beneath to a second separating funnel and extract again with 100 ml of hexane. Once more run off the lower phase and wash the hexane extracts (combined in another separating funnel) three times with 100 ml each time of a mixture of ethanol-water (1: 1) until neutral pH is reached. U.K.
6.1.3. Pass the hexane solution through anhydrous sodium sulphate (50 g), wash with 20 ml hexane and evaporate in a rotary evaporator at 30 °C under reduced pressure until dryness. U.K.
6.2. Separation of steroidal hydrocarbon fraction U.K.
6.2.1. Take the residue to the fractioning column with the aid of two 1-ml portions of hexane, run the sample onto the column by allowing the solution level to drop to the top of the sodium sulphate and start the chromatographic elution with hexane at a flow rate of 1 ml/min approximately. Discard the first 25 to 30 ml of eluate and then collect the following 40 ml fraction. After collection, transfer this fraction to a 100-ml round bottomed flask (4.3). U.K.
Note 7: U.K.
The first fraction contains saturated hydrocarbons (Figure 1 a) and the second fraction the steroidal ones. Further elution provides squalene and related compounds. To achieve a good separation between saturated and steroidal hydrocarbons, the optimization of fraction volumes is required. For this, the volume of the first fraction should be adjusted so that when the second fraction is analysed the peaks representing the saturated hydrocarbons are low (see Figure 1 c); if they do not appear but the intensity of the standard peak is low, the volume should be reduced. Anyway, a complete separation between the components of the first and second fractions is unnecessary; as there is no overlapping of peaks during GC analysis if GC conditions are ajusted as indicated in 6.3.1. The optimization of the volume of the second fraction if generally not needed as a good separation exists with the further components. Nevertheless, the presence of a large peak at approximately 1,5 minutes lower retention time than the standard is due to squalene, and it is indicative of a bad separation. U.K.
6.2.2. Evaporate the second fraction in a rotary evaporator at 30 °C under reduced pressure until dryness, and immediately dissolve the residue in 0,2 ml of hexane. Keep the solution in the refrigerator until analysis. U.K.
Note 8: U.K.
Residues 6.1.3 and 6.2.2 should not be kept dry and at room temperature. As soon as they are obtained, the solvent should be added and the solutions should be kept in the refrigerator. U.K.
6.3. Gas chromatography U.K.
6.3.1. Working conditions for split injection: U.K.
injector temperature: 300 °C,
detector temperature: 320 °C,
integrator-recorder: the parameters for integration should be fixed so as to give a correct assessment of the areas. Valley-valley integration mode is recommended,
sensitivity: about 16 times the minimum attenuation,
amount of solution injected: 1μl,
oven programming temperatures: initial 235 °C for six minutes and then rising at 2 °C/minute up to 285 °C,
injector with 1: 15 flow divider,
carrier: helium or hydrogen at about 120 kPa pressure.
These conditions may be adjusted in accordance with the characteristics of the chromatograph and the column to give chromatograms meeting the following requirements: internal standard peak within approximately five minutes of the time given in 6.3.2; the internal standard peak should be at least 80 % of the full scale.
The gas chromatographic system must be checked injecting a mixture of the stock solution of cholestadiene (5.6) and n-nonacosane solution (5.8). The cholesta-3,5-diene peak must appear before the n-nonacosane (Figure 1c); if it does not occur two actions can be undertaken: reduce the oven temperature and/or use a less polar column.
6.3.2. Peak identification U.K.
The internal standard peak appears at approximately 19 minutes and the 3,5-stigmastadiene at a relative retention time of approximately 1,29 (see Figure 1b). The 3,5-stigmastadiene occurs with small quantities of an isomer, and usually, both elute together as a single chromatographic peak. Nevertheless, if the column is too polar or shows a high resolving power, the isomer can appear as a small peak before and close to that of stigmasta-3,5-diene (Figure 2). In order to ensure that the stigmastadienes are eluted as one peak, it is advisable to replace the column by one which is either less polar or has a wider internal diameter.
Note 9: U.K.
Stigmastadienes for reference can be obtained from the analysis of a refined vegetable oil by using less amount of sample (1 to 2 g). Stigmastadienes originate a prominent and easily identifiable peak. U.K.
6.3.3. Quantitative analysis U.K.
The stigmastadienes content is determined according to the formula:
| where: | A s = area of stigmastadienes peak (if the peak is resolved into two isomers, sum of areas of the two peaks), A c = area of internal standard (cholestadiene), M c = mass of standard added, in micrograms, M o = mass of oil taken, in grams. |
Detection limit: about 0,01 mg/kg.
Figure 1 U.K.
Gas chromatograms obtained from olive oil samples analysed on a fused silica capillary column (0,25 mm internal diameter by 25 m) coated with 5 %-phenylmethylsilicone, 0,25 μm film thickness. U.K.
First fraction (30 ml) from a virgin oil, spiked with standard.
Second fraction (40 ml) from an olive oil containing 0,10 mg/kg of stigmastadienes.
Second fraction (40 ml) containing a small proportion of the first fraction.
Gas chromatogram obtained from a refined olive oil sample analysed on DB-5 column showing the isomer of 3,5-stigmastadiene.]
[F14ANNEX XVIII U.K. DETERMINATION OF TRIACYLGLYCEROLS WITH ECN 42 (DIFFERENCE BETWEEN HPLC DATA AND THEORETICAL CONTENT)
Textual Amendments
F14 Inserted by Commission Regulation (EC) No 2472/97 of 11 December 1997 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis and Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
1. Scope U.K.
Determination of the composition of triacylglycerols (TAGs) in olive oils, in terms of their equivalent carbon number by differences between the analytical results obtained by high performance liquid chromatography (HPLC) and the theoretical content, calculated starting from the fatty acid composition.
2. Field application U.K.
The standard is applicable to olive oils. The method is applicable to the detection of the presence of small amounts of seed oils (rich in linoleic acid) in every class of olive oils.
3. Principle U.K.
The content of triacylglycerols with ECN42 determined by HPLC analysis and the theoretical content of triacylglycerols with ECN42 (calculated on the basis of GLC determination of fatty acid composition) correspond within a certain limit for pure oils. A difference larger than the values stated in the Regulation for each type of oil points out that the oil contains seed oils.
4. Method U.K.
The method for calculation of theoretical content of triacylglycerols with ECN42 and of the difference between the HPLC data and this one essentially is made by the coordination of analytical data obtained by means of other methods: it is possible to distinguish three phases: determination of fatty acid composition by capillary gas chromatography, calculation of theoretical composition of triacylglycerols with ECN42, HPLC determination of ECN42 triacylglycerols
4.1. Apparatus U.K.
4.1.1. Round bottom flasks, 250 and 500 ml. U.K.
4.1.2. Beakers 100 ml. U.K.
4.1.3. Glass chromatographic column, 21 mm internal diameter, 450 mm length, with cock and normalized cone (female) at the top. U.K.
4.1.4. Separator funnels, 250 ml, with normalized cone (male) at the bottom, suitable to be connected with the top of the column. U.K.
4.1.5. Glass rod, 600 mm length. U.K.
4.1.6. Glass funnel, 80 mm diameter. U.K.
4.1.7. Volumetric flasks, 50 ml. U.K.
4.1.8. Volumetic flasks, 20 ml. U.K.
4.1.9. Rotative evaporator. U.K.
4.1.10. High performance liquid chromatography, allowing thermostatic control of column temperature. U.K.
4.1.11. Injection units for 10 μl delivery. U.K.
4.1.12. Detector: differential refractometer. The full scale sensitivity should be at least 10 -4 units of refractive index. U.K.
4.1.13. Column: stainless steel tube 250 mm length and 4,5 mm internal diameter packed with 5 μm diameter particles of slica with 22 to 23 % carbon in the form of octadecylsilane (note 2). U.K.
4.1.14. Recorder and/or integrator. U.K.
4.2. Reagents U.K.
The reagents should be of analytical purity. Elution solvents should be de-gassed, and may be recycled several times without effect on the separations.
4.2.1. Petroleum ether 40 to 60 °C chromatographic grade. U.K.
4.2.2. Ethil ether, peroxides free, freshly distilled. U.K.
4.2.3. Glass chromatographic elution solvent: mixture petroleum ether/ethil ether 87/13 (v/v). U.K.
4.2.4. Silicagel, 70-230 mesh, type Merck 7734, with water content standardized at 5 % (w/w). U.K.
4.2.5. Glass wool. U.K.
4.2.6. Acetone. U.K.
4.2.7. Acetonitrile. U.K.
4.2.8. HPLC elution solvent: acetonitrile + acetone (proportions to be adjusted to obtain the desired separation; begin with 50:50 mixture). U.K.
4.2.9. Solubilization solvent: acetone. U.K.
4.2.10. Reference triglycerides commercial triglycerides tripalmitin, triolein, etc.) may be used and the retention times thence plotted in accordance with the equivalent carbon number, or alternatively reference chromatograms obtained from soya oil, mixture 30:70 soya oil/olive oil and pure olive oil (see notes 3 and 4 and figure 1, 2, 3, 4). U.K.
4.3. Sample preparation U.K.
As a number of interfering substances can rise false positive results, the sample must always be purified according to IUPAC method 2.507, used for determination of polar substances in oxidised oils.
4.3.1. Chromatographic column preparation U.K.
Fill the column (4.1.3) with about 30 ml of elution solvent (4.2.3), then introduce inside the column some glass wool (4.2.5) pushing it to the bottom of the column by means of the glass rod (4.1.5).
In a 100 ml beaker, suspend 25 g of silicagel (4.2.4) in 80 ml of elution mixture (4.2.3), then transfer it inside the column, by means of a glass funnel (4.1.6).
To ensure the complete transfer of silicagel inside the column, wash the beaker with the elution mixture and transfer the washing portions inside the column, too.
Open the cock and let solvent elute from the column until its level is about 1 cm over the silicagel.
4.3.2. Column chromatography U.K.
Weigh with the accuracy of 0,001 g, 2,5 ± 0,1 g of oil, previously filtered, homogenized and anhydrified, if necessary, in a 50 ml volumetric flask (4.1.7). Solve it in about 20 ml of elution solvent (4.2.3), if necessary, slightly heat it to make the dissolution easily. Cool at room temperature and adjust the volume with elution solvent.
By means of a volumetric pipette, introduce 20 ml of solution inside the column prepared according to 4.3.1, open the cock and let solvent elute to the silicagel layer level.
Then elute with 150 ml of elution solvent (4.2.3), adjusting the solvent rate at about 2 ml/min (150 ml will take about 60 to 70 minutes to pass through the column).
The eluated is recovered in a 250 ml round bottom flask (4.1.1) previously tared in an oven and exactly weighted. Eliminate solvent at reduce pressure (Rotavapor) and weigh the residue that will be used to prepare the solution for HPLC analysis and for methyl ester preparation.
The sample recovery from the column must be 90 % at least for extra virgin, virgin, ordinary refined and olive oil categories, and a minimum of 80 % for lampante and residue olive oils.
4.4. HPLC analysis U.K.
4.4.1. Preparation of the samples for chromatographic analysis U.K.
A 5 % solution of the sample to be analysed is prepared by weighing 0,5 ± 0,001 g of the sample into a 10 ml graduated flask and making up to 10 ml with the solubilization solvent (4.2.9).
4.4.2. Procedure U.K.
Set up the chromatographic system. Pump elution solvent (4.2.8) at a rate of 1,5 ml/min to purge the entire system. Wait until a stable base line is obtained. Inject 10 μl of the sample prepared as in 4.3.
4.4.3. Calculation and expression of results U.K.
Use the area normalization method, i.e. assume that the sum of the areas of the peaks corresponding to TAGs from ECN42 up to ECN52 is equal to 100 %. Calculate the relative percentage of each triglyceride using the formula:
% triglyceride = area of peak × 100 / sum of peak areas.
The results are to be given to within at least two decimal places.
Note 1: U.K.
The elution order can be determined by calculating the equivalent carbon numbers, often defined by the relation ECN = CN-2n, where CN is the carbon number and n is the number of double bounds, it can be calculated more precisely by taking into account the origin of the double bond. If n o , n l and n ln are the numbers of double bonds attributed to oleic, linoleic and linolenic acids respectively, the equivalent carbon number can be calculated by means of the relation of the formula: U.K.
ECN = CN - d o n o - d l n l - d ln n ln ,
where the coefficient do, d l and d ln can be calculated by means of the reference triglycerides. Under the conditions specified in this method, the relation obtained will be close in:
ECN = CN - (2,60 n o ) - (2,35 n l ) - (2,17 n ln )
Note 2: U.K.
| Examples: | Lichrosorb (Merck) RP 18 Art 50333 |
| Lichrosphere or equivalent (Merck) 100 CH18 Art 50377. |
Note 3: U.K.
With several reference triglycerides, it is also possible to calculate the resolution with respect to triolein: U.K.
α = RT' / RT triolein
by use of the reduced retention time RT' = RT - RT solvent.
The graph of log α against f (number of double bonds) enables the retention values to be determined for all the triglycerides of fatty acids contained in the reference triglycerides — see figure 2.
Note 4: U.K.
The efficiency of the column should permit clear separation of the peak of trilinoein from the peaks of the triglycerides with an adjacent RT. The elution is carried out up to ECN52 peak. U.K.
Note 5: U.K.
A correct measure of the areas of all peaks of interest for the present determination is ensured if the second peak corresponding to ECN50 is 50 % of full scale of the recorder. U.K.
4.5. Calculation of triacylglycerols composition U.K.
4.5.1. Determination of fatty acid composition U.K.
Fatty acid composition is carried out by means of the EEC gas chromatographic method reported in Annex X A of Regulation (EEC) No 2568/91, by means of a capillary column. The methyl esters preparation is carried out according to Annex X B (sodium methylate alcohol solution).
4.5.2. Fatty acids for calculation U.K.
Glycerides are grouped by their equivalent carbon number (ECN), taking into account the following equivalencies between ECN and fatty acids. Only fatty acids with 16 and 18 carbon atoms were taken in consideration, because only these are important for olive oil.
| Fatty acid (FA) | Abbreviation | Molecular weight (MW) | ECN |
|---|---|---|---|
| Palmatic acid | P | 256,4 | 16 |
| Palmatoleic acid | Po | 254,4 | 14 |
| Stearic acid | S | 284,5 | 18 |
| Oleic acid | O | 282,5 | 16 |
| Linoleic acid | L | 280,4 | 14 |
| Linolenic acid | Ln | 278,4 | 12 |
4.5.3. Conversion of area % into moles for all fatty acids U.K.
4.5.4. Normalization of fatty acids to 100 % U.K.
The result gives the percentage of each fatty acid in moles % in the overall (1,2,3-) position of the TAGs.
Then the sum of the saturated fatty acids P and S (SFA) and the unsaturated fatty acids Po, O, L and Ln (UFA) are calculated:
| moles % SFA = moles % P + moles % S | (3) |
| moles UFA = 100 - moles % SFA |
4.5.5. Calculation of the fatty acid composition in 2- and 1,3- positions of TAGs U.K.
The fatty acids are distributed to three pools as follows: two identical for 1- and 3- positions and one for 2- position, with different coefficients for the saturated (P and S) and unsaturated acids (Po, O, L and Ln).
4.5.5.1. Saturated fatty acids in 2- position [P(2) and S(2)] U.K.
| moles % P(2) = moles % P (1,2,3) * 0,06 | (4) |
| moles % S(2) = moles % S (1,2,3) * 0,06 |
4.5.5.2. Unsaturated fatty acids in 2- position [Po(2), O(2), L(2) and Ln(2)]: U.K.
4.5.5.3. Fatty acids in 1,3-positions [P(1,3), S(1,3), Po(1,3) O(1,3), L(1,3) and Ln(1,3)]: U.K.
4.5.6. Calculation of triacylglycerols U.K.
4.5.6.1. TAGs with one fatty acid (AAA, here LLL, PoPoPo) U.K.
4.5.6.2. TAGs with two fatty acids (AAB, here PoPoL, PoLL) U.K.
4.5.6.3. TAGs with three different fatty acids (ABC, here OLLn, PLLn, PoOLn, PPoLn) U.K.
4.5.6.4. Triacylglycerides with ECN42 U.K.
The following triglycerides with ECN42 are calculated according equation 7, 8 and 9 in order of expected elution in HPLC (normally only three peaks).
LLL
PoLL and the positional isomer LPoL
OLLn and the positional isomers OLnL and LnOL
PoPoL and the positional isomer PoLPo
PoOLn and the positional isomers OPoLn and OLnPo
PLLn and the positional isomers LLnP and LnPL
PoPoPo
SLnLn and the positional isomer LnSLn
PPoLn and the positional isomers PLnPo and PoPLn
The triacylglycerides with ECN42 are given by the sum of the nine triacylglycerols including their positional isomers. The results to be given with at least two decimal places.
5. Evaluation of the results U.K.
The calculated theoretical content and the content determined by the HPLC analysis are compared. If the difference between HLPC data minus theoretical data is greater than the values states for the appropriate oil category in the Regulation, the sample contains seed oil.
Note: U.K.
Results are given to within one decimal figure. U.K.
6. Example (The numbers refer to the sections in the text of the method) U.K.
4.5.1. Calculation of moles % fatty acids from GLC data (area %) U.K.
The following data are obtained for the fatty acid composition by GLC:
| FA MW | P 256,4 | S 284,5 | Po 254,4 | O 282,5 | L 280,4 | Ln 278,4 |
|---|---|---|---|---|---|---|
| area % | 10,0 | 3,0 | 1,0 | 75,0 | 10,0 | 1,0 |
4.5.3. Conversion of area % into moles for all fatty acids U.K.
4.5.4. Normalization of fatty acids to 100 % U.K.
Sum of the saturated and unsaturated fatty acids in the 1,2,3- position of TAGs
| moles % SFA = 10,888 % + 2,944 % = 13,831 % | See formula (3) |
| moles % UFA = 100,000 % — 13,831 % = 86,169 % | See formula (3) |
4.5.5. Calculation of the fatty acid composition in 2- and 1,3- positions of the TAGs U.K.
4.5.5.1. Saturated fatty acids in 2- position [P(2) and S(2)] U.K.
| moles % P(2) = 10,888 % * 0,06 = 0,653 moles % | See formula (4) |
| moles % S(2) = 2,944 % * 0,06 = 0,177 moles % | See formula (4) |
4.5.5.2. Unsaturated fatty acids in 1,3-position [Po(1,3), O(1,3), L(1,3) and Ln(1,3)] U.K.
4.5.5.3. Fatty acids in 1,3-positions [P(1,3), S(1,3), Po(1,3), O(1,3), L(1,3) and Ln(1,3)] U.K.
4.5.6. Calculation of triacylglycerols U.K.
From the calculated fatty acid composition in sn-2- and sn-1,3- positions (see above):
| FA in | 1,3-pos. | 2-pos. |
|---|---|---|
| P | 16,005 % | 0,653 % |
| S | 4,327 % | 0,177 % |
| Po | 1,015 % | 1,263 % |
| O | 68,522 % | 85,295 % |
| L | 9,205 % | 11,458 % |
| Ln | 0,927 % | 1,154 % |
| Sum | 100,0 % | 100,0 % |
the following triacylglycerols are calculated:
LLL
PoPoPo
PoLL with 1 positional isomer
SLnLn with 1 positional isomer
PoPoL with 1 positional isomer
PPoLn with 2 positional isomers
OLLn with 2 positional isomers
PLLn with 2 positional isomers
PoOLn with 2 positional isomers.
4.5.6.1. TAGs with one fatty acid (LLL, PoPoPo) See formula (7) U.K.
4.5.6.2. TAGs with two fatty acids (PoLL, SLnLn, PoPoL) See formula (8) U.K.
4.5.6.3. TAGs with three different fatty acids (PoPLn, OLLn, PLLn, PoOLn) See formula (9) U.K.
[F15Note: U.K.
La = lauric acid; My = myristic acid; P = palmitic acid; St = stearic acid; O = oleic acid; L = linoleic acid; Ln = linolenic acid.] ] U.K.
Textual Amendments
[F16ANNEX XIX U.K. DETERMINATION OF ALIPHATIC ALCOHOLS CONTENT BY CAPILLARY GAS CHROMATOGRAPHY
Textual Amendments
F16 Inserted by Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
1. OBJECT U.K.
The procedure describes a method for the determination of aliphatic alcohols content in oils and fats.
2. PRINCIPLE OF THE METHOD U.K.
The fatty substance, with 1-eicosanol added as internal standard, is saponified with ethanolic potassium hydroxide and then the unsaponifiable matter extracted with ethyl ether. The alcoholic fraction is separated from the unsaponifiable matter by chromatography on a basic silica gel plate; the alcohols recovered from the silica gel are transformed into trimethylsilyl ethers and analysed by capillary gas chromatography.
3. EQUIPMENT U.K.
3.1. 250 ml round-bottomed flask fitted with a reflux condenser having ground-glass joints. U.K.
3.2. 500 ml separating funnel. U.K.
3.3. 250 ml round-bottomed flasks. U.K.
3.4. Chromatographic tank for thin-layer chromatographic analysis, for glass plates of dimensions 20 x 20 cm. U.K.
3.5. Ultraviolet lamp having a wavelength of 366 or 254 nm. U.K.
3.6. 100 μl and 500 μl microsyringes. U.K.
3.7. A cylindrical filter funnel with a G3 porous septum (porosity 15 to 40 μm) of diameter approximately 2 cm and a depth of some 5 cm, with an attachment suitable for filtration under vacuum and a 12/21 male ground glass joint. U.K.
3.8. 50 ml vacuum conical flask with a 12/21 ground-glass female joint which can be fitted to the filter funnel (3.7). U.K.
3.9. A 10 ml test tube with a tapering bottom and a sealing stopper. U.K.
3.10. Gas chromatograph for use with a capillary column, and provided with a splitting system composed of: U.K.
Thermostatic chamber for columns (column oven) to hold the temperature desired with a precision of ± 1 °C.
A temperature-adjustable injection unit with a persilanised glass vaporising element.
A flame ionisation detector and converter-amplifier.
Recorder-integrator for operation with the converter-amplifier (3.10.3), with response time not exceeding one second and with variable paper-speed.
3.11. Glass or fused silica capillary column, of length 20 to 30 m, internal diameter 0,25 to 0,32 mm, with SE-52 or SE-54 liquid phase or equivalent, with a film thickness between 0,10 and 0,30 μm. U.K.
3.12. Microsyringe for gas chromatography, of 10 μl capacity with hardened needle. U.K.
3.13. Analytical balance sensitive to 1 mg (with 0,1 mg display). U.K.
4. REAGENTS U.K.
4.1. Potassium hydroxide, approximately 2 N ethanolic solution: 130 g potassium hydroxide (minimum concentration 85 %) is dissolved, with cooling, in 200 ml distilled water and then made up to one litre with ethanol. The solution should be stored in a well-stoppered opaque glass bottle. U.K.
4.2. Ethyl ether, pure for analysis. U.K.
4.3. Anhydrous sodium sulphate, analytical purity. U.K.
4.4. Glass plates coated with silica gel, without fluorescence indicator, thickness 0,25 mm (commercially available ready for use). U.K.
4.5. Potassium hydroxide, approximately 0,2 N ethanolic solution; 13 g of potassium hydroxide are dissolved in 20 ml of distilled water and made up to one litre with ethanol. U.K.
4.6. Benzene, for chromatography (see 5.2.2). U.K.
4.7. Acetone, for chromatography (See 5.2.2). U.K.
4.8. Hexane, for chromatography (see 5.2.2). U.K.
4.9. Ethyl ether, for chromatography (see 5.2.2). U.K.
4.10. Chloroform, for chromatography. U.K.
4.11. Reference solution for thin-layer chromatography: cholesterol or phytosterols, 5 % solution in chloroform. U.K.
4.12. 0,2 % solution of 2', 7'-dichlorofluorescein in ethanol. Make slightly basic by adding a few drops of 2 N alcoholic potassium hydroxide solution. U.K.
4.13. Anhydrous pyridine, for chromatography. U.K.
4.14. Hexamethyl disilazane. U.K.
4.15. Trimethylchlorosilane. U.K.
4.16. Standard solutions of trimethylsilyl ethers of aliphatic alcohols from C 20 to C 28 . They may be prepared from mixtures of pure alcohols at the time they are required for use. U.K.
4.17. A 0,1 % (m/v) solution of 1-eicosanol in chloroform (internal standard). U.K.
4.18. Carrier gas: hydrogen or helium, gas-chromatographic purity. U.K.
4.19. Auxiliary gas: nitrogen, gas-chromatographic purity. U.K.
5. PROCEDURE U.K.
5.1. Preparation of the unsaponifiables U.K.
5.1.1. Using a 500 μl microsyringe place, into a 250 ml round-bottom flask, a volume of 0,1 % 1-eicosanol solution in chloroform (4.17) containing a quantity of 1-eicosanol approximately equal to 10 % of the aliphatic alcohols content in that portion of sample to be taken for analysis. For example, to 5 g of sample add 250 μl of the 0,1 % 1-eicosanol solution if olive oil and 1 500 μl if olive pomace oil. U.K.
Evaporate to dryness in current of nitrogen and then weigh accurately 5 g of the dry filtered sample into the same flask.
5.1.2. Add 50 ml of 2 N potassium hydroxide ethanolic solution, fit the reflux condenser and heat the apparatus to slight boiling on a steam bath, stirring continuously throughout the heating process until saponification has taken place (the solution becomes clear). Continue heating for a further 20 minutes and then add 50 ml of distilled water through the condenser. The condenser is then disconnected and the flask cooled to approximately 30 °C. U.K.
5.1.3. The contents of the flask are quantitatively transferred to a separating funnel of 500 ml capacity by adding distilled water several times, using a total of around 50 ml distilled water. Add approximately 80 ml of ethyl ether, shake vigorously for approximately 30 seconds and allow to settle (Note 1). U.K.
Separate off the lower aqueous phase collecting it in a second separating funnel. Two further extractions are effected on the aqueous phase, in the same manner, using each time 60 to 70 ml ethyl ether.
Note 1: U.K.
Emulsions may be eliminated by adding, using as a spray, small quantities of ethyl alcohol or methyl alcohol. U.K.
5.1.4. The ethyl ether extracts are combined in a separating funnel and washed with distilled water (50 ml at a time) until the washing water gives a neutral reaction. U.K.
Discard the washing water, dry with anhydrous sodium sulphate and filter, into a flask of 250 ml capacity which has been weighed beforehand, the funnel and filter being washed with small quantities of ethyl ether which are added to the total.
5.1.5. Distil the ether down to a few ml, then bring to dryness under a slight vacuum or in a current of nitrogen, completing drying in an oven at 100 °C for approximately a quarter of an hour, and then weigh after cooling in a desiccator. U.K.
5.2. Separation of alcoholic fractions U.K.
5.2.1. Preparation of basic TLC plates: the silica gel plates (4.4) are immersed completely, in 0,2 N potassium hydroxide solution (4.5) for 10 seconds, and then left to dry for two hours under an extractor hood and finally placed in an oven at 100 °C for one hour. U.K.
Remove from the oven and keep in a calcium chloride desiccator until required for use (plates treated in this way must be used within 15 days).
Note 2: U.K.
When basic silica gel plates are used to separate the alcoholic fraction there is no need to treat the unsaponifiables with alumina. It follows that all acid compounds (fatty acids and others) are retained at the origin thereby obtaining both aliphatic alcohol and terpenic alcohol bands which are both separated distinctly from the sterol band. U.K.
5.2.2. Place a 65/35 by volume hexane/ethyl ether mixture in the plate-developing chamber to a depth of approximately 1 cm (3) . U.K.
Close the chamber using an appropriate cover and leave for half an hour to allow equilibration between vapour and liquid. Strips of filter paper dipping into the eluent may be affixed to the inside surfaces of the tank to reduce the development time by approximately one third and obtain more uniform, regular elution of the components.
Note 3: U.K.
The developing solution must be replaced for each analysis in order to obtain reproducible developing conditions. U.K.
5.2.3. An approximately 5 % solution of unsaponifiable matter (5.1.5) in chloroform is prepared and 0,3 ml of the solution is streaked as a uniform strip of minimum thickness, using the 100 μl microsyringe, on a TLC plate at approximately 2 cm from the bottom of the TLC plate. Aligned with the origin, 2 to 3 μl of the aliphatic alcohols reference solution (4.11) are spotted for the identification of the aliphatic alcohols band after development has been completed. U.K.
5.2.4. Place the plate inside the development tank as stated in 5.2.2. The ambient temperature should be maintained between 15 and 20 °C. Immediately close the chamber with the cover and allow to elute until the solvent front reaches approximately 1 cm from the upper edge of the plate. U.K.
The plate is then removed from the development chamber and the solvent evaporated under a hot air current or the plate is left for a while under the extractor hood.
5.2.5. The plate is sprayed lightly and evenly with the solution of 2', 7'-dichlorofluorescein when the plate is observed under ultra violet light. The aliphatic alcohols band can be identified through being aligned with the stain obtained from the reference solution: mark the limits of the band with a black pencil; outlining the band of aliphatic alcohols and the band immediately above that, which is the terpenic alcohols band, together. U.K.
Note 4: U.K.
The aliphatic alcohols band and the terpenic alcohols band are to be grouped together in view of the possible migration of some aliphatic alcohols into the triterpenic alcohols band. U.K.
5.2.6. Using a metal spatula scrape off the silica gel in the marked area. Place the finely comminuted material removed into the filter funnel (3.7). Add 10 ml of hot chloroform, mix carefully with the metal spatula and filter under vacuum, collecting the filtrate in the conical flask (3.8) attached to the filter funnel. U.K.
Wash the pomace in the flask three times with ethyl ether (approximately 10 ml each time) collecting the filtrate in the same flask attached to the funnel. Evaporate the filtrate to a volume of 4 to 5 ml, transfer the residual solution to the previously weighed 10 ml test tube (3.9), evaporate to dryness by mild heating in a gentle flow of nitrogen, make up again using a few drops of acetone, evaporate again to dryness, place in an oven at 105 °C for approximately 10 minutes and then allow to cool in a desiccator and weigh.
The pomace inside the test tube is composed of the alcoholic fraction.
5.3. Preparation of the trimethylsilyl ethers U.K.
5.3.1. The reagent for silylation, consisting of a mixture of 9:3:1 by volume (Note 5) of pyridine-hexamethyldisilazane-trimethylchlorosilane in the proportion of 50 μl for each milligram of aliphatic alcohols, is added to the test tube containing the alcoholic fraction, avoiding all absorption of moisture (Note 6). U.K.
Note 5: U.K.
Solutions which are ready for use are available commercially. Other silanising reagents such as, for example, bis-trimethylsilyl, trifluor acetamide + 1 % trimethyl chlorosilane, which has to be diluted with an equal volume of anhydrous pyridine, are also available. U.K.
Note 6: U.K.
The slight opalescence which may form is normal and does not cause any interference. The formation of a white floc or the appearance of a pink colour are indicative of the presence of moisture or deterioration of the reagent. If these occur the test must be repeated. U.K.
5.3.2. Stopper the test tube, shake carefully (without overturning) until the aliphatic alcohols are completely dissolved. Stand for at least 15 minutes at ambient temperature and then centrifuge for a few minutes. The clear solution is ready for gas chromatographic analysis. U.K.
5.4. Gas chromatography analysis U.K.
5.4.1. Preliminary operations, column packing U.K.
5.4.1.1. Fit the column in the gas chromatograph, attaching the inlet end to the injector connected to the splitting system and the outlet end to the detector. Carry out a general check of the gas chromatography assembly (tightness of gas fittings, efficiency of the detector, efficiency of the splitting system and of the recording system, etc.). U.K.
5.4.1.2. If the column is being used for the first time it is recommended that it should be subjected to conditioning. A little carrier gas is passed through the capillary column and then the gas chromatography assembly is switched on and gradually heated until a temperature not less than 20 °C above the operating temperature (see Note 7) is attained. That temperature is held for not less than two hours and then the assembly is brought to the operating conditions (regulation of gas flow, split flame ignition, connection to the electronic recorder, adjustment of the temperature of the capillary column oven, the detector and the injector, etc.) and the signal is adjusted to a sensitivity not less than twice the highest level contemplated for the execution of the analysis. The course of the base line must be linear, without peaks of any kind, and must not drift. A negative straight-line drift indicates leakage from the column connections; a positive drift indicates inadequate conditioning of the column. U.K.
Note 7: U.K.
The conditioning temperature shall be at least 20 °C less than the maximum temperature contemplated for the liquid phase employed. U.K.
5.4.2. Choice of operating conditions U.K.
5.4.2.1. The guideline operating conditions are as follows: U.K.
column temperature: the initial isotherm is set at 180 °C for eight minutes and then programmed at 5 °C/minute to 260 °C and a further 15 minutes at 260 °C,
temperature of evaporator: 280 °C,
temperature of detector: 290 °C,
linear velocity of carrier gas: helium 20 to 35 cm/s, hydrogen 30 to 50 cm/s,
splitting ratio: 1:50 to 1:100,
sensitivity of instrument: 4 to 16 times the minimum attenuation,
sensitivity of recording: 1 to 2 mV fs,
paper speed: 30 to 60 cm/h,
quantity of substance injected: 0,5 to 1 μl of TMSE solution.
The above conditions may be modified according to the characteristics of the column and of the gas chromatograph to obtain chromatograms satisfying the following conditions:
alcohol C 26 retention time shall be 18 ± 5 minutes,
the alcohol C 22 peak shall be 80 ± 20 % of the full scale value for olive oil and 40 ± 20 % of the full scale value for seed oil.
5.4.2.2. The above requirements are checked by repeated injection of the standard TMSE mixture of alcohols and the operating conditions are adjusted to yield the best possible results. U.K.
5.4.2.3. The parameters for the integration of peaks shall be set so that a correct appraisal of the areas of the peaks considered is obtained. U.K.
5.4.3. Analytical procedure U.K.
5.4.3.1. Using the microsyringe of 10 μl capacity draw in 1 μl of hexane followed by 0,5 μl of air and subsequently 0,5 to 1 μl of the sample solution; raise the plunger of the syringe further so the needle is emptied. Push the needle through the membrane of the injection unit and after one to two seconds inject rapidly, then slowly remove the needle after some five seconds. U.K.
5.4.3.2. Recording is effected until the TMSE of the aliphatic alcohols present have been eluted completely. The base line shall always correspond to the requirements of 5.4.1.2. U.K.
5.4.4. Peak identification U.K.
The identification of individual peaks is effected according to the retention times and by comparison with the standard TMSE mixture, analysed under the same conditions.
A chromatogram of the alcoholic fraction of a virgin olive oil is shown in Figure 1.
5.4.5. Quantitative evaluation U.K.
5.4.5.1. The peak areas of 1-eicosanol and of the aliphatic alcohols C 22 , C 24 , C 26 and C 28 are calculated by electronic integration. U.K.
5.4.5.2. The contents of each aliphatic alcohol, expressed in mg/ U.K.
1 000
g fatty substance, are calculated as follows:
where:
=
area of the alcohol peak x
=
area of 1-eicosanol
=
mass of 1-eicosanol in milligrams
=
mass of sample drawn for determination, in grams.
6. EXPRESSION OF THE RESULTS U.K.
The contents of the individual aliphatic alcohols in mg/ 1 000 g of fatty substance and the sum of the ‘ total aliphatic alcohols ’ are reported.
APPENDIX Determination of the linear velocity of the gas
1 to 3 μl of methane or propane are injected into the gas chromatograph set at normal operating conditions and the time taken for the methane or propane to flow through the column from the instant of injection to the instant the peak elutes (tM) is measured using a stop clock.
The linear velocity in cm/s is given by L/tM, where L is the length of the column in centimetres and tM is the measured time in seconds.
=
Eicosanol
=
Decosanol
=
Tricosanol
=
Tetracosanol
=
Pentacosanol
=
Hexacosanol
=
Heptacosanol
=
Octacosanol]
[F1After elution of the sterol esters the chromatogram trace must not show any significant peaks (triglycerides).]
[F3Tasters may refrain from tasting if they note some extremely intense negative attribute when smelling the oil, in which case they must note this exceptional circumstance on the profile sheet.]
[F16In these cases in particular, a 95/5 by volume benzene/acetone eluent mixture must be used to obtain distinct band separation.]
Textual Amendments
F1 Substituted by Commission Regulation (EC) No 702/2007 of 21 June 2007 amending Commission Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis.
F3 Substituted by Commission Regulation (EC) No 640/2008 of 4 July 2008 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis.
F16 Inserted by Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff.
Options/Help
Print Options
PrintThe Whole Regulation
PrintThe Annexes only
You have chosen to open the Whole Regulation
The Whole Regulation you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
You have chosen to open Schedules only
Y Rhestrau you have selected contains over 200 provisions and might take some time to download. You may also experience some issues with your browser, such as an alert box that a script is taking a long time to run.
Would you like to continue?
Mae deddfwriaeth ar gael mewn fersiynau gwahanol:
Y Diweddaraf sydd Ar Gael (diwygiedig):Y fersiwn ddiweddaraf sydd ar gael o’r ddeddfwriaeth yn cynnwys newidiadau a wnaed gan ddeddfwriaeth ddilynol ac wedi eu gweithredu gan ein tîm golygyddol. Gellir gweld y newidiadau nad ydym wedi eu gweithredu i’r testun eto yn yr ardal ‘Newidiadau i Ddeddfwriaeth’.
Gwreiddiol (Fel y’i mabwysiadwyd gan yr UE): Mae'r wreiddiol version of the legislation as it stood when it was first adopted in the EU. No changes have been applied to the text.
Pwynt Penodol mewn Amser: This becomes available after navigating to view revised legislation as it stood at a certain point in time via Advanced Features > Show Timeline of Changes or via a point in time advanced search.
Gweler y wybodaeth ychwanegol ochr yn ochr â’r cynnwys
Rhychwant ddaearyddol: Indicates the geographical area that this provision applies to. For further information see ‘Frequently Asked Questions’.
Dangos Llinell Amser Newidiadau: See how this legislation has or could change over time. Turning this feature on will show extra navigation options to go to these specific points in time. Return to the latest available version by using the controls above in the What Version box.
Rhagor o Adnoddau
Gallwch wneud defnydd o ddogfennau atodol hanfodol a gwybodaeth ar gyfer yr eitem ddeddfwriaeth o’r tab hwn. Yn ddibynnol ar yr eitem ddeddfwriaeth sydd i’w gweld, gallai hyn gynnwys:
- y PDF print gwreiddiol y fel adopted version that was used for the EU Official Journal
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- pob fformat o’r holl ddogfennau cysylltiedig
- slipiau cywiro
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill
Llinell Amser Newidiadau
Mae’r llinell amser yma yn dangos y fersiynau gwahanol a gymerwyd o EUR-Lex yn ogystal ag unrhyw fersiynau dilynol a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig.
Cymerir dyddiadau fersiynau’r UE o ddyddiadau’r dogfennau ar EUR-Lex ac efallai na fyddant yn cyfateb â’r adeg pan ddaeth y newidiadau i rym ar gyfer y ddogfen.
Ar gyfer unrhyw fersiynau a grëwyd ar ôl y diwrnod ymadael o ganlyniad i newidiadau a wnaed gan ddeddfwriaeth y Deyrnas Unedig, bydd y dyddiad yn cyd-fynd â’r dyddiad cynharaf y daeth y newid (e.e. ychwanegiad, diddymiad neu gyfnewidiad) a weithredwyd i rym. Am ragor o wybodaeth gweler ein canllaw i ddeddfwriaeth ddiwygiedig ar Ddeall Deddfwriaeth.
Rhagor o Adnoddau
Defnyddiwch y ddewislen hon i agor dogfennau hanfodol sy’n cyd-fynd â’r ddeddfwriaeth a gwybodaeth am yr eitem hon o ddeddfwriaeth. Gan ddibynnu ar yr eitem o ddeddfwriaeth sy’n cael ei gweld gall hyn gynnwys:
- y PDF print gwreiddiol y fel adopted fersiwn a ddefnyddiwyd am y copi print
- slipiau cywiro
liciwch ‘Gweld Mwy’ neu ddewis ‘Rhagor o Adnoddau’ am wybodaeth ychwanegol gan gynnwys
- rhestr o newidiadau a wnaed gan a/neu yn effeithio ar yr eitem hon o ddeddfwriaeth
- manylion rhoi grym a newid cyffredinol
- pob fformat o’r holl ddogfennau cysylltiedig
- dolenni i ddeddfwriaeth gysylltiedig ac adnoddau gwybodaeth eraill